Abstract
Background
TRA-8 is a murine agonist monoclonal antibody to death receptor 5 (DR5), which is able to trigger apoptosis in DR5 positive human tumor cells without the aid of crosslinking. It has demonstrated cytotoxicity in vitro and in vivo antitumor efficacy to a wide range of solid tumors in murine xenograft models. Tigatuzumab is a humanized IgG1 monoclonal antibody derived from TRA-8.
Methods
A phase I trial of tigatuzumab in patients with relapsed/refractory carcinomas (n = 16) or lymphoma (n = 1) was designed to determine the maximal tolerated dose (MTD), pharmacokinetics, immunogenicity, and safety. Three to six (3–6) patients were enrolled in successive escalating cohorts at doses ranging from 1 to 8 mg/kg weekly.
Results
Seventeen (17) patients enrolled, 9 in the 1-, 2-, and 4-mg/kg dose cohorts (3 in each cohort) and 8 in the 8-mg/kg dose cohort. Tigatuzumab was well tolerated with no DLTs observed, and the MTD was not reached. There were no study-drug–related grade 3 or 4, renal, hepatic, or hematologic toxicities. Plasma half-life was 6–10 days, and no anti-tigatuzumab responses were detected. Seven (7) patients had stable disease, with the duration of response ranging from 81 to 798 days.
Conclusions
Tigatuzumab is well tolerated, and the MTD was not reached. The high number of patients with stable disease suggests antitumor activity.
Key words: antibody, apoptosis, cancer, immunotherapy, targeted therapy
Introduction
The tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL), a member of the TNF superfamily of cytokines, is a type 2 membrane protein that is expressed in the majority of normal tissues and can undergo protease cleavage, resulting in a soluble form able to bind to TRAIL receptors.1,2 TRAIL has strong apoptosis-inducing activity against cancer cells in vitro and potent antitumor activity against tumor xenografts of various cancers in vivo.3–5 Unlike other death-inducing ligands of the TNF superfamily, TRAIL has been of particular interest in the development of cancer therapies, as it preferentially induces apoptosis of tumor cells, having little or no effect on normal cells.6 Five receptors for TRAIL have been identified, two of which, death receptor (DR)4 (TRAIL-R1) and DR5 (TRAIL-R2), have a cytoplasmic death domain and are able to trigger apoptosis in tumor cells via downstream caspase activation7,8; the other three receptors lack a cytoplasmic death domain and do not mediate apoptosis.4,5 Expression of either DR4 or DR5 is frequently detected in human cancers, including colon, gastric, pancreatic, ovarian, breast, and non-small-cell lung cancer with low or no expression in normal tissues.9,10 Agonistic monoclonal antibodies that specifically bind to DR4 and DR5 have been produced and represent a new generation of cancer therapy, as they are able to directly induce apoptosis of targeted tumor cells.3,10 The use of an agonistic monoclonal antibody against the death receptors instead of TRAIL could be advantageous; TRAIL targets multiple receptors, including both death receptors and decoy receptors. In addition, TRAIL has a much shorter circulating half-life, compared with monoclonal antibodies, and this can impact dose and schedule parameters. The very short half-life of TRAIL requires large and frequent dosing, compared with monoclonal antibodies. Further, there is considerable experience in the clinical use of monoclonal antibodies, therefore providing guidance in the development of single-agent and combination regimens.
The murine anti-DR5 monoclonal antibody, TRA-8 (mTRA-8), was selected from a series of anti-DR5 monoclonal antibodies based on its specificity, ability to trigger apoptosis in vitro without the use of crosslinking reagents, and lack of toxicity to human hepatocytes.11, It was shown to have potent in vitro cytotoxicity to a variety of human tumor cell lines and in vivo antitumor efficacy in murine xenograft models of human cancers.12 Its in vitro cytotoxicity and in vivo antitumor efficacy can be substantially enhanced in combination with a variety of chemotherapeutic agents and/or radiation.12,13 Tigatuzumab (CS-1008) is a humanized monoclonal antibody composed of the CDR regions of mTRA-8, as well as the variable region framework and constant regions of human immunoglobulin IgG-1 mAb58'CL. Tigatuzumab mediates a very similar pattern of in vitro cytotoxicity and in vivo antitumor efficacy as mTRA-8 and was well tolerated in preclinical toxicology studies in monkeys (unpublished results).
Here, we detail the first phase I dose-escalation trial of tigatuzumab in order to characterize its safety and tolerability as a single agent in patients with relapsed or refractory solid tumors and to determine the maximum tolerated dose (MTD), dose-limiting toxicity (DLT), pharmacokinetics, and immunogenicity in a weekly schedule of administration.
