Abstract
Background
CpG administration abolishes airway inflammation and remodeling in acute models of allergic airway disease.
Methods
Herein, we investigated the therapeutic effect of CpG in a chronic fungal model of asthma. TLR9+/+ and TLR9–/– mice were sensitized to soluble Aspergillus fumigatus antigens and challenged with live A. fumigatus conidia. Mice were treated with intraperitoneal (IP) or intranasal (IN) CpG, or left untreated 14–28 days after conidium challenge. All features of allergic airway disease were attenuated in TLR9+/+ mice treated with IN CpG, including airway hyperresponsiveness (AHR), mucus production, and peribronchial fibrosis.
Results
TLR9–/– mice treated with IN CpG exhibited attenuated airway remodeling but not AHR. Whole-lung IL-12 levels were significantly elevated in both TLR9+/+ and TLR9–/– mice receiving IN CpG but not in either group receiving IP CpG. Whole-lung IL-10 levels were significantly elevated in IN CpG-treated TLR9+/+ mice but not in TLR9–/– mice receiving IN CpG. Increased whole-lung transcript and protein levels of the scavenger receptors SR-A and MARCO were observed in TLR9–/– mice compared with TLR9+/+ mice, possibly accounting for the CpG responsiveness in the knockout group.
Conclusions
Together, these data show that IN CpG has a therapeutic effect during established fungal asthma, which is TLR9 dependent and independent.
Key Words: Aspergillus, Asthma, CpG therapy, MARCO, SR-A, TLR9
Introduction
Allergic asthma is characterized by Th2 inflammation, which drives physiologic and structural remodeling events in the lung [1,2]. Recent strategies to treat allergic airway disease have focused on limiting or reducing Th2 inflammation. One immunotherapeutic strategy being developed for clinical therapy in asthma involves the use of hypomethylated oligodeoxynucleotides (ODNs) containing CpG motifs, which mimic either bacterial or viral DNA [3,4,5]. Prophylactic, systemic CpG administration in acute allergic airway models driven by ovalbumin (OVA), Aspergillus antigen, or house dust mite elicits major protective effects [6,7,8]. Further, exogenously administered CpG has been shown to inhibit and/or reverse airway remodeling in an OVA model of allergic asthma even when administered after OVA challenge and establishment of airway disease [9,10,11]. The mechanism through which CpG inhibits or reverses allergic airway disease is attributed, in part, to the inhibition of Th2 cytokines such as IL-4, IL-5, IL-9, and IL-13 and migration of Th2 cells into the lung environment [6,9,12,13,14]. B cells and plasmacytoid dendritic cells (DCs) respond to CpG and generate Th1-type proinflammatory cytokines, interferons, and chemokines. Certain CpG motifs have also been demonstrated to activate natural killer (NK) cells and induce plasmacytoid DCs to secrete IFN-α [15,16]. Some controversy exists as to which Th1-type cytokines mediate the therapeutic effects of CpG in allergic airway disease. The beneficial effects of CpG in experimental allergic airway disease are partially inhibited after the immunoneutralization of IFN-α, IFN-β, and IL-12 [6]. Subsequent studies identified that neither IL-12 nor IFN-γ were necessary for the therapeutic effect observed with CpG in allergic asthma models [17]. More recently, the mechanisms behind the inhibitory effects of CpG have been identified as involving the regulation of IL-5 through IL-10 synthesis [18]. Thus, CpG administration prior to or during acute allergic airway disease markedly attenuates features of this disease via its immunomodulatory effects.
Although CpG is assumed to work via Toll-like receptor 9 (TLR9), emerging evidence suggests that this ligand might exert immunomodulatory effects in a TLR9-independent manner [19,20,21]. TLR9-independent CpG activation occurs through Src kinase signaling mechanisms, which produce tyrosine phosphorylation events leading to actin polymerization and chemokine generation [21]. In neutrophils, TLR9-independent, but MyD88-dependent mechanisms have been identified as critical to the response to CpG in vitro [20]. Macrophage receptor with collagenous structure (MARCO) and scavenger receptor-A (SR-A) have been recently identified as receptors for CpG and are expressed on lung macrophages and DCs [22,23,24]. Whether CpG exerts immunomodulatory effects in a TLR9-independent manner during experimental allergic airway disease has not been addressed previously.
In the present study, we investigated the therapeutic effect of CpG in a chronic fungal asthma model. While previous studies of CpG in experimental asthma have investigated various modes of CpG delivery in wild-type (TLR9+/+) mice with allergic airway disease, our present investigation examined the effects of both the systemic (intraperitoneal, IP) and local (intranasal, IN) administration of CpG on TLR9+/+ and TLR9–/– mice with established Aspergillus fumigatus conidium-induced fungal asthma. We recently reported that A. fumigatus-sensitized TLR9–/– mice exhibit a very severe form of fungal asthma characterized by fungal growth and profound tissue remodeling after challenging these mice with swollen conidia [25]. In the present study, IN CpG but not IP CpG administration to TLR9+/+ mice 14–28 days after conidium challenge ameliorated AHR and airway remodeling at the day-28 time point. IN CpG but not IP CpG treatment over the same time ameliorated airway remodeling but not AHR in TLR9–/– mice with fungal asthma. While IN CpG raised whole-lung levels of IL-12 in both TLR9+/+ and TLR9–/– mice, only the TLR9+/+ group exhibited an increase in whole-lung levels of IL-10 when CpG was delivered in this manner. Responses to IN CpG in TLR9–/– mice were consistent with the markedly increased expression of MARCO and SR-A in the lungs of TLR9–/– mice with fungal asthma. Thus, TLR9-dependent and -independent recognition of CpG in the lung are responsible for reversing airway remodeling in mice during chronic fungal asthma.
Materials and Methods
Mice
Homozygous, female TLR9-gene-deficient (TLR9–/–) mice on BALB/c background were bred at the University of Michigan. Female, wild-type BALB/c (TLR9+/+) mice at 6–8 weeks of age were purchased from the Jackson Laboratory (Bar Harbor, Me., USA). Both groups of mice were maintained in a specific pathogen-free facility for the duration of the study. Prior approval for mouse usage was obtained from the University Laboratory of Animal Medicine at the University of Michigan Medical School.
A. fumigatus Growth and Isolation
Lyophilized A. fumigatus strain 13073 conidia (American Type Culture Collection, Manassas, Va., USA) stored in PBS with 0.1% BSA and glycerol were aseptically inoculated on Sabouraud dextrose agar plates (Teknova, Hollister, Calif., USA) and were cultured for 10 days at 37°C until mature conidia were visually apparent (i.e. dark green coloration). Conidia were washed from culture plates using 50 ml of sterile PBS with 0.1% Tween 80, strained through sterile gauze to remove hyphal contamination, centrifuged, and finally reconstituted to a concentration of 1.7 × 108 conidia/ml. Conidia were then incubated for 6 h at 37°C to allow them to become swollen prior to intratracheal (IT) injection.
CpG Treatments in a Chronic Fungal Asthma Model
TLR9+/+ and TLR9–/– mice were sensitized with a commercially available preparation of soluble A. fumigatus antigens as described in detail previously [26]. Seven days after a third IN challenge, each mouse received 5 × 106 swollen conidia suspended in 30 μl of PBS Tween 80 (0.1%; vol/vol) via IT administration as previously described [27]. Beginning on day 14 after conidium challenge, both groups of TLR9 received either 5 μg of CpG (HyCult Biotechnology, Uden, The Netherlands) dissolved in 20 μl of distilled water for IN instillation or 200 μl of distilled water for IP injection every other day until day 28 after conidium challenge. The CpG used herein is a 20-mer synthetic ODN that has the following sequence: 5′-tccatgacgttcctgatgct-3′ and contains a CpG-DNA motif, which mimics the immunostimulatory effects of bacterial DNA.
On day 28 after IT A. fumigatus swollen conidium challenge, bronchial hyperresponsiveness was assessed in a Buxco™ plethysmograph (Buxco, Troy, N.Y., USA) [26]. Briefly, sodium pentobarbital (Butler, Columbus, Ohio, USA; 0.04 mg/g of mouse body weight) was used to anesthetize mice prior to their intubation and ventilation with a Harvard pump ventilator (Harvard Apparatus, Reno, Nev., USA). Once baseline airway resistance was established, 420 mg/kg of methacholine was administered intravenously through the tail vein, and airway hyperresponsiveness (AHR) was monitored for approximately 2 min. The peak increase in airway resistance was then recorded. After the assessment of AHR, blood was removed for immunoglobulin analysis and whole lungs were dissected from each mouse and snap frozen in liquid nitrogen or fixed in 10% formalin for genomic, proteomic, and histological analyses.
