Abstract
Background
Acute palliative care units (APCUs) are new programs aimed at integrating palliative and oncology care. Few outcome studies from APCUs are available.
Objectives
We examined the frequency, survival, and predictors associated with home discharge and death in our APCU.
Methods
All patients discharged from the APCU between September 1, 2003 and August 31, 2008 were included. Demographics, cancer diagnosis, discharge outcomes, and overall survival from discharge were retrieved retrospectively.
Results
The 2568 patients admitted to APCU had the following characteristics: median age, 59 years (range, 18–101); male, 51%; median hospital stay, 11 days; median APCU stay, 7 days; and median survival 21 days (95% confidence interval [CI] 19–23 days). Five hundred ninety-two (20%), 89 (3%), and 1259 (43%) patients were discharged to home, health care facilities, and hospice, respectively, with a median survival of 60, 29, and 14 days, respectively (p < 0.001). Nine hundred fifty-eight (33%) patients died during admission (median stay, 11 days). Compared to hospice transfers, home discharge (hazard ratio = 0.35, 95% CI 0.30–0.41, p < 0.001) was associated with longer survival in multivariate analysis, with a 6-month survival of 22%. Multivariate logistic regression revealed that male gender, specific cancer primaries, and admissions from oncology units were associated with death in the APCU, while younger age and direct admissions to the APCU were associated with home discharge.
Conclusions
Our APCU serves patients with advanced cancer with diverse clinical characteristics and survival, and discharged home a significant proportion with survival greater than 6 months. Results from this simultaneous care program suggest a pattern of care different from that of traditional hospice and palliative care services.
Introduction
With disease progression, patients with advanced cancer frequently experience worsening symptoms, functional deterioration, and emotional distress. Many of these patients require palliative care for symptom management, psychosocial support, and transition to the end of life. Palliative care in advanced cancer requires an in-depth knowledge of symptom control, specialized skills to facilitate communication and complex decision-making, and close collaborations with oncologists and the interprofessional palliative care team to provide comprehensive cancer care.1 Acute palliative care units (APCUs) are novel programs that are integrated into tertiary acute care facilities.2–5 APCUs provide intensive palliative care for inpatients and emotional support for their families, with the aim of enhancing quality of life, facilitating transition to end-of-life care, and assisting with hospital discharges.
Discharge planning represents one of the most important and complex decisions for patients admitted to the APCU. Patients admitted under palliative care generally have a median survival of weeks,6–10 with a sizable proportion of patients dying in the hospital. Among patients discharged alive, the decision regarding discharge location, such as home, outpatient hospices, inpatient hospice care, or long-term care facilities, depends on patient preferences, performance status, and prognosis, along with various clinical, psychosocial, logistic, and financial factors.
The M. D. Anderson Cancer Center is a 520-bed National Cancer Institute-designated comprehensive cancer center. In 2003, it opened a 12-bed APCU to serve patients with advanced cancer. Staffed by an interdisciplinary team of physicians, nurses, social workers, physiotherapists, pharmacists, and a chaplain, the APCU aims to provide symptom management for patients with advanced cancer and severe physical and/or psychosocial distress, to provide emotional support for their families, and to facilitate transition of care.11 Given that the timing of referral to palliative care varies significantly with patients presenting at different stages of disease, the process of transitioning to the end-of-life may range from months over multiple APCU admissions to days during a single and sole admission. Patients who are not candidates for anti-neoplastic therapy are generally encouraged to consider hospice care upon discharge, while those who remain eligible for anti-neoplastic treatments or refuse hospice care are discharged home with palliative care and oncology outpatient clinics follow-up. Intravenous chemotherapy is generally not administered in the APCU, although patients who improved during the APCU stay and were deemed appropriate for chemotherapy may be transferred back to oncology units for further treatments.
To our knowledge, no studies to date have reported survival after APCU discharge in patients with advanced cancer, and only one study has examined the predictors of location of discharge for palliative care patients.12 A better understanding of the factors associated with discharge outcomes would allow clinicians to distinguish patients who are appropriate for home discharge from those who have a high likelihood of dying in the hospital, and facilitate clinical decision-making. Using a retrospective design, we examined the frequencies, survival times, and predictors associated with home discharges and death in hospitalized patients with cancer who required an APCU stay.
Patients and Methods
Subjects
The Institutional Review Board at M. D. Anderson Cancer Center approved this study and waived the requirement for informed consent. All patients with cancer admitted to the APCU at M. D. Anderson Cancer Center between September 1, 2003 and August 31, 2008 were included in this retrospective study. These dates were chosen to correspond to the institution's fiscal year. In addition to patients discharged directly from our APCU, we included patients discharged from oncology units following an APCU stay during the same admission.
Patient characteristics and discharge outcomes
We retrospectively reviewed patient demographics (age, gender, race, and religion), cancer diagnosis, durations of the entire hospitalization and APCU stays, and survival from time of admission from institutional databases, electronic health records, and Tumor Registry Vital Statistics Database. For patients with multiple cancer diagnoses, the cancer diagnosis most responsible for hospitalization was used for analysis. In this study, home hospice and inpatient hospice were both included in the “hospice” category, and long-term care facilities and other healthcare facilities were combined into the “institutions” category. We also collected information regarding administration of chemotherapy during hospitalization that included an APCU stay.