Patients and Methods
Trial design and objectives
This single-institution trial was designed to determine the MTD and to document the safety profile for tigatuzumab in patients with relapsed or refractory solid tumors or lymphomas (with no leukemic component). In addition, the trial characterized the pharmacokinetics, immunogenicity, and preliminary antitumor effects of tigatuzumab in patients.
Patient eligibility
Patients were eligible if they were ≥18 years in age and had histologic proof of metastatic solid tumors or lymphomas (with no leukemic component); expression of DR5 in the tumor cells was not required. Patients were required to be refractory to, or have relapsed after, standard systemic therapy. The number of previous systemic therapies was not limited. Patients recently treated with chemotherapy, immunotherapy, radiation therapy, or experimental drugs were eligible only after 4 weeks had elapsed since the most recent therapy and there was resolution of any toxicity associated with that previous therapy. All patients were required to have a life expectancy of at least 12 weeks and an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–2. The following laboratory values, completed ≤7 days before treatment, were required: a total absolute neutrophil count ≥1.5 × 109/L (1500/μL); a platelet count ≥100 × 109/L (100,000/μL); a hemoglobin level ≥9 g/dL; a total bilirubin count ≤1.5 × upper limit of normal (ULN); a serum creatinine level <1.5 mg/dL (or creatinine clearance >60 mL/min); and an aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP) level <2.5 × ULN (in patients with liver metastasis <4.5 × ULN). Investigators were required to conduct this trial according to good clinical practice guidelines and the Declaration of Helsinki.
Patients were excluded from protocol accrual for the following conditions: brain metastasis; an uncontrolled seizure disorder; spinal cord compression; carcinomatous meningitis; myocardial infarction; severe or unstable angina pectoris; coronary or peripheral artery bypass graft; New York Heart Association class III or IV congestive heart failure; cerebrovascular accident; transient ischemic attack; pulmonary embolism; deep vein thrombosis; clinically significant pulmonary disease; active infection; HIV-positive patients; chronic diarrhea; inflammatory bowel disease; partial bowel obstruction; a medical or psychiatric condition that might interfere with achieving the trial objectives; and pregnancy or lactation.
Given the institutional (University of Alabama at Birmingham) conflict of interest (i.e., intellectual property), institutional review board (IRB) review and approval were provided by the University of Pennsylvania IRB (Philadelphia, PA). All patients gave written informed consent to participate in the trial.
Trial design
This trial was an open-label, phase I, dose-escalation study with 3–6 patients at each dose level. Cohorts of patients received tigatuzumab at escalating dose levels of 1, 2, 4, and 8 mg/kg administered weekly by intravenous (i.v.) infusion. A treatment cycle was defined as 3 weekly infusions. Dose escalation to the next cohort did not occur until the first 3 patients in the previous cohort had completed 1 cycle of treatment without drug-related DLTs; dose escalation was continued until the MTD was identified or the dose of 8 mg/kg was reached. If a DLT was observed in the first 3 patients enrolled at a dose level, the cohort was expanded to 6 patients; the trial allowed dose escalation if no DLTs were seen in these additional 3 patients. DLTs were defined as follows: grade 3 or 4 nonhematologic toxicities (except alopecia, fatigue, anorexia, nausea, or fever without neutropenia); failure to recover to baseline toxicity after delaying the next dose by more than 2 weeks; grade 3 or 4 neutropenia complicated by fever ≥38.5°C, infection, or grade 4 neutropenia of at least 7 days in duration; and grade 4 thrombocytopenia, or grade 3 thrombocytopenia complicated by hemorrhage. The MTD was defined as the highest dose at which 0 of 3 or 1 of 6 patients experienced DLTs during the first cycle of therapy. The trial allowed enrollment of additional patients (up to 10) at the highest dose level (8 mg/kg) if the MTD was not reached, or at the MTD to further elucidate study-drug safety and pharmacokinetics.
Drug formulation and administration
Tigatuzumab was supplied to the study site by Daiichi Sankyo Pharma (Edison, NJ) as single-use vials containing 5 mg of sterile, preservative-free, monoclonal antibody. The lyophilized material of the vials was reconstituted with 1.2 mL of 0.9% sodium chloride USP. Tigatuzumab was diluted in a 0.9% sodium chloride injection and was administered as a continuous i.v. infusion, using a rate-regulating device. The initial dose was delivered over 90 minutes; if there were no infusion-associated adverse events with the first infusion, subsequent infusions were delivered over 30–60 minutes. The doses administered in this trial were calculated based on the protein content measured by the Lowry method.
