Abstract
Cytochrome P450 2D6 (CYP2D6) has been identified as the major autoantigen in type 2 autoimmune hepatitis (AIH). However, because of a lack of appropriate animal models, the etiology of AIH is still poorly understood. We generated a mouse model for AIH using the human CYP2D6 as a triggering molecule for autoimmunity. We infected wild-type FVB mice with an adenovirus expressing human CYP2D6 (Ad-2D6) to break self-tolerance to the mouse CYP2D6 homologues. Ad-2D6-infected mice showed persistent features of liver damage including hepatic fibrosis, cellular infiltrations, focal-to-confluent necrosis and generation of anti-CYP2D6 antibodies, which predominantly recognized the identical immunodominant epitope recognized by LKM-1 antibodies from AIH patients. Interestingly, Ad-2D6 infection of transgenic mice expressing the human CYP2D6 (CYP2D6 mice) resulted in delayed kinetics and reduced severity of liver damage. However, the quantity and quality of anti-CYP2D6 antibodies was only moderately reduced in CYP2D6 mice. In contrast, the frequency of CYP2D6-specific CD4 and CD8 T cells was dramatically decreased in CYP2D6 mice, indicating the presence of a strong T cell tolerance to human CYP2D6 established in CYP2D6 mice, but not in wild-type mice. CYP2D6-specific T cells reacted to human CYP2D6 peptides with intermediate homology to the mouse homologues, but not to those with high homology, indicating that molecular mimicry rather than molecular identity breaks tolerance and subsequently causes severe persistent autoimmune liver damage. The CYP2D6 model provides a platform to investigate mechanisms involved in the immunopathogenesis of autoimmune-mediated chronic hepatic injury and evaluate possible ways of therapeutic interference.
Key Words: Autoimmune hepatitis, Mouse model, Molecular mimicry
Introduction
The etiology of autoimmune hepatitis (AIH) is poorly understood, although the major autoantigen, cytochrome P450 2D6 (CYP2D6), has been identified and immunodominant epitopes mapped. One reason might be the lack of an appropriate animal model that reflects the pathogenic processes occurring in patients suffering from AIH. Experimental hepatitis was often reported to be only transient and many current models for autoimmune liver disease depend on a rather complex disease induction protocol (for review, see [1] and [2]).
Therefore, we generated a mouse model for human AIH using the natural human autoantigen CYP2D6 as a triggering molecule for initiation of an autoimmune process. CYP2D6 has been identified as the major autoantigen recognized by liver-kidney microsomal antibodies 1 (LKM-1) in type 2 AIH at the end of the 1980s [3,4]. Since then, many autoreactive epitopes have been identified on the B cell [5,6,7,8,9,10] as well as T cell level [11,12,13]. The immunodominant epitope DPAQPPRD is recognized by LKM-1 antibodies of the majority of type 2 AIH patients [8,10,14,15]. The three-dimensional structure of CYP2D6 reveals that the immunodominant as well as most of the subdominant epitopes are located at the surface of the molecule and are therefore easily accessible by patient LKM-1 antibodies [9,16].
Interestingly, an amino acid sequence homology has been documented between DPAQPPRD of human CYP2D6 and the infected cell protein 4 of herpes simplex virus 1 [8], indicating the existence of molecular mimicry between the major autoantigen in type 2 AIH and a human pathogen. However, no association of herpes simplex virus 1 infection and type 2 AIH could be demonstrated to date.
