Abstract
In recent years, ion-selective electrodes based on polymer membranes have been shown to exhibit detection limits that are often in the nanomolar concentration range, and thus drastically lower than traditionally accepted. Since potentiometry is less dependent on scaling laws that other established analytical techniques, their performance in confined sample volumes is explored here. Solid-contact silver-selective microelectrodes, with a sodium-selective microelectrode as a reference, were inserted into a micropipette tip used as a 50-μl sample. The observed potential stabilities, reproducibilities and detection limits were attractive and largely matched that for large 100-ml samples. This should pave the way for further experiments to detecting ultra-small total ion concentrations by potentiometry, especially when used as a transducer after an amplification step in bioanalysis.
Introduction
Miniaturized analytical systems and the quest to detect ever smaller quantities of analytes are becoming increasingly important. Miniaturized sensors have been successfully explored in chemical analysis, including in vivo applications as well as online monitoring bioactive substances [1–4]. Also due to their compact size, high sensitivity and comparably low cost, microsensors are an attractive system for small volume applications to detect ultra-small amounts of compounds.
Potentiometry with ion-selective microelectrodes is a very promising method for trace level analysis in confined samples. The direct relationship in potentiometry between analyte activity and observed potential suggests that no scaling laws exist, that is, there is no expected deterioration of the signal or detection limit as the sample volume is reduced. This is a rather unique expected result, and one that has the promise of elevating potentiometry to a preferred method when dealing with ultra-miniaturized systems.
Potentiometric microelectrodes have been known for a long time. Miniaturization of conventional liquid-based membrane electrodes involves silanized pulled glass microcapillaries that are filled with the membrane cocktail and inner solution using techniques described elsewhere [5, 6]. Microelectrodes made from silanized glass microcapillaries have been utilized for the determination of many types of ions, but lowering their detection limit to the submicromolar concentration range has only recently been attempted [7]. As shown earlier with electrodes of larger size [8], one of the most common sources of the lower detection limit formation comes from the leaching of primary ions from the inner filling solution since the inner side contains a rather concentrated solution of the primary ion. Such fluxes can be reduced if primary ions in the inner filling solution are buffered with certain ligands, and thus, their concentration remains constant [9–12]. However, for each individual experiment one must find an appropriate composition of inner filling solution, which can be tedious and inconvenient. Elimination of the fluxes can also be performed by blocking the outer surface of the membrane [13] or by using capillaries of low porosity [14]. It was also shown that a lower detection limit could be achieved by variation of the membrane composition, the thickness of diffusion layers in both aqueous and organic phases and by altering diffusion coefficients in the organic phase. Very recently, optimized liquid contact microelectrodes with detection limits in the nanomolar concentration range were successfully used to distinguish ultra-trace levels (100 picomolar concentrations) in 3-μL volumes from the background electrolyte, demonstrating the detectability of 300 attomoles of material [15].
Difficulties arising from undesired ion fluxes may also be avoided by using membrane electrodes with a solid inner contact. This design of electrodes is attractive because here is no need for any optimization of the inner filling solution compositon, and the possibility of working without any liquid components. At first, attractive lower detection limits with such types of electrodes were not achieved, mainly because of the interference of oxygen [16, 17] as well as the presence of an internal water film at the boundary between solid contact and membrane [18, 19].
The problem of potential instability has been addressed by the use of conducting polymers as intermediate layer that makes the electron-to-ion transfer better defined. Different types of conducting polymers have been explored, such as poly(pyrrole) [20–22], polyaniline [23], polyalkylthiophene [17, 24–26], and poly(3,4-ethylenedioxythiophene) [27, 28]. The elimination of the water layer can also be achieved with the right choice of membrane matrix. It has been shown that the copolymer methylmethacrylate–decylmethacrylate (MMA-DMA) as a matrix for the membrane provided nanomolar and subnanomolar detection limits without apparent water layer formation [17]. Recently, a recipe for making solid contact electrodes has been described by illustrating a simple procedure for the fabrication of solid-contact ion-selective electrodes with low detection limits [26].
The development of solid-contact microelectrodes with low detection limit gives the possibility not only to create robust, easy to handle systems but also to reduce the working volume and apply highly sensitive microelectrodes for the detection of very small absolute amounts of substances. Attempts to make solid-contact ion-selective microelectrodes have already been reported in the literature [29, 30]. The preparation of the internal contact involves either electropolymerization of a conducting polymer onto the metal surface or solution casting. These promising approaches for solid contact microelectrode construction have so far not yet been applied to the detection of trace level concentrations in small volumes. In this work we develop and characterize silver-selective solid-contact microelectrodes and present them as promising ion-selective systems that can be applied for detection of femtomole amounts of silver ions in miniaturized sensing systems.