Assessments
Baseline assessments included: signed informed consent; medical history and physical examination; serum chemistries (i.e., total bilirubin, serum creatinine, AST, ALT, ALP, lactate dehydrogenase, glucose, sodium, potassium, chloride, bicarbonate, calcium, phosphorus, total protein, uric acid, and creatinine kinase); serum immunoglobulins; urine; hematology analyses [i.e., complete blood counts (CBCs) with differential and platelet counts); a 12-lead electrocardiogram (ECG); human antitigatuzumab antibodies; and radiographic scans, including a fluorescein deoxy glucose (FDG-PET) scan.
Safety
Patients were monitored throughout the trial by physical examination, laboratory analyses, and assessment of adverse events. Adverse events were graded according to the National Cancer Institute–Common Toxicity Criteria (NCI-CTC), version 3. Complete physical examination, CBC with differential and platelet counts, and serum chemistries (as a baseline assessment) were conducted weekly for as long as the patient was receiving tigatuzumab. Physical examinations, CBC with differential and platelet counts, and serum chemistries (as a baseline assessment) were repeated at the end-of-study visit (30–45 days after the last dose of tigatuzumab), when feasible.
Efficacy
Antitumor responses were measured by using Response Evaluation Criteria In Solid Tumors,14 the Cotswold criteria for Hodgkin's lymphoma,15 and the International Workshop Standardized Response Criteria for patients with non-Hodgkin's lymphoma (NHL).16 Radiologic assessments, such as computed tomography (CT) and/or magnetic resonance imaging (MRI), were performed at baseline and on week 7 (before infusion of the seventh dose of tigatuzumab); patients who continued in the trial (with stable disease, partial response, or complete response) had radiologic assessments every 6 weeks until disease progression.
Tigatuzumab pharmacokinetics
Blood samples for pharmacokinetics were obtained pretherapy and 1, 3, 6, 12, 24, 48, 96, and 168 hours after the first infusion, and prior to study-drug infusion for subsequent cycles to determine study-drug accumulation. After the collection of samples, sera were separated and stored at −80°C until assay at a central laboratory. A validated sandwich-enzyme–linked immunosorbent assay (ELISA) method was used to measure tigatuzumab concentrations in sera. In brief, microtiter plates precoated with histidine-labeled soluble DR5 were incubated at 37°C for 1 hour with diluted serum samples (1:100) in triplicate. Bound tigatuzumab was detected by using a secondary horseradish peroxidase (HRP)-conjugated goat-antihuman Fc antibody incubated at 37°C for 1 hour, which was then incubated with TMB-soluble reagent at an ambient temperature for 20 minutes. The absorbance was measured with a SPECTRAmax (Molecular Devices, Sunnyvale, CA) 340PC spectrophotometer at 450 nm. Standard and control samples of tigatuzumab in normal human sera were included in each analytic run. The intra- and interassay precision and accuracy of the quality control samples in all the analytic runs were within ±10%.
Noncompartmental pharmacokinetic parameters, including maximum plasma or serum concentration (Cmax), concentration at the end of infusion (Ceoi), area under the curve to the last collection point (AUC0–last), area under the curve for dosing interval (AUC0–τ), area under the curve extrapolated to infinity (AUC0–∞), time of maximum concentration (Tmax), elimination-rate constant (kel), terminal half-life (t½), total clearance (CL), and volume of distribution at steady state (Vss), were calculated by using the WinNonlin version 4.0 program (Pharsight Corp., Mountain View, CA). Compartmental and population pharmacokinetic analysis were also conducted by using different models, such as the one- or two-compartmental model, with zero- or first-order input.
Human anti-tigatuzumab response
Blood samples were collected prior to dosing with tigatuzumab on days 1, 21, and 49, and then, every 6 weeks during treatment. An additional sample was collected 3 months after the cessation of tigatuzumab, when possible. Human antitigatuzumab antibodies were measured by a validated ELISA assay. Briefly, serum samples were added to a tigatuzumab-coated plate and incubated at 37°C for 1 hour, followed by incubation with biotinylated-tigatuzumab at 37°C for 1 hour. Plates were then incubated with streptavidin-HRP conjugate at 37°C for 1 hour, followed by incubation with TMB-soluble reagent at an ambient temperature for 20 minutes. The absorbance was measured with a SPECTRAmax 340PC spectrophotometer at 450 nm. Antibody titers were assessed for positive samples, based on dilutions with signals, above a cut-off point determined from negative control values.
Statistics
Descriptive statistics (i.e., mean, standard deviation, coefficient of variation, standard error of the mean, sample size, number missing, median, geometric mean, geometric coefficient of variation, minimum, and maximum) were used in the analysis of observations from this trial.
