Abstract
Context
Working memory deficits are considered a core feature of schizophrenia. Several recent integrative papers have offered mechanistic computational and neurobiological models of the origins of this cognitive deficit.
Objective
To test predictions of these models using a new experimental paradigm from the basic science literature that makes it possible to determine whether patients with schizophrenia show: 1) deficits in working memory storage capacity, 2) deficits in the precision of working memory representations, and 3) an amplification of these deficits as the retention interval increases.
Design
Case control design. All subjects performed a color working memory test where they were asked to recall 3 or 4 items after a 1 or 4 second delay. All subjects also received a standard measure of intelligence and the MATRICS battery.
Setting
A tertiary care research outpatient clinic.
Patients
A total of 31 clinically stable patients with a DSM IV diagnosis of schizophrenia or schizoaffective disorder and 26 healthy volunteers participated. The two groups were similar in age, gender, and ethnicity distributions.
Main Outcome measures
We examined two outcome measures: 1) the number of items stored in working memory, and 2) the precision of the working memory representations.
Results
Patients showed a clear reduction in the number of items stored in working memory. Patients did not differ from controls in the precision of their working memory representations. There was no evidence of delay-related amplification of impairment in either capacity or precision.
Conclusions
Patients do not show the type of imprecision or delay-dependent amplification of impairment that are predicted on the basis of current models of the neurobiology of the illness. The models need to be revised to account for a pure reduction in the number of items that patients are able to store in working memory.
Introduction
Working memory (WM) has been a major focus of recent schizophrenia research, driven by robust behavioral evidence of patient impairment and neuroimaging evidence suggesting abnormalities in neural activity during the performance of WM tasks.1–4 This clinical literature has been motivated by basic cognitive science models suggesting that WM is a critical building block of many higher cognitive functions.5,6 Further, there is an extensive basic neuroscience literature suggesting that WM involves dopaminergic activity in prefrontal cortex, and the known abnormalities in dopaminergic function in schizophrenia would seem to be consistent with deficits in WM. 4, 7–11 More recently, findings from post-mortem neuropathological studies of patients with schizophrenia as well as genetic findings have implicated abnormalities in the neural circuitry involved in WM. 12–15
Several investigators have recently proposed integrative theoretical accounts of the biological origins of cognitive impairment in schizophrenia. Each account involves an effort to translate the behavioral implications of basic biological findings. Lisman et al 12 provide a circuit-based account of the implications of genetic findings involving the dopamine, glutamate, and GABA systems. They emphasize the cascading impact of reductions in inhibitory function needed to tune and focus cortical processing, with a particular focus on memory and sensory processing. Durstewitz and Seamans16 explicitly address WM and propose that D1 hypofunction would result in “highly unstable representations” leading to “an inability to hold and manipulate information.” Rolls et al17 address much of the same evidence from the standpoint of computational modeling, concluding that NMDA receptor hypofunction would result in a neural environment where the “stability of the attractor state is reduced, resulting in difficulty maintaining a short-term memory.” 17p701 Further, reductions in prefrontal dopamine function “could be measured as a decreased signal to noise ratio and impaired short-term memory performance”. 17p707
While these accounts primarily address basic biological mechanisms, they lead to testable predictions about the types of cognitive impairment that would be expected in schizophrenia. Further, it is much easier to test these behavioral predictions than the predictions these models make about cellular activity in patients. For example, both Durstewitz and Rolls imply that WM representations should be prone to accelerated decay due to network instability. Further, Rolls, Durstewitz and perhaps Lisman suggest that WM representations in patients will have a poor signal-to-noise ratio, which should be evident behaviorally in the form of reduced memory precision. Here we ask whether these theoretically motivated claims, rooted in neurobiological evidence, accurately reflect the WM performance of schizophrenia patients. To preview, we will argue that these theoretical accounts are largely at odds with the accumulated behavioral literature, and we will present evidence from a new paradigm that provides direct evidence that visual WM representations are neither less precise nor more prone to decay in schizophrenia. Instead, patients exhibit a reduction in the number of items they can concurrently maintain in WM.
The overall pattern of WM findings in the schizophrenia literature does not provide much support for the idea that WM representations are less stable in patients, leading to faster decay. In a meta-analytic review of the WM literature, including 65 separate effect-size estimates with retention intervals that ranged from one to 30 seconds, Lee and Park2 concluded that the extent of patient impairment did not vary with length of delay interval. That is, the WM impairment in schizophrenia is just as pronounced at a one-second delay as it is at longer delays, arguing against instability of the representations during the retention interval. However, relatively few studies have parametrically varied the retention interval, and these conclusions rely on comparisons across studies. Moreover, most studies used categorical response alternatives (e.g., same vs. different), which may have made it difficult to observe gradual declines in precision over time. Thus, it is possible that the methods employed have not been optimal to document representational instability.
A few studies have provided evidence of reduced WM precision in schizophrenia patients.18–23 In these studies, perceptual parameters or encoding durations were adjusted at a short retention interval to equate patient and control performance. Patients required more discriminable stimuli to reach the same level of performance at short retention intervals, which may indicate that their WM representations were less precise. In addition, some of these studies found greater rates of decline in the patients as the retention interval increased.18–20, 24 However, the threshold estimation procedures in these studies can lead to biased threshold estimates when subjects occasionally fail to encode the stimuli, either due to attention lapses or low WM capacity25. Thus, the findings of these studies may reflect a higher rate of all-or-none failures of encoding rather than instability or imprecision of the WM representations.