Experimental
Chemicals
The ionophore o-xylylenebis(N, N-diisobutyldithiocarbamate) (Copper (II) ionophore (I)) was synthesized and purified as described by Kamata [31]. Poly(vinyl chloride) (PVC), bis(2-ethylhexyl) sebacate (DOS), sodium ionophore X, the lipophilic salt tetradodecylammonium tetrakis(4-chlorophenyl)borate (ETH 500), sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate (NaTFPB) were purchased in selectophore or puriss grade from Fluka (Buchs, Switzerland). Other chemicals used included methylene chloride (Fisher, New Jersey, USA), chloroform (Mallinckrodt Baker, Inc., Phillipsburg, USA), ethyl alcohol (VWR International, Inc, Illinois, USA), hydrochloric acid and ammonium hydroxide (Mallinckrodt Baker), ethylenediaminetetraacetic acid disodim salt (EDTA; Fluka), and iron (III) chloride (97%) (Sigma-Aldrich, Montana, USA). Poly(3-octylthiophene) (POT) was synthesized following the procedure of H. Jarvinen et al. [32] and purified according to the Patent Application [33] briefly described as follows: Synthesis of POT involves utilization of ferric chloride (FeCl3) so it was crucial to purify POT before use. POT was first purified by liquid extraction with EDTA. 0.5 g of POT was dissolved in 100 ml of chloroform to which 50 ml of 5 % EDTA (aqueous solution at pH 8–10, adjusted with ammonia) was added. The mixture was vigorously stirred for 4 h at the temperature 40 °C followed by transfer into a separation funnel to settle. The separated chloroform solution of POT was precipitated from a mixture of ethanol –hydrochloric acid (9: 1) solution under vigorous stirring, followed by filtration and drying. Aqueous solutions were prepared by dissolving the appropriate salts in Nanopure purified water. Synthesis of the methylmethacrylate–(MMA-DMA) copolymer matrix was prepared as described previously [34]. The monomers methylmethacrylate, 99.5% (MMA) and n-decylmethacrylate 99% DMA were obtained from Polysciences, Inc. (Warrington, PA), the initiator 2,2,-azobis(isobutyronitrile) 98% (AIBN) from Aldrich (Milwaukee, USA). The AIBN was recrystallized from warm methanol prior to use. Anhydrous ethylacetate and 1,4–dioxin reagent grade were obtained from Fisher and used as received.
Membranes
The membranes were prepared by dissolving 50 mg of components in 0.8 ml of either THF or methylene chloride: copper (II) ionophore (I) (17 mmol/kg), lipophilic cation-exchanger (7 mmol/kg), ETH 500 (12 mmol/kg), MMA-DMA: PVC/DOS = 9: 1 by mass (when THF was used), or MMA-DMA (when methylene chloride was used). The membrane cocktail was degassed by sonication for 1 min prior to coating the microelectrodes.
Microelectrodes
Two configurations were explored for microelectrode fabrication:
Configuration A
A gold wire of 0.5 cm length and 100 μm diameter used as a solid contact was soldered to a 10 cm long copper wire with a 1 mm diameter. The copper wire was sealed in a polypropylene tube to separate the interface between gold and copper materials so the length of protruded gold was about 0.3 mm. The gold tips were carefully left in dilute H2SO4 for 15 min, then rinsed with deionized water. The tips were rinsed with ethanol, then with methylene chloride and put onto clean microscope glass slides. A solution of POT was prepared by dissolving 0.2 mg of the conducting polymer in 40 μL of chloroform. A drop of POT solution was deposited onto the slide and the microelectrode tip was immersed into the solution and left until the solvent had evaporated. For each microelectrode this procedure was performed twice. The microelectrodes were left to dry for 2–3 minutes. The final color of the wire after exposure to the polymer solution must be black. The existence of any red spaces indicates a thinner layer of POT on the wire, and application of POT coating should be continued until the black color is achieved. Next, the membrane cocktail was applied by coating the microelectrode along its length. This coating step was repeated 3 times at 15-minute intervals. After the third coating, the microelectrodes were left to dry on the slides for 2–3 hours until the THF had fully evaporated.
Configuration B
A 2 cm long gold wire (200 μm dia.) was used as a solid contact and soldered to a copper wire for electric contact. Before use the gold wires were thoroughly cleaned with diluted sulfuric acid and rinsed with water, then acetone and left for 3 min in chloroform. The solution of POT was prepared as described above and applied along the length of gold wire at least three times or until the color of the wire became black. After the gold wires were fully covered with POT they were left to dry. The wires were then inserted into a polypropylene tip so that they were level with the end of the micropipette tip. Finally the membrane cocktail was applied on top of the wire covered with POT for three times at 15 min intervals.
The following conditioning protocol was used for both configurations: two day of conditioning in 10−3 M AgNO3 solution, followed by 1 day in 10−9 M AgNO3 + 10−5 M NaNO3.
EMF Measurements
Potentials were monitored with a PCI MIO16XE data acquisition board (National Instruments, Austin, TX) utilizing a four-channel high Z interface (WPI, Sarasota, FL) at room temperature (22 °C) in stirred solutions, in a galvanic cell of the type:
Ag/AgCl/3 M KCl/1M LiOAc/sample solution/ISE-membrane/POT/Au
with a double-junction reference electrode (type 6.0729.100, Methrom AG, Herisau, Switzerland). The calibration and selectivity measurements were performed in a 100 ml polypropylene beaker pretreated overnight in 0.1 M HNO3. Only the very tips of the electrodes were immersed into the sample solution. All measurements were performed in a Faraday cage.
For small volume measurements a sodium-selective liquid-contact electrode was used as reference electrode, which was constructed as follows: the membrane cocktail was poured into the back of the vertically positioned micropipette tip, the end of which was capped with a glass slide to prevent cocktail escaping from the tip. The cocktail was left to evaporate for 1 day. Then the obtained membrane inside of the micropipette tip was conditioned in 10−3 M NaNO3 for one day. For potentiometric measurements the double junction (10−2 M NaCl) was used.
Selectivity measurements
Before performing selectivity measurements, the ISE membranes were conditioned in 10−3 M NaNO3 for two days. For each ion a calibration curve was recorded and the selectivity coefficients were calculated using the separate solution method. EMF values were corrected for liquid junction potentials (4.4 mV) according to the Henderson equation for measurements in 100-ml samples where a regular reference electrode was used. Activity coefficients were calculated by the Debye-Huckel approximation.
Reproducibility experiments
The reproducibility experiments consisted of alternatively reading the potential values in solutions of AgNO3 switching between 10−9 and 10−8 M as well as between 10−8 and 10−7 M. The tests were performed with the microelectrode of configuration A in a plexiglass cell with two orifices. The total volume for a liquid occupying the cell was 16 mL. The microelectrode and reference electrode were fixed in the cell so that only the very tip of the microelectrode was immersed into the solution. The potential was monitored in a stagnant solution (stopped-flow). After recording the potential, the previous solution was flushed away through the outlet and the new solution was introduced. The lag time between the measurement of two subsequent solutions in the 16 ml volume cell was about 1 min for reproducibility experiments between 10−8 and 10−7 M and 2 min between 10−9 and 10−8 M of AgNO3; the potential was not recorded during this period.