Statistical analysis
Among 2568 patients, 244 (10%) had more than one admission to the APCU. For these individuals, only one admission per patient was chosen for analysis using a simple randomization scheme. This is because many of the statistical tests and models require the assumption of independence among subjects. As a result, 334 readmissions were excluded from analysis.
We summarized baseline demographics, admission characteristics, and discharge outcomes using descriptive statistics, including medians, means, standard deviations, ranges, and frequencies together with 95% confidence intervals (CI). The χ2 test for trend was used to determine whether there was a yearly trend of discharge locations.
Survival analyses were plotted by using the Kaplan-Meier method, and survival curves were compared by the log-rank test.13,14 Overall survival time was calculated from the time of hospital discharge to the date of death from any cause or the date at which the patient was last known alive. Multivariate analysis was performed by using the Cox proportional hazards model with backward elimination.
To identify factors on admission associated with hospital death, we compared the baseline and admission characteristics between patients who died at the end of admission to those who were discharged alive. Comparisons were made using the Student's t test for continuous variables that were normally distributed (i.e., age), the Mann-Whitney test for continuous, nonparametric variables (e.g., admission length), and Pearson's χ2 test for categorical variables (e.g., race, religion). Variables associated with a p value of 0.20 or less in univariate analysis were then fitted in a logistic regression model to identify factors associated with death during hospitalization. A two-sided p value of less than 0.05 was considered to be statistically significant. We performed a similar analysis to determine factors associated with home discharge.
Recursive partitioning was performed on a random selection of 1500 patients to identify optimal patient classifications among significant factors identified in the multivariate Cox model selection for hospital death. Once the optimal classifications were determined, the remaining 1068 patients were used to estimate the odds of a hospital death between those classifications. Similar analyses were performed for home discharge.
The Statistical Package for the Social Sciences (SPSS version 16.0, SPSS Inc., Chicago, Illinois) software and R (R version 2.3.1, The R Foundation for Statistical Computing, Vienna, Austria) were used for statistical analysis.
Results
The characteristics of the 2568 patients who required admission to the APCU during the study period are shown in Table 1. A total of 728 (28%) patients were admitted to and discharged directly from the APCU; the remainder had both an oncology unit and APCU stay.
Table 1.
Characteristics of Two Thousand Five Hundred Sixty-Eight Patients Admitted to the APCU
| Number (%)a | |
|---|---|
| Median age, years (range) | 59 (18–101) |
| Female | 1255 (48.9%) |
| Race | |
| Caucasian | 1717 (66.9%) |
| Black | 416 (16.2%) |
| Hispanic | 306 (11.9%) |
| Others | 129 (5.0%) |
| Religionb | |
| Christian | 2095 (93.9%) |
| Non-Christian | 135 (6.1%) |
| Cancer diagnosis | |
| Breast | 206 (8.0%) |
| Central nervous system | 22 (0.9%) |
| Dermatologic | 98 (3.8%) |
| Endocrine | 22 (0.9%) |
| Gastrointestinal | 506 (19.7%) |
| Genitourinary | 266 (10.4%) |
| Gynecologic | 216 (8.4%) |
| Head and neck | 152 (5.9%) |
| Hematologic | 292 (11.4%) |
| Sarcoma | 105 (4.1%) |
| Unknown primary | 91 (3.5%) |
| Respiratory | 592 (23.1%) |
| Admission type | |
| Direct admission to APCU | 728 (28.3%) |
| Transfers to APCU from oncology units | 1840 (71.7%) |
| Median admission duration | |
| MDA hospital stay in days (Q1–Q3) | 11 (8–17) |
| APCU stay in days (Q1–Q3) | 7 (4–10) |
| Number of admissions | |
| 1 | 2324 (90.5%) |
| 2 | 183 (7.1%) |
| 3 | 42 (1.6%) |
| 4 | 13 (0.5%) |
| 5 | 5 (0.2%) |
| 9 | 1 (0.04%) |
Unless otherwise stated.
Information regarding religion was unavailable for 338 patients.
APCU, acute palliative care unit; MDA, M. D. Anderson Cancer Center.
Among the 2902 admissions, the in-hospital mortality rate was 33%. The location of discharge for the 2568 patients admitted to the APCU is shown in Table 2. Among patients discharged alive, 592 (20%), 855 (29%), 404 (14%), 56 (2%), and 33 (1%) were sent home, to home hospice, inpatient hospice, long-term care facilitates, and other institutions, respectively. Patients discharged from oncology wards after an APCU stay were significantly more likely to go home compared to patients discharged from the APCU (56% versus 15%, p < 0.001). We did not detect any significant changes in the frequency of discharge location over the 5-year period. Patients with multiple APCU admissions were more likely to be discharged alive compared to those with only one admission (86% versus 62%, p < 0.001).
Table 2.
APCU Admissions by Discharge Outcome
| Home | Hospice | Other institutions | Died during admission | Total | |
|---|---|---|---|---|---|
| Discharge outcome by admission (n = 2902) | |||||
| Discharged from APCU | 561 (20%) | 1247 (44%) | 86 (3%) | 958 (33%) | 2852 (98%) |
| Discharged from an oncology unit | 31 (62%) | 12 (24%) | 3 (6%) | 4 (8%) | 50 (2%) |
| Discharge outcome by patient (n = 2568)a | |||||
| Discharged from APCU | 382 (15%) | 1158 (46%) | 76 (3%) | 911 (36%) | 2527 (98%) |
| Discharged from an oncology unit | 22 (54%) | 12 (29%) | 3 (7%) | 4 (10%) | 41 (2%) |
Three hundred thirty-four readmissions were excluded for patients with multiple visits. This sample is used for statistical analysis.