The novel CYP2D6 mouse model is based on the concept of molecular mimicry as a potential mechanism for the initiation and/or acceleration of autoimmunity. The molecular mimicry concept [17,18,19] first requires an infection by a pathogen which shares a structural relatedness with components of the host in order to cause an aggressive immune response with the goal of eliminating the invading pathogen as fast as possible. Second, the activated antipathogen immune reactivity is then directed against host structures that share a structural similarity to the pathogen, resulting in autoimmunity. Several associations between pathogen infections and human autoimmune diseases have been made in the past [20,21]. However, definite proof for molecular mimicry being responsible for autoimmune diseases is rare. One of the few examples of postinfectious autoimmunity due to molecular mimicry was demonstrated for the Guillain-Barré syndrome, where an association of infection with Campylobacter jejuni sharing a structural homology of the lipo-oligosaccharide with the peripheral nerve GM1 ganglioside could be convincingly reproduced in an animal model [22]. In addition, convincing evidence has been established for Streptococcus pyogenes-induced acute rheumatic fever, where the lysoganglioside of the host shares a structural similarity with N-acetyl-β-D-glucosamine, the dominant epitope of the group A streptococcal carbohydrate [23]. For autoimmune liver disease, it has been recently demonstrated that environmental factors, such as the bacterium Novosphingobium aromaticivorans and chemical xenobiotics, confer molecular mimicry to the immunodominant structure in the major autoantigen in primary biliary cirrhosis PDC-E2 containing the prosthetic group lipoic acid [24,25]. Among chemical xenobiotics, the cosmetic and food additive 2-octynoic acid shows a high structural similarity to lipoic acid, and injection of 2-octynoic acid coupled to bovine serum albumin (BSA) resulted in the generation of primary biliary cirrhosis-like disease in wild-type C57BL/6 mice [26].
Several animal models have been generated that used virus infections paired with a transgenic expression of target antigens. In many cases the target antigen was identical to the triggering antigen. For example in the RIP-LCMV mouse model for type 1 diabetes (T1D), the glycoprotein (GP) or the nucleoprotein (NP) of the lymphocytic choriomeningitis virus (LCMV) was expressed in transgenic RIP-LCMV mice under the control of the rat-insulin promoter (RIP), specifically in the β-cells of the islets of Langerhans, and LCMV itself was used as a trigger [27,28]. However, the success of this model depended on the thymic expression of the transgene. LCMV-NP was found to be expressed in the thymus with the result that high-affinity NP-specific CD8 T cells were deleted in the thymus and T1D development was rather slow (1–6 months) and required CD4 T cell help. In contrast, LCMV infection of RIP-LCMV-GP mice resulted in the rapid (10–14 days) development of T1D in a CD4 T cell-independent manner [29]. The frequency of autoreactive T cells plays a central part in the development of autoimmune diseases. Interestingly, sequential infections by heterologous viruses influence such frequencies massively [30]. It has been shown that molecular mimicry between heterologous viruses that share similar epitopes might skew the T cell repertoire in a way that results in an acceleration of autoimmune disease [31]. In particular, infection of mice with Pichinde virus (PV) that shares a subdominant epitope with LCMV fails to induce T1D in the RIP-LCMV model because the frequency of LCMV/PV-specific T cells is too low. In contrast, sequential infection of mice with LCMV followed by PV massively accelerates the autodestructive process of the pancreatic β-cells [31,32]. These data indicate that in the RIP-LCMV model, infection with a pathogen that shares a similar, but not identical, epitope with a specific target in the host is not sufficient for the initiation of autoimmune disease, but can accelerate an already ongoing autoimmune destructive process by enhancing the number of autoreactive T cells. However, such an outcome is influenced by a variety of factors including the nature of the epitope, the strength of tolerance, the location of the target structure and the tropism of the pathogen.
Intuitively, one would assume that tolerance might be stronger to identical structures than to structures that just share a certain degree of similarity. Self-reactive lymphocytes with high avidity are more likely to be deleted or functionally silenced by central and/or peripheral tolerance mechanisms. Thus, perfect mimicry between identical structures might fail in inducing autoimmunity because of efficient tolerance mechanisms. In contrast, imperfect mimicry between similar, but not identical, structures might on the one hand circumvent tolerance, but on the other hand result in the generation of lymphocytes with only low to intermediate avidity.
Methods
The CYP2D6 model offers the possibility to evaluate the roles of molecular mimicry and molecular identity as inducers and/or accelerators of autoimmune diseases in more detail. An adenovirus expressing the human CYP2D6 (Ad-2D6) is used as a trigger for inducing autoimmune liver damage in either wild-type FVB mice or in transgenic CYP2D6 mice. Wild-type mice only express the mouse homologues of human CYP2D6, such as Cyp2D9, Cyp2D11, Cyp2D22 and Cyp2D26, which display up to 75% amino acid sequence homology to human CYP2D6 (NCBI databank). Cyp2D22 has been suggested as the functional orthologue of human CYP2D6 because of a certain similarity in substrate specificity [33,34,35]. In contrast to wild-type mice, transgenic CYP2D6 mice express, in addition to the mouse Cyp isoenzymes, the human CYP2D6 under its own promoter, predominantly in the liver. Thus, the CYP2D6 model allows for distinguishing molecular mimicry (Ad-2D6 infection of wild-type mice) from molecular identity (Ad-2D6 infection of CYP2D6 mice).
