Abstract
The infralimbic division of the medial prefrontal cortex (IL) has been implicated in the consolidation and retention of extinction memories. However, the effects of IL lesions on the retention of extinction memory are inconsistent. In the present experiments, we examined whether rat strain influences the effects of IL lesions on extinction. In Experiment 1, Sprague-Dawley (SD) or Long-Evans (LE) rats received a standard auditory fear conditioning procedure, which was followed by an extinction session; freezing served as the index of conditional fear. Our results reveal that focal IL lesions impair the retention of extinction in SD, but not LE rats. In addition to the strain difference in sensitivity to IL lesions, LE rats exhibited significantly higher levels of contextual fear before the outset of extinction training than SD rats. In a second experiment we thus examined whether contextual fear influenced the sensitivity of extinction to IL lesions in LE rats. Long-Evans rats received the same conditioning as in Experiment 1, and then were either merely exposed to a novel context or administered unsignaled shocks in that context, followed by extinction and test sessions. Our results reveal that LE rats with IL lesions showed normal extinction regardless of the levels of contextual fear manifest before extinction. Thus, we conclude that rat strain is an important variable that influences the role of infralimbic cortex in fear extinction.
Keywords: extinction, strain, fear, medial prefrontal cortex, rat
Extinction is a form of new learning in which presentation of a conditioned stimulus (CS) in the absence of the US reduces conditional responding to that CS (Bouton, 2002, 2004; Bouton, Westbrook, Corcoran, & Maren, 2006; Pavlov, 1927). Considerable interest has emerged in the neurobiological mechanisms of extinction, insofar as impairments in extinction may contribute to a variety of anxiety disorders, including post-traumatic stress disorder (PTSD) (Bouton, Mineka, & Barlow, 2001; Rothbaum & Davis, 2003). One brain structure that has been implicated in the extinction of learned fear is the medial prefrontal cortex (mPFC), specifically the infralimbic cortex (IL) (Quirk, Garcia, & Gonzalez-Lima, 2006; Quirk & Mueller, 2008). Robust projections from IL to inhibitory interneurons located in the intercalated nuclei (ITC) of the amygdala (McDonald, Mascagni, & Guo, 1996) make it perfectly positioned to regulate amygdala output after extinction learning (Likhtik, Popa, Apergis-Schoute, Fidacaro, & Pare, 2008).
In support of this possibility, several studies indicate that IL manipulations influence the extinction of fear. For example, intra-IL infusions of protein synthesis inhibitors (Santini, Ge, Ren, Pena de Ortiz, & Quirk, 2004), NMDA receptor antagonists (Burgos-Robles, Vidal-Gonzalez, Santini, & Quirk, 2007; Laurent & Westbrook, 2008), or sodium channel blockers (Sierra-Mercado, Corcoran, Lebron-Milad, & Quirk, 2006), prior to extinction leads to poor retrieval of extinction memory the following day without affecting acquisition of extinction per se. Intra-IL infusions of NMDA receptor antagonists (Burgos-Robles et al., 2007; Sotres-Bayon, Diaz-Mataix, Bush, & LeDoux, 2009) or MAPK inhibitors (Hugues, Chessel, Lena, Marsault, & Garcia, 2006; Hugues, Deschaux, & Garcia, 2004) immediately after extinction also leads to impaired extinction retrieval, suggesting that consolidation of extinction memory involves post-training molecular cascades in IL. Physiological correlates of extinction have been observed in IL (Burgos-Robles et al., 2007; Herry & Garcia, 2002; Hugues & Garcia, 2007; Milad & Quirk, 2002) and electrical stimulation of IL enhances extinction (Milad & Quirk, 2002; Milad, Vidal-Gonzalez, & Quirk, 2004). Moreover, inhibitory interneurons in the amygdala that receive input from the IL are involved in the expression of extinction (Likhtik et al., 2008).
Despite mounting evidence for a role for IL in fear extinction, studies examining the effect of IL lesions on extinction in rats have yielded inconsistent results. Although several studies have found impaired retention of extinction with IL lesions (Lebron, Milad, & Quirk, 2004; Morgan, Romanski, & LeDoux, 1993; Quirk, Russo, Barron, & Lebron, 2000), other studies have not (Farinelli, Deschaux, Hugues, Thevenet, & Garcia, 2006; Garcia, Chang, & Maren, 2006; Gewirtz, Falls, & Davis, 1997). Several factors could account for this discrepancy, such as lesion size, the nature of the behavioral task, and the strain of rat. Studies employing focal IL lesions have typically found impairments in extinction retrieval (Lebron et al., 2004; Quirk et al., 2000), while larger lesions in mPFC including IL and prelimbic cortex (PrL) tend not to affect extinction (Farinelli et al., 2006; Garcia et al., 2006; Gewirtz et al., 1997; Morgan, Schulkin, & LeDoux, 2003). Because IL and PrL have opposite influences on the expression of learned fear (Corcoran & Quirk, 2007; Vidal-Gonzalez, Vidal-Gonzalez, Rauch, & Quirk, 2006), larger lesions including IL and PrL may produce different results than IL lesions alone. In addition, studies using suppression of bar pressing to index fear (Lebron et al., 2004; Quirk et al., 2000) reveal IL lesion effects on extinction, while those using freezing or startle (Farinelli et al., 2006; Garcia et al., 2006; Gewirtz et al., 1997; Morgan et al., 1993) tend not to indicate an IL impairment. Moreover, the majority of studies that find effects of IL lesions on extinction used albino rats as subjects (Lebron et al., 2004; Morgan et al., 1993; Quirk et al., 2000); we have failed to observe an effect of IL lesions on extinction in hooded rats (Garcia et al., 2006). Therefore, it is possible that different strains of rat may influence IL involvement in extinction. Consistent with this, a recent study in mice revealed strain differences in fear conditioning and extinction that interact with pharmacological manipulations of extinction learning (Hefner et al., 2008). We therefore designed the present experiments to directly compare the effects of focal IL lesions on the extinction of conditioned freezing to an auditory CS in Sprague-Dawley (SD) and Long-Evans (LE) rats.