Small volume measurements
The experiments for small volume measurements were performed in a micropipette tip with a sample volume of 50 μl. The silver-selective microelectrode as indicator and the sodium-selective electrode as reference electrode were inserted into the micropipette tip through holes previously drilled into the sides of the material. Both microelectrodes fitted snugly into the holes avoiding any leaking of solution. The whole system was glued down to a solid support and connected appropriately for measurements.
Results and Discussion
As shown recently [35, 36], the copper (II) ionophore (I) possesses a very high selectivity toward silver ions. Recently, solid-contact silver-selective macroelectrodes based on this ionophore have been prepared and studied [26]. A detection limit of 2 × 10−9 M for the potentiometric detection of silver ions was demonstrated using poly(3-octylthiophene) as a solid contact and copolymer MMA-DMA as a membrane matrix [26]. Here, our goal was to establish a similar system at the microscale to be applied in small sample volumes. Two configurations were used and compared: the first one contained 10 wt% PVC/DOS (1:2) blended with MMA-DMA, the second one consisted of MMA-DMA as a matrix only. The choice of having additional PVC/DOS was based on preliminary studies that yielded nanomolar detection limits and faster response times (data not shown). Also, the addition of PVC/DOS with THF as a solvent makes application of membrane cocktail easier to be accomplished since THF evaporates slower and permits smoother coating. Moreover, addition of plasticizer reduces the resistance over pure MMA-DMA.
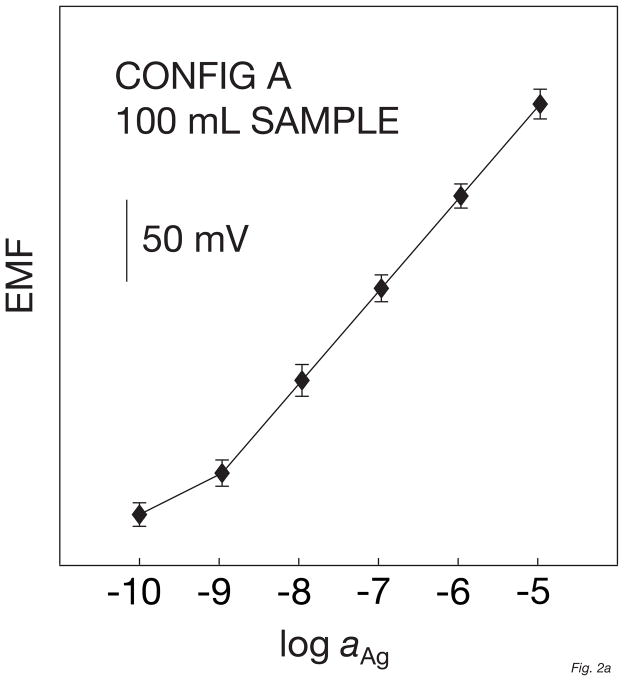
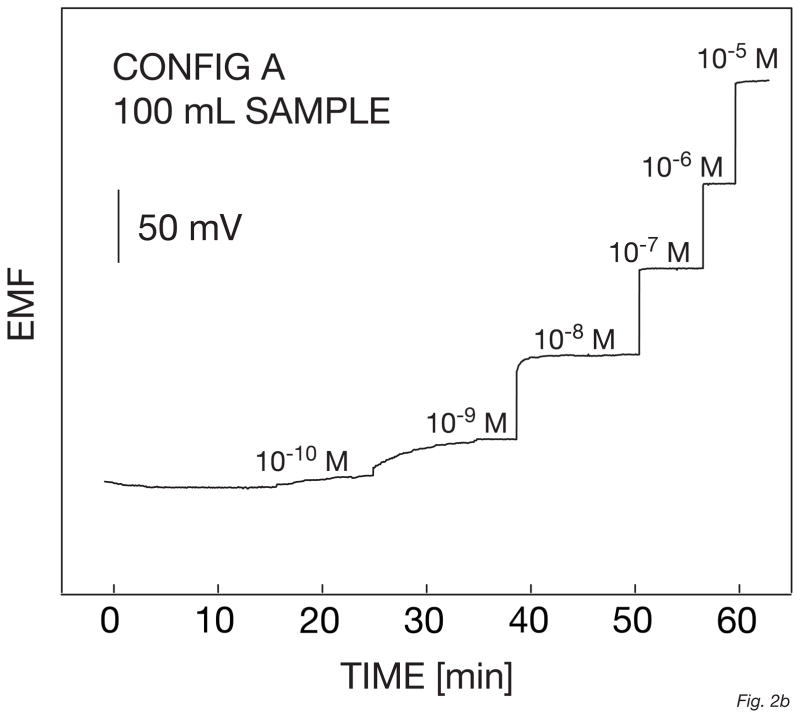
The electrodes based on this configuration A showed a Nernstian response function in the linear range between 10−9 M and 10−5 M (Figure 1a), with a detection limit of 6.3 × 10−10 M. This is somewhat better than the results observed for the corresponding macroelectrodes [26]. The time response traces for these microelectrodes are shown in Figure 1b. The t95% between 10−9 and 10−8 M of silver nitrate is quite fast, not exceeding 5 min, probably due to the addition of 10% PVC/DOS to the matrix.
Fig. 1.
Calibration curves (A) and corresponding time traces (B) for 6 solid-contact silver-selective microelectrodes of configuration A, obtained by successive increase of the silver nitrate concentration in a constant background of 10−5 M NaNO3. Error bars: standard deviations of the potentials from the 6 electrodes.