Results
Patient characteristics
As seen in Table 1, 17 patients (7 male and 10 female) were enrolled in the trial. Patient age ranged from 31 to 88 years (median, 57), and the majority of patients were white (12 whites and 5 blacks). The patients had a variety of cancer types, with colon cancer (n = 4) and cancer of the head and neck (n = 3) being the most common. Most patients had been heavily pretreated, with the mean number of prior treatment regimens at 2.2 (range, 1–3). The ECOG PS was 0 in 8 patients; 1 in 8 patients, and 2 in 1 patient.
Table 1.
Patient Characteristics
| Patient | Dose mg/kg (week) | Number of doses | Age | Gender | Diagnosis | PS | Prior regimens | Response | Duration (days) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 99 | 57 | M | Hepatocellular carcinoma | 0 | 3 | SD | 798 |
| 2 | 1 | 6 | 62 | F | Follicular lymphoma | 0 | 2 | PD | — |
| 3 | 1 | 30 | 58 | M | Carcinoma of head and neck | 0 | 1 | SD | 232 |
| 4 | 2 | 5 | 43 | F | Carcinoma of unknown primary | 0 | 3 | PD | — |
| 5 | 2 | 6 | 61 | M | Esophageal carcinoma | 2 | 3 | PD | — |
| 6 | 2 | 12 | 43 | F | Colon carcinoma | 0 | 2 | SD | 84 |
| 7 | 4 | 23 | 64 | F | Colon carcinoma | 1 | 3 | SD | 169 |
| 8 | 4 | 4 | 31 | F | Cervical carcinoma | 1 | 2 | PD | — |
| 9 | 4 | 12 | 52 | F | Cholangiocarcinoma | 0 | 2 | SD | 82 |
| 10 | 8 | 12 | 44 | M | Colon carcinoma | 1 | 2 | SD | 81 |
| 11 | 8 | 5 | 48 | F | Carcinoma of unknown primary | 0 | 2 | PD | — |
| 12 | 8 | 4 | 72 | F | Colon carcinoma | 1 | 3 | PD | — |
| 13 | 8 | 3 | 60 | M | Carcinoma of head and neck | 1 | 2 | PD | — |
| 14 | 8 | 2 | 88 | F | Pancreatic carcinoma | 1 | 2 | PD | — |
| 15 | 8 | 6 | 69 | M | Carcinoma of head and neck | 1 | 1 | PD | — |
| 16 | 8 | 16 | 52 | M | Hepatocellular carcinoma | 1 | 2 | SD | 126 |
| 17 | 8 | 6 | 50 | F | Breast carcinoma | 0 | 3 | PD | — |
M, male; F, female; SD, stable disease; PD, progressive disease; PS, performance status.
Phase I dose escalation
Three (3) patients per cohort were enrolled in the first three cohorts (1, 2, and 4 mg/kg), and 8 patients were enrolled in the highest dose cohort (8 mg/kg). No DLTs associated with tigatuzumab were seen, and the MTD was not reached. Of the 17 patients enrolled, 11 received at least 2 cycles of tigatuzumab (6 weekly doses of study drug). All patients received full doses at their assigned dosing level and schedule. As of December 2008, the total exposure to tigatuzumab ranged from 1000 mg (patient 4 in the 2-mg/kg dose cohort) to 11,400 mg (patient 1 in the 1-mg/kg dose cohort).
Toxicity
Tigatuzumab was very well tolerated at the doses and schedule used in the trial. There were no infusion reactions. Table 2 details the adverse events that occurred in 2 or more patients. None of these adverse events were classified as related to the study drug, and in general, they were attributed to signs and symptoms of progressive disease. For example, the grade 3 nausea and vomiting was a result of worsening abdominal ascites. One (1) patient with pancreatic cancer in the 8-mg/kg dose group discontinued treatment due to adverse events (weakness/asthenia); however, this was due to disease progression. There were no laboratory abnormalities of grade 3 or 4 severity. There was 1 death during the trial, which occurred 20 days after the last dose of tigatuzumab; this was as a result of radiologically proven disease progression. Thus, no drug-related DLTs were noted, and the MTD was not reached.
Table 2.