Materials and Methods
Experiment 1: Do strain differences influence the effects of IL lesions on fear extinction?
Subjects
Two strains of rats were used in this experiment: 48 male Long-Evans (LE) rats (250-330 g; Blue Spruce) from a commercial supplier (Harlan Sprague Dawley, USA) and 48 male Sprague-Dawley (SD) rats (250-330 g) from another commercial supplier (Hilltop, USA). They were singly-housed with 14-h light/10-h dark cycle (lights on at 7:00 am), and allowed food and water ad libitum. During the first five days, they were handled for 10 sec to habituate them to the experimenter. All experiments were carried out in accordance with guidelines approved by the University of Michigan University Committee on Use and Care of Animals.
Surgery
Rats received pre-conditioning bilateral infralimbic cortex lesions (IL; AP: +2.8 mm; ML: ±0.5 mm; DV: −5.2 mm relative to bregma) or sham surgeries for control groups (SH-E and SH-NE; extinction and no-extinction, respectively). In both cases, rats were anesthetized with sodium pentobarbital (Nembutal, 65 mg/kg, ip), treated with atropine (0.04 mg/kg, i.p.) and placed in a stereotaxic frame for electrolytic lesions with stainless-steel electrodes insulated with Epoxylite except for 0.3 mm at the tip. Lesions were made with anodal, constant direct current (0.3 mA, 5 sec), and the incision was closed with stainless-steel wound clips. The rats were allowed to recover for 7 days.
Behavioral apparatus
Eight identical observation chambers (30 × 24 × 21 cm; MED-Associates) were used in all experiments. The chambers were constructed of aluminum (side walls) and Plexiglas (rear wall, ceiling, and hinged front door) and were situated in sound-attenuating cabinets located in a brightly lit and isolated room. The floor of each chamber consisted of 19 stainless steel rods (4 mm in diameter) spaced 1.5 cm apart (center-to-center). Rods were wired to a shock source and solid-state grid scrambler (MED-Associates) for the delivery of footshock US. A speaker mounted outside a grating in one wall of the chamber was used for the delivery of acoustic CS.
Each conditioning chamber rested on a load-cell platform that was used to record chamber displacement in response to each rat's motor activity and acquired on-line using Threshold-Activity software (MED-Associates). The output of each chamber's load cell was set to a gain that was optimized for detecting freezing behavior (somatomotor immobility, except that necessitated by breathing). Load-cell amplifier output (−10 to +10 V) from each chamber was digitized. Absolute values of the load-cell voltages were then computed and multiplied by 10 to yield a scale that ranged from 0 to 100. For each chamber, load-cell voltages were digitized at 5 Hz, yielding one observation every 200 msec. Freezing was quantified by computing the number of observations for each rat that had a value less than the freezing threshold (load-cell activity = 10). We score an observation as freezing if it fell within a continuous group of at least five observations that were all less than the freezing threshold. Thus, freezing was only scored if the rat was immobile for at least 1 sec (Maren, 1998).
Two distinct contexts were used in Experiment 1 and 2. For the first context (context A), a 15 W houselight mounted opposite the speaker was turned on, and room lights remained on. The chambers were cleaned with a 1% acetic acid solution. To provide a distinct odor, stainless steel pans containing a thin layer of this solution were placed underneath the grid floors before the rats were placed inside. Ventilation fans in each chest supplied background noise (65 dB). Rats were transported to this context in white plastic boxes. For the second context (context B), all room and chamber houselights were turned off. A pair of 40 W red lights provided illumination. Additionally, the doors on the sound-attenuating cabinets were closed, the ventilation fans were turned off, and the chambers were cleaned with 1% ammonium hydroxide solution. Also, stainless steel pans containing a thin layer of the same solution were placed underneath the grid floors before the rats were placed inside to provide a distinct odor. Rats were transported to this context in black plastic boxes.
Procedure
Rats were submitted to three phases of training: fear conditioning, extinction, and extinction retention test. In each phase, trials began 3 min after being placed in the chambers. All phases were conducted in context A. There were 16 animals in each group for each strain (IL, SH-E, and SH-NE; LE and SD).
On Day 1, rats received five conditioning trials consisted of tones (30 sec, 80 dB, 4k Hz) that coterminated with footshocks (1 mA, 0.5 sec) (variable ITI ranging from 90-150 sec, with an average = 120 sec). On Day 2, rats received 20 tone-alone presentations for fear extinction (IL and SH-E). For no-extinction controls (SH-NE), rats were placed in the chamber for the same amount of time but were not exposed to the tone CS. On Day 3, all rats were exposed to another 20 CS-alone presentations for retention test.
Freezing was determined during each 30 sec tone period during conditioning, extinction, and retention test. Baseline freezing to context was assessed during the minutes preceding the first CS presentation.
