Abstract
Background
Acute kidney injury (AKI) is common in patients undergoing cardiac surgery and is associated with a high rate of death, long-term sequelae and healthcare costs. We conducted a systematic review of randomized controlled trials for strategies to prevent or treat AKI in cardiac surgery.
Methods
We screened Medline, Scopus, Cochrane Renal Library, and Google Scholar for randomized controlled trails in cardiac surgery for prevention or treatment of AKI in adults.
Results
We identified 70 studies that contained a total of 5,554 participants published until November 2008. Most studies were small in sample size, were single-center, focused on preventive strategies, and displayed wide variation in AKI definitions. Only 26% were assessed to be of high quality according to the Jadad criteria. The types of strategies with possible protective efficacy were dopaminergic agents, vasodilators, anti-inflammatory agents, and pump/perfusion strategies. When analyzed separately, dopamine and N-acetylcysteine did not reduce the risk for AKI.
Conclusions
This summary of all the literature on prevention and treatment strategies for AKI in cardiac surgery highlights the need for better information. The results advocate large, good-quality, multicenter studies to determine whether promising interventions reliably reduce rates of acute renal replacement therapy and mortality in the cardiac surgery setting.
Key Words: Acute kidney injury, prevention; Cardiac surgery; Healthcare costs
Introduction
Acute kidney injury (AKI) is a frequent and important complication in hospitalized patients, occurring in up to 5% of all patients [1]. The incidence of AKI is especially high in patients undergoing cardiac surgery, reaching 50% by some definitions [2]. The mortality rate in this population is 1–5% in patients who develop AKI and up to 24% in patients who require acute renal replacement therapy for AKI [3]. In addition, the mortality rate in cardiac surgery patients with renal injury increases progressively with the degree of renal impairment [4], and AKI is an independent predictor of mortality after cardiac surgery [5]. AKI doubles the total postoperative cost of cardiac surgery patients and nearly doubles intensive care unit costs [2]. Thus, for many reasons any reduction in risk of AKI would be beneficial, but methods to prevent AKI in cardiac surgery patients have not been established.
There are several reasons to conduct clinical trials and study AKI after cardiac surgery. The timing of injury is known; the injury is homogenous in nature relative to other populations in which AKI is frequently studied; and about 800,000 patients undergo cardiac surgery worldwide each year, allowing for large sample sizes and providing a unique opportunity for controlled interventions [6]. The predominant causes of AKI are hypoperfusion and inflammation due to cardiopulmonary bypass (CPB). CPB has also been shown to cause AKI due to non-pulsatile flow causing vasoconstriction and ischemic renal injury [7]. However, even patients undergoing surgery off CPB (‘off-pump’) are at risk for AKI, suggesting alternative mechanisms for injury.
Given the large population of cardiac surgery patients and the substantial impact of AKI in this population, efforts to treat AKI through various interventions have been attempted. However, no single agent has been shown to prevent AKI in cardiac surgery. Previous systematic reviews have examined AKI in cardiac surgery, but these have focused on individual interventions only [8,9]. Other reviews have examined AKI in the broader perioperative period of cardiothoracic and abdominal surgery, which use disparate surgical techniques and may introduce more heterogeneity into the study sample [10]. We conducted this review to evaluate the conduct and outcomes of clinical trials of AKI prevention and treatment in cardiac surgery, to highlight the strengths and limitations of the current evidence, and to guide an agenda for future research.
Methods
We conducted, analyzed, and reported this systematic review in accordance with consensus guidelines [11].
Data Sources
We screened Medline (1950 to November 2008), Scopus (1966–2008), Cochrane Renal Library, and Google Scholar for the relevant studies. Reference lists and bibliographical data from all retrieved articles and reviews were also searched. The terms ‘kidney diseases’, ‘cardiovascular surgical procedures’, ‘cardiopulmonary bypass’, and ‘renoprotection’ were used. The search strategy in Scopus used the terms ‘renal protection’, ’renoprotec’, ‘acute kidney failure’, ‘kidney failure’, ‘kidney diseases’, ‘kidney disease’, ‘cardiovascular surgery’, ‘cardiovascular procedures’, and ‘cardiopulmonary bypass’. An expert librarian was consulted for assistance in conducting a comprehensive search to identify randomized control trials investigating preventive and therapeutic measures for AKI in cardiac surgery. Two reviewers (M.P. and S.N.) independently screened the citations and those considered potentially relevant were retrieved for full-text review.
Study Eligibility and Selection
Articles published as full manuscripts in English were included. The studies were limited to humans and to all adults from age 19 to 80 years and above, with no upper age limit specified. Randomized controlled trials (RCTs) involving patients undergoing cardiac surgery (coronary artery bypass grafting, CABG; valve surgery, or combined CABG/valve surgery; elective, emergent, or not specified) were included. Studies that assessed kidney injury by methods of serum creatinine or creatinine clearance/glomerular filtration rate were eligible. Eligible interventions included methods of prevention or treatment of AKI administered anytime before, during, or after surgery. These included medical therapies as well as procedure-based therapies such as CPB modification and early renal replacement therapy. Healthcare service interventions such as level of care preceding or following surgery were not eligible.
Comparison was with no therapy, placebo, or with standard care for the institution, such as maximal hydration. Review outcomes were incidence of AKI (defined by individual study authors using one of several definitions for AKI) or change in serum creatinine, creatinine clearance, or GFR, incidence of acute renal replacement therapy and mortality. Renal outcomes were abstracted regardless of whether they were a primary or secondary trial outcome.
Patients with all degrees of renal function prior to surgery were included. Studies describing outcomes for patients who were on renal replacement therapy prior to surgery or who had received a kidney transplant were excluded from analysis.
Data Extraction
We used a comprehensive data collection form to record study characteristics: type of surgery (CABG, valve surgery, combined CABG/valve, elective, urgent), demographics of the participants (age, sex), and baseline mean serum creatinine or GFR. We characterized the timing of the intervention as preoperative (commencing outside operating room), intraoperative (commencing after anesthesia induction, initiated within 30 min before or after CPB), or postoperative (commencing after surgery, outside the operating room). Interventions were described by principal agent, route, and dose administered and were grouped according to their principal mechanism of action as follows: interventions that increase renal blood flow (vasodilators); interventions that induce natriuresis or diuresis; anti-inflammatory interventions, and interventions that work through other mechanisms of actions. Outcomes for creatinine, creatinine clearance/GFR, and incidence of acute renal replacement therapy and mortality were recorded for each study. Data extraction was performed independently by two reviewers (M.P. and S.N.) and disagreements were resolved by consensus.
Quality Assessment
All RCTs were evaluated for study quality using the Jadad score [12]. This score awards one point each for randomization, appropriateness of randomization, blinding, appropriateness of blinding, and description of withdrawal and dropouts, with a maximum score of 5. As studies involving pump strategies do not uniformly describe blinding techniques, we confirmed the quality characteristics of these studies using randomization, allocation concealment, blinded outcome assessment, and intention-to-treat analysis [8]. Patients excluded and lost to follow-up were recorded. Using these criteria, we classified the studies as good, moderate, and low quality (table 1).
Table 1.