Adverse Events Observed in at Least 2 Patients Receiving Tigatuzumab
| |
Number of events (NCI-CTC grade) cohorts |
|
|||
|---|---|---|---|---|---|
| Adverse event | 1 mg/kg (n = 3) | 2 mg/kg (n = 3) | 4 mg/kg (n = 3) | 8 mg/kg (n = 8) | Overall |
| Nausea | 2 (1, 1) | — | 3 (1, 1, 1) | 3 (2, 2, 3) | 8 |
| Fatigue | 1 (1) | 1 (1) | 2 (1, 2) | 3 (1, 2, 2) | 7 |
| Cough | 3 (1, 1, 1) | 2 (1, 2) | 2 (1, 1) | 1 (1) | 8 |
| Anemia | 1 (2) | 1 (2) | 1 (2) | 2 (2, 2) | 5 |
| Pyrexia | 1 (1) | — | 1 (1) | 3 (1, 1, 1) | 5 |
| Vomiting | — | — | 1 (1) | 3 (1, 2, 3) | 4 |
| Dyspnea | 1 (1) | — | 1 (2) | 2 (2, 3) | 4 |
| Diarrhea | 1 (1) | — | — | 2 (1, 1) | 3 |
| Asthenia | 1 (2) | — | 1 (1) | 1 (3) | 3 |
| Arthralgia | 1 (1) | — | 2 (1, 2) | — | 3 |
| Back pain | — | 1 (1) | 2 (2, 2) | — | 3 |
| Rash | — | — | 1 (1) | 2 (1, 1) | 3 |
| Constipation | 2 (1, 1) | — | 1 (1) | — | 3 |
| Edema peripheral | 1 (2) | — | — | 1 (1) | 2 |
| Decreased appetite | — | — | 1 (1) | 1 (1) | 2 |
| Fluid retention | — | 1 (1) | — | 1 (1) | 2 |
| Pleural effusion | — | — | 1 (3) | 1 (3) | 2 |
| Pulmonary embolism | — | — | 1 (3) | 1 (3) | 2 |
| Sinus congestion | 1 (1) | 1 (1) | — | — | 2 |
| Paresthesia | — | 1 (1) | 1 (1) | — | 2 |
| Depression | 1 (1) | — | 1 (2) | — | 2 |
| Bone pain | 1 (1) | — | — | 1 (1) | 2 |
| Abdominal pain: lower | — | — | 1 (2) | 1 (2) | 2 |
| Abdominal pain: upper | — | — | 1 (2) | 1 (3) | 2 |
NCI-CTC, National Cancer Institute–Common Toxicity Criteria.
Antitumor effects
There were no objective responses in this trial. As seen in Table 1, 7 patients had stable disease lasting longer than 81 days. Patient 1 is notable in that he had stable disease for 26 months. This 57-year-old male entered the trial with progressive metastatic hepatocellular carcinoma, having previously failed treatment with doxorubicin (as a single agent), paclitaxel and carboplatin (given in combination), and docetaxel (as a single agent). He had histologically proven lesions in the liver and the right humerus, with severe pain in the right humerus and right-upper quadrant of the abdomen. The patient became pain free within 6 weeks of initiation of treatment with tigatuzumab and was asymptomatic with stable disease for 26 months.
Tigatuzumab pharmacokinetics
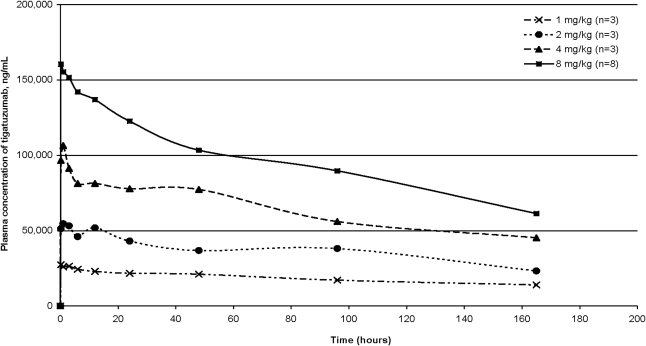
Pharmacokinetic results are shown in Figure 1 and Table 3. Dose-proportional pharmacokinetics were seen with the administration of tigatuzumab. Peak and trough plasma levels of tigatuzumab showed a dose-dependent increase (Table 3) and were maintained for at least 150 hours. The data demonstrated a half-life of 6–10 days for tigatuzumab, with approximately a 3-fold accumulation of the antibody at steady state when given on a weekly schedule, as compared with plasma concentrations observed during the first week of treatment. The plasma-clearance rate was similar to other humanized monoclonal antibodies, and the volume of distribution approximated the normal plasma volume.
FIG. 1.
Mean plasma tigatuzumab levels following initial infusion (week 1) for patients in four dose cohorts.
Table 3.