APCU, acute palliative care unit.
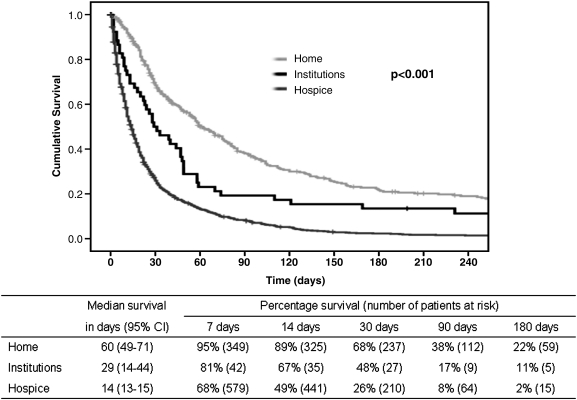
Figure 1 shows that overall survival from time of discharge was significantly influenced by discharge location (p < 0.001, log-rank test). Specifically, home discharge (without home hospice) was associated with longer survival than transfers to hospice or to other institutions (Fig. 1). Patients discharged to home hospice (median survival 19 days, 95% CI 17–21 days) had longer survival than those sent to inpatient hospice (median survival 6 days, 95% CI 5–7 days; p < 0.001, log-rank test). In multivariate analysis, the location of discharge was a significant determinant of survival (Table 3). The median duration of hospital admission for patients who died in hospital was 11 days (Q1–Q3 7–17 days).
FIG. 1.
Overall survival by discharge location. Kaplan-Meier survival curves for patients who were discharged to home, hospices, or transferred to other institutions are shown. The log-rank test was used to compare the survival times between groups.
Table 3.
Cox Regression Survival Analysis of One Thousand Six Hundred Fifty-Three Patients Discharged from APCUa
| Hazard ratio (95% CI) | p value | |
|---|---|---|
| Race | 0.002 | |
| White | 1.01 (0.76–1.35) | 0.93 |
| Black | 0.78 (0.57–1.07) | 0.13 |
| Hispanic | 0.78 (0.56–1.09) | 0.14 |
| Others | 1.0 | Ref |
| Religion | 0.01 | |
| Christian | 1.15 (0.83–1.59) | 0.40 |
| Non-Christian | 1.32 (1.09–1.59) | 0.004 |
| Not specified | 1.0 | Ref |
| Cancer diagnosis | <0.001 | |
| Breast | 1.15 (0.90–1.47) | 0.26 |
| Central nervous system | 0.87 (0.48–1.55) | 0.63 |
| Dermatologic | 1.12 (0.81–1.55) | 0.48 |
| Endocrine | 0.75 (0.39–1.47) | 0.40 |
| Gastrointestinal | 1.04 (0.87–1.24) | 0.65 |
| Genitourinary | 0.77 (0.62–0.97) | 0.02 |
| Gynecologic | 0.78 (0.63–0.98) | 0.03 |
| Head and neck | 0.97 (0.75–1.24) | 0.79 |
| Hematologic | 1.77 (1.39–2.24) | <0.001 |
| Sarcoma | 0.79 (0.58–1.07) | 0.13 |
| Unknown primary | 1.02 (0.74–1.41) | 0.90 |
| Respiratory | 1.0 | Ref |
| Year of admission | 0.001 | |
| 2003 | 1.46 (1.12–1.89) | 0.005 |
| 2004 | 1.23 (0.97–1.63) | 0.08 |
| 2005 | 1.64 (1.26–2.12) | <0.001 |
| 2006 | 1.36 (1.03–1.79) | 0.03 |
| 2007 | 1.0 | Ref |
| Direct APCU admission | 0.84 (0.74–0.95) | 0.006 |
| Discharge outcome | <0.001 | |
| Home | 0.35 (0.30–0.41) | <0.001 |
| Hospice | 0.43 (0.32–0.59) | <0.001 |
| Other institutions | 1.0 | Ref |
Based on survival from time of discharge. The 915 patients who died during admission were not included in this analysis. Age, gender, race, religion, cancer diagnosis, year of admission, direct APCU admission, MDA admission length, APCU admission length, discharge outcome and chemotherapy use during admission were included in Cox regression model.
APCU, acute palliative care unit; CI, confidence interval; MDA, M. D. Anderson Cancer Center.