Histology
Histological verification of lesion location was performed after behavioral testing. Rats were intracardially perfused with 0.9% saline followed by a 10% formalin solution. After extraction from the skull, brains were post-fixed in 10% formalin solution for two days, at which time the solution was replaced with a 10% formalin and 30% sucrose solution until sectioning. Sections (45 μm thick) were cut on a cryostat (−20°C), and wet mounted on glass microscope slides with 70% ethanol. After drying, sections were stained with 0.25% thionin for visualization of lesions.
Data analysis
All behavioral data are expressed as means and standard error of the means (SE) and analyzed by analysis of variance (ANOVA). Post hoc comparisons in the form of Fisher's PLSD tests were performed after a significant F ration.
Experiment 2: Does contextual fear influence the role of the IL in extinction in LE rats?
Subjects
The subjects were 48 adult male Long-Evans rats (250-330 g) obtained and housed as described in Experiment 1.
Surgery and behavioral apparatus
The surgical procedures and behavioral apparatus were identical to those described in Experiment 1.
Procedure
All procedures were identical to those described in Experiment 1, except that one day after conditioning (context A), rats were placed in a novel context (context B) and were either administered five unsignaled footshocks (SHOCK; 0.5s, 1.0 mA, ITI = 4 min) or were not shocked (NO-SHOCK). On Days 3 and 4, the rats were extinguished and tested, respectively, in context B. There were 12 animals in each group (IL and SH; SHOCK and NO-SHOCK). Both contexts were counterbalanced in all groups.
Histology and data analysis
Histology and data analyses were performed as described in Experiment 1.
Results
Experiment 1: Do strain differences influence the effects of IL lesions on fear extinction?
In this experiment, we examined the influence of focal electrolytic IL lesions on the extinction of conditioned freezing to an auditory CS in SD and LE rats. We used a conditioning and extinction procedure that has previously been shown to be sensitive to IL lesions in SD rats (Lebron et al., 2004; Quirk et al., 2000). As in previous studies, lesions of the IL were made prior to fear behavioral training.
Histology
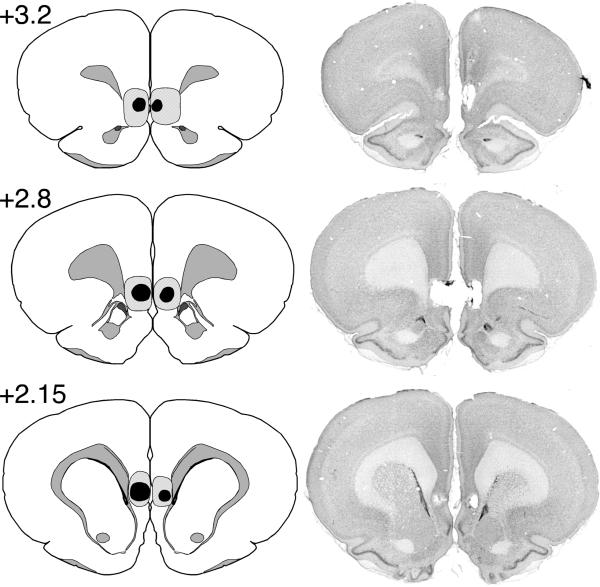
Maximum and minimum IL lesions and a representative IL lesion are shown in Figure 1. Only rats with focal bilateral IL lesions were included in the final data analyses. Animals were included if their lesion produced substantial IL damage in at least two of three coronal sections (+3.2 mm, +2.8 mm, and +2.15 mm relative to bregma). For the LE strain, three rats in the IL group were excluded, and one rat without an LE lesion was collapsed into the SH-E group. This yielded the following group sizes: IL (n = 13), SH-E (n = 17), and SH-NE (n = 16). For the SD strain, two rats in the IL group were excluded and three rats died during surgery. This yielded the following group sizes: IL (n = 14), SH-E (n = 14), and SH-NE (n = 15).
Figure 1.
Schematic illustration of the extent of minimal (black area) and maximal (shaded area) infralimbic (IL) cortical lesions in three coronal planes. The photomicrographs illustrate a representative IL lesion.
Behavior
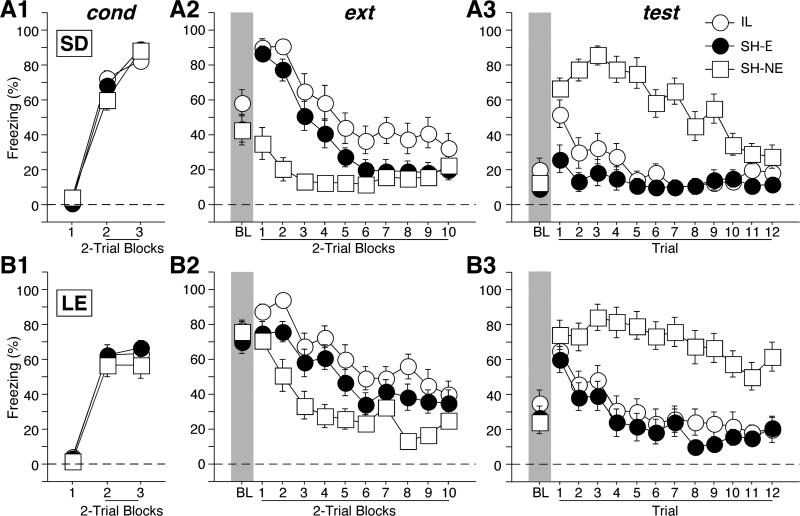
Freezing behavior during the tone CS across all behavioral phases is shown in Figure 2, with SD and LE strains in the upper and lower panels, respectively. Freezing behavior was low before the first conditioning trial (Figures 2A1 and 2B1), and then increased in magnitude thereafter. There was a significant main effect of strain [F(1,83) = 15.1, p = 0.0002], a significant main effect of trial block [F(2,166) = 623.9, p < 0.0001], and a significant interaction between strain and trial block [F(2,166) = 17.5, p < 0.0001]. Planned comparison revealed that between strains, there was a significant difference in freezing behavior only on the last trial block [F(1,87) = 36.3, p < 0.0001], suggesting that at the end of conditioning, SD rats spent more time freezing than LE rats.