Serum IgE and IgG2a Analyses
In TLR9+/+ and TLR9–/– groups, serum levels of IgE and IgG2a were analyzed on day 28 after conidium challenge using complementary capture and detection antibody pairs for IgE (PharMingen, San Diego, Calif., USA). Immunoglobulin ELISAs were performed according to the manufacturer's directions. Duplicate serum samples were diluted to 1:20 for IgE determination. Immunoglobulin levels were then determined from optical density readings at 492 nm, and immunoglobulin concentrations were calculated from a standard curve generated using recombinant IgE or IgG2a (the standard curves ranged from 0 to 1,000 ng/ml).
Whole-Lung RNA Isolation and TaqMan Analysis
Total RNA was isolated from homogenized mouse lungs using TRIzol reagent (Invitrogen/Life Technologies, Carlsbad, Calif., USA). Purified RNA was treated with DNAse and reverse transcribed into cDNA using TaqMan reverse transcription reagents (Applied Biosystems, Foster City, Calif., USA). For quantitative TaqMan analysis, a total of 0.2 μg of total RNA was reverse transcribed to yield cDNA, and predeveloped TaqMan gene expression assays were used to quantify IFN-α, IFN-β, CXCL10, MARCO, SR-AI/II, TLR2, TLR3, and TLR9 according to the manufacturer's (Applied Biosystems) instructions. GAPDH was analyzed as an internal control. Gene expression was normalized to GAPDH before the fold change in gene expression was calculated. The fold changes in transcript expression were calculated via the comparison of gene expression in naïve whole-lung samples, which were assigned a value of 1 to that in whole-lung samples from mice with chronic fungal asthma.
Whole-Lung Proteomic Analysis
Murine IL-4, IL-5, IL-10, IL-13, IFN-γ, TGF-β, TNF-α, IL-12p70, CXCL10, CCL11, CXCL9, CCL21, CCL6, CCL2, CCL3, CCL5, CCL22, CCL17, MARCO, and SR-A levels were determined in 50-μl samples from whole-lung homogenates using a standardized sandwich ELISA technique previously described in detail or through a bead-based multiple target sandwich ELISA system (Bio-Plex, Bio-Rad Laboratories, Hercules, Calif., USA). Recombinant murine cytokines and chemokines (R&D Systems, Rochester, Minn., USA) were used to generate the standard curves from which the sample concentrations were derived. The limits of ELISA detection for each cytokine were consistently >50 pg/ml for sandwich ELISA and >1 pg/ml for Bio-Plex. The cytokine and chemokine levels in each sample were normalized to total protein levels measured using the Bradford assay.
Whole-Lung Histological Analysis
Whole lungs from A. fumigatus-sensitized TLR9+/+ and TLR9–/– mice prior to and at various times after A. fumigatus conidium challenge were fully inflated with 10% formalin, dissected, and placed in fresh 10% formalin for 24 h. Routine histological techniques were used to paraffin embed the entire lung, and 5-μm sections of whole lung were stained with periodic acid-Schiff (PAS) and Masson trichrome.
MARCO and SR-A Immunohistochemistry
Paraffin-embedded whole-lung samples were analyzed using routine immunohistochemical techniques for the presence of either MARCO or SR-A. Goat anti-mouse MARCO and SR-A polyclonal antibodies were obtained from R&D Systems (Minneapolis, Minn., USA). Briefly, 5-μm histological sections were dewaxed with xylene, rehydrated in graded concentrations of ethanol, and stained with the mouse HRP-DAB cell and tissue staining kit according to the manufacturer's instructions (R&D Systems). Other histological samples were immunostained with control antibodies (IgG isotype controls and HRP substrate).
Statistical Analysis
All results are expressed as means ± SEM. A Student's t test or analysis of variance (ANOVA) and a Student-Newman-Keuls multiple comparison test were used to determine statistical significance on a normal distribution between TLR9+/+ and TLR9–/– mice between CpG treatment groups on day 28 after the conidium challenge; p < 0.05 was considered statistically significant.
Results
IN CpG Attenuated AHR in TLR9+/+ but Not in TLR9–/– Mice
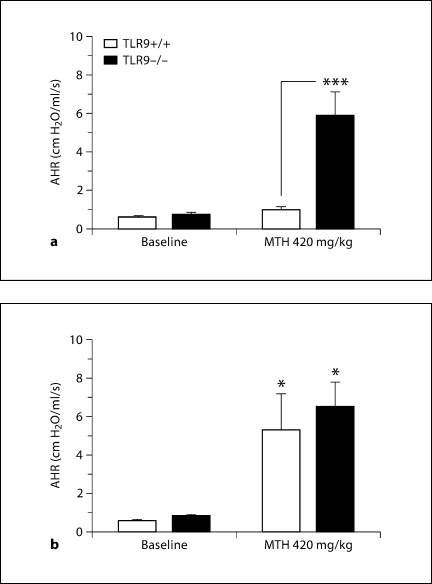
In a previous study on AHR in TLR9+/+ and TLR9–/– mice [27], absence of TLR9 had no effect on methacholine-induced AHR since both wild-type and knockout mice showed similar airway responses to methacholine. Previous reports have shown that CpG inhibits AHR in experimental asthma [8]. Our present results indicate that IN CpG from days 14 to 28 after conidium challenge, but not IP CpG treatment over the same time period, decreased AHR in TLR9+/+ mice assessed on day 28 after conidium challenge. In addition, neither IN nor IP CpG affected allergic airway hyperreactivity in TLR9–/– mice (fig. 1). Thus, local but not systemic CpG administration inhibited AHR in TLR9+/+ mice and the inhibitory effects of IN CpG required TLR9 expression.
Fig. 1.
IN CpG treatment attenuated AHR in TLR9+/+ but not TLR9–/– mice. Airway resistance analysis in TLR9+/+ and TLR9–/– Aspergillus-sensitized and conidium-challenged mice treated with IN CpG (a) or IP CpG (b) on alternate days from day 14 to day 28 after conidium challenge. Peak increases in airway resistance or hyperresponsiveness were determined at each time point after the intravenous injection of methacholine (MTH). Airway resistance was 1.89 ± 0.11 cm H2O/ml/s in untreated TLR9+/+ mice and 16.96 ± 3.86 cm H2O/ml/s after intravenous MTH administration. Means ± SEM; n = 5/group/time point. ∗ p ≤ 0.05; ∗∗∗ p ≤ 0.001, vs. the appropriate baseline measurement or the indicated treatment group following MTH challenge.
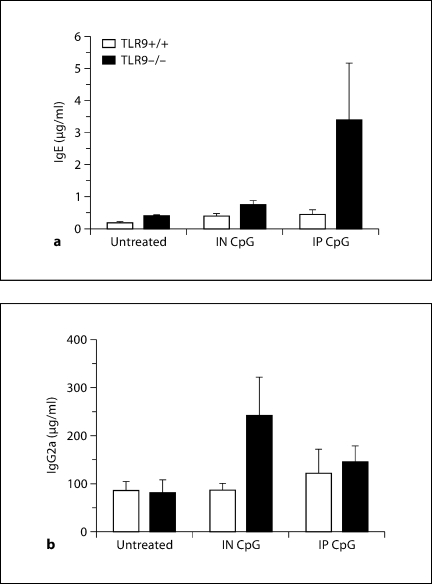
Exogenous CpG Altered Circulating Immunoglobulin Levels in TLR9–/– but Not in TLR9+/+ Mice
We next assessed serum levels of IgE and IgG2a to determine whether the CpG therapies affected these immunoglobulins on day 28 after conidium challenge. Elevation of serum IgE levels is a characteristic feature of atopy and allergic disease due to Aspergillus hypersensitivity [28,29]. Aspergillus-sensitized and -challenged TLR9–/– mice treated with IP CpG did not exhibit any significant increase in serum IgE levels compared with TLR9+/+ mice. No differences in serum IgE levels between the untreated and IN CpG-treated TLR9+/+ and TLR9–/– groups were observed (fig. 2a). IN CpG-treated TLR9–/– mice also did not demonstrate a significant change in serum IgG2a levels compared with TLR9+/+ mice (fig. 2b). No differences in serum IgG2a levels between the untreated and IP CpG-treated TLR9+/+ and TLR9–/– groups were observed. It is important to note that neither IP nor IN CpG treatments in TLR9+/+ mice produced any noticeable effect on serum immunoglobulin levels.