Characteristics of included randomized controlled trials
| Source | Intervention | Trial type | Inclusion of CKD patients | Number of patients | Mean age years | Male % | ladad score1 |
|---|---|---|---|---|---|---|---|
| Anti-inflammatory | |||||||
| Adabagetal. [18], 2008 | NAC | Prevention | Yes | 102 | 71 | 100 | 5 |
| Amano et al. [19], 1994 | Glutathione | Prevention | No | 19 | 57.5 | NR | 3 |
| Barr and Kolodner [22], 2008 | NAC, fenoldopam | Prevention | Yes | 79 | 74.2 | 65.8 | 4 |
| Bolcaletal. [25], 2006 | Leukodepletion | Prevention | Yes | 50 | 56.9 | 72 | 3 |
| Burns et al. [28], 2005 | NAC | Prevention | Yes | 295 | 69.1 | 78.7 | 5 |
| Fischer et al. [41], 2005 | NAC | Prevention | NR | 40 | 66 | 77.5 | 5 |
| Gerrahetal. [44], 2004 | Aspirin | Prevention | Yes | 94 | 68.5 | 78 | 1 |
| Haaseetal. [45], 2007 | NAC | Prevention | Yes | 60 | 68.6 | 73.3 | 5 |
| Loefetal. [54], 2004 | Dexamethasone | Prevention | No | 20 | 63.7 | 85 | 3 |
| McBride et al. [58], 2004 | Methylprednisolone | Prevention | Yes | 36 | 61.45 | 97.2 | 2 |
| Ristikankare et al. [67], 2006 | NAC | Prevention | Yes | 77 | 70.5 | 80.5 | 5 |
| Sisillo et al. [73], 2008 | NAC | Prevention | Yes | 254 | 72.5 | 49 | 5 |
| Tang et al. [78], 2002 | Leukodepletion | Prevention | Yes | 44 | 63.5 | 85 | 3 |
| Wijeysundera et al. [81], 2007 | NAC | Prevention | Yes | 177 | 73.5 | 59.5 | 5 |
| Natriuretics/diuretics | |||||||
| Chen et al. [33], 2007 | Nesiritide | Prevention | Yes | 36 | 77.5 | 61.5 | 4 |
| Hayashida et al. [47], 2000 | ANP | Prevention | NR | 18 | 60.3 | 80 | 2 |
| Mahesh et al. [55], 2008 | Furosemide | Prevention | Yes | 42 | 71.3 | 73.8 | 4 |
| Mentzer et al. [59], 2007 | Nesiritide | Prevention | Yes | 272 | 63.9 | 78.5 | 5 |
| Meyer et al. [60], 1997 | Urodilatin | Treatment | Yes | 14 | 59.4 | NR | 3 |
| Nuutinen and Hollmen [64], 1976 | Furosemide | Prevention | NR | 45 | 35.1 | 53.3 | 1 |
| Sezaietal. [70], 2000 | ANP | Prevention | NR | 40 | 63.5 | 87.5 | 3 |
| Sezaietal. [71], 2007 | ANP | Prevention | No | 124 | 67.25 | 70.2 | 3 |
| Sirivella et al. [72], 2000 | Mannitol, furosemide, DA | Treatment | Yes | 100 | 71 | 62.5 | 3 |
| Smith et al. [74], 2008 | Mannitol | Prevention | Yes | 47 | 74.7 | 72.3 | 5 |
| Sward et al. [77], 2004 | ANP | Treatment | Yes | 59 | 69.7 | 71 | 5 |
| Yallopetal. [84], 2008 | Mannitol | Prevention | No | 40 | 63.2 | 75 | 5 |
| Vasodilators | |||||||
| Abe et al. [17], 1993 | PGE1 | Prevention | NR | 20 | 55 | NR | 3 |
| Amano et al. [20], 1995 | Diltiazem | Prevention | NR | 23 | 54.4 | NR | 2 |
| Berendes et al. [23], 1997 | Dopexamine | Prevention | NR | 44 | 61.5 | 59.1 | 3 |
| Bergman et al. [24], 2002 | Diltiazem | Prevention | Yes | 24 | 72.5 | 92 | 5 |
| Boveetal. [27], 2005 | DA, fenoldopam | Prevention | Yes | 80 | 68.5 | 72.5 | 4 |
| Caimmietal. [29], 2003 | Fenoldopam | Prevention | Yes | 160 | 69 | 66.3 | 2 |
| Carcoana et al. [30], 2003 | DA, mannitol | Prevention | Yes | 100 | 64 | 72 | 5 |
| Cogliati et al. [34], 2007 | Fenoldopam | Prevention | Yes | 193 | 70 | 0.6 | 5 |
| Colsonetal. [35], 1990 | ACE inhibitor | Prevention | Yes | 18 | 58 | 100 | 3 |
| Costa et al. [36], 1990 | DA, nitroprusside | Prevention | Yes | 36 | 58.6 | NR | 3 |
| Dehneetal. [37], 2001 | Dopexamine | Prevention | NR | 36 | 63.4 | 100 | 2 |
| Durai et al. [39], 2000 | DA, mannitol | Prevention | No | 36 | 53.7 | 63.9 | 3 |
| Gatotetal. [43],2004 | DA | Prevention | Yes | 82 | 65 | NR | 5 |
| Halpenny et al. [46], 2001 | Fenoldopam | Prevention | No | 31 | 64 | 24.3 | 3 |
| Kayaetal. [48], 2007 | Sodium nitroprusside | Prevention | Yes | 240 | 61.1 | 63.7 | 5 |
| Kramer et al. [50], 2002 | Theophylline | Prevention | No | 56 | 60.4 | 75 | 4 |
| Lassnigg et al. [52], 2000 | DA, furosemide | Prevention | Yes | 123 | 63.3 | 68.3 | 4 |
| Lema et al. [53], 1998 | DA, phenylephrine | Prevention | Yes | 17 | 65 | 85 | 2 |
| Monaco et al. [61], 2005 | DA | Prevention | Yes | 67 | 65.8 | 70.7 | 2 |
| Morgeraetal. [62], 2002 | Prostacyclin | Prevention | NR | 34 | 61.5 | 91.2 | 3 |
| Mylesetal. [63], 1993 | DA | Prevention | Yes | 52 | 61.6 | 63 | 4 |
| Piper et al. [66], 2003 | DA, diltiazem | Prevention | Yes | 60 | 67.7 | 66.7 | 5 |
| Ryckwaert et al. [68], 2001 | ACE inhibitor | Prevention | Yes | 14 | 63.2 | 92.9 | 4 |
| Sumerayetal. [76], 2001 | DA | Prevention | Yes | 36 | 63.4 | 91.7 | 5 |
| Tang et al. [80], 1999 | DA | Prevention | No | 40 | 58.7 | 60 | 2 |
| Witczaketal. [82], 2008 | Nifedipine | Prevention | Yes | 20 | 66.8 | 80 | 5 |
| Woo et al. [83], 2002 | DA | Prevention | Yes | 42 | 65.5 | 58.5 | 3 |
| Yavuzetal. [85], 2002 | DA | Prevention | No | 22 | 56.1 | 91 | 2 |
| Yavuzetal. [86], 2002 | DA, diltiazem | Prevention | No | 60 | 59.3 | 86.7 | 2 |
| Operative | |||||||
| Ascioneetal. [21], 1999 | Off-pump | Prevention | Yes | 50 | 61.6 | 90 | 2 |
| Carrier et al. [31], 2003 | Off-pump | Prevention | Yes | 65 | 70 | 76.9 | 3 |
| Celiketal. [32], 2005 | Off-pump | Prevention | Yes | 60 | 67.1 | 51.7 | 3 |
| Kocakulaket al. [49], 2005 | Off-pump pulsatile | Prevention | NR | 40 | 53.7 | 77.5 | 2 |
| Masoumi et al. [57], 2008 | Off-pump | Prevention | NR | 124 | 58.9 | 83 | 3 |
| Onoratietal. [65], 2007 | Off-pump pulsatile | Prevention | Yes | 100 | 68 | 93 | 3 |
| Sajjaetal. [69], 2007 | Off-pump | Prevention | Yes | 116 | 60.3 | 88.8 | 3 |
| Strakaetal. [75], 2004 | Off-pump | Prevention | Yes | 388 | 62.5 | 81.5 | 3 |
| Tang et al. [79], 2002 | Off-pump | Prevention | Yes | 40 | 66 | 80 | 3 |
| Other | |||||||
| Boldtetal. [26], 2008 | Albumin | Prevention | Yes | 50 | 82.5 | 50 | 2 |
| Demirkilic et al. [38], 2004 | Early CVVHDF | Treatment | Yes | 61 | 60.5 | NR | 2 |
| Durmazetal. [40], 2003 | RRT | Prevention | Yes | 44 | 56.2 | 79.4 | 2 |
| Gandhi et al. [42], 2007 | Insulin | Prevention | NR | 371 | 63 | 69 | 5 |
| Kulkaetal. [51], 1996 | Clonidine | Prevention | NR | 50 | 57.5 | 78 | 3 |
| Marathias et al. [56], 2006 | Hydration with 0.5% normal saline | Prevention | Yes | 64.1 | 95 | 2 | |
Multicenter trials: Burns et al. [28], Kaya et al. [48], Mentzer et al. [59], Meyer et al. [60], and Sward et al. [77]. Industry sponsor: Barr and Kolodner [22], Chen et al. [33], Gandhi et al. [42], Hal-penny et al. [46], and Kramer et al. [50],
NAC = N-Acetylcysteine; ANP = atrial natriuretic peptide; DA = dopamine; ACE = angiotensin-converting enzyme; NR = not reported; RRT = renal replacement therapy; CVVHDF = continuous veno-venous hemodiafiltration.