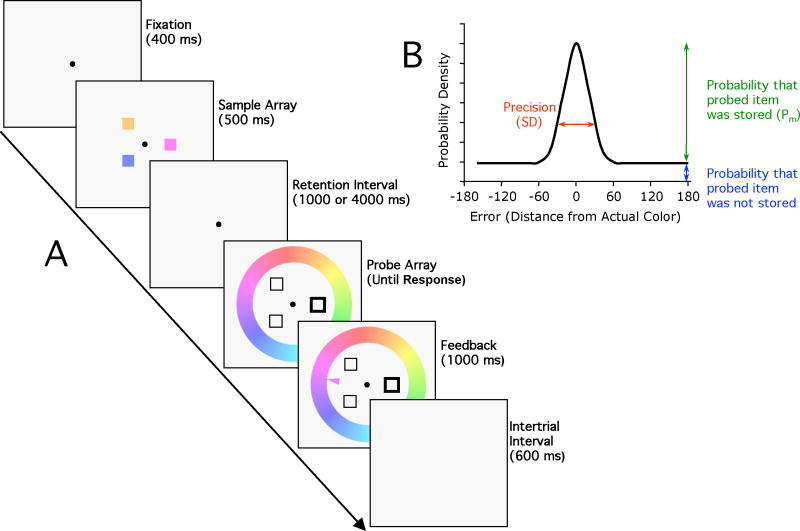
To provide a powerful test of WM instability in schizophrenia, a task must be able to directly measure the precision of WM representations, the number of representations that are stored in WM, and the decline in the number and/or precision of these representations with increasing delays. A new paradigm and analytic approach developed by Zhang and Luck 25,26 can separately measure each of these aspects of WM performance. As illustrated in Figure 1A, participants are first shown a sample array of 3–4 different colors for 500 ms. After a 1- or 4-second blank delay interval, one of the previous color locations is cued. Participants then indicate the color previously presented at the cued location by clicking on a color wheel displaying the entire range of possible colors.
Figure 1.
A. Stimuli from the color recall task. B. Model of performance. When the probed item is present in memory, the reported color is most likely to be at the original value, and the probability declines with distance from the original value. When the probed item is absent from memory, subjects report a randomly selected color, which adds a constant offset to the distribution of responses. The precision of the memory, when a memory was present, is inversely related to the width of the bell-shaped portion of the distribution, which is quantified as the standard deviation (SD). The height of the tails reflects the probability that the probed item was absent from memory, and the probability that the probed item was in memory (Pm) is 1 minus the probability that the probed item was absent from memory.
If the cued item is present in WM, the recalled color should be close to the color of the originally presented item, with a bell-shaped distribution of errors (see Figure 1B). If the cued item was not stored in WM, however, the response will be a random guess, leading to a flat distribution of errors. The observed data represent a mixture of these two types of trials, but it is possible to decompose this mixture, yielding two parameters that represent the two critical performance dimensions: (1) Pm (probability in memory) represents the probability that the cued item was stored in WM and was available at time of test; (2) SD (standard deviation) represents the width of the bell curve, which is inversely related to the precision of the WM representation for trials on which it was actually present in memory. Thus, reductions in WM capacity should be evident in lower Pm values, whereas reduced WM precision should be reflected in larger SD values. Most critically, a significant reduction in Pm in the absence of a difference in SD would indicate that the capacity reduction in schizophrenia cannot be explained on the basis of impaired WM precision. It should be noted that Pm would also be reduced if subjects accidentally reported the color of one of the uncued items; the frequency of this type of error can be assessed by examining the distribution of responses around each of the uncued colors.
The inclusion of two delay intervals also makes it possible to determine whether WM representations are less stable in patients than in controls, which would yield a reduction in Pm or an increase in SD over time. We chose delay intervals of 1 and 4 seconds because healthy young adults begin to show a decline in performance sometime between 4 and 10 seconds.25 If WM representations are unstable in patients, they should exhibit a decline at an earlier delay than do control subjects. We did not go beyond 4 seconds because longer delays may lead to an inability of patients to stay on task, artifactually producing the appearance of a WM decline.
In our view, recent theoretical accounts lead to strong predictions that patients should demonstrate reduced WM precision (i.e., an increased SD) and that the patient impairment of Pm and/or SD should be amplified as delay interval increases: each of these predictions is contradicted by the data presented here.
Methods
Participants
Thirty-one patients meeting DSM-IV27 criteria for schizophrenia (15 paranoid, 8 undifferentiated, 2 disorganized, 2 residual) or schizoaffective disorder (N=4), and 26 matched healthy control subjects participated in this study. Demographic information is summarized in Table 1. Groups were virtually identical in age and parental education, and did not differ in sex or ethnicity (Chi-square P>0.4 in both cases). However, patients had significantly fewer years of education than controls (P=0.005, independent-samples t-test).
Table 1.