The performance of the microelectrodes of this first configuration was only observed when the conditioning procedure described in the experimental part was strictly adhered to, likely because the addition of 10% of PVC/DOS. Additionally, the sensing membrane surface of solid-contact microelectrodes of this configuration was about a factor of 2.5 larger in comparison with microelectrodes of configuration B. This made it inconvenient to work in small volumes because of the risk of membrane damage. Similarly, irreproducibilities in the potential were observed due to the microelectrode changing its shape upon introducing a new solution to the small volume cell. Therefore, microelectrodes of the configuration A showed excellent results in a large beaker (100 ml) but did not behave satisfactorily in small cells.
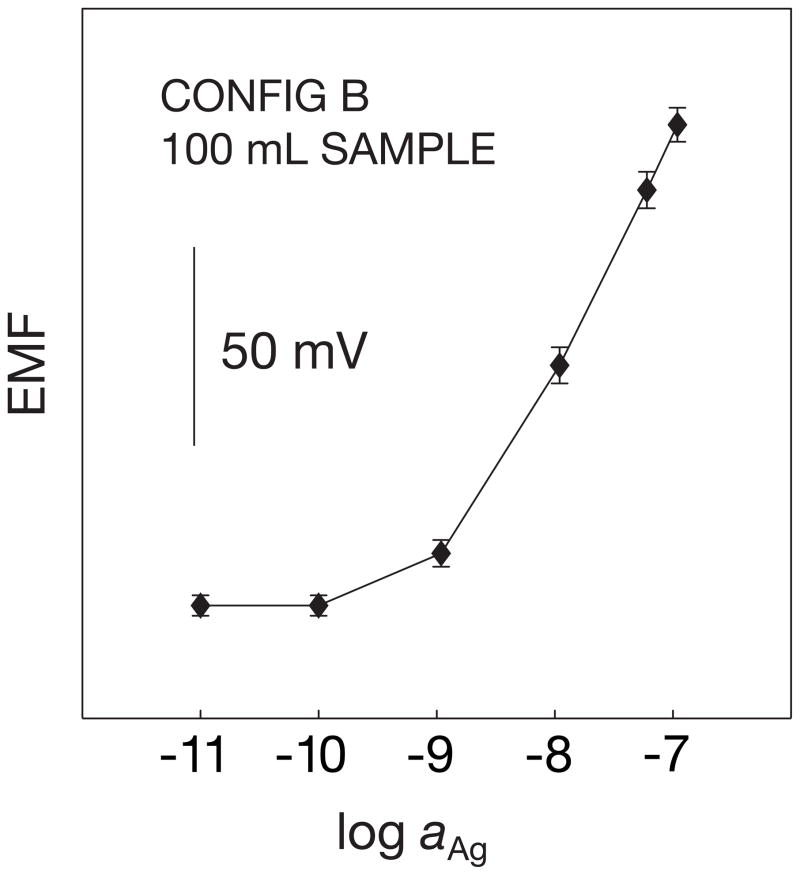
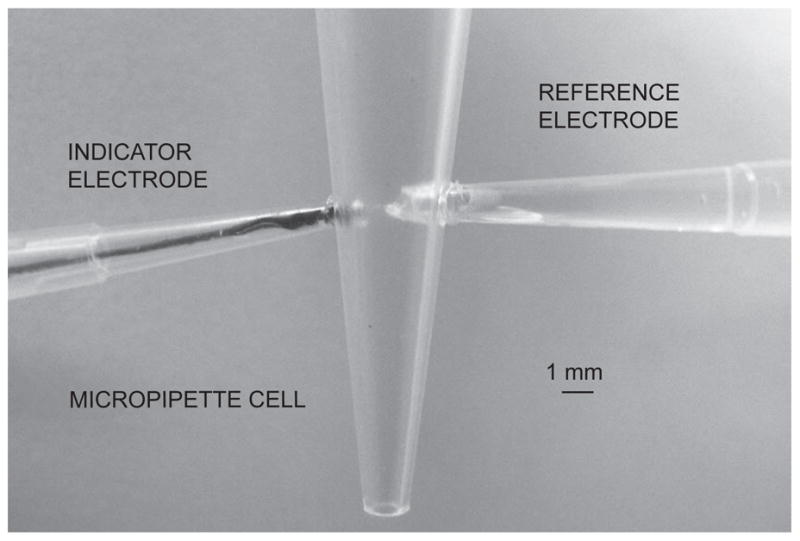
Microelectrodes of configuration B (see Figure 2 for experimental setup in the 50-μl micropipette cell) showed a similar Nernstian response slope in the range of 10−9 M to 10−5 M with a nanomolar detection limit, high potential stability and a long life time of more than one month (Figure 3). For this configuration it was observed that the conditioning protocol was less dependent on the matrix composition since only MMA-DMA was used. This is attributed to the significantly lower diffusion coefficients of this matrix relative to traditional plasticized PVC [37]. In addition, since this configuration was flat and less surface was exposed, it was an easy-to-handle and more robust system, and chosen for exploration in small volume experiments.
Fig. 2.
Photograph of the setup of configuration B in the 50-μl micropipette cell.
Fig. 3.
Calibration curves (A) and corresponding time traces (B) for 4 solid-contact silver-selective microelectrodes of configuration B, obtained by successive increase of the silver nitrate concentration in 10−5 M NaNO3. Error bars: standard deviations of the potentials from the 4 electrodes.