Jadad score awards one point for randomization, appropriateness of randomization, blinding, appropriateness of blinding, and description of withdrawal and dropouts, with a maximum score of 5.
Data Analysis
All trials were two- or three-arm interventions and administered parallel in design. Three-arm trials involved two separate interventions analyzed against a single control group. Outcomes were reviewed separately for prevention and treatment cohorts. Overall results for each intervention class were mathematically pooled using techniques that accounted for within- and between- study heterogeneity [13,14]. For studies that reported a continuous outcome (e.g. change in creatinine, creatinine clearance, or eGFR), we compared the standard difference in means in treatment and control groups. For trials that only reported continuous outcomes, we converted the standard difference in means to log odds ratios via the following formula: log odds ratio = π × standard difference/square root [3]. The variance calculation was based on the following: log odds SE = square root (π ^ 2 × standard difference SE ^ 2/3). The log odds variance was equal to the log odds SE ^ 2. This allowed pooling of studies that only reported continuous outcomes with those that reported categorical outcomes. We formally assessed heterogeneity of treatment effects between studies with the Cochrane Q and the I2 statistics. Publication bias was not assessed due to high statistical heterogeneity [15,16]. All analyses were performed using Comprehensive Meta Analysis Software Version 2.0 (Englewood, N.J., USA).
Results
Retrieval of Studies and Study Characteristics
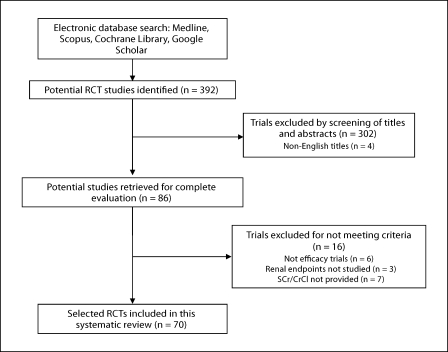
Our search of Medline yielded a total of 169 citations for individual review. Scopus retrieved 194 citations for review. Additional searches of the Cochrane Renal Library (Issue 4, 2008), PubMed, and Google Scholar produced 29 additional citations for review. We also studied reference lists and bibliographical data from all retrieved articles and reviews for any additional relevant material (fig. 1). A total of 70 studies (5,554 patients) met eligibility criteria [17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86] (table 1).
Fig. 1.
Flowchart of study selection.
Twenty-one different countries were represented, with the highest number of studies coming from the UK (n = 9), Turkey (n = 9), Germany (n = 9), USA (n = 7), and Italy (n = 7). Sixty-four studies involved CABG, and 27 involved valvular surgeries. Study size ranged widely from 14 to 388 patients. Nine studies comprised groups of 20 patients or fewer; 17 studies had more than 100 patients, and only 6 studies had more than 200 patients. The majority (93%), including the larger studies, were single-center studies and only 5 trials included more than one center (table 1, footnote). Five studies described an industry sponsor. Sixty-six studies examined prevention strategies and 4 examined treatment strategies. Patients with preoperative chronic kidney disease (CKD) were excluded from 10 studies. Definitions of AKI were not uniform. Criteria for initiation of acute renal replacement therapy were not standardized across studies.
Thirty studies were designed to analyze the effects of interventions that primarily increase renal blood flow (vasodilators). These interventions included dopamine, dopexamine, fenoldopam, angiotensin-converting enzyme inhibitors (captopril, enalaprilat), diltiazem, prostacyclin, nifedipine, PGE-1, sodium nitroprusside and theophylline. All of the studies amongst the vasodilator cohort were designed to evaluate effects on prevention of AKI and none of the studies in this cohort evaluated effects on treatment of an established AKI.
Twelve studies were designed to analyze the effects of interventions that primarily induce natriuresis or diuresis or both. These interventions included atrial natriuretic peptide, brain natriuretic peptide, urodilatin, and diuretic agents (loop diuretics and mannitol). Ten studies amongst this natriuretic cohort were designed to evaluated effects on prevention of AKI and 3 studies were designed to evaluate effects on treatment of an established AKI.
Fourteen studies were designed to analyze the effects of interventions that primarily counteract inflammation (anti-inflammatory agents). These interventions included N-acetylcysteine, aspirin, glutathione, corticosteroids, and leukodepletion. All of the studies amongst this anti-inflammatory cohort were designed to evaluate effects on prevention of AKI.
Interventions that have been studied previously but could not be assigned to one of the above 3 cohorts included clonidine, albumin infusion, isotonic saline infusion, insulin therapy, early continuous veno-venous hemofiltration and interventions such as the off-pump technique and pulsatile technique. Amongst these only 1 study addressing the role of continuous veno-venous hemofiltration was designed to treat an established AKI and all other interventions were studied in a prevention setting.
Quality
There were 18 studies of good quality, 15 studies of moderate quality, and 37 studies of low quality. Forty-seven studies (67%) had 5% or fewer patients excluded or lost to follow-up, with 34 (49%) of these studies having 0% excluded. Fifteen studies (21%) had >5% excluded or lost to follow-up, with a range of 6–29%. Seven studies did not report patients excluded or lost to follow-up. All studies assessed kidney function uniformly between the intervention and control groups. The frequency of assessing kidney function was variable between studies, ranging from a frequency of every 8 h (as in a good-quality study [77]) to measurements on days 1, 5, and 15 [69], with 9 studies not reporting the method of assessment.
AKI
The incidence of AKI as a dichotomous outcome was reported in 22 studies (2,674 patients). AKI was not uniformly defined in these studies. Continuous endpoints were reported in 43 studies (2,148 patients). Amongst the prevention cohort trials, there were 9 interventions that commenced preoperatively, 53 administered intraoperatively, and 5 postoperatively (table 2).
Table 2.