Results
The CYP2D6 Mouse Model for AIH
First, we infected wild-type FVB mice with Ad-2D6 to break self-tolerance to the mouse CYP2D6 homologues. Within days after infection, the mice had features of acute liver damage such as transiently elevated serum aminotransferases and focal infiltrations. However, these features occurred in Ad-2D6-infected mice as well as in mice infected with a control adenovirus expressing the green fluorescent protein (Ad-GFP) [36]. In contrast, at later times only Ad-2D6-infected mice displayed persistent features characteristic for liver damage associated with AIH. These features included massive hepatic fibrosis, ‘fused’ liver lobules, disorganized architecture, cellular infiltrations and focal to confluent necrosis [36]. Interestingly, the severity of liver damage was reduced and the onset of disease was delayed in Ad-2D6-infected transgenic CYP2D6 mice. These data indicate that mice expressing the human CYP2D6 in the liver established a certain degree of tolerance or ignorance to the challenge with the identical triggering molecule when compared to wild-type mice that only express the similar mouse Cyp isoenzymes.
Fibrosis
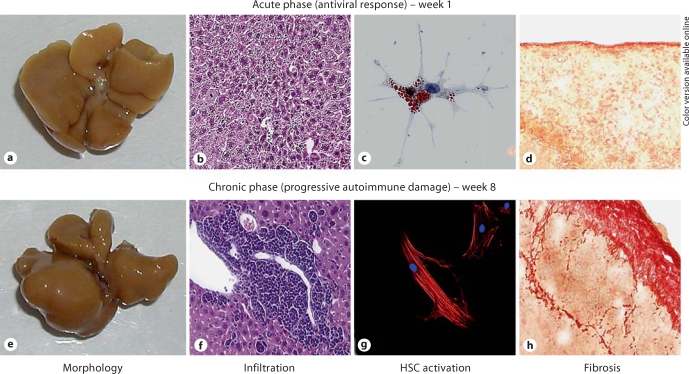
The development of hepatic fibrosis was restricted to Ad-2D6-infected mice and was found predominantly in the subcapsular area, resulting in the fusion of individual lobules [36]. After 4–8 weeks of Ad-2D6 infection, periportal fibrosis became apparent, which correlated with cellular infiltration and overall liver damage, and was similar to carbon tetrachloride (CCl4)-induced fibrosis in control mice at weeks 2–4 [Hintermann and Christen, manuscript in preparation]. However, subcapsular fibrosis was less frequent in CCl4-treated mice. Staining for α-smooth muscle actin revealed activation of hepatic stellate cells in tissue sections of both CCl4-treated and virus-infected CYP2D6 mice. Furthermore, isolation of hepatic stellate cells revealed an enhanced activation status in CCl4-treated mice and virus-infected CYP2D6 mice [Hintermann and Christen, manuscript in preparation]. Our data indicate that virus-infected CYP2D6 mice display subcapsular and periportal fibrosis, and hepatic stellate cell-activation similar to CCl4-treated animals. An overview of the morphological features as well as cellular infiltration and fibrosis is shown in figure 1.
Fig. 1.
Acute and chronic phases of Ad-2D6-induced AIH in the CYP2D6 mouse model. In the acute phase directly after Ad-2D6 infection, the liver still maintains a normal morphology (a), but small focal infiltrations are visible (H&E staining of a 5-μm liver section, b). Isolated hepatic stellate cells have a naïve phenotype (Oil red O staining of cultured hepatic stellate cells, c) and no significant subcapsular fibrosis is detectable (Sirius red staining of a 5-μm liver section, d). In the chronic phase of autoimmune liver damage, the morphology of the liver changes and individual lobes become fused (e). Large clusters of infiltrating cells become apparent predominantly in the perivascular region (H&E staining of a 5-μm liver section, f). Isolated hepatic stellate cells are activated and express α-smooth muscle actin [anti-α-smooth muscle actin antibody (red) and DAPI (blue) staining, g] and extensive subcapsular and perivascular fibrosis are found (Sirius red staining of a 5-μm liver section, h) ([36]; Hintermann and Christen, manuscript in preparation). Color in online version only.