Fig. 2.
CpG treatment altered systemic immunoglobulin levels in TLR9–/– mice alone. ELISA analysis of serum IgE (a) and IgG2a (b) from Aspergillus-sensitized and conidium-challenged TLR9+/+ and TLR9–/– mice either untreated, or treated with IN or IP CpG. Serum was removed from all groups of mice on day 28 after conidium challenge. Means ± SEM; n = 10/group/time point.
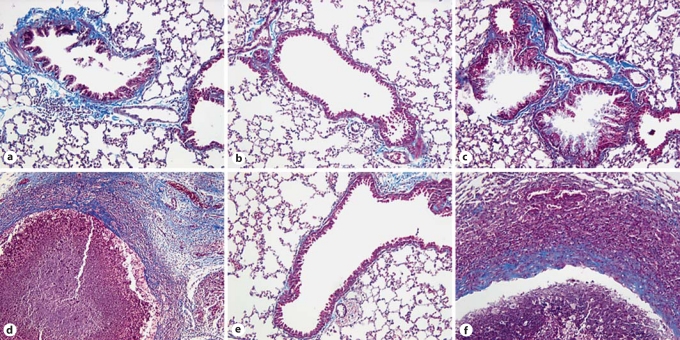
IN CpG but Not IP CpG Treatment of TLR9+/+ and TLR9–/– Asthmatic Mice Ameliorates Peribronchial Fibrosis and Fungal Growth
We have previously reported that TLR9–/– mice were unable to control the growth of A. fumigatus leading to prominent fungal masses in the lungs of these mice on day 28 after conidium challenge [25]. Trichrome-stained lung sections from TLR9+/+ and TLR9–/– mice on day 28 after IT conidium challenge revealed that fibrosis was prominent around airways and fungal masses were prominent in control (fig. 3a, d; fibrotic tissue is stained light blue) and IP CpG-treated groups (fig. 3c, f), whereas peribronchial fibrosis was absent in both the TLR9+/+ and TLR9–/– groups that received IN CpG (fig. 3b, e). Most remarkable, there was the therapeutic effect of IN CpG on the fibrotic fungal masses in TLR9–/– mice; no fibrotic fungal masses were detected in IN CpG-treated TLR9–/– mice, and the histological appearance of whole- lung sections in this group of mice was similar to that of IN CpG-treated TLR9+/+ mice. Thus, these data indicate that IN CpG but not IP CpG reversed the peribronchial fibrotic remodeling in TLR9+/+ and TLR9–/–mice, and eliminated fungus from TLR9–/– mice.
Fig. 3.
Peribronchial fibrosis and perifungal fibrotic capsules were markedly decreased or absent following IN but not IP CpG treatment in TLR9+/+ and TLR9–/– mice. Whole-lung tissue sections from Aspergillus-sensitized and challenged TLR9+/+ and TLR9–/– mice were stained with Masson trichrome stain. Representative panels of whole-lung sections from TLR9+/+ (a–c) and TLR9–/– mice (d–f) given no treatment (a, d), IN CpG (b, e), or IP CpG (c, f) are shown. Of note, no fungal masses were detected in whole-lung sections from IN CpG-treated TLR9–/– mice. Collagen deposition is stained blue in these photomicrographs (original magnification: ×200).
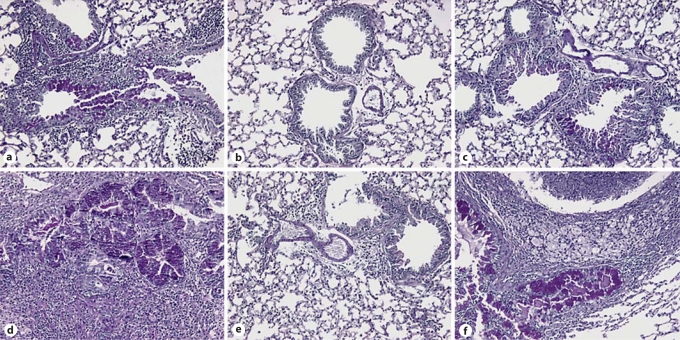
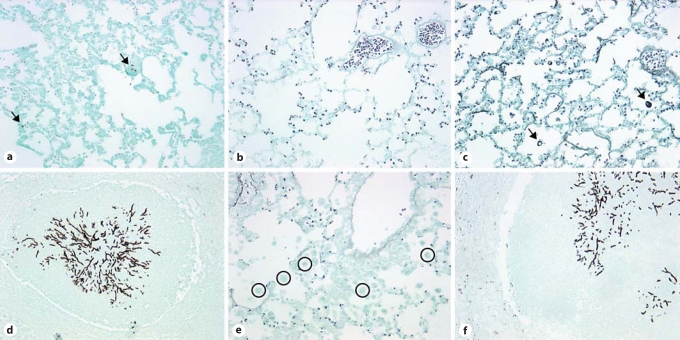
IN CpG but Not IP CpG Treatment Reduced Mucus Production and Fungal Growth in Both TLR9+/+ and TLR9–/– Mice
Following our observations of route-specific effects of CpG administration on lung inflammation and remodeling, changes in airway mucus secretion through PAS stain of lung histological sections were examined next. PAS-stained lung sections from TLR9+/+ and TLR9–/– mice on day 28 after IT conidium challenge demonstrated enhanced mucus production in untreated (fig. 4a, d) and IP CpG-treated groups (fig. 4c, f), but markedly decreased mucus secretion was observed in lungs from IN CpG-treated mice (fig. 4b, e). Further, Gomori methenamine silver (GMS)-stained lung sections from TLR9+/+ and TLR9–/– mice on day 28 after IT conidium challenge demonstrated fungal growth and macrophage engulfment of conidia in untreated (fig. 5a, d) and IP CpG-treated groups (fig. 5c, f), but markedly reduced fungal growth and macrophage phagocytosis of conidia was observed in lungs from IN CpG-treated mice (fig. 5b, e). Thus, these data indicated that mucus metaplasia and fungal growth were markedly attenuated by IN CpG treatment in both TLR9+/+ and TLR9–/– mice.
Fig. 4.
Mucus was markedly reduced following IN but not IP CpG treatment in TLR9+/+ and TLR9–/– mice. Mouse whole-lung tissue sections from Aspergillus-sensitized and challenged TLR9+/+ and TLR9–/–mice were stained with PAS. Representative whole lung sections from TLR9+/+ (a–c) and TLR9–/– mice (d–f) that were given no treatment (a, d), IN CpG (b, e), or IP CpG (c, f) are shown. Mucus stains purple using the PAS staining procedure (original magnification: ×200).
Fig. 5.
Fungal growth is markedly reduced following IN but not IP CpG treatment in TLR9+/+ and TLR9–/– mice. Mouse whole-lung tissue sections from Aspergillus-sensitized and challenged TLR9+/+ and TLR9–/–mice were stained with GMS. Aspergillus conidia and hyphae stain black using the GMS staining procedure. Representative whole-lung sections from TLR9+/+ (a–c) and TLR9–/– mice (d–f) that were given no treatment (a, d), IN CpG (b, e), or IP CpG (c, f) are shown. Black arrows highlight conidia engulfed by macrophages. Open circles denote macrophages that have not engulfed conidia. No macrophages containing engulfed conidia were visible in TLR9+/+ mice given IN CpG treatment. Original magnification: ×200 (a, c, d, f) and ×400 (b, e).