Demographic and Cognitive features of the study groups
| Patients | Controls | |
|---|---|---|
| Age | 43.4 ± 8.4 (range 25–54) | 43.5 ± 9.4 (range 22–54) |
| Male: Female | 20:11 | 14:12 |
| Ethnicitya: AA: A: C: O | 9:1:19:2 | 9:0:17:0 |
| Education (years) | 13.1 ± 2.3 | 14.8 ± 2.1 |
| Parental educationc | 13.3 ± 3.3b | 13.3 ± 2.8 |
| WASI IQ | 96.3 ± 13.3b | 113.9 ± 12.8 |
| WRAT-4 | 95.6 ± 12.5b | 101.8 ± 16.4 |
| WTAR | 98.7 ± 15.1b | 105.8 ± 15.5 |
| MATRICS Total T score | 32.3 ± 11.6b | 50.3 ± 13.2 |
AA= African American, A=Asian, C= Caucasian, O = other
Data unavailable for 1 patient.
Average over mother’s and father’s years of education.
The patients were clinically stable outpatients. At the time of testing, patients were mildly/moderately symptomatic with a total score of 37.7 ± 8.0 (mean ± stdev) on the Brief Psychiatric Rating Scale (BPRS, range 24–65), 36.2 ± 14.4 on the Scale for the Assessment of Negative Symptoms (SANS, 14–72), 2.6 ± 2.5 on the Calgary Depression Scale (0–12).28–30 All patients were receiving antipsychotic medication; one was treated with a first-generation antipsychotic, 29 with second-generation antipsychotics, and one with both. Eighteen patients received clozapine, either alone or in combination with other second-generation antipsychotics. Nineteen patients also received mood-stabilizing medications, and nine received anxiolytic medication. Patients were on stable medications for a minimum of 4 weeks prior to testing. Control participants were recruited from the community and had no current Axis 1 or 2 diagnosis as established by the SCID, 31,32 had no family history of psychosis, and were not taking any psychotropic medication. All participants provided informed consent for a protocol approved by the University of Maryland School of Medicine Institutional Review Board.
Neuropsychological testing
All participants completed the Wechsler Abbreviated Scale of Intelligence (WASI), the Wide Range Achievement Test Reading (WRAT 4), the Wechsler Test of Adult Reading (WTAR), and the MATRICS battery.33–36 Patients tended to score lower than controls on the WASI (P<0.001, independent-samples t-test), WRAT (P=0.12), WTAR (P=0.09) and MATRICS battery (P<0.001; see Table 1).
Stimuli and Task
Stimuli were presented on a CRT monitor with a grey background (Figure 1). Each trial commenced with a fixation circle that remained visible throughout the trial. After 400 ms, a sample array consisting of three or four colored squares was presented for 500 ms. Each square subtended 2×2° of visual angle and was presented at one of eight possible positions on an invisible circle with a 4.5° radius. A delay of either 1 or 4 seconds followed. The probe array was then presented, surrounded by a color wheel (8.2° radius, 2.2° thick) consisting of 180 equally spaced equiluminant color values that covered the entire spectrum (see reference 26 for details). The sample array colors were randomly selected from this set with a minimum distance of 24 degrees between any two colors. The orientation of the color wheel varied randomly across trials. The probe array consisted of outlined squares at the sample locations. One of the outlined squares was thicker than the others, indicating the item to be recalled. Subjects reported the color remembered at this location by mouse-clicking on the appropriate location in the color wheel. The probe array and color wheel remained visible until a response was made. After the response, a feedback arrow indicated the correct location on the color wheel for 1000 ms. After a 600-ms intertrial interval, the next trial began.
The three- and four-item versions of the task were tested in separate sessions on separate days, in counterbalanced order. The 1- and 4-second delay intervals were equally likely and were randomly intermixed within each session, with 150 trials presented at each delay in each session.
Each session began with two control tasks, one testing motor precision (20 trials) and one testing color perception precision (30 trials). To minimize memory requirements in these control tasks, the colored squares and color wheel were presented simultaneously and remained visible until a response was made. In the motor control task, one square was always white, and a thin white bar was presented at a random location on the color wheel. The task was to mouse-click on the white bar. In the sensory control task, one colored square was outlined, indicating that its color should be reported by clicking on the color wheel. After each response, an arrow indicated the correct location. Subjects were given no instructions regarding the use of verbal coding, but previous research indicates that verbal representations do not contribute significantly to the performance of tasks such as this. 37
Participants also performed a 60-trial change localization task to obtain a second measure of short-term memory for comparison, using the method of Gold et al. 38 (Experiment 5). Participants viewed an array of four colored squares for 100 ms. After a 900-ms delay, the four squares reappeared, and the task was to mouse-click on the one square that had changed color.
Data Analysis
Raw data consisted of the degree of error on each trial, i.e., the distance between the reported color and the original color value. Trials on which the probed item was not encoded into memory will yield a uniform distribution of error. In contrast, in trials on which the probed item was encoded, the recalled value will tend to be near the original color, and the error will follow a von Mises distribution (the circular analog of the Gaussian distribution). The two types of trials are mixed together in the data. As described by Zhang and Luck 25,26, a maximum likelihood algorithm39 was used to derive Pm, the probability that the probed item was present in memory, and SD, which is inversely related to the precision of the representation when the probed item was present in memory. Pm is inversely related to the height of the tails of the distribution, and SD is related to the width of the von Mises portion of the distribution. We estimated the total number of items that were present in WM at the time of test (storage capacity, K) by multiplying Pm by the set size (SS, 3 or 4).