Study details by intervention
| Source | Intervention | Control | Timing of intervention | Baseline renal function1 | Outcomes |
|---|---|---|---|---|---|
| Anti-inflammatory | |||||
| Adabagetal. [18], 2008 | NAC p.o., 600 mg b.i.d. × 14 doses | Placebo | Pre | 1.9 ±0.7/40 ±10 | AKI, D |
| Amano et al. [19], 1994 | Glutathione i.v., 200 mg/kg | Placebo | I | 1.1±0.2/NR | Mean SCr |
| Barr and Kolodner [22] 2008 | Fenoldopam i.v., 0.1 μg/kg/min | Placebo + NAC or Fenoldopam + NAC | I | NR/34 ± 2 | Mean CrCl |
| Bolcaletal. [25], 2006 | Leukodepletion | CPB alone | I | 1.8±0.6/NR | Mean SCr, R |
| Burns et al. [28], 2005 | NAC i.v., 600 mg × 4 doses | 5% dextrose | I | 1.2±0.4/NR | AKI, R, D |
| Fischer et al. [41], 2005 | NAC i.v., 100 mg/kg, 20 mg/kg/h infusion | Placebo | I | 1.0±0.4/NR | Mean SCr |
| Gerrahetal. [44], 2004 | Aspirin p.o., 100 mg daily until surgery | Placebo | Pre | 2.8 ±1.6/31 ±14 | Mean S r |
| Haaseetal. [45], 2007 | NAC i.v., 300 mg/kg over 24 h | 5% dextrose | I | 1.0 ±0.3/78 ±24 | AKI, R |
| Loefetal. [54], 2004 | Dexamethasone i.v., 1 mg/kg plus 0.5 mg/kg | Placebo | I | 1.0 ±0.2/104 ±10 | Mean CrCl |
| McBrideetal. [58], 2004 | Methylprednisolone i.v., 30 mg/kg | Placebo | I | 1.1 ±0.2 | Mean SCr |
| Ristikankare et al. [67] 2006 | NAC i.v., 150 mg/kg, 50 mg/kg, 100 mg/kg+ | Placebo | I | 1.5±0.4/NR | AKI, R, D |
| Sisillo et al. [73], 2008 | NAC i.v., 1, 200 mg every 12 h × 4 boluses | Placebo | I | 1.3 ±0.4/46 ±8 | AKI, D |
| Tang et al. [78], 2002 | Leukodepletion | CPB alone | I | 1.1±0.2/NR | Mean SCr |
| Wijeysundera et al. [81] 2007 | NAC i.v., 100 mg/kg bolus, 20 mg/kg/h infusion | 5% dextrose | I | 1.4 ±0.4/44 ±11 | AKI, R |
| Diuretics | |||||
| Maheshetal. [55], 2008 | Furosemide i.v., 4 mg/h | Normal saline | I | 1.1 ±0.3/65 ±34 | AKI, R, D |
| Nuutinen and Hollmen [64], 1976 | Furosemide i.v., varying doses w/UOP <40 ml/h | Placebo | I | NR/89±17 | Mean CrCl |
| Sirivella et al. [72], 2000 | Furosemide, ethacrynic acid, bumetanide | Osmitrol, furosemide, DA | Post | 1.8 NR/NR | R |
| Smith et al. [74], 2008 | Mannitol i.v., 0.5 g/kg | Hartmann's solution | I | 1.8 ±0.3/33 ±10 | Mean SCr |
| Yallopetal. [84], 2008 | Mannitol i.v., 5 ml/kg, 10% solution | Hartmann's solution | I | 1.1±0.2/NR | Mean SCr |
| Vasodilators | |||||
| Abe et al. [17], 1993 | PGE-1 i.v., 0.02 μg/kg-min | Normal saline | I | NR/89 ± 8 | Mean CrCl |
| Amano et al. [20], 1995 | Diltiazem i.v., 0.1 mg/kg bolus, 2 mcg/kg/min inf. | Placebo | I | NR/90 ± 10 | Mean CrCl |
| Berendes et al. [23], 1997 | Dopexamine i.v., 0.5, 1.0, 2.0 μg/kg/min | Placebo | Pre | NR/NR | Mean CrCl |
| Bergman et al. [24], 2002 | Diltiazem i.v., 0.25 mg/kg bolus, 1.7 [g/kg/min infusion | Placebo | I | 1.8±0.1/NR | Mean SCr |
| Boveetal. [27], 2005 | Fenoldopam i.v., 0.05 μg/kg/min | DA i.v., 2.5 μg/kg/min | I | 1.6 ±0.7/50 ±21 | AKI, R, D |
| Caimmietal. [29], 2003 | Fenoldopam i.v., 0.1–0.3 μg/kg/min | DA or dobutamine i.v., renal doses+ | I | 1.8 ±0.3/51 ±22 | Mean SCr |
| Carcoana et al. [30], 2003 | DA ± mannitol i.v., 2 μg/kg/min or 1 g/kg | Placebo | I | 1.1 ±0.2/96 ±27 | Mean SCr |
| Chen et al. [33], 2007 | Nesiritide i.v., 0.005 μg/kg/min | Placebo | I | 1.7±0.6/40±11 | AKI, R, D |
| Cogliati et al. [34], 2007 | Fenoldopam i.v., 0.1 μg/kg/min | Placebo | I | 1.8 ±0.4/39 ±10 | AKI, R |
| Colsonetal. [35], 1990 | Captopril p.o., 100 mg b.i.d. x 2 days | Placebo | Pre | 1.2 ±0.2/107 ±12 | Mean CrCl |
| Costa et al. [36], 1990 | DA i.v., 2.5 μg/kg/min | Placebo; DA; nitroprusside | I | NR/37±10 | Mean CrCl |
| Dehneetal. [37], 2001 | Dopexamine i.v., 1 μg/kg/min | Placebo | I | NR/61±13 | Mean CrCl |
| Durai et al. [39], 2000 | DA i.v., 0.3 μg/kg/min | No treatment; 20% mannitol i.v., 1 mg/kg/h | I | 1.1±0.4/NR | Mean SCr |
| Gatotetal. [43], 2004 | DA i.v., 3–5 μg/kg/min | Normal saline | Post | 1.1±0.2/NR | Mean SCr |
| Halpenny et al. [46], 2001 | Fenoldopam i.v., 0.1 μg/kg/min | Placebo | Post | 1.1 ±0.2/107 ±36 | Mean CrCl |
| Hayashida et al. [47], 2000 | ANP i.v., 0.05 μg/kg/min | Control | I | NR/48 ± 7 | Mean CrCl |
| Kaya et al. [48], 2007 | SNP i.v., 0.1 mg/kg/h | Normal saline | I | 1± 0.2/77 ±21 | AKI |
| Kramer et al. [50], 2002 | Theophylline i.v., 0.25 mg/kg/h | Normal saline | I | 0.8 NR/NR | AKI |
| Lassnigg et al. [52], 2000 | DA i.v., 2 μg/kg/min+ | Normal saline; furosemide 0.5 μg/kg/min | I | 1.0 ±0.2/96 ±37 | AKI, R, D |
| Lema et al. [53], 1998 | DA i.v., 2 μg/kg/min | Phenylephrine | I | 1.5 ±0.4/74 ±37 | Mean SCr |
| Mentzeretal. [59], 2007 | Nesiritide i.v., 0.01 μg/kg/min | Placebo | I | 1.1 ±0.4/80 ±29 | Mean SCr, D |
| Meyer et al. [60], 1997 | Urodilatin i.v., 20 ng/kg/min x 7 days | Placebo | Post | 2.7 ±0.6 | Mean SCr, R, D |
| Monaco et al. [61], 2005 | DA i.v., 3 μg/kg/min | Placebo | Pre | 1.9 ±0.2 | Mean SCr, R, D |
| Morgera et al. [62], 2002 | Prostacyclin i.v., 2 ng/kg/min | Placebo | I | NR/96 ± 22 | AKI |
| Mylesetal. [63], 1993 | DA i.v., 200 μg/min | 5% dextrose | I | 1.0±0.2/NR | Mean SCr |
| Piper et al. [66], 2003 | DA i.v., 2.5 μg/kg/min | Normal saline; diltiazem i.v., 2 μg/kg/min | Post | NR/74 ± 34 | Mean CrCl |
| Ryckwaert et al. [68], 2001 | Enalaprilat i.v., 1 mg every 6 h over 2 days | Placebo | I | 1.2 ±0.2/70 ±14 | Mean CrCl |
| Sezaietal. [70], 2000 | Human ANP i.v., 0.03–0.05 μg/kg/min | Placebo | I | NR/91±20 | AKI |
| Sezaietal. [71], 2007 | Human ANP i.v., 0.02 g/dl/min | NR | I | NR/NR | R, D |
| Sumerayetal. [76], 2001 | DA i.v., 2.5 μg/kg/min | 5% dextrose | I | NR/77 ± 3 | Mean CrCl |
| Swardetal. [77], 2004 | Recombinant hANP i.v., 50 ng/kg/min | NR | treatment | 1.2±0/NR | R, D |
| Tangetal. [80], 1999 | DA i.v., 2.5–4.0 μg/kg/min | Placebo | I | 1.3±0.1/NR | Mean SCr |
| Witczaketal. [82], 2008 | Nifedipine i.v., 0.25–0.60 μg/kg/min | Placebo | I | 2.7 ±1.0/35 ±10 | Mean CrCl, R |
| Woo et al. [83], 2002 | DA i.v., 3 μg/kg/min x 48 h | NR | I | 1.1±0.2/NR | Mean SCr |
| Yavuzetal. [85], 2002 | DA i.v., 2 μg/kg/min | Placebo | Pre | NR/71±35 | Mean CrCl |
| Yavuzetal. [86], 2002 | DA i.v., 200 μg/kg/min | Placebo/diltiazem; diltiazem and DA | I | NR/77 ±49 | Mean CrCl |
| Operative | |||||
| Ascioneetal. [21], 1999 | Off-pump | On-pump | I | NR/91±26 | Mean CrCl |
| Carrier et al. [31], 2003 | Off-pump | On-pump | I | NR/NR | AKI, D |
| Celiketal. [32], 2005 | Off-pump | On-pump | I | 1.4±0.3/NR | Mean SCr, R |
| Kocakulak et al. [49], 2005 | Pulsatile flow | Continuous flow | I | 1.5±1.1/NR | Mean SCr |
| Masoumi et al. [57], 2008 | Off-pump | On-pump | I | NR/NR | AKI, D |
| Onoratietal. [65], 2007 | Pulsatile flow | Standard IABP | I | 1.2 ±0.4/80 ±25 | AKI |
| Sajjaetal. [69], 2007 | Off-pump | On-pump | I | 1.5 ±0.5/52 ±9 | AKI, R |
| Strakaetal. [75], 2004 | Off-pump | On-pump | I | NR/NR | R, D |
| Tangetal. [79], 2002 | Off-pump | Pulsatile CPB | I | 1.1±0.2/NR | Mean SCr |
| Other | |||||
| Boldt et al. [26], 2008 | Albumin 5% i.v., 500 ml | HES 6% i.v., 500 ml | I | 1.3 ±0.4/47 ±20 | Mean SCr |
| Demirkilic et al. [38], 2004 | Early CVVHDF | Standard CVVHDF | Post | >3 NR/NR | D |
| Durmazetal. [40], 2003 | Postoperative prophylactic hemodialysis | Standard indications for renal replacement therapy | Post | 3.4±0.8/NR | AKI, D |
| Gandhi et al. [42], 2007 | Insulin i.v., continuously | Insulin i.v., intermittently | I | NR/NR | AKI |
| Kulkaetal. [51], 1996 | Clonidine i.v., 4 μg/kg | Placebo | Pre | NR/94 ± 19 | Mean CrCl |
| Marathias et al. [56], 2006 | 0.5% normal saline i.v., 1 ml/kg/h over 12 h | Fluid restriction | Pre | 3.3 ±0.5/26 ±2 | AKI, R |
NAC = N-Acetylcysteine; ANP = atrial natriuretic peptide; DA = dopa-mine; NR = not reported; RRT = renal replacement therapy; CVVHDF = continuous veno-venous hemodiafiltration; AKI = acute kidney injury; D = death; R = acute renal replacement therapy; Pre = preoperative; Post = postoperative; I = intraoperative; CrCl = creatinine clearance; SCr = serum creatinine; CPB = cardiopulmonary bypass.