B Cell Response
All Ad-2D6-infected mice generated high titers of anti-CYP2D6 antibodies. The enhanced tolerance to human CYP2D6 and its mouse homologues in CYP2D6 mice seemed to have only a minor effect on the generation of CYP2D6-specififc antibodies since the anti-CYP2D6 antibodies reached very high levels in both CYP2D6 as well as wild-type FVB mice [36]. Antibody formation was only moderately reduced in transgenic CYP2D6 mice compared to wild-type FVB mice. In contrast, mice infected with a control adenovirus neither developed liver damage nor generated anti-CYP2D6 antibodies. Epitope mapping revealed that such anti- CYP2D6 antibodies predominantly recognized the identical immunodominant linear epitope WDPAQPPRD that is also recognized by LKM-1 antibodies from AIH type 2 patients [8,10,14,15,36]. Importantly, anti- CYP2D6 antibodies generated in CYP2D6 and wild-type FVB mice both reacted to the same major B cell epitope WDPAQPPRD. These data indicate that B cell tolerance induction does not account for the observed differences in disease kinetics and severity between wild-type and transgenic mice.
T Cell Response
We analyzed the T cell-mediated immune response by stimulating lymphocytes with 61 overlapping 20-mer peptides covering the entire human CYP2D6. T cells produced interferon-γ after stimulation with peptides only if isolated from Ad-2D6-infected mice. The frequency of CYP2D6-specific T cells was more than three times higher in lymphocytes isolated from the liver than from the spleen, indicating either an organ-specific proliferation or a selective retention process in the liver [Holdener and Christen, manuscript in preparation]. Importantly, the frequency of CYP2D6-specific CD4 as well as CD8 cells was dramatically decreased in transgenic CYP2D6 mice, indicating the presence of a strong T cell tolerance to human CYP2D6 established in transgenic CYP2D6 expressing the identical target antigen compared to wild-type FVB mice expressing the mouse homologues only [Holdener and Christen, manuscript in preparation]. T cell epitope mapping further revealed that CYP2D6-specific T cells reacted to human CYP2D6 peptides with intermediate homology to the mouse homologues, but not to those with high homology or identity. Our data indicate that molecular mimicry rather than molecular identity breaks tolerance on both the B cell and T cell level, subsequently causing severe and persistent autoimmune liver damage.
Conclusion
In summary, the CYP2D6 mouse model for AIH uses a true human autoantigen, which seems to be important for establishing a chronic model that comes close to the immunopathogenesis of autoimmune liver disease as observed in patients with AIH. Figure 2 shows a schematic overview of the observed liver damage over time after infection. After an initial antiviral immune response, which lasts for 1–2 weeks, chronic AIH develops over the following weeks and months.
Fig. 2.
Schematic overview on the development of AIH in the CYP2D6 model. The antiviral phase includes acute inflammation, transient elevation of serum aminotransferases, cytokine and chemokine release, focal infiltration, and initial activation of hepatic stellate cells (HSC). The chronic progressive autoimmune phase is characterized by high titer anti-CYP2D6 antibodies, CYP2D6-specific CD4 and CD8 T cells, strong hepatic infiltration and extensive fibrosis. The degree and onset of liver damage is dependent on the degree of immunologic tolerance that is more efficiently broken in conditions of molecular mimicry than identity ([36]; Hintermann and Christen, manuscript in preparation; Holdener and Christen, manuscript in preparation).
Mechanistically, the CYP2D6 model revealed that tolerance is sturdier in mice expressing an autoantigen (i.e. human CYP2D6) that is identical to the environmental trigger (i.e. human CYP2D6 expressed by an infecting adenovirus) than in mice that only express similar autoantigens (i.e. the mouse Cyp isoenzymes). In particular, the condition of molecular mimicry was more effective in breaking T cell tolerance since almost no T cell reactivity to human CYP2D6 was found in Ad-2D6-infected CYP2D6 mice. In contrast, B cell tolerance collapsed in both wild-type and transgenic mice. The data confirm earlier studies indicating that B cell tolerance is not as robust as T cell tolerance [37]. The CYP2D6 model provides a platform for investigating mechanisms involved in the immunopathogenesis of autoimmune-mediated chronic hepatic injury as seen in human AIH and evaluating possible ways of therapeutic interference.