Pharmacokinetic Parametersa Week 1 of Tigatuzumab Infusion
| CS-1008 (mg/kg) | Cmax (μg/mL) | Cmin—168 hours (μg/mL) | AUC0–168 (μg/mL · hr) | Tmax (Hours) | T½(Hours) | Cl (mL/hours) | VD (mL) |
|---|---|---|---|---|---|---|---|
| 1 | 29.6 ± 2.7 | 14.0 ± 3.6 | 3140.5 ± 182.9 | 2.4 ± 1.6 | 227.8 ± 5.4 | 11.7 ± 1.0 | 3875.3 ± 1105.9 |
| 2 | 56.7 ± 10.2 | 30.2 ± 9.1 | 6220.9 ± 1072.1 | 6.4 ± 6.0 | 163.5 ± 46.2 | 18.5 ± 5.8 | 4256.9 ± 1521.8 |
| 4 | 110.2 ± 16.4 | 48.8 ± 11.5 | 10753.8 ± 876.9 | 2.2 ± 0.7 | 154.9 ± 25.7 | 14.4 ± 1.2 | 3177.4 ± 3177.4 |
| 8 | 167.0 ± 41.4 | 58.6 ± 23.9 | 16,081.0 ± 4244.9 | 1.8 ± 0.4 | 149.4 ± 38.6 | 20.1 ± 4.7 | 4174.9 ± 741.5 |
Pharmacokinetic parameters were calculated by the noncompartmental method, using the WinNonlin version 4.0 program (Pharsight Corp., Mountain View, CA).
Cmax, maximum plasma-serum concentration; Cmin, minimum plasma-serum concentration; AUC, area under the concentration versus time curve; T½, serum half-life; Cl, clearance; VD, volume of distribution.
Human anti-tigatuzumab immune response
No detectable human anti-tigatuzumab antibody response was detected on any of the analyzed samples (Table 4).
Table 4.
Human Anti-Tigatuzumab Response
| Patient | Last day in the trial | HAHAa/samples tested |
|---|---|---|
| 1 | 798 | Negative/22 |
| 2 | 37 | Negative/3 |
| 3 | 232 | Negative/10 |
| 4 | 33 | Negative/2 |
| 5 | 43 | Negative/4 |
| 6 | 84 | Negative/4 |
| 7 | 169 | Negative/5 |
| 8 | 33 | Negative/3 |
| 9 | 82 | Negative/4 |
| 10 | 81 | Negative/4 |
| 11 | 42 | Negative/3 |
| 12 | 42 | Negative/2 |
| 13 | 16 | Negative/2 |
| 14 | 14 | Negative/1 |
| 15 | 43 | Negative/3 |
| 16 | 126 | Negative/5 |
| 17 | 44 | Negative/4 |
Serum samples were collected prior to the first dose of tigatuzumab and 1, 21, and 49 days after the first dose. Then, serum samples were collected every 6 weeks until patients were taken off the study.
HAHA, Human anti-human antibodies.
Discussion
TRA-8, the parent murine antibody for tigatuzumab, has been studied extensively and been shown to demonstrate potent in vitro cytotoxicity and in vivo antitumor efficacy, which can be amplified by combination therapy with chemotherapeutic agents and/or radiation. These preclinical studies used human tumor cell lines and murine xenograft animal models for cancers of the breast,12 colon,17 pancreas,18,19 ovary,20 cervix,21 and brain.22,23 In addition, this antibody has considerable cytotoxicity to fresh human ovarian cancer tissue slices without the addition of crosslinking reagents or effector cells.20 Antitumor efficacy was demonstrated in subcutaneous12 and orthotopic xenograft models.18,19 The enhancement of TRA-8-mediated cytotoxicity was observed with a wide range of FDA-approved chemotherapy agents.
Conclusions
These observations support the hypothesis that tigatuzumab, particularly in combination with chemotherapy, may have antitumor efficacy in a variety of human cancers. This phase I trial shows that tigatuzumab, at doses as high as 8 mg/kg/week, is very well tolerated and did not induce DLTs, but did result in long-term disease stabilization (longer than 81 days) in 41% of patients with aggressive, treatment-resistant solid tumors. No adverse events or serious adverse events were related to tigatuzumab. Interest in this strategy of anti-DR5 in the treatment of human cancer is evidenced by an array of additional anti-DR5 reagents in various stages of preclinical and clinical development. These include lexatumumab,24 apomab,25 LBy135,26 WD-1,27 and AMG655.28,29 A consistent finding from all these studies is the considerable variability in the sensitivity of various tumor cell lines to anti-DR5–mediated cytotoxicity. Each tumor type appears to have some cell lines that trigger caspase-dependent apoptosis on exposure to small doses of agonistic antibody (5–200 ng of antibody) and other cell lines that do not trigger apoptosis at doses >1000 ng. The combination of antibody and chemotherapy usually enhances the degree of apoptosis and can partially reverse resistance in some cell lines.12,13,17–19,23,26,28,29 There is considerable ongoing effort to determine a possible biomarker in tumor specimens that might predict those patients most likely to benefit from such therapy. Based on the safety profile of tigatuzumab in the phase I trial and the results of preclinical studies that have shown additive or synergistic antitumor activity when added to standard chemotherapy agents, clinical trials of tigatuzumab, in combination with chemotherapy, for the treatment of DR5-positive epithelial tumors have been initiated.