Figure 2.
Percentage of freezing (mean ± SEM) during conditioning (cond), extinction (ext), and retention testing (test) for (A) Sprague-Dawley (SD) and (B) Long-Evans (LE) rats. Prior to conditioning, rats received infralimbic (IL) cortical lesions or sham surgery (SH-E). A sham control group that did not receive extinction (SH-NE) was also included. IL lesions produced a transient impairment in extinction recall in the earliest test trial of the test session in SD, but not LE rats.
Despite the fact that SD rats acquired higher levels of freezing at the end of conditioning, LE rats showed significantly higher freezing to the conditioning context before the first CS trial during extinction (Figures 2A2 and 2B2; BL periods). There was a significant main effect of strain [F(1,83) = 18.8, p < 0.0001]. Moreover, the effects of IL lesions across different trial blocks differed in the two strains (Figures 2A2 and 2B2; tone CS periods). There was a significant main effect of strain [F(1, 83) = 8.7, p = 0.0042], a significant main effect of group [F(2,83) = 23.6, p < 0.0001], a significant twoway interaction between group and trial blocks [F(18, 747) = 5.0, p < 0.0001], and a significant three-way interaction among strain, group, and trial blocks [F(18, 747) = 3.3, p < 0.0001]. Post hoc analyses revealed that LE rats showed higher freezing than SD rats [p < 0.05]. Moreover, rats with IL lesions showed the highest level of freezing and SHNE the lowest; SH-E rats exhibits intermediate level of freezing [all ps < 0.05]. Planned comparisons revealed that at the end of extinction, all groups in both strains showed equivalent and low freezing levels [F(5,83) = 1.5, p = 0.2], demonstrating that despite different rates of decrease in freezing levels across strains and lesions, all groups showed good within-session extinction toward the end.
Freezing behavior during the first 12 CSs of the test session is shown in Figures 2A3 and 2B3. Similar to the extinction session, LE rats showed significantly higher freezing to the context than SD rats before the first test trial. There was a significant main effect of strain [F(1,83) = 10.2, p = 0.002] (Figures 2A3 and 2B3; BL periods). Moreover, the effects of IL lesions across different trial blocks differed in the two strains (Figures 2A3 and 2B3; tone CS periods). There was a significant main effect of strain [F(1, 83) = 7.0, p = 0.01], a significant main effect of group [F(2,83) = 46.0, p < 0.0001], a significant two-way interaction between group and trial blocks [F(22, 913) = 5.5, p < 0.0001], and a significant three-way interaction among strain, group, and trial blocks [F(22, 913) = 2.8, p < 0.0001]. Planned comparisons revealed that during the first tone CS trial, there was a significant difference in freezing behavior across all groups [F(5,83) = 5.5, p = 0.0002]. There was a strain difference in spontaneous recovery with control LE rats showing more spontaneous recovery than SD rats [p < 0.05], suggesting that LE rats are more resistant to extinction than SD rats. Moreover, the effects of IL lesions also differed between the two strains during the first tone CS trial. Planned comparisons also revealed that for the SD strain, IL and SH-NE rats showed equivalent freezing levels [p = 0.15] that were significantly higher than SH-E animals [both ps < 0.05]. This indicates that SD rats with IL lesions failed to retrieve the extinction memory during the first test trial. However, for LE rats, there was no significant difference in freezing levels among all groups [all ps > 0.05].
The effect of IL lesions on the recall of extinction in SD rats was transient. Planned comparisons revealed that during the second tone CS trial, there was a significant difference in freezing behavior across all groups [F(5,83) = 11.5, p < 0.0001]. However, for both the SD and LE rats, IL and SH-E animals showed equivalent and significantly lower freezing than their SH-NE controls, respectively [all ps < 0.05]. For SD rats, there were no longer differences in freezing levels among the groups by the 11th trial [all ps > 0.05], while for LE rats, IL and SH-E animals showed equivalent and significantly lower freezing than SH-NE [both ps < 0.05] until the last trial in test session. Thus, IL lesions only impaired the retrieval of extinction memory in SD rats, and this impairment was most pronounced in the earliest trials of the extinction session.
Experiment 2: Does contextual fear influence the role of the IL in extinction in LE rats?
In Experiment 1, we show that focal IL lesions impair extinction retrieval in SD, but not LE rats. Interestingly, LE rats exhibited much higher levels of contextual fear prior to the outset of extinction training, and this may have impaired both extinction learning and IL function (Akirav & Maroun, 2007; Correll, Rosenkranz, & Grace, 2005; Izquierdo, Wellman, & Holmes, 2006; Maren & Chang, 2006). We therefore hypothesized that the effect of IL lesions on extinction recall may be influenced by the degree of contextual fear at the outset of extinction training. If so, reducing contextual fear in LE rats prior to extinction might facilitate extinction in control rats and unmask an effect of IL lesions. To test this hypothesis, rats were exposed to a novel context 24 hours after conditioning; half of them received unsignaled footshocks to increase contextual fear, and the other half were merely exposed to the context to reduce any generalized fear to the extinction context. All rats were then returned to the exposed context for extinction and retention test.