Serum creatinine ± SD/creatinine clearance ± SD. Serum creatinine in mg/dl (converted from μmol/1) rounded to the nearest 10th, creatinine clearance in ml/min rounded to nearest 1. For further details of interventions, refer to original references.
The following agents from the vasodilator group were associated with a reduction in AKI: fenoldopam and angiotensin-converting enzyme inhibitors; whereas the other vasodilator agents were noted to have no effect on the incidence of AKI. Anti-inflammatory agents including N-acetylcysteine were not associated with any reduction in AKI. The following agents from the natriuretic and/or diuretic cohort were associated with a reduction in AKI: atrial natriuretic peptide, B-natriuretic peptide, urodilatin; whereas the remaining agents from this group were noted to have no effect on the incidence of AKI.
Amongst the other interventions that were reviewed, the off-pump surgical technique and pulsatile flow techniques were associated with a reduction in the incidence of AKI, whereas interventions such as clonidine, albumin infusion, isotonic saline infusion, and insulin therapy were not associated with a reduction in AKI.
Acute Renal Replacement Therapy
The incidence of acute renal replacement therapy was provided in 21 studies comprising 2,172 patients [18,25,27,28,32,33,34,40,45,52,55,56,60,61,67,69,71,75,77,81,82]. Predefined criteria for acute renal replacement therapy initiation were provided in 7 of these studies and were not uniform across studies [25,27,40,55,56,77,81]. In 1 case, initiation was determined by blinded nephrologists without specific parameters described [81]. In another study, prophylactic hemodialysis was the intervention and thus acute renal replacement therapy parameters were needed for both groups [40]. Other studies did not describe criteria for initiation of acute renal replacement therapy.
None of the individual trials showed a clear benefit in terms of reducing the incidence of acute renal replacement therapy in the cardiac surgery setting and none of the trials were adequately powered to study this outcome.
Mortality
Data on mortality were reported in 18 studies comprising 2,227 patients. As mortality is a competing endpoint for AKI, we considered any study that described both of these outcomes as a composite endpoint; however, no such studies were found.
None of the cohorts (vasodilator agents, anti-inflammatory agents, natriuretic/diuretic agents and agents with other mechanisms of action) demonstrated a reduction in mortality and none of the trials were adequately powered to study this outcome.
Comment
This systematic review demonstrates that a large number of RCTs to prevent or treat AKI after cardiac surgery have been performed over the past 30 years. The majority of trials were small, single-center, methodologically and statistically heterogeneous, rated to be of low methodological quality, and most were not powered to detect differences in hard endpoints such as mortality and acute renal replacement therapy. In addition, the definitions of AKI were quite variable and many trials instead examined continuous changes in kidney function. However, analysis of these existing trials shows that there may be benefits associated with some interventions for the prevention of AKI. This review calls for good-quality, large-population trials of individual or combination of agents.
In general, strategies to prevent AKI were effective if administered preoperatively and intraoperatively. Strategies for treatment of AKI were far less numerous and thus it is difficult to draw conclusions about their efficacy relative to prophylaxis strategies. Our exploratory analyses revealed that most types of prophylactic strategies were protective for AKI. In particular, fenoldopam, ANP/nesiritide and off-pump CABG demonstrated excellent efficacy for the prevention of AKI. There was no evidence for benefit from preoperative administration of dopamine or N-acetylcysteine.
The primary endpoints in the majority of trials contained in this review were continuous changes in creatinine or creatinine clearance/GFR rather than the most clinically important endpoints of acute renal replacement therapy and mortality. Furthermore, in the 22 studies that utilized categorical outcomes for AKI, the definition of AKI was highly variable. Acute renal replacement therapy and/or mortality were considered primary outcomes in only 5 studies; most studies did not have adequate statistical power for non-primary outcomes.
There are many challenges to consider when designing and executing future trials for the prevention or treatment of AKI in cardiac surgery. The first challenge relates to patient selection. If one chooses to study an intervention that has potential adverse side effects, only those at the highest risk of AKI should be enrolled. These may include patients undergoing redo cardiac surgeries or those with CKD. Second, since AKI may be multifactorial after CPB, multiple agents acting through different pathways may need to be administered simultaneously or in succession in order to effectively reduce AKI. Third, the selection of the correct endpoint for these trials is vital. Early phase 1 and 2 trials should measure surrogate endpoints such a AKI defined by serum creatinine using RIFLE or AKIN criteria or changes in novel biomarkers of AKI. Larger phase 3 and 4 studies should examine the ability of the interventions to reduce hard endpoints, such as dialysis, death, length of stay, and long-term events such as cardiovascular events, CKD, and long-term death.
Our study has several strengths. We performed a comprehensive search to compile relevant studies, screening over 500 citations. Article identification, eligibility assessment, and data abstraction were performed independently and in duplicate, to minimize potential biases inherent in these tasks. Through these methods, we included a large number of studies for systematic review and meta-analysis, encompassing a wider scope than previous reviews evaluating a similar question.
Our review also has some limitations. Only studies written in English were included, resulting in the exclusion of four studies (one in Chinese, one in Japanese, and two in Italian). Trials were in general small, underpowered, and of poor methodological quality. Risk factors for AKI, such as CKD, were not consistently reported in a standardized fashion. Trials included in this review had variable definitions of AKI and none of the included trials reported the recently proposed AKIN criteria to define AKI. Future trials should follow these criteria to consistently define AKI and should also have predefined trial criteria for the initiation of renal replacement therapy [89].
Conclusions
AKI in cardiac surgery patients is common and is associated with significant morbidity and mortality. A method of preventing this common complication is urgently needed. Most studies were underpowered to demonstrate a beneficial effect on acute renal replacement therapy and mortality. The beneficial effect on AKI alone is enough impetus for a more thorough investigation into prophylaxis and treatment strategies. Large, good-quality, multicenter trials needed to demonstrate benefits of prevention of AKI and reduction in rates of acute renal replacement therapy and mortality in the cardiac surgery setting.
Acknowledgment
The authors would like to thank Mark Gentry, Clinical Support Librarian and Coordinator, Yale University Cushing/Whitney Medical Library. Dr. Parikh was supported by the NIH grant HL-085757.