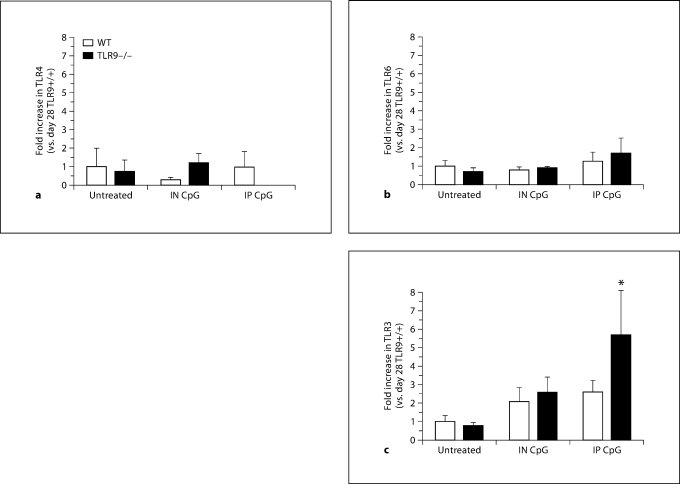
TLR9–/– Mice Exhibited Significantly Increased Whole-Lung TLR3 Transcript Levels following IP CpG Treatment
We next examined whether IN or IP CpG altered whole-lung TLR expression in TLR9+/+ and TLR9–/– mice. Transcript levels of TLR4, TLR6, and TLR3 were analyzed in whole-lung tissues on day 28 after conidium challenge. CpG treatment did not alter levels of TLR4 or TLR6 in the lungs of asthmatic mice (fig. 6a, b). IP CpG-treated TLR9–/– mice, however, demonstrated a significant increase in TLR3 levels compared with TLR9+/+ mice (fig. 6c). Thus, these data suggest that IP CpG treatment in TLR9-deficient mice induced TLR3. However, CpG administration to TLR9+/+ mice did not alter whole-lung TLR4 or TLR6 levels in either wild-type or knockout mice.
Fig. 6.
Elevated TLR3 levels were observed in IP CpG-treated TLR9–/– mice. TaqMan analysis of TLR9+/+ and TLR9–/– mouse lung TLR4 (a), TLR6 (b), and TLR3 (c) levels from Aspergillus-sensitized and conidium-challenged TLR9+/+ and TLR9–/– mice that were either untreated, treated with IN CpG, or treated with IP CpG. Means ± SEM; n = 5/group/time point. ∗ p ≤ 0.05 vs. the appropriate untreated control group. WT = Wild type.
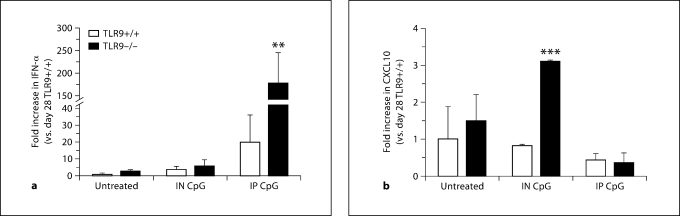
TLR9–/– Mice Exhibit Significantly Increased Whole-Lung IFN-α Transcript Levels following IP CpG Treatment, but Significantly Elevated CXCL10 Transcript Levels following IN CpG Treatment
We next sought to characterize the mechanism through which IN CpG attenuated airway remodeling in TLR9+/+ and TLR9–/– mice and analyzed the transcript levels of several cytokines and chemokines in whole-lung tissues on day 28 after conidium challenge. IP CpG-treated TLR9–/– mice demonstrated an approximately 10-fold increase in IFN-α levels compared with TLR9+/+ mice (fig. 7a). It is significant to note that IFN-α was only induced with IP CpG treatment, but this induction had no effect on any of the characteristics of allergic airway disease. The amount of IFN-α detected in TLR9–/– mice was markedly greater than that detected in untreated or IN CpG-treated mice. Further, CXCL10 transcript levels were significantly increased in IN CpG-treated TLR9–/– mice and were 3-fold greater compared with the TLR9+/+ group (fig. 7b). Thus, these data suggested that the levels of CpG administered to TLR9+/+ mice did not alter whole-lung Th1-type cytokine and chemokine levels. However, the amounts of CpG delivered either by the IN or IP route were sufficient to alter these cytokine and chemokine levels in TLR9–/– mice.
Fig. 7.
Elevated IFN-α levels were observed in IP CpG treated TLR9–/–mice, and CXCL10 levels were increased following IN CpG treatment in TLR9–/– mice. TaqMan analysis of TLR9+/+ and TLR9–/– mouse lung IFN-α (a) and CXCL10 (b) levels from Aspergillus-sensitized and conidium-challenged TLR9+/+ and TLR9–/– mice that were either untreated, treated with IN CpG, or treated with IP CpG. Means ± SEM; n = 5/group/time point. ∗∗ p ≤ 0.01; ∗∗∗ p ≤ 0.001, vs. the appropriate untreated control group.
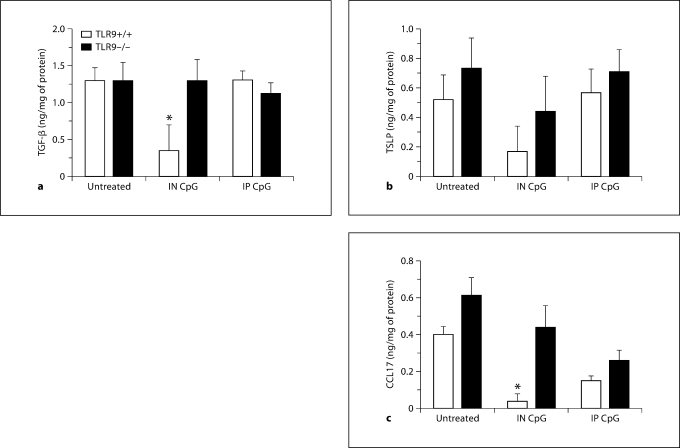
Pro-Allergic Th2 Cytokine and Chemokine Levels Are Suppressed following IN CpG Treatment in TLR9+/+ Mice Only
Whole-lung cytokine and chemokine protein levels were analyzed next in this model of fungal asthma in response to IN and IP CpG treatment on day 28 after conidium challenge. No significant differences in IL-4, IL-5, IL-13, and IFN-γ were observed between the IN and IP CpG groups on day 28 after conidium challenge (table 1). Significant differences in the levels of TGF-β, thymic stromal lymphopoietin (TSLP), and CCL17 (cytokines and chemokines that promote allergic airway disease, differentiation of Th2 cells, and chemotaxis of Th2 cells) were observed following IN CpG treatment. Specifically, whole-lung levels of TGF-β were significantly decreased in TLR9+/+ mice receiving IN CpG compared with untreated and IP CpG-treated TLR9–/– mice (fig. 8a). Further, there was a decrease in whole-lung levels of TSLP in TLR9+/+ mice treated with IN CpG compared with untreated and IP CpG-treated TLR9–/– mice (fig. 8b). Finally, a significant decrease in whole-lung levels of CCL17 was observed in TLR9+/+ mice that received IN CpG compared with untreated and IP CpG-treated TLR9–/– mice (fig. 8c). It is important to note that IN CpG did not inhibit levels of these Th2-type cytokines and chemokines in TLR9–/– mice compared with untreated TLR9–/– mice. Further, IP CpG did not inhibit levels of these Th2-type cytokines and chemokines in either group of mice compared with the appropriate untreated group of mice. Thus, these data indicated that decreased allergic airway disease in IN CpG-treated TLR9+/+ mice with fungal asthma might be a consequence of both decreased levels of TGF-β and lower levels of the Th2-associated cytokines TSLP and CCL17.
Table 1.
Whole-lung cytokine and chemokine levels (ng/mg total protein) on day 28 after swollen conidium challenge in A. fumigatus-sensitized TLR9+/+ and TLR9−/− mice
| Cytokine/chemokine | Untreated |
INCpG |
IPCpG |
|||
|---|---|---|---|---|---|---|
| TLR9+/+ | TLR9−/− | TLR9+/+ | TLR9−/− | TLR9+/+ | TLR9−/− | |
| IL-4 | 0.01 ±0.00 | 0.00 ±0.00∗ | 0.02 ±0.00 | 0.00 ±0.00∗∗ | 0.01 ±0.00 | 0.02 ±0.00 |
| IL-5 | ND | ND | ND | ND | ND | ND |
| IL-13 | 0.08 ±0.01 | 0.04 ±0.00∗ | 0.00 ±0.00 | 0.00 ±0.00 | 0.00 ±0.00 | 0.00 ±0.00 |
| IFN-γ | ND | ND | ND | ND | ND | ND |
p < 0.05
p < 0.01, compared with untreated TLR9+/+ mice. ND = Not detected.
Fig. 8.