Overall, selectivity experiments gave similar results as with solid-contact macroelectrodes [26]. As required for the determination of unbiased selectivity coefficients the microelectrodes were not placed in contact with primary ions during the conditioning time. The selectivity coefficients and corresponding electrodes slopes for the interfering ions were found as log KAg,Napot = −8.5 ± 0.3 (33.5 ± 0.3 mV slope); log KAg,Kpot = −9.5 ± 0.5 (22.4 ± 0.4 mV slope); log KAg,Hpot = −9.3 ± 0.4 (41.3 ± 0.2 mV slope); log KAg,Capot = −10.9 ± 0.4 (14.3 ± 0.5 mV slope); and log KAg,Cupot = −10.7 ± 0.3 (30.6 ± 0.3 mV slope). The electrode slopes to interfering ions were mostly sub-Nernstian, indicating that the selectivity coefficients are still biased to some extent, although they seem sufficiently attractive for practical use. A fast response upon initial contact to the 10−5 M of silver ions was observed, which is indicative of a slower diffusion coefficient in the membrane phase relative to regular plasticized PVC. Due to lower diffusion coefficients the primary ions saturate the outer surface of membrane upon initial contact very quickly and therefore do not exhibit the sluggish response behavior normally observed with unconditioned membranes at these concentrations [38].
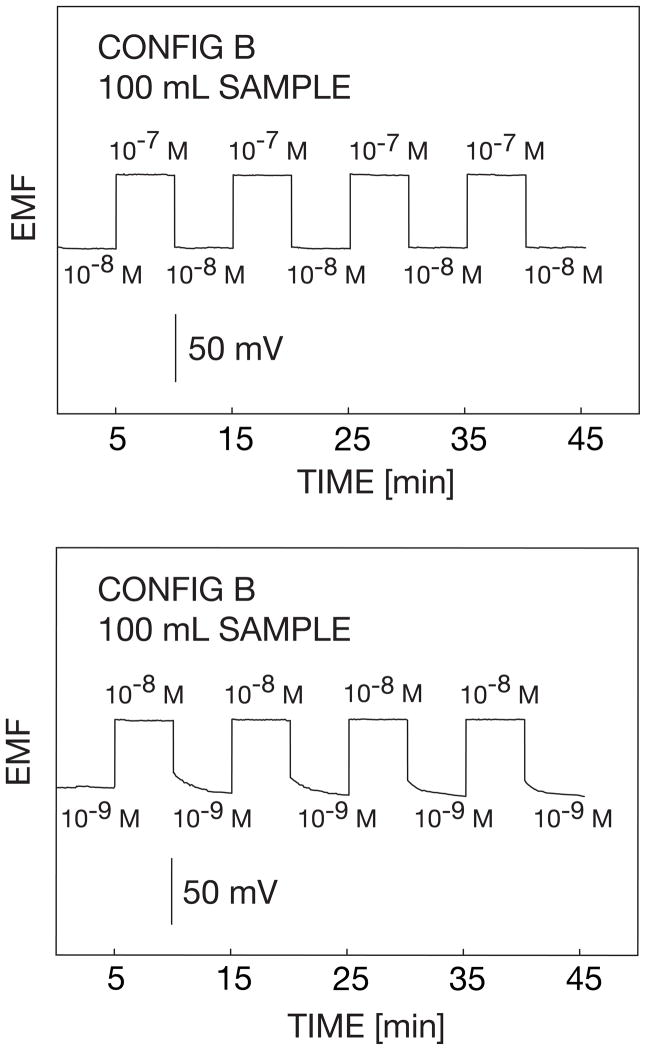
The reproducibility of the microelectrodes was evaluated with reversibility experiments (Figure 4). Here the microelectrode was alternatively exposed to 10−9 M and 10−8 M solutions of AgNO3 in a background of 10−5 M NaNO3. The transition from 10−7 M to 10−8 M occurred immediately while the transition from 10−9 M to 10−8 M was slower. This confirms that the electrode was around its detection limit [39]. The reversibility experiment between 10−7 M and 10−8 M is expected to be much faster since the activity change is within the Nernstian response range of the electrode. For both cases, however, it can be noted that the sensor possessed reproducible characteristics.
Fig. 4.
Reproducibilities of the observed potentials for configuration B upon alternating the silver concentration between the indicated values. The potential was not recorded during the time when the solution was replaced between concentrations (ca. 1–2 min).
The system used for measurements in small volumes was described above (see Experimental). Another ion-selective electrode was chosen as a reference electrode since it possesses good reproducibility and stability. Since the microelectrode fabricated for silver measurements had high discrimination of sodium ions, the measurement of silver ions was conducted in a constant background of sodium ions without loss of detection limit. Thus, the electrode chosen as a reference was a sodium-selective electrode in analogy to recent work [15].