Histology
The criteria are the same as described in Experiment 1. On the basis of the histological results, 6 of 24 IL rats were excluded. This yielded the following group sizes: IL-SHOCK (n = 8), IL-NOSHOCK (n=10), SH-SHOCK (n = 12), and SH-NOSHOCK (n = 12).
Behavior
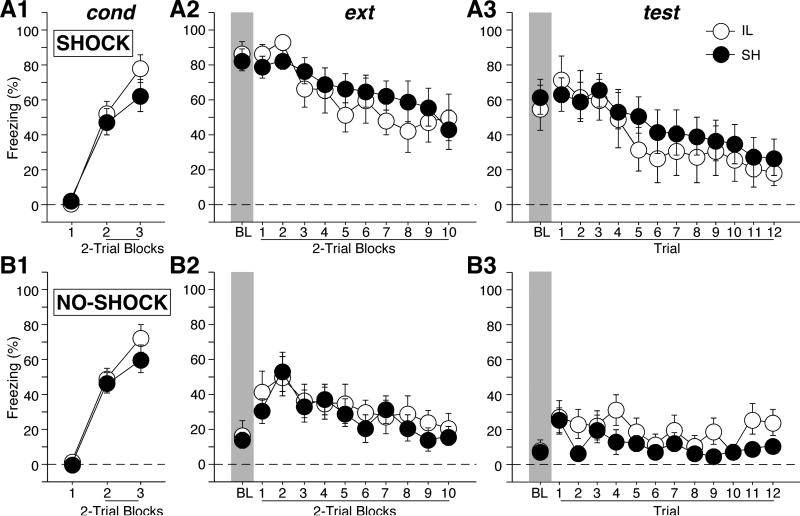
Freezing behavior during the conditioning session is shown in Figure 3. Freezing behavior was low before the first conditioning trial (Figures 3A1 and 3B1), and then increased in magnitude thereafter. There was an equivalent increase in freezing across trials in all groups [shock × lesion × trial, F(2,76) < 1].
Figure 3.
Percentage of freezing (mean ± SEM) during conditioning (cond), extinction (ext), and retention testing (test) in Long-Evans rats. Rats were divided into two groups for which contextual fear was either high (SHOCK) or low (NO-SHOCK) prior to extinction training. Prior to conditioning, rats received either infralimbic (IL) cortical lesions or sham surgery (SH-E). IL lesions did not produce an extinction recall impairment under either condition.
Freezing behavior during the extinction session is shown in Figures 3A2 and 3B2. As intended, rats that were shocked in the extinction context prior to extinction training exhibited significantly higher levels of freezing than rats that were merely exposed to the context (Figures 3A2 and 3B2; BL periods). There was a significant main effect of shock [F(1,38) = 115.0, p < 0.0001]. Moreover, this difference persisted across the extinction session, with shocked rats exhibiting higher levels of fear to the CS after extinction commenced (Figures 3A2 and 3B2; tone CS periods). Again, there was a significant main effect of shock during the extinction trials [F(1,38) = 20.5, p < 0.0001]. Rats with IL lesions showed equivalent fear to SH rats throughout the session [both Fs < 1].
Freezing behavior during the first 12 tones during the retention test is shown in Figures 3A3 and 3B3. Contrary to our hypothesis, there was no evidence of greater spontaneous recovery of fear in rats with IL lesions when contextual fear was reduced prior to extinction training. There was a significant main effect of shock during both the BL [F(1,38) = 37.5, p < 0.0001] and the tone CS periods [F (1,38) = 11.8, p = 0.0014]. However, there was no effect of IL lesion in either group [all Fs < 1]. Thus, extinction recall in LE rats with IL lesions is independent of contextual fear before extinction.
Discussion
In the present study, we directly examined the effects of focal IL lesions on the retrieval of extinction memory in SD and LE rats with freezing as the behavioral index of fear. Our results reveal that IL lesions produce a transient impairment in the retrieval of extinction memory in SD, but not LE rats (Experiment 1). The failure of LE rats with IL lesions to show an impairment in extinction recall was not related to their higher levels of contextual fear. Reducing contextual fear in LE rats prior to extinction did not uncover an effect of IL lesions (Experiment 2). Thus, under identical extinction protocols, behavioral measures, and lesion procedures, only SD rats exhibited an impairment in extinction after IL lesions. We therefore conclude that rat strain is an important factor in determining the role of the IL in the long-term retention of extinction.
Although IL lesions in SD rats impaired extinction recall, this effect was transient. Consistent with earlier studies (Lebron et al., 2004; Quirk et al., 2000), SD rats with IL lesions exhibited savings and consequently acquired extinction at a faster rate than non-extinguished controls during the test session. Despite mounting evidence that the IL is involved in long-term extinction (Quirk & Mueller, 2008), it is well known that the IL is not the only brain structure involved in extinction memory. The savings of extinction memory in SD rats and the normal recall in LE rats of the extinction training after permanent IL lesions may reflect compensation by other brain structures such as the amygdala (Bouton et al., 2006; Davis, Walker, & Myers, 2003; Quirk & Mueller, 2008). Fear extinction is impaired by antagonizing NMDA receptors (Falls, Miserendino, & Davis, 1992) or inhibiting GABAA receptor insertion (Lin, Mao, & Gean, 2009) in the amygdala, suggesting that local plasticity in the amygdala is critical for extinction memory. The hippocampus may also play a role in maintaining extinction memories. Extinction-related changes in neuronal activity in the lateral amygdala (Quirk, Repa, & LeDoux, 1995; Repa et al., 2001) are modulated by the hippocampus (Hobin, Goosens, & Maren, 2003; Maren & Hobin, 2007) and hippocampal inactivation impairs extinction learning (Corcoran, Desmond, Frey, & Maren, 2005).