IN CpG inhibited Th2-associated cytokine levels in a TLR9-dependent manner in chronic fungal asthma. ELISA or Bio-Plex of TGF-β (a), TSLP (b), and CCL17 (c) in whole-lung samples from Aspergillus-sensitized and conidium-challenged TLR9+/+ and TLR9–/– mice. Both groups of mice were left untreated or treated with IN CpG, or IP CpG on alternate days from day 14 to day 28 after conidium challenge. Means ± SEM; n = 5/group/time point. ∗ p ≤ 0.05 vs. the appropriate untreated control group.
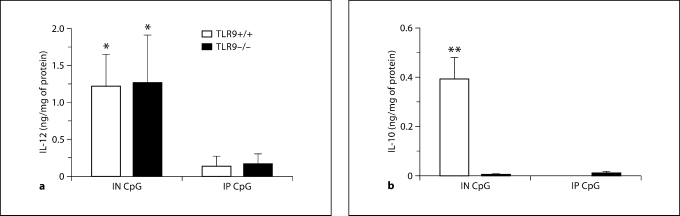
Asthmatic Mice Treated with IN CpG Exhibited Increased IL-12 Levels Regardless of TLR9 Expression, but IL-10 Induction following IN CpG Was TLR9-Dependent
Changes in other cytokines, namely IL-12 and IL-10, were observed following CpG treatment in this model on day 28 after conidium challenge. A significant elevation in IL-12 levels was observed in both TLR9+/+ and TLR9–/– mice during IN CpG treatment compared with IP CpG treatment (fig. 9a). We also observed a significant elevation in whole-lung IL-10 levels in IN CpG-treated TLR9+/+ mice, but levels of this cytokine were not altered in TLR9–/– mice (fig. 9b). No immunoreactive IL-10/IL-12 was detected at this time point in untreated mice, indicating that IN CpG drove the expression of lung IL-12 in both TLR9+/+ and TLR9–/– mice, but elicited lung IL-10 levels in only TLR9+/+ mice.
Fig. 9.
TLR9-independent induction of IL-12 and TLR9-dependent induction of IL-10 following IN CpG treatment in chronic fungal asthma. ELISA or Bio-Plex of IL-12 (a) and IL-10 (b) in whole-lung samples from Aspergillus-sensitized and conidium-challenged TLR9+/+ and TLR9–/– mice. Both groups of mice were left untreated or treated with IN or IP CpG on alternate days from day 14 to day 28 after conidium challenge. Note that the levels of IL-12 and IL-10 in untreated TLR9+/+ and TLR9–/– mice were below the limits of detection for these assays. Means ± SEM; n = 10/group/time point. ∗ p ≤ 0.05; ∗∗ p ≤ 0.01, vs. the appropriate IP CpG group.
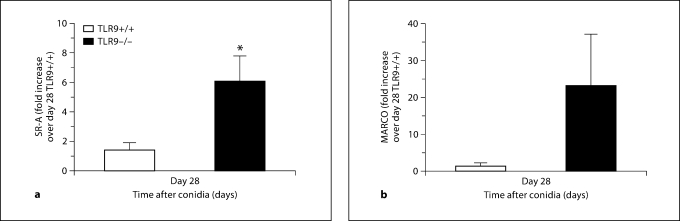
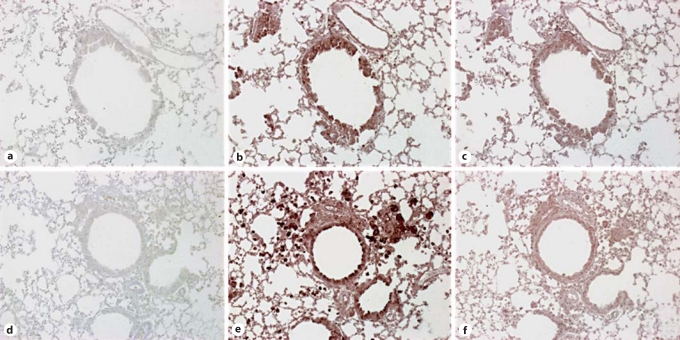
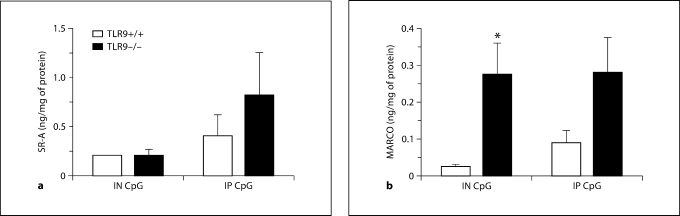
SR-A and MARCO Levels Were Elevated in TLR9–/– Mice with Chronic Fungal Asthma
Given the altered histological and Th1-type cytokine/chemokine profile in CpG-treated TLR9–/– mice, we next sought to identify alternative receptors through which CpG was exerting its therapeutic effects and examined the transcript and protein levels of the scavenger receptors MARCO and SR-A on day 28 after challenge. Results indicated a significant elevation in SR-A transcript levels, but no significant elevation in transcript levels of MARCO in untreated TLR9–/– mice compared with TLR9+/+ mice (fig. 10). Further, immunohistochemical analysis of whole-lung tissue sections revealed that both SR-A and MARCO were more abundantly expressed in the epithelium and mononuclear cells in untreated TLR9–/–mice compared with TLR9+/+ mice on day 28 after conidium challenge (fig. 11). To determine the effect of CpG treatment on whole-lung levels of SR-A and MARCO, ELISA was employed. Lower levels of MARCO and SR-A were measured in whole-lung samples from IN CpG-treated TLR9+/+ mice compared with IP CpG-treated TLR9+/+ mice (fig. 12). SR-A levels were lower in IN CpG-treated TLR9–/– mice compared with IP CpG-treated TLR9–/–mice, but MARCO levels were similar between the two treatment groups of TLR9–/–mice (fig. 12). Notably, significantly greater levels of MARCO were present in whole-lung samples from IN CpG-treated TLR9–/– mice compared with similarly treated TLR9+/+ mice (fig. 12). Thus, greater transcript and protein levels of both SR-A and MARCO were present in TLR9–/– mice possibly accounting for the responsiveness of these mice to CpG.
Fig. 10.
Scavenger receptor transcript levels were elevated in TLR9–/– mice with chronic fungal asthma. TaqMan analysis of TLR9+/+ and TLR9–/– mouse lung SR-A (a) and MARCO (b) levels in Aspergillus-sensitized and conidium-challenged TLR9+/+ and TLR9–/– mice. Both groups of mice were left untreated and transcript levels for these scavenger receptors were determined from whole-lung samples taken on day 28 after conidium challenge. Means ± SEM; n = 5/group/time point. ∗ p ≤ 0.05 vs. the TLR9+/+ group.
Fig. 11.
Immunohistochemical analysis of SR-A and MARCO expression in whole-lung sections from TLR9+/+ and TLR9–/– mice on day 28 after conidium challenge. Whole-lung tissue sections from Aspergillus-sensitized and challenged TLR9+/+ and TLR9–/– mice were stained using routine immunohistochemical techniques. Representative whole-lung sections from TLR9+/+ (a–c) and TLR9–/– mice (d–f) mice stained with IgG control (a, d), anti-SR-A antibody (b, e), or anti-MARCO antibody (c, f) are shown. Receptor expression stains brown with this immunohistochemical procedure (original magnification: ×200).
Fig. 12.
Immunoreactive levels of MARCO and SR-A remain elevated in TLR9–/– mice compared with TLR9+/+ mice after CpG treatment. ELISA analysis of SR-A (a) and MARCO (b) in whole-lung samples from Aspergillus-sensitized and conidium-challenged TLR9+/+ and TLR9–/– mice. Both groups were treated with IN CpG or IP CpG on alternate days from day 14 to day 28 after conidium challenge. Means ± SEM; n = 5/group/time point. ∗ p ≤ 0.05 vs. IN CpG TLR9+/+ group.