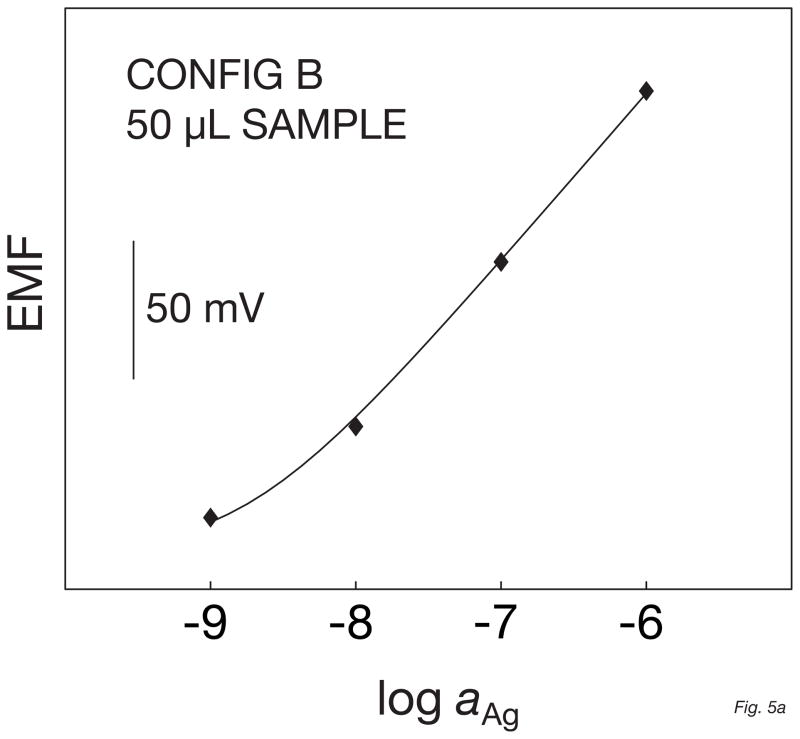
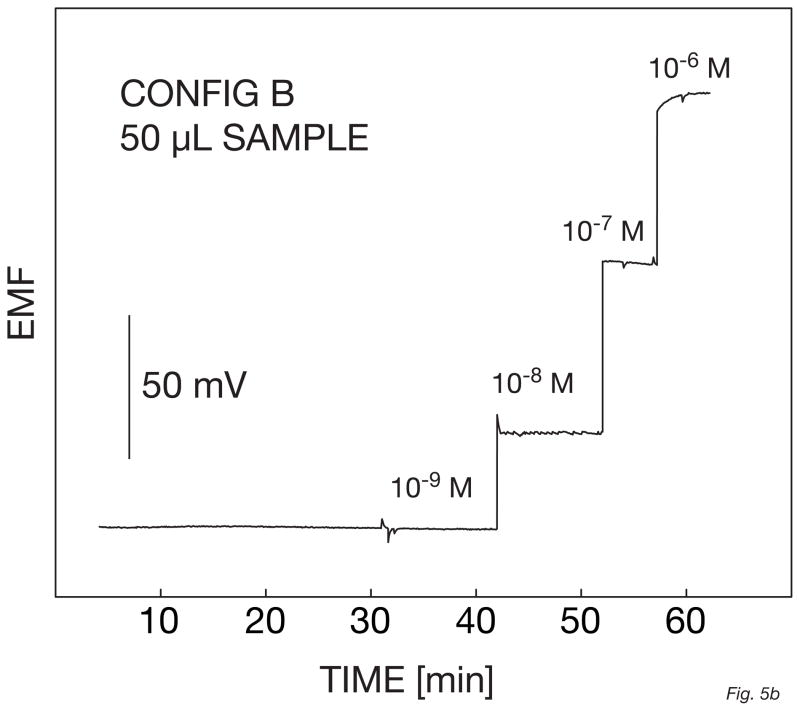
The electrode responses were first evaluated in a 100-ml beaker using a sodium selective electrode as a reference before performing the measurements in a 50-μl volume. The background potential for the micropipette tip used as the small volume cell was found to be about 20 mV higher than in the beaker, suggesting a loss in detection limit of half an order of magnitude. This is likely because of some contamination in the micropipette tip since the measuring volume has been drastically reduced. Otherwise the performance of the electrode in this system was similar to that in the beaker, with a Nernstian response slope between 10−9 and 10−6 M of AgNO3 and with a detection limit of 4 × 10−9 M. This was less good that in the beaker but still in the nanomolar range (Figure 5a and b). However, since this was measured in a 50-μl cell, the amount of silver ions present at the detection limit is as low as 100 femtomoles. As recently demonstrated with liquid inner contact microelectrodes, this amount may be further reduced by performing the measurements in even smaller sample volumes [15]. This opens opportunities for the application of ion selective electrodes to the detection of ultra-small amounts of ions with a simple technique that does not require sophisticated instrumentation.
Fig. 5.
Calibration curves (A) and corresponding time traces (B) for a solid-contact silver-selective microelectrode of configuration B, obtained in a 50-μl sample volume by successive increase of the silver nitrate concentration in a 10−5 M NaNO3 background.
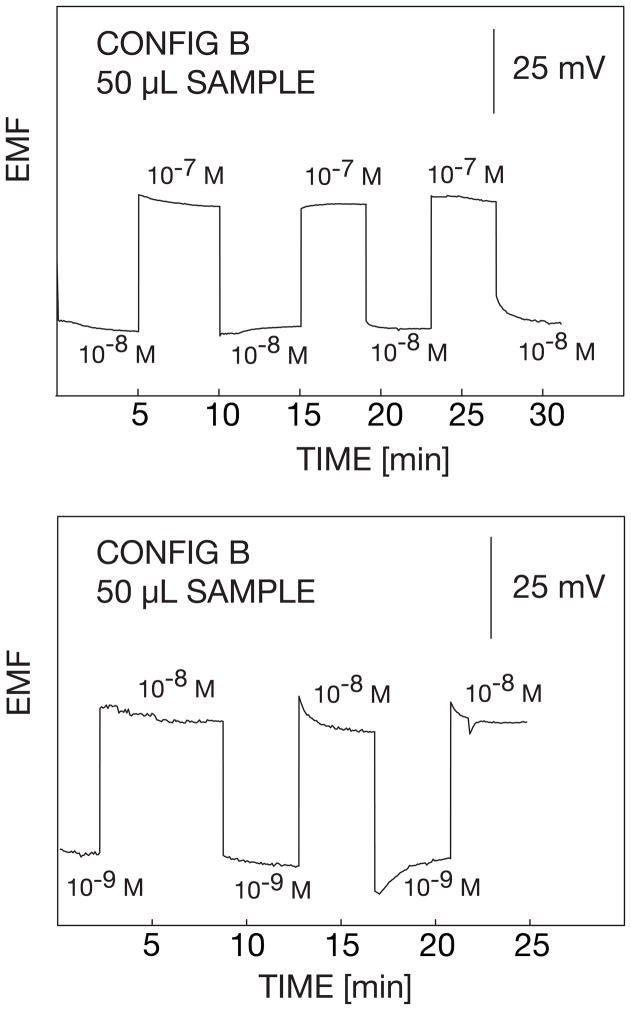
The reproducibility of the electrodes in the 50-μl volume was also evaluated. The concentrations were alternated between 10−9 to 10−8 M and 10−8 to 10−7 M AgNO3. As shown in Figure 6, the changes in the potential were reproducible with a standard deviation of nearly 1 mV between measurements.. This is a strong indication that the microelectrode has a good reproducibility and can be used for continuous measurements with acceptable precision.