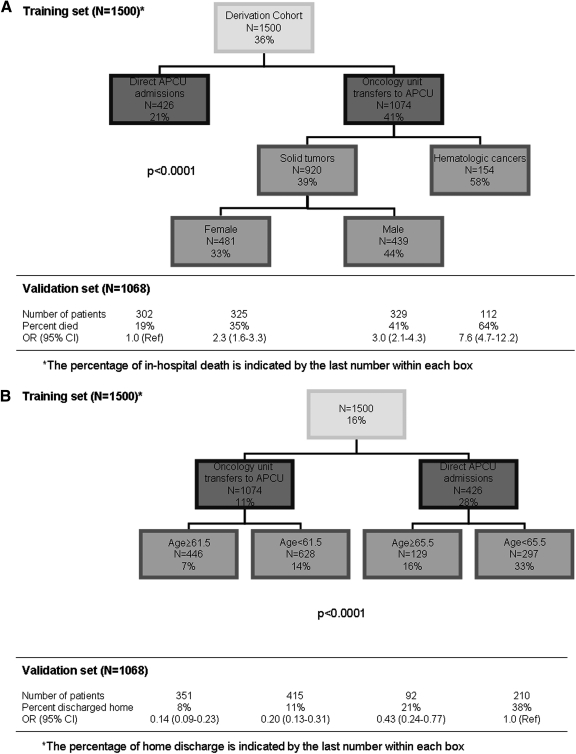
We identified factors associated with in-hospital death in our cohort. In univariate analysis, male gender, specific cancer diagnoses (particularly hematologic malignancies), chemotherapeutic agent use, transfers to APCU from oncology units, and shorter APCU stay were associated with an increased risk of in-hospital mortality (Table 4). In multivariate analysis, male sex, hematologic malignancy, and transfers to APCU from oncology units were independently associated with an increased chance of dying in the hospital (Table 5). Figure 2A shows a recursive partitioning model that provides a risk of in-hospital death during admission. Based on this algorithm, patients with hematologic malignancies and transferred to APCU from oncology units have a significantly higher risk of in-hospital mortality compared to patients admitted directly to the APCU (odds ratio [OR] = 7.6, 95% CI 4.7–12.2).
Table 4.
Factors associated with Death and Home Discharge in Two Thousand Five Hundred Sixty-Eight APCU Patients by Univariate Analysisa
| Alive (n = 1653) | Dead (n = 915) | p value | Non-home (n = 2164) | Home (n = 404) | p value | |
|---|---|---|---|---|---|---|
| Median age (range) | 59 (19–101) | 59 (18–96) | 0.70 | 60 (18–101) | 55 (20–89) | <0.001 |
| Gender | ||||||
| Female | 850 (67.7%) | 405 (32.3%) | 0.001 | 1048 (83.5%) | 207 (16.5%) | 0.30 |
| Male | 803 (61.2%) | 510 (38.8%) | 1116 (85.0%) | 197 (15.0%) | ||
| Race | ||||||
| White | 1092 (63.6%) | 625 (36.4%) | 0.71 | 1461 (85.1%) | 256 (14.9%) | 0.28 |
| Black | 275 (66.1%) | 141 (33.9%) | 343 (82.5%) | 73 (17.5%) | ||
| Hispanic | 202 (66.0%) | 104 (34.0%) | 257 (84.0%) | 49 (16.0%) | ||
| Others | 84 (65.1%) | 45 (34.9%) | 103 (79.8%) | 26 (20.2%) | ||
| Religionb | ||||||
| Christian | 1344 (64.2%) | 751 (35.8%) | 0.95 | 1773 (84.6%) | 322 (15.4%) | 0.096 |
| Non-Christian | 87 (64.4%) | 48 (35.6%) | 107 (79.3%) | 28 (20.7%) | ||
| Cancer types | ||||||
| Breast | 142 (68.9%) | 64 (31.1%) | <0.001 | 173 (84.0%) | 33 (16.0%) | 0.034 |
| Neurologic | 18 (81.8%) | 4 (18.2%) | 20 (90.9%) | 2 (9.1%) | ||
| Dermatologic | 59 (60.2%) | 39 (38.9%) | 86 (87.8%) | 12 (12.2%) | ||
| Endocrine | 11 (50.0%) | 11 (50.0%) | 18 (81.8%) | 4 (18.2%) | ||
| Gastrointestinal | 334 (66.0%) | 172 (34.0%) | 423 (83.6%) | 83 (16.4%) | ||
| Genitourinary | 178 (66.9%) | 88 (33.1%) | 226 (85.0%) | 40 (15.0%) | ||
| Gynecologic | 169 (78.2%) | 47 (21.8%) | 171 (79.2%) | 45 (20.8%) | ||
| Head and neck | 113 (74.3%) | 39 (25.7%) | 118 (77.6%) | 34 (22.4%) | ||
| Hematologic | 126 (43.2%) | 166 (56.8%) | 264 (90.4%) | 28 (9.6%) | ||
| Sarcoma | 73 (69.5%) | 32 (30.5%) | 86 (81.9%) | 19 (18.1%) | ||
| Unknown primary | 57 (62.6%) | 34 (37.4%) | 80 (87.9%) | 11 (12.1%) | ||
| Respiratory | 373 (63.0%) | 219 (37.0%) | 499 (84.3%) | 93 (15.7%) | ||
| Chemotherapy agentsc | ||||||
| Given | 158 (53.9%) | 135 (46.1%) | <0.001 | 250 (85.3%) | 43 (14.7%) | 0.60 |
| Not given | 1495 (65.7%) | 780 (34.3%) | 1914 (84.1%) | 361 (15.9%) | ||
| Admission type | ||||||
| Direct APCU admission | 579 (79.5%) | 149 (20.5%) | <0.001 | 511 (70.2%) | 217 (29.8%) | <0.001 |
| Oncology unit transfers | 1074 (58.4%) | 766 (41.6%) | 1653 (89.8%) | 187 (10.2%) | ||
| Year of admission | ||||||
| 2003 | 311 (64.3%) | 173 (35.7%) | 0.94 | 401 (82.9%) | 83 (17.1%) | 0.36 |
| 2004 | 358 (63.9%) | 202 (36.1%) | 480 (85.7%) | 80 (14.3%) | ||
| 2005 | 343 (66.0%) | 177 (34.0%) | 431 (82.9%) | 89 (17.1%) | ||
| 2006 | 325 (64.2%) | 181 (35.8%) | 437 (86.4%) | 69 (13.6%) | ||
| 2007 | 316 (63.5%) | 182 (36.5%) | 415 (83.3%) | 83 (16.7%) | ||
| Median MDA admission length (Q1–Q3) | 12 (8–17) | 11 (7–17) | 0.058 | 12 (8–18) | 9 (6–14) | <0.001 |
| Median APCU admission length (Q1–Q3) | 8 (6–10) | 5 (3–8) | <0.001 | 7 (4–10) | 7 (5–9) | 0.61 |
Comparisons were made between patients who died in the APCU and those discharged alive, and also between patients who were discharged home and those who died or transferred to hospices/other institutions; the χ2 test was used for categorical variables; Student's t test for age; and Mann-Whitney test was used for median hospital admission and APCU admission lengths.