K and SD were analyzed in ANOVAs with factors of Group, SS, and Delay length. K and SD during the sensory control task were analyzed by two-factor ANOVAs (Group × SS). Performance in the motor control task was too accurate to allow estimates of SD, and we therefore compared the average response error between groups with an independent-samples t-test.
Pearson correlations were established, separately for patients and controls, between participants’ task performance (K and SD scores) and their WASI IQ scores, total MATRICS battery scores, and capacity estimate (K) from the change localization task.
Results
Motor and sensory control tasks
The mean error of responses in the motor control task (clicking on a thin white bar) was close to zero and did not differ between patients and controls [t(55)=0.01, P>0.9, independent-samples t-test], indicating that patients were able to control the mouse just as well as control participants. In the sensory control task, the precision of color matching was lower for patients than control participants, and this was confirmed by a main effect of Group [F(1,55)=5.42, P<0.02]. This difference did not interact with SS (P>0.9). K was essentially at ceiling for both groups at both SSs in the sensory control condition, indicating that both groups of subjects understood the task and could report the color of the cued item when it was visible at the time of report.
Number of items represented in WM (K)
Figure 2 shows the observed distribution of response errors in each condition and the model fits. The model provided an excellent fit to the data, accounting for 99% of the variance in both patient and control participants (adjusted R2 for the pooled data of each group). As seen in Figure 3A, patients exhibited lower memory capacity (K) than controls at both SS3 and SS4, with a similar between-group difference at the 1- and 4-second delay intervals. Overall, K was slightly higher at SS4 than at SS3, which probably reflects a ceiling on performance for some subjects at SS3. K remained constant across the 1- and 4-second delays in the control group, as was previously observed with healthy college students25, and there was also no sign of a decline in patients. These impressions were statistically supported by a main effect of Group [F(1,55)=4.22, P<0.05] and a main effect of SS [F(1,55)=7.42, P<0.01], but no significant main effect (P>0.5) or interaction (P>0.2) involving Delay. The between-group difference was somewhat larger at SS4 than SS3, such that controls displayed a steeper increase in K from SS3 to SS4, but the Group by SS interaction fell short of significance (P=0.13). The overall effect size for the between group K difference was .56, very close to the meta-analytic mean effect size of .459 for visuospatial WM.2
Figure 2.
Observed distribution of recall error (difference between original value and reported value; symbols) and model fits (lines) for the sensory control task, the 1-second delay condition, and the 4-second delay condition).
Figure 3.
The number of task stimuli represented in short-term memory (K) and the precision of the representations (SD) in patients and control subjects in the sensory control task (Sensory), and the color recall task at 1 s delay (Short) and 4 s delay (Long). The graphs represent averages (± SEM) over 31 patients with schizophrenia and 26 control participants.
One possible explanation of the reduced K in patients is that they had difficulty binding the colors to their locations, causing them to report the color of one of the wrong items. We assessed this possibility by examining the distribution of responses relative to the unprobed colors, treating each unprobed item as if it were the probed item and estimating Pm and SD. We found that the distribution of responses around the unprobed items was essentially flat; Pm for the unprobed items was near zero (< 0.003) and did not differ significantly between groups (P>0.3). Thus, the reduced K observed for patients in the main analysis was not a consequence of reporting the color of the wrong item.
Precision of stored representations (SD)
As seen in Figure 3B, WM precision was very similar for patients and controls at both SSs and at each delay interval, and the mean SD value was nearly identical for patients (26.86) and controls (26.13) averaged across conditions. Indeed, there were no significant main effects or interactions involving Group [P>0.4]. A main effect of SS [F(1,55)=6.58, P<0.02] reflected somewhat lower memory precision at SS4 than SS3 in all participants. Most importantly, there was no significant main effect (P>0.5) or interaction (P>0.2) involving Delay. Thus, the precision achieved at a 1- second delay was fully maintained over the 4-second interval, which matches findings from healthy college-age subjects. 25
Performance Correlations
To determine whether estimates of WM capacity from the color wheel paradigm are similar to those observed with more conventional visual WM tasks, we examined the correlation between K estimates derived from the color wheel paradigm (averaged over delays and SSs) and from the change localization task. As expected, K values derived from the change localization task were significantly decreased in patients relative to controls [t(54)=3.51, P<0.001]. K scores for the two tasks were strongly correlated in both controls (R=0.63, P<0.001) and patients (R=0.65, P<0.001). Thus, both tasks appear to be measuring a similar ability in both groups.