Fig. 6.
Potential reproducibilities for configuration B in a 50-μl sample volume upon alternating the silver concentration between the indicated values. The potential was not recorded during the time when the solution was replaced between concentrations (ca. 30 s for lower to higher concentrations and up to 3–5 min for higher to lower concentrations).
Conclusions
Two configurations of solid-contact silver-selective microelectrodes were fabricated and compared based on a solvent polymeric membrane and poly(3-octylthiophene) as a conducting polymer. Both types of microelectrodes exhibit excellent selectivity toward silver ions with a nanomolar detection limit, good reproducibility and stability. The more robust second configuration was applied for measurements in 50-μl sample volumes. The microelectrode in the 50-μl cell showed reproducible behavior and possibility of detecting as small as 100 femtomoles of silver ions. This demonstrates that solid contact microelectrodes can be successfully fabricated to exhibit attractive behavior at trace levels in confined samples. It is anticipated that such measurement systems may find elegant use as transducers for metal labeled biological and clinical assays with performance characteristics that will match or even surpass that of available electrochemical techniques.
Acknowledgments
The authors are grateful to the National Institutes of Health (EB002189) for financial support of this research.
References
- 1.Ward WK, Jansen LB, Anderson E, Reach G, Klein JC, Wilson GS. A new amperometric glucose microsensor: in vitro and short-term in vivo evaluation. Biosens Bioelectron. 2002;17:181–189. doi: 10.1016/s0956-5663(01)00268-8. [DOI] [PubMed] [Google Scholar]
- 2.Wang J, Tian B. Gold ultramicroelectrodes for on-site monitoring of trace lead. Electroanalysis. 1993;5:809–814. [Google Scholar]
- 3.Chen T, Friedman KA, Lei I, Heller A. In situ assembled mass-transport controlling micromembranes and their application in implanted amperometric glucose sensors. Anal Chem. 2000;72:3757–3763. doi: 10.1021/ac000348c. [DOI] [PubMed] [Google Scholar]
- 4.Schlue WR, Kilb W, Günzel D. Ultramicroelectrodes for membrane research. Electrochim Acta. 1997;42:3197–3205. [Google Scholar]
- 5.Ammann D. Ion-selective microelectrodes: principles, design, and application. Springer; Berlin, New York: 1986. [Google Scholar]
- 6.Thomas RC. Ion-selective intracellular microelectrodes: how to make and use them. Academic Press; London, New York: 1978. [Google Scholar]
- 7.Sodergard M, Csoka B, Nagy G, Ivaska A. Lowering the detection limit of solvent polymeric ion-selective membrane electrodes. An experimental study with calcium-selective micropipette electrodes. Anal Lett. 2003;36:2909–2923. [Google Scholar]
- 8.Mathison S, Bakker E. Effect of transmembrane electrolyte diffusion on the detection limit of carrier-based potentiometric ion sensors. Anal Chem. 1998;70:303–309. [Google Scholar]
- 9.Ion AC, Bakker E, Pretsch E. Potentiometric Cd2+ - selective electrode with a detection limit in the low ppt range. Anal Chim Acta. 2001;440:71–79. [Google Scholar]
- 10.Ceresa A, Bakker E, Hattendorf B, Gunther D, Pretsch E. Potentiometric polymetric membrane electrodes for measurements of environmental samples at trace levels: new requirements for selectivities and measuring protocols, and comparison with ICPMS. Anal Chem. 2001;72:343–351. doi: 10.1021/ac001034s. [DOI] [PubMed] [Google Scholar]
- 11.Sokalski T, Ceresa A, Fibbioli M, Zwickl T, Bakker E, Pretsch E. Lowering limit of solvent polymeric ion-selective membrane electrodes. 2. Influence of composition of sample and internal electrolyte solution. Anal Chem. 1999;71:1210–1214. [Google Scholar]
- 12.Qin W, Zwickl T, Pretsch E. Improved detection limits and unbiased selectivity coefficients by using ion-exchange resins in the inner reference solution of ion-selective polymeric membrane electrodes. Anal Chem. 2000;72:3236–3240. doi: 10.1021/ac000155p. [DOI] [PubMed] [Google Scholar]
- 13.Vigassy T, Gyurcsanyi RE, Pretsch E. Influence of incorporated lipophilic particles on ion fluxes through polymeric ion-selective membranes. Electroanalysis. 2003;15:375–382. [Google Scholar]
- 14.Vigassy T, Huber CG, Wintringer R, Pretsch E. Monolithic capillary-based ion-selective electrodes. Anal Chem. 2005;77:3966–3970. doi: 10.1021/ac050424h. [DOI] [PubMed] [Google Scholar]
- 15.Malon A, Vigassy T, Bakker E, Pretsch E. Potentiometry at trace levels in confined samples: ion-selective electrodes with subfemtomole detection limits. J Am Chem Soc. 2006;128:8154–8155. doi: 10.1021/ja0625780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cattrall RW, Drew DM, Hamilton IC. Alkylphosphoric acid esters for use in coated-wire calcium-selective electrodes. I. Response characteristics. Anal Chim Acta. 1975;76:269–277. [Google Scholar]
- 17.Sutter J, Radu A, Peper S, Bakker E, Pretsch E. Solid-contact polymeric membrane electrodes with detection limits in the subnanomolar range. Anal Chim Acta. 2004;523:53–59. [Google Scholar]