Information regarding religion was unavailable for 338 patients.
Chemotherapy administered during hospitalization that included an APCU stay.
APCU, acute palliative care unit; MDA, M. D. Anderson Cancer Center.
Table 5.
Factors Associated with Death in APCU or Home Discharge in Two Thousand Five Hundred Sixty-Eight Patients by Multivariate Logistic Regression
| |
Death in APCU |
Home discharge |
||
|---|---|---|---|---|
| Odds ratio (95% CI) | p value | Odds ratio (95% CI) | p value | |
| Age (per year) | — | — | 0.98 (0.97–0.99) | <0.001 |
| Male | 1.26 (1.04–1.52) | 0.018 | — | — |
| Cancer | <0.001 | |||
| Breast | 0.83 (0.58–1.19) | 0.31 | NS | NS |
| Neurologic | 0.37 (0.12–1.12) | 0.078 | ||
| Dermatologic | 1.0 (0.64–1.56) | 1.0 | ||
| Endocrine | 1.77 (0.74–4.21) | 0.20 | ||
| Gastrointestinal | 0.91 (0.70–1.17) | 0.45 | ||
| Genitourinary | 0.75 (0.55–1.03) | 0.077 | ||
| Gynecologic | 0.49 (0.33–0.72) | <0.001 | ||
| Head and neck | 0.61 (0.40–0.91) | 0.016 | ||
| Hematologic | 1.96 (1.39–2.49) | <0.001 | ||
| Sarcoma | 0.75 (0.47–1.18) | 0.22 | ||
| Unknown primary | 1.02 (0.64–1.63) | 0.92 | ||
| Respiratory | 1.0 | Ref | ||
| Direct APCU admission | 0.38 (0.31–0.47) | <0.001 | 3.78 (3.01–4.74) | <0.001 |
APCU, acute palliative care unit; CI, confidence interval; NS, not significant;—denotes variables not included in regression model.
FIG. 2.
Recursive partitioning models for predicting in-hospital death and home discharge. Training sets for predictive models for (A) in-hospital death and (B) home discharge are shown, with validation sets provided below each flow diagram. The per-centage within each box indicates the proportion of patients who experienced the event of interest (i.e., death or home discharge).
Given that patients discharged to home with follow-up (but not to home hospice) had a significantly longer survival than the rest, we determined factors that were associated with home discharge. In univariate analysis, younger age, specific cancer primaries, direct admissions to APCU, and shorter hospital stay were associated with home discharge (Table 4). Multivariate logistic regression analysis revealed that only younger age and direct admission to the APCU were independently associated with home discharge (Table 5). A recursive partitioning model that incorporates age and APCU admission type provided an estimation of the risk of home discharge (Figure 2B). Older patients transferred to APCU from oncology units were much less likely to be discharged home compared to younger patients admitted directly to the APCU (odds ratio [OR] = 0.14, 95% CI 0.09–0.23).
Discussion
Our APCU served a heterogeneous patient population, with diverse clinical characteristics and outcomes. While 33% of the patients died during admission, 20% of the patients were discharged home, with a 6-month survival of 22%. We identified a number of determinants of in-hospital death and home discharge. The APCU delivers palliative care customized to the individuals' needs, and facilitates simultaneous care.
In the United States, approximately half of all hospitals provide palliative care services.15 This proportion approaches 90% among teaching hospitals, with one third of these facilities equipped with palliative care inpatient units.16 Over the last few years, a distinction has been made between traditional palliative care units and APCUs.2–5 First, APCUs provide simultaneous care within tertiary care institutions, where acutely ill patients receive intensive symptom support. This model of care is parallel to intensive care units (ICUs), which focus on life-support measures. Second, APCUs play a key role in coordinating discharge planning, as a majority of APCU patients are discharged alive. In the process, APCUs serve as critical links between acute care units, hospices, and the community for patients with far advanced cancer.17,18 Third, the specialized expertise and knowledge accumulated by the interprofessional APCU team, coupled with the infrastructure of academic centers, facilitate active research and education.