Discussion
Herein, we characterized the asthmatic phenotype in A. fumigatus-sensitized and conidium-challenged TLR9+/+ and TLR9–/– mice after treatment with IP or IN CpG 14–28 days after conidium challenge. We observed that IN CpG but not IP CpG therapy significantly enhanced whole-lung IL-12 and IL-10 levels, and significantly inhibited airway inflammation and hyperresponsiveness, lung remodeling, and Th2-associated cytokine levels in a model of allergic asthma in TLR9+/+ mice. Surprisingly, we observed that IN CpG but not IP CpG therapy also significantly increased serum IgG2a levels and whole-lung levels of CXCL10 and IL-12. Compared with untreated TLR9–/– mice, IN CpG-treated TLR9–/– mice exhibited attenuated fungal growth and airway remodeling but not AHR. One explanation for the CpG responsiveness of A. fumigatus-sensitized and conidium-challenged TLR9–/– mice might be derived from the observation that transcript and protein levels of the scavenger receptors MARCO and SR-A were dramatically elevated in these mice compared with similarly sensitized and challenged TLR9+/+ mice. Thus, these data demonstrated that therapeutic responses to CpG during experimental fungal asthma were dependent upon the site of CpG administration, and CpG had TLR9-independent therapeutic effects in this model of fungal asthma, possibly due to CpG activation via MARCO and SR-A receptors.
Previous studies have demonstrated that the adjuvant or Th1-type cytokine response evoked by CpG appears to depend on the site of administration [30], with mucosal delivery being identified as the route promoting the optimal immunostimulatory effect [31,32]. Although the IN delivery of CpG promotes inflammatory changes in non-allergic mice [33], its delivery via this route in allergic mice has been shown to markedly attenuate the pulmonary allergic inflammatory response in wild-type mice [reviewed in ref. [34]]. The therapeutic effect of CpG-ODN treatment in the experimental fungal asthma model examined herein was dependent on the site of administration since IN delivery of CpG provided a therapeutic effect in both wild-type and knockout mice, whereas the IP delivery of CpG did not. This difference in therapeutic outcome could be explained by the direct modulation of CpG-responsive cell types, such as epithelial cells, macrophages, and DCs, which are responsible for fungus-induced allergic inflammation. In fact, previous studies involving the priming of mice with Aspergillus antigens and CpG in a model of invasive aspergillosis have demonstrated that CpG promotes DC activation within the lung and the production of IL-12 and IFN-γ by these cells [35]. Thus, the route of administration of CpG proved to be important for its therapeutic effect in experimental fungal asthma.
Immunostimulatory sequences containing CpG motifs induce IFNs and IFN-inducible genes such as CXCL10 both in experimental models of allergic airway disease [reviewed in ref. [34]] and clinical asthma [36]. CpG directly activates monocytes, macrophages, and DCs to secrete IFN-α/IFN-β, IL-6, IL-12, and TNF-α leading to NK cell activation and secretion of IFN-γ [32,34]. Administration of neutralizing monoclonal antibodies against type I cytokines such as IFN-α have been found to attenuate the inhibitory effect of CpG-ODN on airway inflammation and Th2 cell migration into the lung [6]. In the present study, IN CpG treatment in TLR9+/+ mice was associated with significantly increased whole-lung levels of IL-12, but this mode of CpG delivery did not alter whole-lung levels of IFN-α and CXCL10. Given that IP CpG increased whole-lung IFN-α but not IL-12 levels in TLR9+/+ mice with fungal asthma, it is likely that the therapeutic effects of CpG were mediated, in part, via IL-12 and not IFN-α or CXCL10 in TLR9+/+ mice. Surprisingly, more dynamic changes were noted in Th1-type mediators in TLR9–/– mice with fungal asthma. IN CpG significantly enhanced serum levels of IgG2a, a Th1- associated immunoglobulin, and whole-lung levels of CXCL10 and IL-12. IP CpG significantly enhanced whole-lung levels of IFN-α. The latter finding in IP CpG-treated TLR9–/– mice might reflect the fact that TLR3 levels were significantly elevated in whole-lung samples from this group of mice, but appears to confirm that this type-1 IFN does not mediate the therapeutic effects of CpG. Thus, the administration of CpG to TLR9-sufficient and -deficient mice with fungal asthma dynamically altered levels of Th1-type factors and mediators although only IN CpG enhanced the expression of whole-lung IL-12.
Shifting the immune response away from Th2-type cytokine generation has been shown to effectively reduce asthmatic symptoms both experimentally and clinically [5]. TGF-β and CCL17 have well-described roles in Th2-mediated airway inflammation [37], and both are prominently expressed and appear to have clear roles in the experimental asthma model studied herein [26,38]. The cytokine TSLP has not been examined in this model previously, but it is known to activate DCs and promotes the differentiation of Th2 T cells and secretion of Th2 cytokines and chemokines [39,40]. We observed that whole-lung levels of TGF-β were reduced IN CpG-treated TLR9+/+ mice and these findings are consistent with previous CpG studies in models of allergic airway disease [8,10,17]. Inhibitory effects of IN CpG on whole-lung TSLP and CCL17 were also apparent in TLR9+/+ mice alone, consistent with reduced AHR and remodeling in these mice. Changes in IL-10 following IN CpG were also TLR9 dependent. The inhibitory effects of IL-10 on allergic inflammation are well known and include regulation of eosinophilia [8], inhibition of the proliferation of various structural cell types [41], and regulation of airway inflammation through its inhibitory action on nitric oxide, a potent mediator of AHR [42,43]. Recent studies have shown that the adoptive transfer of IL-10-overexpressing DCs into OVA-sensitized and challenged mice ameliorates allergic asthma symptoms [44]. The contribution of macrophage-derived IL-10 driven by CpG activation of TLR9 has also shown to be an important therapeutic modality of CpG in allergic airway inflammation [45]. Thus, IN CpG inhibited whole-lung Th2 cytokines and chemokines and enhanced whole-lung IL-10 levels in a TLR9-dependent manner, and these effects were consistent with the amelioration of all features of chronic fungal asthma in TLR9+/+ mice.
The macrophage class A scavenger receptors SR-A and MARCO recognize CpG and promote IL-12 and nitric oxide synthesis [22,24]. SR-A levels were increased in an OVA-induced asthma model in wild-type mice, and both SR-A- and MARCO-deficient mice exhibited increased AHR and eosinophilic airway inflammation in this same model with a concomitant increase in the recruitment of DCs in the lung [46]. Recent studies have demonstrated that targeting scavenger receptors in a model of allergy produces a shift from a Th2 to a Th1 immune response [47]. In the present study, elevated SR-A and MARCO transcript and protein levels were observed in the whole lung from TLR9–/– mice compared with similar samples from TLR9+/+ mice. We interpret these findings to indicate that scavenger receptors, such as SR-A and MARCO, play a compensatory role in the TLR9-deficient mouse and recognize CpG. This interpretation is consistent with the enhanced Th1-type factors and mediators observed in TLR9–/– mice following CpG treatment. However, the full therapeutic effect of CpG required the presence of TLR9 indicating that SR-A and MARCO could not fully compensate for the absence of this TLR. Future studies will address the identification and characterization of the TLR9-expressing cell, which is so critically important in the regulation of AHR.
While CpG therapies have proven very effective in experimental studies [48], clinical trials involving hypomethylated CpG-ODNs targeting TLR9 have failed to reproduce results observed in the laboratory. Specifically, clinical investigations into the efficacy of CpG in asthma have found that CpG increases in IFN-γ and IFN-inducible genes in asthmatics but has no impact on the characteristic AHR in these patients [36]. Extrapolating the experimental findings in the present study to the clinical situation, it is plausible that the failure of CpG to work clinically is due in part to polymorphisms in TLR9 resulting in loss of function. Our previous study of the role of TLR9 during chronic fungal asthma showed that the absence of this receptor led to a severe form of allergic airway disease characterized by fungal growth [25] and thus it is possible that similar changes in severity related to the expression and function of TLR9 occur in clinical asthma. Investigations into the role of TLR9 polymorphisms in CpG-induced responses between individuals found major interindividual differences in CpG-induced IFN-α production, however, these were not associated with common TLR9 variants [49]. As mentioned above, CpG treatment in patients drove a Th1 response without improvement in AHR [36]. Again, one might speculate that this scenario is similar to what we observed in TLR9–/– mice given IN CpG, in which the presence of CpG recognizing scavenger receptors accounted for changes in Th1 cytokines without any attenuation of Th2 mechanisms driving AHR.