References
- 1.Chertow GM, Levy EM, Hammermeister KE, Grover F, Daley J. Independent association between acute renal failure and mortality following cardiac surgery. Am J Med. 1998;104:343–348. doi: 10.1016/s0002-9343(98)00058-8. [DOI] [PubMed] [Google Scholar]
- 2.Dasta JF, Kane-Gill SL, Durtschi AJ, Pathak DS, Kellum JA. Costs and outcomes of acute kidney injury (AKI) following cardiac surgery. Nephrol Dial Transplant. 2008;23:1970–1974. doi: 10.1093/ndt/gfm908. [DOI] [PubMed] [Google Scholar]
- 3.Chertow GM, Christiansen CL, Cleary PD, Munro C, Lazarus JM. Prognostic stratification in critically ill patients with acute renal failure requiring dialysis. Arch Intern Med. 1995;155:1505–1511. [PubMed] [Google Scholar]
- 4.Zanardo G, Michielon P, Paccagnella A, et al. Acute renal failure in the patient undergoing cardiac operation. Prevalence, mortality rate, and main risk factors. J Thorac Cardiovasc Surg. 1994;107:1489–1495. [PubMed] [Google Scholar]
- 5.Lassnigg A, Schmidlin D, Mouhieddine M, et al. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J Am Soc Nephrol. 2004;15:1597–1605. doi: 10.1097/01.asn.0000130340.93930.dd. [DOI] [PubMed] [Google Scholar]
- 6.Nalysnyk L, Fahrbach K, Reynolds MW, Zhao SZ, Ross S. Adverse events in coronary artery bypass graft (CABG) trials: a systematic review and analysis. Heart. 2003;89:767–772. doi: 10.1136/heart.89.7.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abu-Omar Y, Ratnatunga C. Cardiopulmonary bypass and renal injury. Perfusion. 2006;21:209–213. doi: 10.1191/0267659106pf870oa. [DOI] [PubMed] [Google Scholar]
- 8.Wijeysundera DN, Beattie WS, Djaiani G, et al. Off-pump coronary artery surgery for reducing mortality and morbidity: meta-analysis of randomized and observational studies. J Am Coll Cardiol. 2005;46:872–882. doi: 10.1016/j.jacc.2005.05.064. [DOI] [PubMed] [Google Scholar]
- 9.Landoni G, Biondi-Zoccai GG, Marino G, et al. Fenoldopam reduces the need for renal replacement therapy and in-hospital death in cardiovascular surgery: a meta-analysis. J Cardiothorac Vasc Anesth. 2008;22:27–33. doi: 10.1053/j.jvca.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 10.Zacharias M, Conlon NP, Herbison GP, Sivalingam P, Walker RJ, Hovhannisyan K: Interventions for protecting renal function in the perioperative period. [update of Cochrane Database Syst Rev 2005;3:CD003590.] [DOI] [PubMed]
- 11.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet. 1999;354:1896–1900. doi: 10.1016/s0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- 12.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 13.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terrin N, Schmid CH, Lau J, Olkin I. Adjusting for publication bias in the presence of heterogeneity. Stat Med. 2003;22:2113–2126. doi: 10.1002/sim.1461. [DOI] [PubMed] [Google Scholar]
- 16.Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ. 2006;333:597–600. doi: 10.1136/bmj.333.7568.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abe K, Fujino Y, Sakakibara T. The effect of prostaglandin E1 during cardiopulmonary bypass on renal function after cardiac surgery. Eur J Clin Pharmacol. 1993;45:217–220. doi: 10.1007/BF00315386. [DOI] [PubMed] [Google Scholar]
- 18.Adabag AS, Ishani A, Koneswaran S, et al. Utility of N-acetylcysteine to prevent acute kidney injury after cardiac surgery: a randomized controlled trial. Am Heart J. 2008;155:1143–1149. doi: 10.1016/j.ahj.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 19.Amano J, Suzuki A, Sunamori M. Salutary effect of reduced glutathione on renal function in coronary artery bypass operation. J Am Coll Surg. 1994;179:714–720. [PubMed] [Google Scholar]
- 20.Amano J, Suzuki A, Sunamori M, Tofukuji M. Effect of calcium antagonist diltiazem on renal function in open heart surgery. Chest. 1995;107:1260–1265. doi: 10.1378/chest.107.5.1260. [DOI] [PubMed] [Google Scholar]
- 21.Ascione R, Lloyd CT, Underwood MJ, Gomes WJ, Angelini GD. On-pump versus off-pump coronary revascularization: evaluation of renal function. Ann Thorac Surg. 1999;68:493–498. doi: 10.1016/s0003-4975(99)00566-4. [DOI] [PubMed] [Google Scholar]
- 22.Barr LF, Kolodner K. N-acetylcysteine and fenoldopam protect the renal function of patients with chronic renal insufficiency undergoing cardiac surgery. Crit Care Med. 2008;36:1427–1435. doi: 10.1097/CCM.0b013e31816f48ba. [DOI] [PubMed] [Google Scholar]
- 23.Berendes E, Mollhoff T, Van Aken H, et al. Effects of dopexamine on creatinine clearance, systemic inflammation, and splanchnic oxygenation in patients undergoing coronary artery bypass grafting. Anesth Analg. 1997;84:950–957. doi: 10.1097/00000539-199705000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Bergman AS, Odar-Cederlof I, Westman L, Bjellerup P, Hoglund P, Ohqvist G. Diltiazem infusion for renal protection in cardiac surgical patients with preexisting renal dysfunction. J Cardiothorac Vasc Anesth. 2002;16:294–299. doi: 10.1053/jcan.2002.124136. [DOI] [PubMed] [Google Scholar]
- 25.Bolcal C, Akay HT, Bingol H, et al. Leukodepletion improves renal function in patients with renal dysfunction undergoing on-pump coronary bypass surgery: a prospective randomized study. Thorac Cardiovasc Surg. 2007;55:89–93. doi: 10.1055/s-2006-924571. [DOI] [PubMed] [Google Scholar]
- 26.Boldt J, Brosch C, Rohm K, Lehmann A, Mengistu A, Suttner S. Is albumin administration in hypoalbuminemic elderly cardiac surgery patients of benefit with regard to inflammation, endothelial activation, and long-term kidney function? Anesth Analg. 2008;107:1496–1503. doi: 10.1213/ane.0b013e31818370b2. [DOI] [PubMed] [Google Scholar]
- 27.Bove T, Landoni G, Calabro MG, et al. Renoprotective action of fenoldopam in high-risk patients undergoing cardiac surgery: a prospective, double-blind, randomized clinical trial. Circulation. 2005;111:3230–3235. doi: 10.1161/CIRCULATIONAHA.104.509141. [DOI] [PubMed] [Google Scholar]
- 28.Burns KE, Chu MW, Novick RJ, et al. Perioperative N-acetylcysteine to prevent renal dysfunction in high-risk patients undergoing CABG surgery: a randomized controlled trial. JAMA. 2005;294:342–350. doi: 10.1001/jama.294.3.342. [DOI] [PubMed] [Google Scholar]
- 29.Caimmi PP, Pagani L, Micalizzi E, et al. Fenoldopam for renal protection in patients undergoing cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2003;17:491–494. doi: 10.1016/s1053-0770(03)00155-1. [DOI] [PubMed] [Google Scholar]
- 30.Carcoana OV, Mathew JP, Davis E, et al. Mannitol and dopamine in patients undergoing cardiopulmonary bypass: a randomized clinical trial. Anesth Analg. 2003;97:1222–1229. doi: 10.1213/01.ANE.0000086727.42573.A8. [DOI] [PubMed] [Google Scholar]
- 31.Carrier M, Perrault LP, Jeanmart H, Martineau R, Cartier R, Page P. Randomized trial comparing off-pump to on-pump coronary artery bypass grafting in high-risk patients. Heart Surg Forum. 2003;6:E89–E92. [PubMed] [Google Scholar]