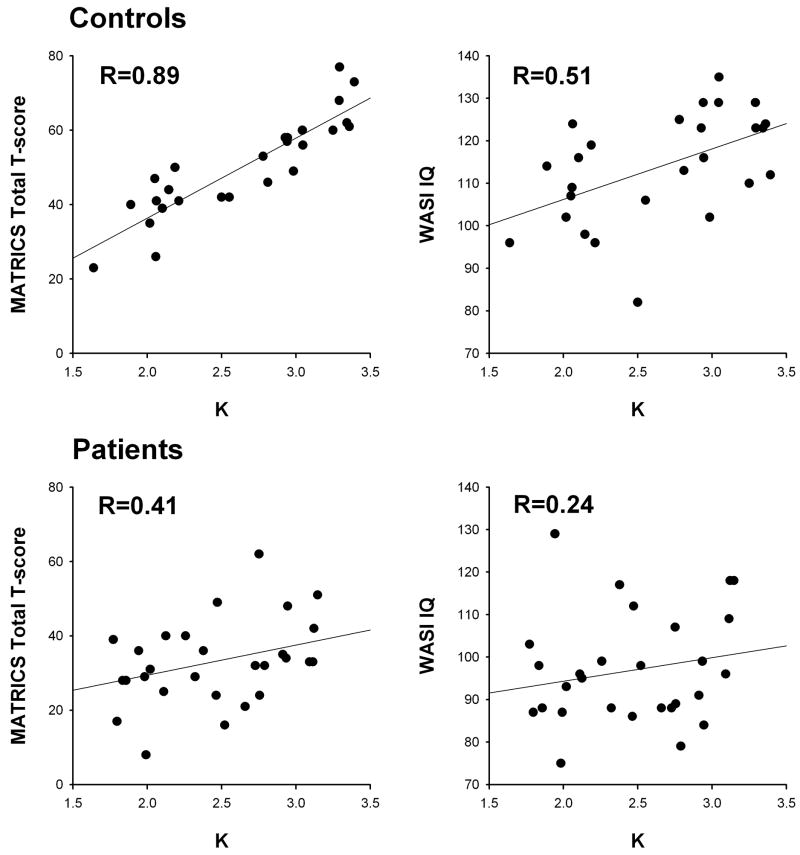
The correlations between K from the color wheel paradigm (averaged over delays and SSs), WASI IQ scores, and the overall T score from the MATRICS battery are shown in Figure 4. The Control participants displayed a remarkable correlation between K and total MATRICS score (R=0.89, P<0.001) and a moderate correlation between K and WASI IQ scores (R=0.51, P<0.01). SD correlated significantly with total MATRICS score (R=−0.46, P<0.02) but not WASI IQ (R=0.28, p=0.17). These correlations were attenuated in patients. The correlation of K with total MATRICS scores was significant (R=0.41, P<0.03) but significantly weaker than in controls (Fisher’s z-transformation test for difference in correlation: z=7.01, P<0.001). The correlation of K with WASI IQ was R=0.24 (P=0.2) in patients. In both patients and controls, similar but smaller magnitude correlations were observed with the MATRICS WM domain score as for the overall T score (R=0.72, P< 0.001 for controls; R=0.46, P<0.01 for patients). SD was not significantly correlated with either the total MATRICS score (R=−0.12, P>0.5) or WASI IQ (R=−0.18, P>0.3) in patients.
Figure 4.
Scatterplots and regression lines showing the relationship between WM capacity from the color wheel task (K, averaged over delays and set sizes) and MATRICS battery Total T score (left column) and IQ scores from the WASI (right column). Controls are shown in the top row, patients in the bottom row.
Discussion
These results provide several important insights into the nature of WM impairment in schizophrenia that constrain models linking cognitive deficits to the underlying neurobiological abnormalities. Patients show clear reductions in the number of items that can be stored in WM but no evidence that their WM representations are less precise or less stable than those of healthy individuals. Although it is possible that schizophrenia leads to less stable and less precise representations of other types of stimuli, the present results demonstrate that WM capacity reduction can and does occur in the absence of impairments in WM precision.
We observed no evidence for delay-related magnification of patient WM impairment: the patient deficit was equally robust at the 1-s and 4-s retention intervals. When combined with the lack of a reduction in precision, this absence of a magnification of impairment at a longer delay provides convincing evidence against the proposal that WM representations are unstable or inherently more noisy in schizophrenia. Moreover, this finding is consistent with earlier meta-analytic results showing a lack of delay dependency.2 However, the present results go beyond previous visual object WM studies by using a task that involves a fine-grained report, making it possible to separately measure the capacity and the precision of WM.
It is possible, of course, that evidence of instability could be obtained at longer delay intervals. In healthy college-age subjects, increasing the delay interval to 10 seconds resulted in a decline in capacity but no significant decline in precision 25, and it is possible that patients would show a decline in precision or a sharper decline in capacity at longer intervals. However, visual WM representations are typically used for periods ranging from a few hundred milliseconds to a few seconds in most real-world tasks.40 If schizophrenia involves a meaningful level of WM instability that is important for other cognitive operations, then it should be evident by a 4-second delay interval. Further, any impairments observed at long delay intervals could reflect the contribution of long-term memory systems or intermittent failures in goal maintenance rather than accelerated decay of WM representations.
Consistent with prior studies in healthy subjects, we observed a remarkably robust relationship between WM capacity and measures of general intellectual and cognitive ability.41–43 Indeed, the degree of covariation exceeded the levels typically documented in the literature. It will remain for future studies to determine if this is due to the unusual measurement accuracy offered by the color wheel paradigm or an unusual group of healthy participants. Note, the lower level of correlation seen in the patient cohort is more typical of the magnitude of relationship between WM capacity and cognitive ability in healthy populations.41 However, it is intriguing that the relationship between WM capacity and general ability is different in patients than in healthy subjects. Perhaps, as capacity is pathologically decreased, different systems are engaged in a compensatory fashion.