Although the present data indicate that SD rats are more likely to exhibit extinction impairments after IL lesions, not all investigators have observed extinction impairments in this strain of rat (Farinelli et al., 2006; Gewirtz et al., 1997; Morgan et al., 2003). Hence, it is likely the factors other than strain also influence the sensitivity of extinction to IL lesions. One possibility is that extinction impairments are related to lesion size. In the studies that did not report extinction impairments after IL lesions in SD rats, the lesions were quite extensive and included both the IL and PrL. There is emerging evidence that the PrL is involved in the expression of conditioned fear (Corcoran & Quirk, 2007; Vidal-Gonzalez et al., 2006), and damage to this area might therefore mask extinction deficits after IL lesions.
The observation of strain differences in the effect of IL lesions on extinction is not surprising in the light of several reports of strain differences in fear-motivated behavior (Balogh & Wehner, 2003; Brinks, de Kloet, & Oitzl, 2008; Capone, Venerosi, Puopolo, Alleva, & Cirulli, 2005; Glowa & Hansen, 1994; Hefner et al., 2008; Lopez-Aumatell et al., 2009; Neophytou et al., 2000; Rex, Sondern, Voigt, Franck, & Fink, 1996; Waddell, Dunnett, & Falls, 2004). There are also many reports of strain differences in the effects of a variety of pharmacological challenges and brain lesions on defensive behaviors (Gerlai, 1998; Hefner et al., 2008; Restivo, Passino, Middei, & Ammassari-Teule, 2002; Solecki, Turek, Kubik, & Przewlocki, 2009). On one hand, strain differences in the involvement of the medial prefrontal cortex in extinction might raise doubts about the use of rats to model human extinction circuits. On the other hand, differences between strains, which are presumably due to genetic differences, provide a powerful model for understanding individual differences in anxiety-related behavior in both rodents and humans (Caldarone et al., 1997; Yang et al., 2008). Indeed, individual differences in mPFC-amygdala connectivity are related to variability in trait anxiety in humans (Kim & Whalen, 2009) and a genetic variant common to both mice and humans influences extinction learning (Soliman et al., 2010). Together with the present work, these studies indicate that genetic background is a key determinant of the structure and function of neural circuits involved in fear conditioning and extinction.
Acknowledgements
This work was supported by a grant from the National Institutes of Health (R01MH065961).
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bne
References
- Akirav I, Maroun M. The role of the medial prefrontal cortex-amygdala circuit in stress effects on the extinction of fear. Neural Plast. 2007;2007:30873. doi: 10.1155/2007/30873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balogh SA, Wehner JM. Inbred mouse strain differences in the establishment of long-term fear memory. Behav Brain Res. 2003;140(1-2):97–106. doi: 10.1016/s0166-4328(02)00279-6. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol Psychiatry. 2002;52(10):976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context and behavioral processes in extinction. Learn Mem. 2004;11(5):485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Mineka S, Barlow DH. A modern learning theory perspective on the etiology of panic disorder. Psychol Rev. 2001;108(1):4–32. doi: 10.1037/0033-295x.108.1.4. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biol Psychiatry. 2006;60(4):352–360. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Brinks V, de Kloet ER, Oitzl MS. Strain specific fear behaviour and glucocorticoid response to aversive events: modelling PTSD in mice. Prog Brain Res. 2008;167:257–261. doi: 10.1016/S0079-6123(07)67019-8. [DOI] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ. Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron. 2007;53(6):871–880. doi: 10.1016/j.neuron.2007.02.021. [DOI] [PubMed] [Google Scholar]
- Caldarone B, Saavedra C, Tartaglia K, Wehner JM, Dudek BC, Flaherty L. Quantitative trait loci analysis affecting contextual conditioning in mice. Nat Genet. 1997;17(3):335–337. doi: 10.1038/ng1197-335. [DOI] [PubMed] [Google Scholar]
- Capone F, Venerosi A, Puopolo M, Alleva E, Cirulli F. Behavioral responses of 129/Sv, C57BL/6J and DBA/2J mice to a non-predator aversive olfactory stimulus. Acta Neurobiol Exp (Wars) 2005;65(1):29–38. doi: 10.55782/ane-2005-1537. [DOI] [PubMed] [Google Scholar]
- Corcoran KA, Desmond TJ, Frey KA, Maren S. Hippocampal inactivation disrupts the acquisition and contextual encoding of fear extinction. J Neurosci. 2005;25(39):8978–8987. doi: 10.1523/JNEUROSCI.2246-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Quirk GJ. Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. J Neurosci. 2007;27(4):840–844. doi: 10.1523/JNEUROSCI.5327-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll CM, Rosenkranz JA, Grace AA. Chronic cold stress alters prefrontal cortical modulation of amygdala neuronal activity in rats. Biol Psychiatry. 2005;58(5):382–391. doi: 10.1016/j.biopsych.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Myers KM. Role of the amygdala in fear extinction measured with potentiated startle. Ann N Y Acad Sci. 2003;985:218–232. doi: 10.1111/j.1749-6632.2003.tb07084.x. [DOI] [PubMed] [Google Scholar]