- 32.Celik JB, Gormus N, Topal A, Okesli S, Solak H. Effect of off-pump and on-pump coronary artery bypass grafting on renal function. Ren Fail. 2005;27:183–188. [PubMed] [Google Scholar]
- 33.Chen HH, Sundt TM, Cook DJ, Heublein DM, Burnett JC., Jr Low dose nesiritide and the preservation of renal function in patients with renal dysfunction undergoing cardiopulmonary-bypass surgery: a double-blind placebo-controlled pilot study. Circulation. 2007;116:134–138. doi: 10.1161/CIRCULATIONAHA.106.697250. [DOI] [PubMed] [Google Scholar]
- 34.Cogliati AA, Vellutini R, Nardini A, et al. Fenoldopam infusion for renal protection in high-risk cardiac surgery patients: a randomized clinical study. J Cardiothorac Vasc Anesth. 2007;21:847–850. doi: 10.1053/j.jvca.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 35.Colson P, Ribstein J, Mimran A, Grolleau D, Chaptal PA, Roquefeuil B. Effect of angiotensin converting enzyme inhibition on blood pressure and renal function during open heart surgery. Anesthesiology. 1990;72:23–27. doi: 10.1097/00000542-199001000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Costa P, Ottino GM, Matani A, et al. Low-dose dopamine during cardiopulmonary bypass in patients with renal dysfunction. J Cardiothorac Anesth. 1990;4:469–473. doi: 10.1016/0888-6296(90)90293-o. [DOI] [PubMed] [Google Scholar]
- 37.Dehne MG, Klein TF, Muhling J, Sablotzki A, Osmer C, Hempelmann G. Impairment of renal function after cardiopulmonary bypass is not influenced by dopexamine. Ren Fail. 2001;23:217–230. doi: 10.1081/jdi-100103493. [DOI] [PubMed] [Google Scholar]
- 38.Demirkilic U, Kuralay E, Yenicesu M, et al. Timing of replacement therapy for acute renal failure after cardiac surgery. J Card Surg. 2004;19:17–20. doi: 10.1111/j.0886-0440.2004.04004.x. [DOI] [PubMed] [Google Scholar]
- 39.Dural O, Ozkara A, Celebioglu b, Kanbak M, Ciliv G, Aypar U. Comparative study of dopamine and mannitol effects on renal function during cardiopulmonary bypass by using N-acetyl-beta-D-glucosaminidase assay. Turk J Med Sci. 2000;30:453–457. [Google Scholar]
- 40.Durmaz I, Yagdi T, Calkavur T, et al. Prophylactic dialysis in patients with renal dysfunction undergoing on-pump coronary artery bypass surgery. Ann Thorac Surg. 2003;75:859–864. doi: 10.1016/s0003-4975(02)04635-0. [DOI] [PubMed] [Google Scholar]
- 41.Fischer UM, Weissenberger WK, Warters RD, Geissler HJ, Allen SJ, Mehlhorn U. Impact of cardiopulmonary bypass management on postcardiac surgery renal function. Perfusion. 2002;17:401–406. doi: 10.1191/0267659102pf610oa. [DOI] [PubMed] [Google Scholar]
- 42.Gandhi GY, Nuttall GA, Abel MD, et al. Intensive intraoperative insulin therapy versus conventional glucose management during cardiac surgery: a randomized trial. Ann Intern Med. 2007;146:233–243. doi: 10.7326/0003-4819-146-4-200702200-00002. [DOI] [PubMed] [Google Scholar]
- 43.Gatot I, Abramov D, Tsodikov V, et al. Should we give prophylactic renal-dose dopamine after coronary artery bypass surgery? J Card Surg. 2004;19:128–133. doi: 10.1111/j.0886-0440.2004.04044.x. [DOI] [PubMed] [Google Scholar]
- 44.Gerrah R, Ehrlich S, Tshori S, Sahar G. Beneficial effect of aspirin on renal function in patients with renal insufficiency postcardiac surgery. J Cardiovasc Surg. 2004;45:545–550. [PubMed] [Google Scholar]
- 45.Haase M, Haase-Fielitz A, Bagshaw SM, et al. Phase II, randomized, controlled trial of high-dose N-acetylcysteine in high-risk cardiac surgery patients. Crit Care Med. 2007;35:1324–1331. doi: 10.1097/01.CCM.0000261887.69976.12. [DOI] [PubMed] [Google Scholar]
- 46.Halpenny M, Lakshmi S, O'Donnell A, O'Callaghan-Enright S, Shorten GD. Fenoldopam: renal and splanchnic effects in patients undergoing coronary artery bypass grafting. Anaesthesia. 2001;56:953–960. doi: 10.1046/j.1365-2044.2001.02220.x. [DOI] [PubMed] [Google Scholar]
- 47.Hayashida N, Chihara S, Kashikie H, et al. Effects of intraoperative administration of atrial natriuretic peptide. Ann Thorac Surg. 2000;70:1319–1326. doi: 10.1016/s0003-4975(00)01658-1. [DOI] [PubMed] [Google Scholar]
- 48.Kaya K, Oguz M, Akar AR, et al. The effect of sodium nitroprusside infusion on renal function during reperfusion period in patients undergoing coronary artery bypass grafting: a prospective randomized clinical trial. Eur J Cardiothorac Surg. 2007;31:290–297. doi: 10.1016/j.ejcts.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 49.Kocakulak M, Askin G, Kucukaksu S, Tarcan O, Piskin E. Pulsatile flow improves renal function in high-risk cardiac operations. Blood Purif. 2005;23:263–267. doi: 10.1159/000085174. [DOI] [PubMed] [Google Scholar]
- 50.Kramer BK, Preuner J, Ebenburger A, et al. Lack of renoprotective effect of theophylline during aortocoronary bypass surgery. Nephrol Dial Transplant. 2002;17:910–915. doi: 10.1093/ndt/17.5.910. [DOI] [PubMed] [Google Scholar]
- 51.Kulka PJ, Tryba M, Zenz M. Preoperative alpha2-adrenergic receptor agonists prevent the deterioration of renal function after cardiac surgery: results of a randomized, controlled trial. Crit Care Med. 1996;24:947–952. doi: 10.1097/00003246-199606000-00012. [DOI] [PubMed] [Google Scholar]
- 52.Lassnigg A, Donner E, Grubhofer G, Presterl E, Druml W, Hiesmayr M. Lack of renoprotective effects of dopamine and furosemide during cardiac surgery. J Am Soc Nephrol. 2000;11:97–104. doi: 10.1681/ASN.V11197. [DOI] [PubMed] [Google Scholar]
- 53.Lema G, Urzua J, Jalil R, et al. Renal protection in patients undergoing cardiopulmonary bypass with preoperative abnormal renal function. Anesth Analg. 1998;86:3–8. doi: 10.1097/00000539-199801000-00002. [DOI] [PubMed] [Google Scholar]
- 54.Loef BG, Henning RH, Epema AH, et al. Effect of dexamethasone on perioperative renal function impairment during cardiac surgery with cardiopulmonary bypass. Br J Anaesth. 2004;93:793–798. doi: 10.1093/bja/aeh266. [DOI] [PubMed] [Google Scholar]
- 55.Mahesh B, Yim B, Robson D, Pillai R, Ratnatunga C, Pigott D. Does furosemide prevent renal dysfunction in high-risk cardiac surgical patients? Results of a double-blinded prospective randomised trial. Eur J Cardiothorac Surg. 2008;33:370–376. doi: 10.1016/j.ejcts.2007.12.030. [DOI] [PubMed] [Google Scholar]
- 56.Marathias KP, Vassili M, Robola A, et al. Preoperative intravenous hydration confers renoprotection in patients with chronic kidney disease undergoing cardiac surgery. Artif Organs. 2006;30:615–621. doi: 10.1111/j.1525-1594.2006.00270.x. [DOI] [PubMed] [Google Scholar]
- 57.Masoumi M, Saidi MR, Rostami F, Sepahi H, Roushani D. Off-pump coronary artery bypass grafting in left ventricular dysfunction. Asian Cardiovasc Thorac Ann. 2008;16:16–20. doi: 10.1177/021849230801600105. [DOI] [PubMed] [Google Scholar]