Note, these data do not and cannot contradict the biological findings reviewed by Lisman, Durstewitz, and Rolls. Instead they contradict the postulated links from biology to behavior. One of the challenges facing the field is the need to accurately translate the implications of findings across levels of evidence (from genes, to cells, to systems, to behavior) so that progress in one area serves to drive progress in another. Such progress requires that models at more basic levels be constrained by an accurate understanding of the behavioral endpoints that characterize the illness. These are the targets that need to be “hit” by models and theories. In our view, the recent biological accounts discussed above are at odds with much of the behavioral literature, and clearly at odds with the data presented here.
Before accepting this assertion, it is important to consider the limitations of the present findings. Our patients were stably treated outpatients with chronic schizophrenia, many treated with clozapine. Thus, our results may not generalize to less treatment resistant cohorts or to unmedicated early illness patients. Also, as in most studies of visual WM in schizophrenia, the present study examined WM performance for relatively simple stimuli. Additional mechanisms may come into play for more complex objects,44–46 and the present study would have been unable to detect impairments in these mechanisms. However, the predictions of the biological models are clearest for simple stimuli, for which precision is well defined.
It is also possible that our findings might prove to be specific to WM for color or other ventral stream features. Indeed, the best evidence for impaired WM stability in schizophrenia comes from studies showing delay-dependent drift in spatial memory.19,20,24 Nonetheless, our data demonstrate that WM storage capacity can be impaired without degradation in WM precision in at least one very common WM task. Moreover, the biological models provide no reason to suspect that WM representations would be any more stable for nonspatial information than for spatial information. If further studies confirm that dorsal stream WM representations are unstable in schizophrenia patients but ventral stream WM representations are not, then this will focus future theoretical efforts on the differences in circuitry between these types of representations.
It is also important to question the sensitivity of our methods. That is, might the experimental paradigm simply lack sensitivity to a change in precision? This is unlikely. Zhang and Luck 25 showed that several experimental manipulations significantly impacted the SD measure in healthy individuals. In the present study, SD was significantly smaller in the perceptual matching condition than in the memory conditions, and SD was significantly larger at SS 4 than at SS 3. Moreover, SD correlated significantly with measures of cognitive ability. Thus, the SD measure is sensitive to both experimental manipulations and individual differences. How then can we account for the observation of group differences in SD in the sensory control condition but similar WM precision? That is, how could WM precision be normal in the face of degraded sensory input? We suspect, but cannot prove, a very simple answer. In the control task, the sample array remained on the screen until a response was made. If controls (more so than patients) looked back and forth between the sample array and the color wheel in the sensory control condition, this would have decreased the SD in this condition but not in the WM task, where encoding time was controlled. Unfortunately, we did not record response times or monitor eye movements, the evidence that is needed to confirm the proposed explanation. Note, however, that the purpose of the sensory control condition was to aid in the interpretation of any differences in SD in the memory conditions; because patient and control SDs were nearly identical in the memory conditions (26.86 vs 26.13, averaged over conditions), the sensory control condition was not needed for this purpose.
Left unanswered is the question of the origins of the WM capacity limits in schizophrenia. Might capacity reduction result from a slowed encoding in patients? We consider this unlikely as in previous work we found nearly identical WM performance using 100- and 500-ms sample array exposures.26,37,47 Unfortunately, there is very little understanding of the origins of capacity limits in the basic cognitive neuroscience literature. Neuroimaging studies have implicated the intraparietal sulcus a likely contributor to visual WM capacity limits46, but animal physiology studies have not required subjects to retain multiple items concurrently in WM, and we therefore lack direct knowledge of the circuitry underlying WM capacity limitations. The field therefore has a great need for neurobiological models that can explain the nature of WM deficits in schizophrenia. However, these models must accurately capture the behavioral endpoint, which is characterized primarily by reductions in storage capacity and not by an instability of the WM representations.38,47
Acknowledgments
We gratefully acknowledge the contributions of Sharon August, Samuel Kaiser, and Lindsay Phebus to the conduct of the study. We thank our patient and healthy volunteer participants for their essential contributions.
This work was supported by NIMH R01 MH065034 and R01 MH076226. The NIMH had no role in the design, conduct, analysis, or reporting of the data
Footnotes
These data were presented as a talk at the International Congress on Schizophrenia Research in April 2009.
Conflicts of Interest Notifications: None of the authors have any conflicts of interest or financial disclosures that are relevant to the work presented here.