- Falls WA, Miserendino MJ, Davis M. Extinction of fear-potentiated startle: blockade by infusion of an NMDA antagonist into the amygdala. J Neurosci. 1992;12(3):854–863. doi: 10.1523/JNEUROSCI.12-03-00854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farinelli M, Deschaux O, Hugues S, Thevenet A, Garcia R. Hippocampal train stimulation modulates recall of fear extinction independently of prefrontal cortex synaptic plasticity and lesions. Learn Mem. 2006;13(3):329–334. doi: 10.1101/lm.204806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia R, Chang CH, Maren S. Electrolytic lesions of the medial prefrontal cortex do not interfere with long-term memory of extinction of conditioned fear. Learn Mem. 2006;13(1):14–17. doi: 10.1101/lm.60406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlai R. Contextual learning and cue association in fear conditioning in mice: a strain comparison and a lesion study. Behav Brain Res. 1998;95(2):191–203. doi: 10.1016/s0166-4328(97)00144-7. [DOI] [PubMed] [Google Scholar]
- Gewirtz JC, Falls WA, Davis M. Normal conditioned inhibition and extinction of freezing and fear-potentiated startle following electrolytic lesions of medical prefrontal cortex in rats. Behav Neurosci. 1997;111(4):712–726. doi: 10.1037//0735-7044.111.4.712. [DOI] [PubMed] [Google Scholar]
- Glowa JR, Hansen CT. Differences in response to an acoustic startle stimulus among forty-six rat strains. Behav Genet. 1994;24(1):79–84. doi: 10.1007/BF01067931. [DOI] [PubMed] [Google Scholar]
- Hefner K, Whittle N, Juhasz J, Norcross M, Karlsson RM, Saksida LM, Bussey TJ, Singewald N, Holmes A. Impaired fear extinction learning and cortico-amygdala circuit abnormalities in a common genetic mouse strain. J Neurosci. 2008;28(32):8074–8085. doi: 10.1523/JNEUROSCI.4904-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, Garcia R. Prefrontal cortex long-term potentiation, but not long-term depression, is associated with the maintenance of extinction of learned fear in mice. J Neurosci. 2002;22(2):577–583. doi: 10.1523/JNEUROSCI.22-02-00577.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobin JA, Goosens KA, Maren S. Context-dependent neuronal activity in the lateral amygdala represents fear memories after extinction. J Neurosci. 2003;23(23):8410–8416. doi: 10.1523/JNEUROSCI.23-23-08410.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugues S, Chessel A, Lena I, Marsault R, Garcia R. Prefrontal infusion of PD098059 immediately after fear extinction training blocks extinction-associated prefrontal synaptic plasticity and decreases prefrontal ERK2 phosphorylation. Synapse. 2006;60(4):280–287. doi: 10.1002/syn.20291. [DOI] [PubMed] [Google Scholar]
- Hugues S, Deschaux O, Garcia R. Postextinction infusion of a mitogen-activated protein kinase inhibitor into the medial prefrontal cortex impairs memory of the extinction of conditioned fear. Learn Mem. 2004;11(5):540–543. doi: 10.1101/lm.77704. [DOI] [PubMed] [Google Scholar]
- Hugues S, Garcia R. Reorganization of learning-associated prefrontal synaptic plasticity between the recall of recent and remote fear extinction memory. Learn Mem. 2007;14(8):520–524. doi: 10.1101/lm.625407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Wellman CL, Holmes A. Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. J Neurosci. 2006;26(21):5733–5738. doi: 10.1523/JNEUROSCI.0474-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Whalen PJ. The structural integrity of an amygdala-prefrontal pathway predicts trait anxiety. J Neurosci. 2009;29(37):11614–11618. doi: 10.1523/JNEUROSCI.2335-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent V, Westbrook RF. Distinct contributions of the basolateral amygdala and the medial prefrontal cortex to learning and relearning extinction of context conditioned fear. Learn Mem. 2008;15(9):657–666. doi: 10.1101/lm.1080108. [DOI] [PubMed] [Google Scholar]
- Lebron K, Milad MR, Quirk GJ. Delayed recall of fear extinction in rats with lesions of ventral medial prefrontal cortex. Learn Mem. 2004;11(5):544–548. doi: 10.1101/lm.78604. [DOI] [PubMed] [Google Scholar]
- Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Pare D. Amygdala intercalated neurons are required for expression of fear extinction. Nature. 2008;454(7204):642–645. doi: 10.1038/nature07167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HC, Mao SC, Gean PW. Block of gamma-Aminobutyric Acid-A Receptor Insertion in the Amygdala Impairs Extinction of Conditioned Fear. Biol Psychiatry. 2009;30:30. doi: 10.1016/j.biopsych.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Lopez-Aumatell R, Blazquez G, Gil L, Aguilar R, Canete T, Gimenez-Llort L, Tobena A, Fernandez-Teruel A. The Roman High- and Low-Avoidance rat strains differ in fear-potentiated startle and classical aversive conditioning. Psicothema. 2009;21(1):27–32. [PubMed] [Google Scholar]