Acknowledgments
The University of Alabama at Birmingham has intellectual property and a licensing agreement relevant to tigatuzumab. Authors from the University of Alabama at Birmingham, however, have no relevant personal conflict of interest to declare. Feng (Roger) Luo and Catherine Copigneaux are employees of Daiichi-Sankyo. Slawomir Wojtowicz-Praga was previously employed by Daiichi-Sankyo during the study. All this work was supported by a grant from the National Cancer Institute (5R21 CA115013-02) and Daiichi Sankyo Pharma.
Disclosure Statement
No competing financial interests exist.
References
- 1.Wiley SR. Schooley K. Smolak PJ, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 2.Daniel PT. Krammer PH. Activation induces sensitivity toward APO-1 (CD95)-mediated apoptosis in human B cells. J Immunol. 1994;152:5624. [PubMed] [Google Scholar]
- 3.Buchsbaum DJ. Zhou T. Lobuglio AF. TRAIL receptor-targeted therapy. Future Oncol. 2006;2:493. doi: 10.2217/14796694.2.4.493. [DOI] [PubMed] [Google Scholar]
- 4.de Vries EG. Gietema JA. de Jong S. Tumor necrosis factor-related apoptosis-inducing ligand pathway and its therapeutic implications. Clin Cancer Res. 2006;12:2390. doi: 10.1158/1078-0432.CCR-06-0352. [DOI] [PubMed] [Google Scholar]
- 5.Carlo-Stella C. Lavazza C. Locatelli A, et al. Targeting TRAIL agonistic receptors for cancer therapy. Clin Cancer Res. 2007;13:2313. doi: 10.1158/1078-0432.CCR-06-2774. [DOI] [PubMed] [Google Scholar]
- 6.Ashkenazi A. Pai RC. Fong S, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999;104:155. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Debatin KM. Apoptosis pathways in cancer and cancer therapy. Cancer Immunol Immunother. 2004;53:153. doi: 10.1007/s00262-003-0474-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaudhary PM. Eby M. Jasmin A, et al. Death receptor 5, a new member of the TNFR family, and DR4 induce FADD-dependent apoptosis and activate the NF-kappaB pathway. Immunity. 1997;7:821. doi: 10.1016/s1074-7613(00)80400-8. [DOI] [PubMed] [Google Scholar]
- 9.Tolcher AW. Mita M. Meropol NJ, et al. Phase I pharmacokinetic and biologic correlative study of mapatumumab, a fully human monoclonal antibody with agonist activity to tumor necrosis factor-related apoptosis-inducing ligand receptor-1. J Clin Oncol. 2007;25:1390. doi: 10.1200/JCO.2006.08.8898. [DOI] [PubMed] [Google Scholar]
- 10.Rowinsky EK. Targeted induction of apoptosis in cancer management: The emerging role of tumor necrosis factor-related apoptosis-inducing ligand receptor activating agents. J Clin Oncol. 2005;23:9394. doi: 10.1200/JCO.2005.02.2889. [DOI] [PubMed] [Google Scholar]
- 11.Ichikawa K. Liu W. Zhao L, et al. Tumoricidal activity of a novel anti-human DR5 monoclonal antibody without hepatocyte cytotoxicity. Nat Med. 2001;7:954. doi: 10.1038/91000. [DOI] [PubMed] [Google Scholar]
- 12.Buchsbaum DJ. Zhou T. Grizzle WE, et al. Antitumor efficacy of TRA-8 anti-DR5 monoclonal antibody alone or in combination with chemotherapy and/or radiation therapy in a human breast cancer model. Clin Cancer Res. 2003;9:3731. [PubMed] [Google Scholar]
- 13.DeRosier LC. Buchsbaum DJ. Oliver PG, et al. Combination treatment with TRA-8 antideath receptor 5 antibody and CPT-11 induces tumor regression in an orthotopic model of pancreatic cancer. Clin Cancer Res. 2007;13:5535s. doi: 10.1158/1078-0432.CCR-07-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Therasse P. Arbuck SG. Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 15.Lister TA. Crowther D. Sutcliffe SB, et al. Report of a committee convened to discuss the evaluations and staging of patients with Hodgkin's disease: Cotswolds meeting. J Clin Oncol. 1989;7:1630. doi: 10.1200/JCO.1989.7.11.1630. [DOI] [PubMed] [Google Scholar]