References
- 1.Barch DM. The cognitive neuroscience of schizophrenia. Annual Reviews of Clinical Psychology. 2005;1:321–353. doi: 10.1146/annurev.clinpsy.1.102803.143959. [DOI] [PubMed] [Google Scholar]
- 2.Lee J, Park S. Working memory impairments in schizophrenia: A meta-analysis. Journal of Abnormal Psychology. 2005;114:599–611. doi: 10.1037/0021-843X.114.4.599. [DOI] [PubMed] [Google Scholar]
- 3.Manoach DS. Prefrontal cortex dysfunction during working memory performance in schizophrenia: Reconciling discrepant findings. Schizophrenia Research. 2003;60:285–298. doi: 10.1016/s0920-9964(02)00294-3. [DOI] [PubMed] [Google Scholar]
- 4.Callicott JH, Mattay VS, Verchinski BA, Marenco S, Egan MF, Weinberger DR. Complexity of prefrontal cortical dysfunction in schizophrenia: More than up or down. American Journal of Psychiatry. 2003;160:2209–2215. doi: 10.1176/appi.ajp.160.12.2209. [DOI] [PubMed] [Google Scholar]
- 5.Baddeley AD. Working Memory. Clarendon; Oxford: 1986. [Google Scholar]
- 6.Miyake A, Shah P. Models of Working Memory. Cambridge, UK: Cambridge University Press; 1999. [Google Scholar]
- 7.Tanaka S. Dopaminergic control of working memory and its relevance to schizophrenia: A circuit dynamics perspective. Neuroscience. 2006;139:153–171. doi: 10.1016/j.neuroscience.2005.08.070. [DOI] [PubMed] [Google Scholar]
- 8.Tan HY, Callicott JH, Weinberger DR. Dysfunctional and compensatory prefrontal cortical systems, genes and the pathogenesis of schizophrenia. Cerebral Cortex. 2007;17(Supplement 1):i171–81. doi: 10.1093/cercor/bhm069. [DOI] [PubMed] [Google Scholar]
- 9.Abi-Dargham A, Moore H. Prefrontal DA transmission at D1 receptors and the pathology of schizophrenia. Neuroscientist. 2003;9:404–416. doi: 10.1177/1073858403252674. [DOI] [PubMed] [Google Scholar]
- 10.Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: Version III–The final common pathway. Schizophrenia Bulletin. 2009;35:549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Forti M, Lappin JM, Murray RM. Risk factors for schizophrenia all roads lead to dopamine. European Neuropsychopharmacology. 2007;17(Supplement 2):S101–107. doi: 10.1016/j.euroneuro.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, Grace AA. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends in Neuroscience. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kolluri N, Sun Z, Sampson AR, Lewis DA. Lamina-specific reductions in dendritic spine density in the prefrontal cortex of subjects with schizophrenia. American Journal of Psychiatry. 2005;162:1200–1202. doi: 10.1176/appi.ajp.162.6.1200. [DOI] [PubMed] [Google Scholar]
- 14.Arion D, Unger T, Lewis DA, Levitt P, Mirnics K. Molecular evidence for increased expression of genes related to immune and chaperone function in the prefrontal cortex in schizophrenia. Biological Psychiatry. 2007;62:711–721. doi: 10.1016/j.biopsych.2006.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hashimoto T, Arion D, Unger T, Maldonado-Aviles JG, Morris HM, Volk DW, Mirnics K, Lewis DA. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex in subjects with schizophrenia. Molecular Psychiatry. 2008;13:146–161. doi: 10.1038/sj.mp.4002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Durstewitz D, Seamans JK. The dual-state theory of prefrontal cortex dopamine function with relevance to catechol-o-methyltransferase genotypes and schizophrenia. Biological Psychiatry. 2008;64:739–749. doi: 10.1016/j.biopsych.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 17.Rolls ET, Loh M, Deco G, Winterer G. Computational models of schizophrenia and dopamine modulation in the prefrontal cortex. Nature Reviews Neuroscience. 2008;9:696–709. doi: 10.1038/nrn2462. [DOI] [PubMed] [Google Scholar]
- 18.Lencz T, Bilder RM, Turkel E, Goldman RS, Robinson D, Kane JM, Leiberman JA. Impairments in perceptual competency and maintenance on a visual delayed match-to-sample test in first-episode schizophrenia. Archives of General Psychiatry. 2003;60:238–243. doi: 10.1001/archpsyc.60.3.238. [DOI] [PubMed] [Google Scholar]
- 19.Tek C, Gold J, Blaxton T, Wilk C, McMahon RP, Buchanan RW. Visual perceptual and working memory impairments in schizophrenia. Archives of General Psychiatry. 2002;59:146–153. doi: 10.1001/archpsyc.59.2.146. [DOI] [PubMed] [Google Scholar]
- 20.Badcock JC, Badcock DR, Read C, Jablensky A. Examining encoding imprecision in spatial working memory in schizophrenia. Schizophrenia Research. 2008;100:144–152. doi: 10.1016/j.schres.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 21.Javitt DC, Liederman E, Cienfuegos A, Shelley AM. Panmodal processing imprecision as a basis for dysfunction of transient memory storage systems in schizophrenia. Schizophrenia Bulletin. 1999;25:763–775. doi: 10.1093/oxfordjournals.schbul.a033417. [DOI] [PubMed] [Google Scholar]