In summary, CpG appeared to have site-specific therapeutic effects on the maintenance of chronic fungal airway disease. Specifically, IN CpG but not IP CpG decreased AHR and airway remodeling in TLR9+/+ mice consistent with its inhibitory effects on Th2-associated factors such as TGF-β, TSLP, and CCL17 and its stimulatory effects on IL-12 and IL-10. Surprisingly, IN CpG reduced airway remodeling in TLR9–/– mice, but AHR was not affected in these mice. Together, these data highlight the importance of TLR9-dependent and -independent mechanisms in the modulation of all features of chronic fungal asthma.
Acknowledgements
This work was financially supported, in part, by the NIH (grant HL069865 to C.M.H.). The authors thank Ms. Erica O’Connor and Dr. Priya Kulasekaran for their technical contributions to this study.
References
- 1.Benayoun L, Druilhe A, Dombret MC, Aubier M, Pretolani M. Airway structural alterations selectively associated with severe asthma. Am J Respir Crit Care Med. 2003;167:1360–1368. doi: 10.1164/rccm.200209-1030OC. [DOI] [PubMed] [Google Scholar]
- 2.Jeffery PK. Remodeling and inflammation of bronchi in asthma and chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2004;1:176–183. doi: 10.1513/pats.200402-009MS. [DOI] [PubMed] [Google Scholar]
- 3.Kline JNK, Arthur M. Cpg oligodeoxynucleotides. New Drugs for Asthma, Allergy and COPD. In: Hansel TT, Barnes PJ, editors. Prog Respir Res. vol 31. Basel: Karger; 2001. pp. 229–232. [Google Scholar]
- 4.Racila DM, Kline JN. Perspectives in asthma: Molecular use of microbial products in asthma prevention and treatment. J Allergy Clin Immunol. 2005;116:1202–1205. doi: 10.1016/j.jaci.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 5.Kline JN. Eat dirt: Cpg DNA and immunomodulation of asthma. Proc Am Thorac Soc. 2007;4:283–288. doi: 10.1513/pats.200701-019AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashino S, Wakita D, Zhang Y, Chamoto K, Kitamura H, Nishimura T. Cpg-Odn inhibits airway inflammation at effector phase through down-regulation of antigen-specific Th2-cell migration into lung. Int Immunol. 2008;20:259–266. doi: 10.1093/intimm/dxm138. [DOI] [PubMed] [Google Scholar]
- 7.Banerjee B, Kelly KJ, Fink JN, Henderson JD, Jr, Bansal NK, Kurup VP. Modulation of airway inflammation by immunostimulatory CpG oligodeoxynucleotides in a murine model of allergic aspergillosis. Infect Immun. 2004;72:6087–6094. doi: 10.1128/IAI.72.10.6087-6094.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirose I, Tanaka H, Takahashi G, Wakahara K, Tamari M, Sakamoto T, Kojima S, Inagaki N, Nagai H. Immunomodulatory effects of CpG oligodeoxynucleotides on house dust mite-induced airway inflammation in mice. Int Arch Allergy Immunol. 2008;147:6–16. doi: 10.1159/000128581. [DOI] [PubMed] [Google Scholar]
- 9.Youn CJ, Miller M, Baek KJ, Han JW, Nayar J, Lee SY, McElwain K, McElwain S, Raz E, Broide DH. Immunostimulatory DNA reverses established allergen-induced airway remodeling. J Immunol. 2004;173:7556–7564. doi: 10.4049/jimmunol.173.12.7556. [DOI] [PubMed] [Google Scholar]
- 10.Jain VV, Kitagaki K, Businga T, Hussain I, George C, O'Shaughnessy P, Kline JN. CpG-oligodeoxynucleotides inhibit airway remodeling in a murine model of chronic asthma. J Allergy Clin Immunol. 2002;110:867–872. doi: 10.1067/mai.2002.129371. [DOI] [PubMed] [Google Scholar]
- 11.Jain VV, Businga TR, Kitagaki K, George CL, O'Shaughnessy PT, Kline JN. Mucosal immunotherapy with Cpg oligodeoxynucleotides reverses a murine model of chronic asthma induced by repeated antigen exposure. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1137–L1146. doi: 10.1152/ajplung.00073.2003. [DOI] [PubMed] [Google Scholar]
- 12.Ikeda RK, Miller M, Nayar J, Walker L, Cho JY, McElwain K, McElwain S, Raz E, Broide DH. Accumulation of peribronchial mast cells in a mouse model of ovalbumin allergen induced chronic airway inflammation: modulation by immunostimulatory DNA sequences. J Immunol. 2003;171:4860–4867. doi: 10.4049/jimmunol.171.9.4860. [DOI] [PubMed] [Google Scholar]
- 13.Cho JY, Miller M, Baek KJ, Han JW, Nayar J, Rodriguez M, Lee SY, McElwain K, McElwain S, Raz E, Broide DH. Immunostimulatory DNA inhibits transforming growth factor-beta expression and airway remodeling. Am J Respir Cell Mol Biol. 2004;30:651–661. doi: 10.1165/rcmb.2003-0066OC. [DOI] [PubMed] [Google Scholar]
- 14.Broide DH, Stachnick G, Castaneda D, Nayar J, Miller M, Cho JY, Roman M, Zubeldia J, Hayashi T, Raz E. Systemic administration of immunostimulatory DNA sequences mediates reversible inhibition of Th2 responses in a mouse model of asthma. J Clin Immunol. 2001;21:175–182. doi: 10.1023/a:1011078930363. [DOI] [PubMed] [Google Scholar]
- 15.Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002;20:709–760. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 16.Krieg AM. Antiinfective applications of toll-like receptor 9 agonists. Proc Am Thorac Soc. 2007;4:289–294. doi: 10.1513/pats.200701-021AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kline JN, Krieg AM, Waldschmidt TJ, Ballas ZK, Jain V, Businga TR. CpG oligodeoxynucleotides do not require Th1 cytokines to prevent eosinophilic airway inflammation in a murine model of asthma. J Allergy Clin Immunol. 1999;104:1258–1264. doi: 10.1016/s0091-6749(99)70022-9. [DOI] [PubMed] [Google Scholar]
- 18.Kitagaki K, Jain VV, Businga TR, Hussain I, Kline JN. Immunomodulatory effects of CpG oligodeoxynucleotides on established Th2 responses. Clin Diagn Lab Immunol. 2002;9:1260–1269. doi: 10.1128/CDLI.9.6.1260-1269.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trevani AS, Chorny A, Salamone G, Vermeulen M, Gamberale R, Schettini J, Raiden S, Geffner J. Bacterial DNA activates human neutrophils by a CpG-independent pathway. Eur J Immunol. 2003;33:3164–3174. doi: 10.1002/eji.200324334. [DOI] [PubMed] [Google Scholar]
- 20.Alvarez ME, Fuxman Bass JI, Geffner JR, Calotti PX, Costas M, Coso OA, Gamberale R, Vermeulen ME, Salamone G, Martinez D, Tanos T, Trevani AS. Neutrophil signaling pathways activated by bacterial DNA stimulation. J Immunol. 2006;177:4037–4046. doi: 10.4049/jimmunol.177.6.4037. [DOI] [PubMed] [Google Scholar]
- 21.Sanjuan MA, Rao N, Lai KT, Gu Y, Sun S, Fuchs A, Fung-Leung WP, Colonna M, Karlsson L. CpG-induced tyrosine phosphorylation occurs via a TLR9-independent mechanism and is required for cytokine secretion. J Cell Biol. 2006;172:1057–1068. doi: 10.1083/jcb.200508058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jozefowski S, Sulahian TH, Arredouani M, Kobzik L. Role of scavenger receptor MARCO in macrophage responses to CpG oligodeoxynucleotides. J Leukoc Biol. 2006;80:870–879. doi: 10.1189/jlb.0705357. [DOI] [PubMed] [Google Scholar]
- 23.Pearson AM. Scavenger receptors in innate immunity. Curr Opin Immunol. 1996;8:20–28. doi: 10.1016/s0952-7915(96)80100-2. [DOI] [PubMed] [Google Scholar]