- 18.Fibbioli M, Morf WE, Badertscher M, de Rooij NF, Pretsch E. Potential drifts of solid-contacted ion-selective electrodes due to zero-current ion fluxes through the sensor membrane. Electroanalysis. 2000;12:1286–1292. [Google Scholar]
- 19.Grekovich AL, Markuzina NN, Mikhelson KN, Bochenska M, Lewenstam A. Conventional and solid-contact lithium-selective electrodes based on tris[(N,N-dicyclohexylamide) neutral ionophore. Electroanalysis. 2002;14:551–555. [Google Scholar]
- 20.Gyurcsanyi RE, Nyback AS, Ivaska A, Toth K, Nagy G. Novel polypyrolle based all-solid-state potassium-selective microelectrodes. Analyst. 1998;123:1339–1344. [Google Scholar]
- 21.Sutter J, Lindner E, Gyurcsanyi RE, Pretsch E. A polypyrrole-based solid-contact Pb2+-selective PVC-membrane electrode with a nanomolar detection limit. Anal Bioanal Chem. 2004;380:7–14. doi: 10.1007/s00216-004-2737-4. [DOI] [PubMed] [Google Scholar]
- 22.Konopka A, Sokalski T, Michalska A, Lewenstam A, Maj-Zurawska M. Factors affecting the potentiometric response of all-solid-state solvent polymeric membrane calcium-selective electrode for low-level measurements. Anal Chem. 2004;76:6410–6418. doi: 10.1021/ac0492158. [DOI] [PubMed] [Google Scholar]
- 23.Lindfors T, Ivaska A. Stability of the inner polyaniline solid contact layer in all-solid-state K+ -selective electrodes based on the plasticized poly(vinyl chloride) Anal Chem. 2004;76:4387–4394. doi: 10.1021/ac049439q. [DOI] [PubMed] [Google Scholar]
- 24.Sutter J, Pretsch E. Response behavior of poly(vinyl chloride)- and polyurethane-based Ca2+-selective membrane electrodes with polypyrrole- and poly(3-octylthiophene)-mediated internal solid contact. Electroanalysis. 2006;18:19–25. [Google Scholar]
- 25.Michalska A, Konopka A, Maj-Zurawska M. All-solid-state calcium solvent polymeric membrane electrode for low-level concentration measurements. Anal Chem. 2003;75:141–144. doi: 10.1021/ac025916y. [DOI] [PubMed] [Google Scholar]
- 26.Chumbimuni-Torres K, Rubinova N, Radu A, Kubota LT, Bakker E. Solid contact potentiometric sensors for trace level measurements. Anal Chem. 2006;78:1318–1322. doi: 10.1021/ac050749y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michalska A, Maksymiuk K. All-plastic, disposable, low detection limit ion-selective electrodes. Anal Chim Acta. 2004;523:97–105. [Google Scholar]
- 28.Vazquez M, Bobacka J, Ivaska A, Lewenstam A. Influence of oxygen and carbon dioxide on the electrochemical stability of poly(3,4-ethylenedioxythiophene) used as ion-to-electron transducer in all-solid-state ion-selective electrodes. Sens Actuators B. 2002;82:7–13. [Google Scholar]
- 29.Vazquez M, Bobacka J, Ivaska A, Lewenstam A. Small-volume radial flow cell for all-solid-state ion-selective electrodes. Talanta. 2004;62:57–63. doi: 10.1016/S0039-9140(03)00408-9. [DOI] [PubMed] [Google Scholar]
- 30.Zine N, Bausells J, Ivorra A, Aguilo J, Zabala M, Teixidor F, Masalles C, Vinas C, Errachid A. Hydrogen-selective microelectrodes based on silicon needles. Sens Actuators B. 2003;91:76–82. [Google Scholar]
- 31.Kamata S, Murata H, Kubo Y, Bhale A. Copper (II)-selective membrane electrodes based on o-xylylene bis(dithiocarbamates) as neutral carriers. Analyst. 1989;114:1029–1031. [Google Scholar]
- 32.Jarvinen H, Lahtinen L, Nasman J, Hormi O, Tammi AL. A new method to prepare 3-octylthiophene and poly-(3-octylthiophene) Synth Met. 1995;69:299–300. [Google Scholar]
- 33.Xiao SS, Qiu C, Qiu CX. Method to purify polymers. 20040254336. U S Patent Application. 2004
- 34.Qin Y, Peper S, Bakker E. Plasticizer-free polymer membrane ion-selective electrodes containing a methacrylic copolymer matrix. Electroanalysis. 2002;14:1375–1381. [Google Scholar]
- 35.Szigeti Z, Malon A, Vigassy T, Csokai V, Grun A, Wygladacz K, Ye N, Xu C, Chebny V, Bitter I, Rathore R, Bakker E, Pretsch E. Novel potentiometric and optical silver ion-selective sensors with subnanomolar detection limits. Anal Chim Acta. 2006 doi: 10.1016/j.aca.2006.05.009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wygladacz K, Radu A, Xu C, Qin Y, Bakker E. Fiber-optic microsensor array based on fluorescent bulk optode microspheres for the trace analysis of silver ions. Anal Chem. 2005;77:4706–4712. doi: 10.1021/ac050856s. [DOI] [PubMed] [Google Scholar]
- 37.Heng LY, Toth K, Hall EAH. Ion-transport and diffusion coefficients of non-plasticised methacrylic–acrylic ion-selective membranes. Talanta. 2004;63:73–87. doi: 10.1016/j.talanta.2003.12.051. [DOI] [PubMed] [Google Scholar]
- 38.Bakker E. Determination of Unbiased Selectivity Coefficients of Neutral Carrier-Based Cation-Selective Electrodes. Anal Chem. 1997;69:1061–1069. [Google Scholar]
- 39.Radu A, Telting-Diaz M, Bakker E. Rotating Disk Potentiometry for Inner Solution Optimization of Low-Detection-Limit Ion-Selective Electrodes. Anal Chem. 2003;75:6922–6931. doi: 10.1021/ac0346961. [DOI] [PubMed] [Google Scholar]