The identity and role of APCUs are still evolving given that they are relatively new programs in the spectrum of palliative care services. Few studies to date have characterized the development, structure, and function of APCUs,3,5,19–22 and even fewer have documented the administrative and financial outcomes from these units.5,11,23 To our knowledge, this is the first study to examine the survival outcomes of patients discharged from an APCU. Although median survival in our cohort was short (i.e., 21 days), we found an important difference between patients discharged to home and those transferred to hospice or other institutions. Comparison among patients discharged to various locations revealed significant differences in clinical characteristics, suggesting the heterogeneity of our patient cohort accounted for the diverse survival outcomes.
The decision regarding location of discharge is complex, involving an interplay of factors such as patient and family preference, social support, financial status, symptom severity, prognosis, and the infrastructure of the health care system. Predictive models for discharge outcomes have practical implications for clinical decision-making. For instance, patients who are predicted to go home may benefit from intensive rehabilitation, while those who are likely to die in the hospital could potentially be spared the stressful process of discharge planning. Admission directly to the APCU, as opposed to being transferred from an oncology unit was found to be a predictor of improved survival post discharge (hazard ratio = 0.84) and home discharge (OR = 3.8). This could be explained by the fact that patients admitted to oncology units and then transferred to the APCU are likely more ill than patients admitted directly to the APCU; this finding is consistent with data from a recent study conducted by our group demonstrating the association between transfers from oncology units and in-hospital death.24 Based on our results, we proposed predictive models for in-hospital death and home discharge (Fig. 2).
Another prospective study examined factors associated with home discharge in 100 palliative care patients discharged from an acute care hospital, and found younger age, married status, good performance status, and good cognitive status as key determinants.12 Further studies are required to confirm these findings.
A total of 244 patients had repeated APCU admissions during the study period. While it would be of interest to examine the reasons for readmission, this information is not available due to the retrospective nature of this study. Further research is necessary to better characterize the predictors for readmission. Of the 41 patients who were admitted to the APCU and then transferred to an oncology unit prior to discharge, 4 (8%) died (Table 2). While this could represent a failure of the APCU to determine the most appropriate discharge location, other alternative explanations include transfers against medical advice, and unexpected deaths due to acute events such as thromboembolism. Recently, Thomas et al.25 reported that clinician prediction of survival is one of the key factors associated with hospice discussions. Further studies to develop highly accurate prognostication tools would facilitate clinical decision making.
Gastrointestinal and genitourinary malignancies were common among patients admitted to our APCU, which could be explained by the palliative care referral pattern differences between the various types of oncologists. We found that hematologic malignancy was associated with increased in-hospital mortality. Indeed, studies from our institution and from others have consistently demonstrated that patients with hematologic malignancies have later referrals to palliative care, and higher frequency of critical care unit admissions and ICU deaths.4,26,27 To date, only one prognostic model for in-hospital death specifically for patients with cancer is available, and includes performance status, duration of disease, type of admission, hemoglobin, and lactate dehydrogenase.28 Further development of predictive tools, with highly accurate models such as the Acute Physiology and Chronic Health Evaluation (APACHE) system,29 would ultimately allow clinicians to identify subgroups of patients who may benefit from various interventions, including anti-neoplastic therapies and discharge planning.
Male gender was found to be a factor associated with in-hospital mortality, independent of tumor type. Interestingly, a recent study on survival after hospice admission also revealed male gender to be a poor prognostic indicator.30 Gender differences in comorbidities, patient preferences, and psychosocial needs may explain this observation. Further studies are needed to examine other clinical and sociodemographic factors as potential determinants of in-hospital mortality.
The physical presence of an APCU within a tertiary care cancer center promotes simultaneous oncologic and palliative care.31,32 While palliative care is commonly associated with end-of-life care, it is most effective when incorporated early in cancer management.33–35 The main purpose of the APCU is to provide sophisticated interdisciplinary transition from active care to end-of-life. Specifically, the APCU is a versatile machine designed to deliver personalized medicine tailored to the individual's needs. For patients who are dying, the APCU enables optimal symptom control, with a focus on maximizing comfort measures for the terminally ill. For patients who are likely to go home, the APCU actively treats acute complications and symptoms related to the cancer and its treatments. For patients who are going to hospice, the APCU plays a critical role in facilitating a smooth and rapid transition to end-of-life care, attending to patients' physical and psychosocial needs through inter-professional teamwork. Thus, the APCU facilitates complex decision making and bridges the gap between acute care and the community.
This study has several limitations. First, a number of potential determinants of discharge such as symptom severity, social support and socioeconomic status were not available because of the retrospective nature of this study. Performance status is a particularly important determinant of clinical outcomes and survival, but is not available in this study. It is possible that many of the predictors identified here were merely confounding variables associated with the performance status, if examined. Further efforts to document performance status regularly are warranted. Second, the patients at our comprehensive cancer center may not be representative of patients with cancer and treatments throughout the United States, and thus the results may not be generalizable.
In summary, our APCU serves advanced cancer patients with diverse clinical characteristics and survival outcomes, and facilitates simultaneous care. The emerging field of palliative oncology promotes a high degree of integration between oncology and palliative care, with the goal to improve patient care. Timely incorporation of palliative care strategies in patients with advanced cancer has the potential to optimize symptom management,36 to maximize psychosocial interventions, to enhance coordination of care, and to facilitate patients' transitions through various stages of their disease. APCUs are strategically positioned to not only deliver exemplary simultaneous care, but also provide the impetus and leadership for change through research and education.