- 24.Jozefowski S, Arredouani M, Sulahian T, Kobzik L. Disparate regulation and function of the class A scavenger receptors SR-AI/II and MARCO. J Immunol. 2005;175:8032–8041. doi: 10.4049/jimmunol.175.12.8032. [DOI] [PubMed] [Google Scholar]
- 25.Ramaprakash H, Ito T, Standiford TJ, Kunkel SL, Hogaboam CM. Toll-like receptor 9 modulates immune responses to Aspergillus fumigatus conidia in immunodeficient and allergic mice. Infect Immun. 2009;77:108–119. doi: 10.1128/IAI.00998-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hogaboam CM, Blease K, Mehrad B, Steinhauser ML, Standiford TJ, Kunkel SL, Lukacs NW. Chronic airway hyperreactivity, goblet cell hyperplasia, and peribronchial fibrosis during allergic airway disease induced by Aspergillus fumigatus. Am J Pathol. 2000;156:723–732. doi: 10.1016/S0002-9440(10)64775-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramaprakash H, Ito T, Standiford TJ, Kunkel SL, Hogaboam CM. Toll-like receptor 9 modulates immune responses to Aspergillus fumigatus conidia in immunodeficient and allergic mice. Infect Immun. 2009;77:108–119. doi: 10.1128/IAI.00998-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurup VP, Xia JQ, Crameri R, Rickaby DA, Choi HY, Fluckiger S, Blaser K, Dawson CA, Kelly KJ. Purified recombinant A. fumigatus allergens induce different responses in mice. Clin Immunol. 2001;98:327–336. doi: 10.1006/clim.2000.4993. [DOI] [PubMed] [Google Scholar]
- 29.Nolles G, Hoekstra MO, Schouten JP, Gerritsen J, Kauffman HF. Prevalence of immunoglobulin E for fungi in atopic children. Clin Exp Allergy. 2001;31:1564–1570. doi: 10.1046/j.1365-2222.2001.01186.x. [DOI] [PubMed] [Google Scholar]
- 30.Lipford GB, Sparwasser T, Zimmermann S, Heeg K, Wagner H. CpG-DNA-mediated transient lymphadenopathy is associated with a state of Th1 predisposition to antigen-driven responses. J Immunol. 2000;165:1228–1235. doi: 10.4049/jimmunol.165.3.1228. [DOI] [PubMed] [Google Scholar]
- 31.McCluskie MJ, Davis HL. Oral, intrarectal and intranasal immunizations using CpG and non-CpG oligodeoxynucleotides as adjuvants. Vaccine. 2000;19:413–422. doi: 10.1016/s0264-410x(00)00208-5. [DOI] [PubMed] [Google Scholar]
- 32.McCluskie MJ, Davis HL. CpG DNA as mucosal adjuvant. Vaccine. 1999;18:231–237. doi: 10.1016/s0264-410x(99)00194-2. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz DA, Quinn TJ, Thorne PS, Sayeed S, Yi AK, Krieg AM. CpG motifs in bacterial DNA cause inflammation in the lower respiratory tract. J Clin Invest. 1997;100:68–73. doi: 10.1172/JCI119523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nat Rev Drug Discov. 2006;5:471–484. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 35.Bozza S, Gaziano R, Lipford GB, Montagnoli C, Bacci A, Di Francesco P, Kurup VP, Wagner H, Romani L. Vaccination of mice against invasive aspergillosis with recombinant aspergillus proteins and CpG oligodeoxynucleotides as adjuvants. Microbes Infect. 2002;4:1281–1290. doi: 10.1016/s1286-4579(02)00007-2. [DOI] [PubMed] [Google Scholar]
- 36.Gauvreau GM, Hessel EM, Boulet LP, Coffman RL, O'Byrne PM. Immunostimulatory sequences regulate interferon-inducible genes but not allergic airway responses. Am J Respir Crit Care Med. 2006;174:15–20. doi: 10.1164/rccm.200601-057OC. [DOI] [PubMed] [Google Scholar]
- 37.Schuh JM, Blease K, Kunkel SL, Hogaboam CM. Chemokines and cytokines: axis and allies in asthma and allergy. Cytokine Growth Factor Rev. 2003;14:503–510. doi: 10.1016/s1359-6101(03)00057-1. [DOI] [PubMed] [Google Scholar]
- 38.Schuh JM, Power CA, Proudfoot AE, Kunkel SL, Lukacs NW, Hogaboam CM. Airway hyperresponsiveness, but not airway remodeling, is attenuated during chronic pulmonary allergic responses to aspergillus in CCR4–/– mice. FASEB J. 2002;16:1313–1315. doi: 10.1096/fj.02-0193fje. [DOI] [PubMed] [Google Scholar]
- 39.Ying S, O'Connor B, Ratoff J, Meng Q, Fang C, Cousins D, Zhang G, Gu S, Gao Z, Shamji B, Edwards MJ, Lee TH, Corrigan CJ. Expression and cellular provenance of thymic stromal lymphopoietin and chemokines in patients with severe asthma and chronic obstructive pulmonary disease. J Immunol. 2008;181:2790–2798. doi: 10.4049/jimmunol.181.4.2790. [DOI] [PubMed] [Google Scholar]
- 40.Phipps S, Lam CE, Foster PS, Matthaei KI. The contribution of Toll-like receptors to the pathogenesis of asthma. Immunol Cell Biol. 2007;85:463–470. doi: 10.1038/sj.icb.7100104. [DOI] [PubMed] [Google Scholar]
- 41.Ogawa Y, Duru EA, Ameredes BT. Role of IL-10 in the resolution of airway inflammation. Curr Mol Med. 2008;8:437–445. doi: 10.2174/156652408785160907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ameredes BT, Sethi JM, Liu HL, Choi AM, Calhoun WJ. Enhanced nitric oxide production associated with airway hyporesponsiveness in the absence of IL-10. Am J Physiol Lung Cell Mol Physiol. 2005;288:L868–L873. doi: 10.1152/ajplung.00207.2004. [DOI] [PubMed] [Google Scholar]
- 43.Ameredes BT, Zamora R, Sethi JM, Liu HL, Kohut LK, Gligonic AL, Choi AM, Calhoun WJ. Alterations in nitric oxide and cytokine production with airway inflammation in the absence of IL-10. J Immunol. 2005;175:1206–1213. doi: 10.4049/jimmunol.175.2.1206. [DOI] [PubMed] [Google Scholar]
- 44.Henry E, Desmet CJ, Garze V, Fievez L, Bedoret D, Heirman C, Faisca P, Jaspar FJ, Gosset P, Jacquet AP, Desmecht D, Thielemans K, Lekeux P, Moser M, Bureau F. Dendritic cells genetically engineered to express IL-10 induce long-lasting antigen-specific tolerance in experimental asthma. J Immunol. 2008;181:7230–7242. doi: 10.4049/jimmunol.181.10.7230. [DOI] [PubMed] [Google Scholar]
- 45.Vissers JL, van Esch BC, Jeurink PV, Hofman GA, van Oosterhout AJ. Stimulation of allergen-loaded macrophages by TLR9-ligand potentiates IL-10-mediated suppression of allergic airway inflammation in mice. Respir Res. 2004;5:21. doi: 10.1186/1465-9921-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arredouani MS, Franco F, Imrich A, Fedulov A, Lu X, Perkins D, Soininen R, Tryggvason K, Shapiro SD, Kobzik L. Scavenger receptors SR-AI/II and MARCO limit pulmonary dendritic cell migration and allergic airway inflammation. J Immunol. 2007;178:5912–5920. doi: 10.4049/jimmunol.178.9.5912. [DOI] [PubMed] [Google Scholar]
- 47.Bhatia S, Mukhopadhyay S, Jarman E, Hall G, George A, Basu SK, Rath S, Lamb JR, Bal V. Scavenger receptor-specific allergen delivery elicits IFN-gamma-dominated immunity and directs established Th2-dominated responses to a nonallergic phenotype. J Allergy Clin Immunol. 2002;109:321–328. doi: 10.1067/mai.2002.121143. [DOI] [PubMed] [Google Scholar]
- 48.Klinman DM. Immunotherapeutic uses of CpG oligodeoxynucleotides. Nat Rev Immunol. 2004;4:249–258. doi: 10.1038/nri1329. [DOI] [PubMed] [Google Scholar]
- 49.Berghofer B, Frommer T, Konig IR, Ziegler A, Chakraborty T, Bein G, Hackstein H. Common human Toll-like receptor 9 polymorphisms and haplotypes: association with atopy and functional relevance. Clin Exp Allergy. 2005;35:1147–1154. doi: 10.1111/j.1365-2222.2005.02325.x. [DOI] [PubMed] [Google Scholar]