Disclosure Statement
The authors declare that no financial or other conflict of interest exists in relation to the content of the article.
Acknowledgement
The presented work was supported by NIH grant R21 DK071577 and a grant of the German Research Foundation to U.C.
References
- 1.Christen U, Hintermann E, Jaeckel E. New animal models for autoimmune hepatitis. Semin Liver Dis. 2009;29:262–272. doi: 10.1055/s-0029-1233536. [DOI] [PubMed] [Google Scholar]
- 2.Jaeckel E. Old and new animal models of AIH. In: Manns MP, Lehnert H, Lohse AW, editors. Falk Liver Conference – Immunology and Liver Disease. Basel: Karger; 2009. p. 171. [Google Scholar]
- 3.Manns MP, Johnson EF, Griffin KJ, Tan EM, Sullivan KF. Major antigen of liver kidney microsomal autoantibodies in idiopathic autoimmune hepatitis is cytochrome P450db1. J Clin Invest. 1989;83:1066–1072. doi: 10.1172/JCI113949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zanger UM, Hauri HP, Loeper J, Homberg JC, Meyer UA. Antibodies against human cytochrome P-450db1 in autoimmune hepatitis type II. Proc Natl Acad Sci USA. 1988;85:8256–8260. doi: 10.1073/pnas.85.21.8256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imaoka S, Obata N, Hiroi T, Osada-Oka M, Hara R, Nishiguchi S, Funae Y. A new epitope of CYP2D6 recognized by liver kidney microsomal autoantibody from Japanese patients with autoimmune hepatitis. Biol Pharm Bull. 2005;28:2240–2243. doi: 10.1248/bpb.28.2240. [DOI] [PubMed] [Google Scholar]
- 6.Kerkar N, Choudhuri K, Ma Y, Mahmoud A, Bogdanos DP, Muratori L, Bianchi F, Williams R, Mieli-Vergani G, Vergani D. Cytochrome P4502D6(193–212): a new immunodominant epitope and target of virus/self cross-reactivity in liver kidney microsomal autoantibody type 1-positive liver disease. J Immunol. 2003;170:1481–1489. doi: 10.4049/jimmunol.170.3.1481. [DOI] [PubMed] [Google Scholar]
- 7.Klein R, Zanger UM, Berg T, Hopf U, Berg PA. Overlapping but distinct specificities of anti-liver-kidney microsome antibodies in autoimmune hepatitis type II and hepatitis C revealed by recombinant native CYP2D6 and novel peptide epitopes. Clin Exp Immunol. 1999;118:290–297. doi: 10.1046/j.1365-2249.1999.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manns MP, Griffin KJ, Sullivan KF, Johnson EF. LKM-1 autoantibodies recognize a short linear sequence in P450IID6, a cytochrome P-450 monooxygenase. J Clin Invest. 1991;88:1370–1378. doi: 10.1172/JCI115443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sugimura T, Obermayer-Straub P, Kayser A, Braun S, Loges S, Alex B, Luttig B, Johnson EF, Manns MP, Strassburg CP. A major CYP2D6 autoepitope in autoimmune hepatitis type 2 and chronic hepatitis C is a three-dimensional structure homologous to other cytochrome P450 autoantigens. Autoimmunity. 2002;35:501–513. doi: 10.1080/0891693021000069556. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto AM, Cresteil D, Boniface O, Clerc FF, Alvarez F. Identification and analysis of cytochrome P450IID6 antigenic sites recognized by anti-liver-kidney microsome type-1 antibodies (LKM1) Eur J Immunol. 1993;23:1105–1111. doi: 10.1002/eji.1830230519. [DOI] [PubMed] [Google Scholar]
- 11.Löhr HF, Schlaak JF, Lohse AW, Böcher WO, Arenz M, Gerken G, Meyer zum Büschenfelde KH. Autoreactive CD4+ LKM-specific and anticlonotypic T-cell responses in LKM-1 antibody-positive autoimmune hepatitis. Hepatology. 1996;24:1416–1421. doi: 10.1002/hep.510240619. [DOI] [PubMed] [Google Scholar]