Acknowledgments
We thank Ms. Olubumi Akiwumi for her assistance with database management and Mr. Bryan Tutt for review of the manuscript.
Supported in part by the National Institutes of Health grants, RO1NR010162-01A1, RO1CA122292-01 and RO1C-A124481-01 (E.B.), and the Clinician Investigator Program, Royal College of Physicians and Surgeons of Canada (D.H.).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Lagman R. Walsh D. Integration of palliative medicine into comprehensive cancer care. Semin Oncol. 2005;32:134–138. doi: 10.1053/j.seminoncol.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 2.Lagman R. Rivera N. Walsh D. LeGrand S. Davis MP. Acute inpatient palliative medicine in a cancer center: Clinical problems and medical interventions—A prospective study. Am J Hosp Palliat Care. 2007;24:20–28. doi: 10.1177/1049909106295292. [DOI] [PubMed] [Google Scholar]
- 3.Bruera E. Neumann C. Brenneis C. Quan H. Frequency of symptom distress and poor prognostic indicators in palliative cancer patients admitted to a tertiary palliative care unit, hospices, and acute care hospitals. J Palliat Care. 2000;16:16–21. [PubMed] [Google Scholar]
- 4.Fadul N. Elsayem A. Palmer JL. Zhang T. Braiteh F. Bruera E. Predictors of access to palliative care services among patients who died at a comprehensive cancer center. J Palliat Med. 2007;10:1146–1152. doi: 10.1089/jpm.2006.0259. [DOI] [PubMed] [Google Scholar]
- 5.Elsayem A. Smith ML. Parmley L. Palmer JL. Jenkins R. Reddy S. Bruera E. Impact of a palliative care service on in-hospital mortality in a comprehensive cancer center. J Palliat Med. 2006;9:894–902. doi: 10.1089/jpm.2006.9.894. [DOI] [PubMed] [Google Scholar]
- 6.Rees E. Hardy J. Ling J. Broadley K. A'Hern R. The use of the Edmonton Symptom Assessment Scale (ESAS) within a palliative care unit in the UK. Palliat Med. 1998;12:75–82. doi: 10.1191/026921698674135173. [DOI] [PubMed] [Google Scholar]
- 7.Lam PT. Leung MW. Tse CY. Identifying prognostic factors for survival in advanced cancer patients: A prospective study. Hong Kong Med J. 2007;13:453–459. [PubMed] [Google Scholar]
- 8.Moro C. Brunelli C. Miccinesi G. Fallai M. Morino P. Piazza M. Labianca R. Ripamonti C. Edmonton Symptom Assessment Scale: Italian validation in two palliative care settings. Support Care Cancer. 2006;14:30–37. doi: 10.1007/s00520-005-0834-3. [DOI] [PubMed] [Google Scholar]
- 9.Costantini M. Toscani F. Gallucci M. Brunelli C. Miccinesi G. Tamburini M. Paci E. Di Giulio P. Peruselli C. Higginson I. Addington-Hall J. Terminal cancer patients and timing of referral to palliative care: A multicenter prospective cohort study. Italian Cooperative Research Group on Palliative Medicine. J Pain Symptom Manage. 1999;18:243–252. doi: 10.1016/s0885-3924(99)00084-6. [DOI] [PubMed] [Google Scholar]
- 10.Paci E. Miccinesi G. Toscani F. Tamburini M. Brunelli C. Constantini M. Peruselli C. Di Giulio P. Gallucci M. Addington-Hall J. Higginson IJ. Quality of life assessment and outcome of palliative care. J Pain Symptom Manage. 2001;21:179–188. doi: 10.1016/s0885-3924(01)00263-9. [DOI] [PubMed] [Google Scholar]
- 11.Elsayem A. Swint K. Fisch MJ. Palmer JL. Reddy S. Walker P. Zhukovsky D. Knight P. Bruera E. Palliative care inpatient service in a comprehensive cancer center: Clinical and financial outcomes. J Clin Oncol. 2004;22:2008–2014. doi: 10.1200/JCO.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Fainsinger RL. Demoissac D. Cole J. Mead-Wood K. Lee E. Home versus hospice inpatient care: Discharge characteristics of palliative care patients in an acute care hospital. J Palliat Care. 2000;16:29–34. [PubMed] [Google Scholar]
- 13.Kaplan EL. Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 14.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 15.Goldsmith B. Dietrich J. Du Q. Morrison RS. Variability in access to hospital palliative care in the united states. J Palliat Med. 2008;11:1094–1102. doi: 10.1089/jpm.2008.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Billings JA. Pantilat S. Survey of palliative care programs in United States teaching hospitals. J Palliat Med. 2001;4:309–314. doi: 10.1089/109662101753123913. [DOI] [PubMed] [Google Scholar]
- 17.Byock I. Completing the continuum of cancer care: Integrating life-prolongation and palliation. CA Cancer J Clin. 2000;50:123–132. doi: 10.3322/canjclin.50.2.123. [DOI] [PubMed] [Google Scholar]
- 18.Meyers FJ. Linder J. Simultaneous care: Disease treatment and palliative care throughout illness. J Clin Oncol. 2003;21:1412–1415. doi: 10.1200/JCO.2003.01.104. [DOI] [PubMed] [Google Scholar]