- 22.March L, Cienfuegos A, Goldbloom L, Ritter W, Cowan N, Javitt DC. Normal time course of auditory recognition in schizophrenia, despite impaired precision of the auditory sensory (“echoic”) memory code. Journal of Abnormal Psychology. 1999;108:69–75. doi: 10.1037//0021-843x.108.1.69. [DOI] [PubMed] [Google Scholar]
- 23.Rabinowicz EF, Silipo G, Goldman R, Javitt DC. Auditory sensory dysfunction in schizophrenia: Imprecision or distractibility? Archives of General Psychiatry. 2000;57:1149–1155. doi: 10.1001/archpsyc.57.12.1149. [DOI] [PubMed] [Google Scholar]
- 24.Park S, Holzman PS. Schizophrenics show spatial working memory deficits. Archives of General Psychiatry. 1992;49:975–982. doi: 10.1001/archpsyc.1992.01820120063009. [DOI] [PubMed] [Google Scholar]
- 25.Zhang W, Luck SJ. Sudden death and gradual decay in visual working memory. Psychological Science. 2009;20:423–428. doi: 10.1111/j.1467-9280.2009.02322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang W, Luck SJ. Discrete fixed-resolution representations in visual working memory. Nature. 2008;453:233–235. doi: 10.1038/nature06860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, D.C: Author; 2000. [Google Scholar]
- 28.Overall JE, Gorman DR. The Brief Psychiatric Rating Scale. Psychological Reports. 1962;10:799–812. [Google Scholar]
- 29.Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS) Iowa City, IA: University of Iowa; 1984. [Google Scholar]
- 30.Addington D, Addington J, Maticka-Tyndale E, Joyce J. Reliability and validity of a depression rating scale for schizophrenics. Schizophrenia Research. 1992;6(3):201–208. doi: 10.1016/0920-9964(92)90003-n. [DOI] [PubMed] [Google Scholar]
- 31.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV- Axis I Disorders (SCID-I) Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- 32.Pfohl B, Blum N, Zimmerman M, Stangl D. Structured Interview for DSM-III-R Personality Disorders (SIDP-R) Iowa City, IA: University of Iowa, Department of Psychiatry; 1989. [Google Scholar]
- 33.Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio TX: The Psychological Corporation; 1999. [Google Scholar]
- 34.Wilkinson GS, Robertson GJ. Wide Range Achievement Test (WRAT) 4. Lutz, Florida: Psychological Assessment Resources; 2006. [Google Scholar]
- 35.Wechsler D. Wechsler Test of Adult Reading (WTAR) San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- 36.Nuechterlein KH, Green MF. MATRICS Consensus Cognitive Battery, Manual. Los Angeles, CA: MATRICS Assessment Inc; 2006. [Google Scholar]
- 37.Vogel EK, Woodman GF, Luck SJ. Storages of features, conjunctions and objects in visual working memory. Journal of Experimental Psycholology: Human Perception and Performance. 2001;27(1):92–114. doi: 10.1037//0096-1523.27.1.92. [DOI] [PubMed] [Google Scholar]
- 38.Gold JM, Fuller RL, Robinson BM, McMahon RP. Intact attentional control of working memory encoding in schizophrenia. Journal of Abnormal Psychology. 2006;115(4):658–673. doi: 10.1037/0021-843X.115.4.658. [DOI] [PubMed] [Google Scholar]
- 39.Myung IJ. Tutorial on maximum likelihood estimation. Journal of Mathematical Psychology. 2003;47:90–100. [Google Scholar]
- 40.Luck SJ. Visual short-term memory. In: Luck SJ, Hollingworth A, editors. Visual Memory. New York: Oxford University Press; 2008. pp. 43–85. [Google Scholar]
- 41.Jarrold C, Towse JN. Individual differences in working memory. Neuroscience. 2006;139:39–50. doi: 10.1016/j.neuroscience.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 42.Unsworth N, Engle RW. On the division of short-term and working memory: An examination of simple and complex span and their relation to higher order abilities. Psychological Bulletin. 2007;133:1038–1066. doi: 10.1037/0033-2909.133.6.1038. [DOI] [PubMed] [Google Scholar]
- 43.Colom R, Rebollo I, Abad FJ, Shih PC. Complex span tasks, simple span tasks, and cognitive abilities: A reanalysis of key studies. Memory & Cognition. 2006;34:158–171. doi: 10.3758/bf03193395. [DOI] [PubMed] [Google Scholar]
- 44.Awh E, Barton B, Vogel EK. Visual working memory represents a fixed number of items regardless of complexity. Psychological Science. 2007;18(7):622–8. doi: 10.1111/j.1467-9280.2007.01949.x. [DOI] [PubMed] [Google Scholar]
- 45.Alvarez GA, Cavanagh P. The capacity of visual short-term memory is set both by visual information load and by number of objects. Psychological Science. 2004;15(2):106–111. doi: 10.1111/j.0963-7214.2004.01502006.x. [DOI] [PubMed] [Google Scholar]
- 46.Xu Y, Chun MM. Dissociable neural mechanisms supporting visual short-term memory for objects. Nature. 2006;440:91–95. doi: 10.1038/nature04262. [DOI] [PubMed] [Google Scholar]
- 47.Gold JM, Wilk CM, McMahon RP, Buchanan RW, Luck SJ. Working memory for visual features and conjunctions in schizophrenia. Journal of Abnormal Psychology. 2003;112(1):61–71. [PubMed] [Google Scholar]
- 48.Vogel EK, Woodman GF, Luck SJ. Storages of features, conjunctions and objects in visual working memory. Journal of Experimental Psychology: Human Perception and Performance. 2001;27(1):92–114. doi: 10.1037//0096-1523.27.1.92. [DOI] [PubMed] [Google Scholar]