- 12.Ma Y, Bogdanos DP, Hussain MJ, Underhill J, Bansal S, Longhi MS, Cheeseman P, Mieli-Vergani G, Vergani D. Polyclonal T-cell responses to cytochrome P450IID6 are associated with disease activity in autoimmune hepatitis type 2. Gastroenterology. 2006;130:868–882. doi: 10.1053/j.gastro.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 13.Longhi MS, Hussain MJ, Bogdanos DP, Quaglia A, Mieli-Vergani G, Ma Y, Vergani D. Cytochrome P450IID6-specific CD8 T cell immune responses mirror disease activity in autoimmune hepatitis type 2. Hepatology. 2007;46:472–484. doi: 10.1002/hep.21658. [DOI] [PubMed] [Google Scholar]
- 14.Gueguen M, Boniface O, Bernard O, Clerc F, Cartwright T, Alvarez F. Identification of the main epitope on human cytochrome P450 IID6 recognized by anti-liver kidney microsome antibody. J Autoimmun. 1991;4:607–615. doi: 10.1016/0896-8411(91)90180-k. [DOI] [PubMed] [Google Scholar]
- 15.Kitazawa E, Igarashi T, Kawaguchi N, Matsushima H, Kawashima Y, Hankins RW, Miyakawa H. Differences in anti-LKM-1 autoantibody immunoreactivity to CYP2D6 antigenic sites between hepatitis C virus-negative and -positive patients. J Autoimmun. 2001;17:243–249. doi: 10.1006/jaut.2001.0565. [DOI] [PubMed] [Google Scholar]
- 16.Manns MP, Vogel A. Autoimmune hepatitis, from mechanisms to therapy. Hepatology. 2006;43:S132–S144. doi: 10.1002/hep.21059. [DOI] [PubMed] [Google Scholar]
- 17.Christen U, von Herrath MG. Induction, acceleration or prevention of autoimmunity by molecular mimicry. Mol Immunol. 2004;40:1113–1120. doi: 10.1016/j.molimm.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 18.Damian RT. Molecular mimicry: antigen sharing by parasite and host and its consequences. The American Naturalist. 1964;98:129–149. [Google Scholar]
- 19.Oldstone MB. Molecular mimicry and immune-mediated diseases. FASEB J. 1998;12:1255–1265. doi: 10.1096/fasebj.12.13.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christen U, Herrath MG. Initiation of autoimmunity. Curr Opin Immunol. 2004;16:759–767. doi: 10.1016/j.coi.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Münz C, Lünemann JD, Getts MT, Miller SD. Antiviral immune responses: triggers of or triggered by autoimmunity? Nat Rev Immunol. 2009;9:246–258. doi: 10.1038/nri2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ang CW, Jacobs BC, Laman JD. The Guillain-Barré syndrome: a true case of molecular mimicry. Trends Immunol. 2004;25:61–66. doi: 10.1016/j.it.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Kirvan CA, Swedo SE, Heuser JS, Cunningham MW. Mimicry and autoantibody-mediated neuronal cell signaling in Sydenham chorea. Nat Med. 2003;9:914–920. doi: 10.1038/nm892. [DOI] [PubMed] [Google Scholar]
- 24.Amano K, Leung PS, Rieger R, Quan C, Wang X, Marik J, Suen YF, Kurth MJ, Nantz MH, Ansari AA, Lam KS, Zeniya M, Matsuura E, Coppel RL, Gershwin ME. Chemical xenobiotics and mitochondrial autoantigens in primary biliary cirrhosis: identification of antibodies against a common environmental, cosmetic, and food additive, 2-octynoic acid. J Immunol. 2005;174:5874–5883. doi: 10.4049/jimmunol.174.9.5874. [DOI] [PubMed] [Google Scholar]
- 25.Selmi C, Balkwill DL, Invernizzi P, Ansari AA, Coppel RL, Podda M, Leung PS, Kenny TP, Van De Water J, Nantz MH, Kurth MJ, Gershwin ME. Patients with primary biliary cirrhosis react against a ubiquitous xenobiotic-metabolizing bacterium. Hepatology. 2003;38:1250–1257. doi: 10.1053/jhep.2003.50446. [DOI] [PubMed] [Google Scholar]