- 19.Lagman R. Walsh D. Heintz J. Legrand SB. Davis MP. A day in the life: A case series of acute care palliative medicine—The Cleveland Model. Am J Hosp Palliat Care. 2008;25:24–32. doi: 10.1177/1049909107307375. [DOI] [PubMed] [Google Scholar]
- 20.Goldstein P. Walsh D. Horvitz LU. The Cleveland Clinic Foundation Harry R. Horvitz Palliative Care Center. Support Care Cancer. 1996;4:329–333. doi: 10.1007/BF01788838. [DOI] [PubMed] [Google Scholar]
- 21.Zimmermann C. Seccareccia D. Clarke A. Warr D. Rodin G. Bringing palliative care to a Canadian cancer center: The palliative care program at Princess Margaret Hospital. Support Care Cancer. 2006;14:982–987. doi: 10.1007/s00520-006-0093-y. [DOI] [PubMed] [Google Scholar]
- 22.Santa-Emma PH. Roach R. Gill MA. Spayde P. Taylor RM. Development and implementation of an inpatient acute palliative care service. J Palliat Med. 2002;5:93–100. doi: 10.1089/10966210252785051. [DOI] [PubMed] [Google Scholar]
- 23.Rigby A. Krzyzanowska M. Le LW. Swami N. Coe G. Rodin G. Moore M. Zimmermann C. Impact of opening an acute palliative care unit on administrative outcomes for a general oncology ward. Cancer. 2008;113:3267–3274. doi: 10.1002/cncr.23909. [DOI] [PubMed] [Google Scholar]
- 24.Elsayem A. Mori M. Parsons H, et al. Predictors of inpatient mortality in an acute palliative care unit at a comprehensive cancer center. Supportive Care Cancer. doi: 10.1007/s00520-009-0631-5. (in press). [DOI] [PubMed] [Google Scholar]
- 25.Thomas JM. O'Leary JR. Fried TR. Understanding their options: Determinants of hospice discussion for older persons with advanced illness. J Gen Intern Med. 2009;24:923–928. doi: 10.1007/s11606-009-1030-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng WW. Willey J. Palmer JL. Zhang T. Bruera E. Interval between palliative care referral and death among patients treated at a comprehensive cancer center. J Palliat Med. 2005;8:1025–1032. doi: 10.1089/jpm.2005.8.1025. [DOI] [PubMed] [Google Scholar]
- 27.McGrath P. Are we making progress? Not in haematology! Omega (Westport) 2002;45:331–348. doi: 10.2190/KU5Q-LL8M-FPPA-LT3W. [DOI] [PubMed] [Google Scholar]
- 28.Bozcuk H. Koyuncu E. Yildiz M. Samur M. Ozdogan M. Artaç M. Coban E. Savas B. A simple and accurate prediction model to estimate the intrahospital mortality risk of hospitalised cancer patients. Int J Clin Pract. 2004;58:1014–1019. doi: 10.1111/j.1742-1241.2004.00169.x. [DOI] [PubMed] [Google Scholar]
- 29.Zimmerman JE. Kramer AA. McNair DS. Malila FM. Acute physiology and chronic health evaluation (APACHE) IV: Hospital mortality assessment for today's critically ill patients. Crit Care Med. 2006;34:1297–1310. doi: 10.1097/01.CCM.0000215112.84523.F0. [DOI] [PubMed] [Google Scholar]
- 30.Younis T. Milch R. Abul-Khoudoud N, et al. Length of survival in hospice for cancer patients referred from a comprehensive cancer center. Am J Hosp Palliat Care. 2009;2:281–287. doi: 10.1177/1049909109333928. [DOI] [PubMed] [Google Scholar]
- 31.Byock I. Twohig JS. Merriman M. Collins K. Promoting excellence in end-of-life care: A report on innovative models of palliative care. J Palliat Med. 2006;9:137–151. doi: 10.1089/jpm.2006.9.137. [DOI] [PubMed] [Google Scholar]
- 32.Meyers FJ. Linder J. Beckett L. Christensen S. Blais J. Gandara DR. Simultaneous care: A model approach to the perceived conflict between investigational therapy and palliative care. J Pain Symptom Manage. 2004;28:548–556. doi: 10.1016/j.jpainsymman.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 33.World Health Organization. National Cancer Control Programmes: Policies and Managerial Guidelines. 2nd. Geneva: World Health Organization; 2002. Pain relief and palliative care; pp. 83–91. [Google Scholar]
- 34.Levy MH. Back A. Benedetti C. Billings JA. Block S. Boston B. Bruera E. Dy S. Eberle C. Foley KM. Karver SB. Knight SJ. Misra S. Ritchie CS. Spiegel D. Sutton L. Urba S. Von Roenn JH. Weinstein SM NCCN Clinical Practice Guidelines in Oncology. Palliative Care. www.nccn.org/professionals/physician_gls/f_guidelines.asp. 2008. [accessed September 3, 2009]. www.nccn.org/professionals/physician_gls/f_guidelines.asp [DOI] [PubMed]
- 35.IOM National Cancer Policy Board. Improving Palliative Care for Cancer. 1st. Washington, D.C.: Institute of Medicine; 2001. [Google Scholar]
- 36.Rabow MW. Dibble SL. Pantilat SZ. McPhee SJ. The comprehensive care team: A controlled trial of outpatient palliative medicine consultation. Arch Intern Med. 2004;164:83–91. doi: 10.1001/archinte.164.1.83. [DOI] [PubMed] [Google Scholar]