- 16.Cheson BD. Horning SJ. Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 17.Oliver PG. LoBuglio AF. Zinn KR, et al. Treatment of human cancer xenografts with TRA-8 anti-death receptor 5 antibody alone or in combination with CPT-11. Clin Cancer Res. 2008;14:2180. doi: 10.1158/1078-0432.CCR-07-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Derosier LC. Vickers SM. Zinn KR, et al. TRA-8 anti-DR5 monoclonal antibody and gemcitabine induce apoptosis and inhibit radiologically validated orthotopic pancreatic tumor growth. Mol Cancer Ther. 2007;6:3198. doi: 10.1158/1535-7163.MCT-07-0299. [DOI] [PubMed] [Google Scholar]
- 19.Long JW. Derosier LC. Vickers SM, et al. TRA-8 (TRAIL-R2 antibody)-based combination chemotherapy produces a survival benefit in a pancreatic cancer orthotopic model. J Surg Res. 2007;137:167. [Google Scholar]
- 20.Estes JM. Oliver PG. Straughn JM, Jr., et al. Efficacy of anti-death receptor 5 (DR5) antibody (TRA-8) against primary human ovarian carcinoma using a novel ex vivo tissue slice model. Gynecol Oncol. 2007;105:291. doi: 10.1016/j.ygyno.2006.12.033. [DOI] [PubMed] [Google Scholar]
- 21.Kendrick JE. Straughn JM., Jr. Oliver PG, et al. Anti-tumor activity of the TRA-8 anti-DR5 antibody in combination with cisplatin in an ex vivo human cervical cancer model. Gynecol Oncol. 2008;108:591. doi: 10.1016/j.ygyno.2007.11.039. [DOI] [PubMed] [Google Scholar]
- 22.Kaliberov S. Stackhouse MA. Kaliberova L, et al. Enhanced apoptosis following treatment with TRA-8 anti-human DR5 monoclonal antibody and overexpression of exogenous Bax in human glioma cells. Gene Ther. 2004;11:658. doi: 10.1038/sj.gt.3302215. [DOI] [PubMed] [Google Scholar]
- 23.Fiveash JB. Gillespie GY. Oliver PG, et al. Enhancement of glioma radiotherapy and chemotherapy response with targeted antibody therapy against death receptor 5. Int J Radiat Oncol Biol Phys. 2008;71:507. doi: 10.1016/j.ijrobp.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plummer R. Attard D. Pacey S, et al. Phase 1 and pharmacokinetic study of Lexatumumab in patients with advanced cancers. Clin Cancer Res. 2007;13:6187. doi: 10.1158/1078-0432.CCR-07-0950. [DOI] [PubMed] [Google Scholar]
- 25.Adams C. Totpal K. Lawrence D, et al. Structural and functional analysis of the interaction between the agonistic monoclonal antibody, Apomab, and the proapoptotic receptor, DR5. Cell Death Differ. 2008;15:751. doi: 10.1038/sj.cdd.4402306. [DOI] [PubMed] [Google Scholar]
- 26.Li J. Tang B. Cheng J, et al. Antitumor efficacy of LBY135, an anti-DR5 monoclonal antibody, alone or in combination with chemotherapy in human colon tumor cell lines and xenografts. AACR Meeting Abstracts. 2007. Abstract 4874.
- 27.Wang J. Lin Z. Qiao CX, et al. Characterization of a novel anti-DR5 monoclonal antibody WD1 with the potential to induce tumor cell apoptosis. Cell Mol Immunol. 2008;5:55. doi: 10.1038/cmi.2008.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wall J. Colloton M. Huard J, et al. AMG 655, a monoclonal antibody agonist directed against death receptor 5, induces apoptosis in human colon carcinoma cell lines and its therapeutic potential is enhanced in combination with chemotherapeutic agents. AACR Meeting Abstracts. 2008. Abstract 1326.
- 29.Kaplan-Lefko P. Bush T. Belmontes B, et al. AMG 655, a fully human agonistic antibody against death receptor 5, enhances the anti-tumor activity of gemcitabine in MiaPaCa2/T2, a pancreatic cancer model. AACR Meeting Abstracts. 2008. Abstract 399.