- 26.Wakabayashi K, Lian ZX, Leung PS, Moritoki Y, Tsuneyama K, Kurth MJ, Lam KS, Yoshida K, Yang GX, Hibi T, Ansari AA, Ridgway WM, Coppel RL, Mackay IR, Gershwin ME. Loss of tolerance in C57BL/6 mice to the autoantigen E2 subunit of pyruvate dehydrogenase by a xenobiotic with ensuing biliary ductular disease. Hepatology. 2008;48:531–540. doi: 10.1002/hep.22390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohashi P, Oehen S, Buerki K, Pircher H, Ohashi C, Odermatt B, Malissen B, Zinkernagel R, Hengartner H. Ablation of tolerance and induction of diabetes by virus infection in viral antigen transgenic mice. Cell. 1991;65:305–317. doi: 10.1016/0092-8674(91)90164-t. [DOI] [PubMed] [Google Scholar]
- 28.Oldstone MBA, Nerenberg M, Southern P, Price J, Lewicki H. Virus infection triggers insulin-dependent diabetes mellitus in a transgenic model: role of anti-self (virus) immune response. Cell. 1991;65:319–331. doi: 10.1016/0092-8674(91)90165-u. [DOI] [PubMed] [Google Scholar]
- 29.von Herrath MG, Dockter J, Oldstone MB. How virus induces a rapid or slow onset insulin-dependent diabetes mellitus in a transgenic model. Immunity. 1994;1:231–242. doi: 10.1016/1074-7613(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 30.Brehm MA, Pinto AK, Daniels KA, Schneck JP, Welsh RM, Selin LK. T cell immunodominance and maintenance of memory regulated by unexpectedly cross-reactive pathogens. Nat Immunol. 2002;3:627–634. doi: 10.1038/ni806. [DOI] [PubMed] [Google Scholar]
- 31.Christen U, Edelmann KH, McGavern DB, Wolfe T, Coon B, Teague MK, Miller SD, Oldstone MB, von Herrath MG. A viral epitope that mimics a self antigen can accelerate but not initiate autoimmune diabetes. J Clin Invest. 2004;114:1290–1298. doi: 10.1172/JCI22557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Christen U, Hintermann E, Holdener M, von Herrath MG. Viral triggers for autoimmunity: is the ‘glass of molecular mimicry’ half full or half empty? J Autoimmun. 2010;34:38–44. doi: 10.1016/j.jaut.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blume N, Leonard J, Xu ZJ, Watanabe O, Remotti H, Fishman J. Characterization of Cyp2d22, a novel cytochrome P450 expressed in mouse mammary cells. Arch Biochem Biophys. 2000;381:191–204. doi: 10.1006/abbi.2000.1978. [DOI] [PubMed] [Google Scholar]
- 34.McLaughlin LA, Dickmann LJ, Wolf CR, Henderson CJ. Functional expression and comparative characterization of nine murine cytochromes P450 by fluorescent inhibition screening. Drug Metab Dispos. 2008;36:1322–1331. doi: 10.1124/dmd.108.021261. [DOI] [PubMed] [Google Scholar]
- 35.Yu AM, Haining RL. Expression, purification, and characterization of mouse CYP2d22. Drug Metab Dispos. 2006;34:1167–1174. doi: 10.1124/dmd.105.008870. [DOI] [PubMed] [Google Scholar]
- 36.Holdener M, Hintermann E, Bayer M, Rhode A, Rodrigo E, Hintereder G, Johnson EF, Gonzalez FJ, Pfeilschifter J, Manns MP, Herrath MG, Christen U. Breaking tolerance to the natural human liver autoantigen cytochrome P450 2D6 by virus infection. J Exp Med. 2008;205:1409–1422. doi: 10.1084/jem.20071859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mamula MJ, Lin RH, Janeway CA, Jr, Hardin JA. Breaking T cell tolerance with foreign and self co-immunogens. A study of autoimmune B and T cell epitopes of cytochrome c. J Immunol. 1992;149:789–795. [PubMed] [Google Scholar]