- Maren S. Overtraining does not mitigate contextual fear conditioning deficits produced by neurotoxic lesions of the basolateral amygdala. J Neurosci. 1998;18(8):3088–3097. doi: 10.1523/JNEUROSCI.18-08-03088.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Chang CH. Recent fear is resistant to extinction. Proc Natl Acad Sci U S A. 2006;103(47):18020–18025. doi: 10.1073/pnas.0608398103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Hobin JA. Hippocampal regulation of context-dependent neuronal activity in the lateral amygdala. Learn Mem. 2007;14(4):318–324. doi: 10.1101/lm.477007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Guo L. Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience. 1996;71(1):55–75. doi: 10.1016/0306-4522(95)00417-3. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420(6911):70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Milad MR, Vidal-Gonzalez I, Quirk GJ. Electrical stimulation of medial prefrontal cortex reduces conditioned fear in a temporally specific manner. Behav Neurosci. 2004;118(2):389–394. doi: 10.1037/0735-7044.118.2.389. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Romanski LM, LeDoux JE. Extinction of emotional learning: contribution of medial prefrontal cortex. Neurosci Lett. 1993;163(1):109–113. doi: 10.1016/0304-3940(93)90241-c. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Schulkin J, LeDoux JE. Ventral medial prefrontal cortex and emotional perseveration: the memory for prior extinction training. Behav Brain Res. 2003;146(1-2):121–130. doi: 10.1016/j.bbr.2003.09.021. [DOI] [PubMed] [Google Scholar]
- Neophytou SI, Graham M, Williams J, Aspley S, Marsden CA, Beckett SR. Strain differences to the effects of aversive frequency ultrasound on behaviour and brain topography of c-fos expression in the rat. Brain Res. 2000;854(1-2):158–164. doi: 10.1016/s0006-8993(99)02334-3. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned Reflexes. Oxford University Press; Oxford, UK: 1927. [Google Scholar]
- Quirk GJ, Garcia R, Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry. 2006;60(4):337–343. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33(1):56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Repa JC, LeDoux JE. Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: Parallel recordings in the freely behaving rat. Neuron. 1995;15(5):1029–1039. doi: 10.1016/0896-6273(95)90092-6. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci. 2000;20(16):6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repa JC, Muller J, Apergis J, Desrochers TM, Zhou Y, LeDoux JE. Two different lateral amygdala cell populations contribute to the initiation and storage of memory. Nature Neuroscience. 2001;4(7):724–731. doi: 10.1038/89512. [DOI] [PubMed] [Google Scholar]
- Restivo L, Passino E, Middei S, Ammassari-Teule M. The strain-specific involvement of nucleus accumbens in latent inhibition might depend on differences in processing configural- and cue-based information between C57BL/6 and DBA mice. Brain Res Bull. 2002;57(1):35–39. doi: 10.1016/s0361-9230(01)00634-7. [DOI] [PubMed] [Google Scholar]
- Rex A, Sondern U, Voigt JP, Franck S, Fink H. Strain differences in fear-motivated behavior of rats. Pharmacol Biochem Behav. 1996;54(1):107–111. doi: 10.1016/0091-3057(95)02128-0. [DOI] [PubMed] [Google Scholar]
- Rothbaum BO, Davis M. Applying learning principles to the treatment of post-trauma reactions. Ann N Y Acad Sci. 2003;1008:112–121. doi: 10.1196/annals.1301.012. [DOI] [PubMed] [Google Scholar]
- Santini E, Ge H, Ren K, Pena de Ortiz S, Quirk GJ. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J Neurosci. 2004;24(25):5704–5710. doi: 10.1523/JNEUROSCI.0786-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra-Mercado D, Jr., Corcoran KA, Lebron-Milad K, Quirk GJ. Inactivation of the ventromedial prefrontal cortex reduces expression of conditioned fear and impairs subsequent recall of extinction. Eur J Neurosci. 2006;24(6):1751–1758. doi: 10.1111/j.1460-9568.2006.05014.x. [DOI] [PubMed] [Google Scholar]
- Solecki W, Turek A, Kubik J, Przewlocki R. Motivational effects of opiates in conditioned place preference and aversion paradigm--a study in three inbred strains of mice. Psychopharmacology (Berl) 2009;207(2):245–255. doi: 10.1007/s00213-009-1672-7. [DOI] [PubMed] [Google Scholar]
- Soliman F, Glatt CE, Bath KG, Levita L, Jones RM, Pattwell SS, Jing D, Tottenham N, Amso D, Somerville LH, Voss HU, Glover G, Ballon DJ, Liston C, Teslovich T, Van Kempen T, Lee FS, Casey BJ. A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science. 2010;327(5967):863–866. doi: 10.1126/science.1181886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Diaz-Mataix L, Bush DE, LeDoux JE. Dissociable roles for the ventromedial prefrontal cortex and amygdala in fear extinction: NR2B contribution. Cereb Cortex. 2009;19(2):474–482. doi: 10.1093/cercor/bhn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Mem. 2006;13(6):728–733. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell J, Dunnett C, Falls WA. C57BL/6J and DBA/2J mice differ in extinction and renewal of extinguished conditioned fear. Behav Brain Res. 2004;154(2):567–576. doi: 10.1016/j.bbr.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Yang RJ, Mozhui K, Karlsson RM, Cameron HA, Williams RW, Holmes A. Variation in mouse basolateral amygdala volume is associated with differences in stress reactivity and fear learning. Neuropsychopharmacology. 2008;33(11):2595–2604. doi: 10.1038/sj.npp.1301665. [DOI] [PubMed] [Google Scholar]