- 58.McBride WT, Allen S, Gormley SM, et al. Methylprednisolone favourably alters plasma and urinary cytokine homeostasis and subclinical renal injury at cardiac surgery. Cytokine. 2004;27:81–89. doi: 10.1016/j.cyto.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 59.Mentzer RM, Jr, Oz MC, Sladen RN, et al. Effects of perioperative nesiritide in patients with left ventricular dysfunction undergoing cardiac surgery: the NAPA Trial. J Am Coll Cardiol. 2007;49:716–726. doi: 10.1016/j.jacc.2006.10.048. [DOI] [PubMed] [Google Scholar]
- 60.Meyer M, Wiebe K, Wahlers T, et al. Urodilatin (INN:ularitide) as a new drug for the therapy of acute renal failure following cardiac surgery. Clin Exp Pharmacol Physiol. 1997;24:374–376. doi: 10.1111/j.1440-1681.1997.tb01205.x. [DOI] [PubMed] [Google Scholar]
- 61.Monaco M, Di Tommaso L, Mottola M, Stassano P, Iannelli G. Clinical outcome for on-pump myocardial revascularization in patients with mild renal dysfunction. Thorac Cardiovasc Surg. 2005;53:46–51. doi: 10.1055/s-2004-830457. [DOI] [PubMed] [Google Scholar]
- 62.Morgera S, Woydt R, Kern H, et al. Low-dose prostacyclin preserves renal function in high-risk patients after coronary bypass surgery. Crit Care Med. 2002;30:107–112. doi: 10.1097/00003246-200201000-00017. [DOI] [PubMed] [Google Scholar]
- 63.Myles PS, Buckland MR, Schenk NJ, et al. Effect of ‘renal-dose’ dopamine on renal function following cardiac surgery. Anaesth Intensive Care. 1993;21:56–61. doi: 10.1177/0310057X9302100114. [DOI] [PubMed] [Google Scholar]
- 64.Nuutinen L, Hollmen A. The effect of prophylactic use of furosemide on renal function during open heart surgery. Ann Chir Gynaecol. 1976;65:258–266. [PubMed] [Google Scholar]
- 65.Onorati F, Presta P, Fuiano G, et al. A randomized trial of pulsatile perfusion using an intra-aortic balloon pump versus nonpulsatile perfusion on short-term changes in kidney function during cardiopulmonary bypass during myocardial reperfusion. Am J Kidney Dis. 2007;50:229–238. doi: 10.1053/j.ajkd.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 66.Piper SN, Kumle B, Maleck WH, et al. Diltiazem may preserve renal tubular integrity after cardiac surgery. Can J Anaesth. 2003;50:285–292. doi: 10.1007/BF03017799. [DOI] [PubMed] [Google Scholar]
- 67.Ristikankare A, Kuitunen T, Kuitunen A, et al. Lack of renoprotective effect of i.v. N-acetylcysteine in patients with chronic renal failure undergoing cardiac surgery. Br J Anaesth. 2006;97:611–616. doi: 10.1093/bja/ael224. [DOI] [PubMed] [Google Scholar]
- 68.Ryckwaert F, Colson P, Ribstein J, Boccara G, Guillon G. Haemodynamic and renal effects of intravenous enalaprilat during coronary artery bypass graft surgery in patients with ischaemic heart dysfunction. Br J Anaesth. 2001;86:169–175. doi: 10.1093/bja/86.2.169. [DOI] [PubMed] [Google Scholar]
- 69.Sajja LR, Mannam G, Chakravarthi RM, et al. Coronary artery bypass grafting with or without cardiopulmonary bypass in patients with preoperative non-dialysis dependent renal insufficiency: a randomized study. J Thorac Cardiovasc Surg. 2007;133:378–388. doi: 10.1016/j.jtcvs.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 70.Sezai A, Shiono M, Orime Y, et al. Low-dose continuous infusion of human atrial natriuretic peptide during and after cardiac surgery. Ann Thorac Surg. 2000;69:732–738. doi: 10.1016/s0003-4975(99)01305-3. [DOI] [PubMed] [Google Scholar]
- 71.Sezai A, Hata M, Wakui S, et al. Efficacy of continuous low-dose hANP administration in patients undergoing emergent coronary artery bypass grafting for acute coronary syndrome. Circ J. 2007;71:1401–1407. doi: 10.1253/circj.71.1401. [DOI] [PubMed] [Google Scholar]
- 72.Sirivella S, Gielchinsky I, Parsonnet V. Mannitol, furosemide, and dopamine infusion in postoperative renal failure complicating cardiac surgery. Ann Thorac Surg. 2000;69:501–506. doi: 10.1016/s0003-4975(99)01298-9. [DOI] [PubMed] [Google Scholar]
- 73.Sisillo E, Ceriani R, Bortone F, et al. N-acetylcysteine for prevention of acute renal failure in patients with chronic renal insufficiency undergoing cardiac surgery: a prospective, randomized, clinical trial. Crit Care Med. 2008;36:81–86. doi: 10.1097/01.CCM.0000295305.22281.1D. [DOI] [PubMed] [Google Scholar]
- 74.Smith MN, Best D, Sheppard SV, Smith DC. The effect of mannitol on renal function after cardiopulmonary bypass in patients with established renal dysfunction. Anaesthesia. 2008;63:701–704. doi: 10.1111/j.1365-2044.2007.05408.x. [DOI] [PubMed] [Google Scholar]
- 75.Straka Z, Widimsky P, Jirasek K, et al. Off-pump versus on-pump coronary surgery: final results from a prospective randomized study PRAGUE-4. Ann Thorac Surg. 2004;77:789–793. doi: 10.1016/j.athoracsur.2003.08.039. [DOI] [PubMed] [Google Scholar]
- 76.Sumeray M, Robertson C, Lapsley M, Bomanji J, Norman AG, Woolfson RG. Low dose dopamine infusion reduces renal tubular injury following cardiopulmonary bypass surgery. J Nephrol. 2001;14:397–402. [PubMed] [Google Scholar]
- 77.Sward K, Valsson F, Odencrants P, Samuelsson O, Ricksten SE. Recombinant human atrial natriuretic peptide in ischemic acute renal failure: a randomized placebo-controlled trial. Crit Care Med. 2004;32:1310–1315. doi: 10.1097/01.ccm.0000128560.57111.cd. [DOI] [PubMed] [Google Scholar]
- 78.Tang AT, Alexiou C, Hsu J, Sheppard SV, Haw MP, Ohri SK. Leukodepletion reduces renal injury in coronary revascularization: a prospective randomized study. Ann Thorac Surg. 2002;74:372–377. doi: 10.1016/s0003-4975(02)03715-3. [DOI] [PubMed] [Google Scholar]
- 79.Tang AT, Knott J, Nanson J, Hsu J, Haw MP, Ohri SK. A prospective randomized study to evaluate the renoprotective action of beating heart coronary surgery in low risk patients. Eur J Cardiothorac Surg. 2002;22:118–123. doi: 10.1016/s1010-7940(02)00220-8. [DOI] [PubMed] [Google Scholar]
- 80.Tang AT, El-Gamel A, Keevil B, Yonan N, Deiraniya AK. The effect of ‘renal-dose’ dopamine on renal tubular function following cardiac surgery: assessed by measuring retinol binding protein (RBP) Eur J Cardiothorac Surg. 1999;15:717–721. doi: 10.1016/s1010-7940(99)00081-0. [DOI] [PubMed] [Google Scholar]
- 81.Wijeysundera DN, Beattie WS, Rao V, Granton JT, Chan CT. N-acetylcysteine for preventing acute kidney injury in cardiac surgery patients with pre-existing moderate renal insufficiency. Can J Anaesth. 2007;54:872–881. doi: 10.1007/BF03026790. [DOI] [PubMed] [Google Scholar]
- 82.Witczak BJ, Hartmann A, Geiran OR, Bugge JF. Renal function after cardiopulmonary bypass surgery in patients with impaired renal function. A randomized study of the effect of nifedipine. Eur J Anaesthesiol. 2008;25:319–325. doi: 10.1017/S0265021507003158. [DOI] [PubMed] [Google Scholar]
- 83.Woo EB, Tang AT, el-Gamel A, et al. Dopamine therapy for patients at risk of renal dysfunction following cardiac surgery: science or fiction? Eur J Cardiothorac Surg. 2002;22:106–111. doi: 10.1016/s1010-7940(02)00246-4. [DOI] [PubMed] [Google Scholar]
- 84.Yallop KG, Sheppard SV, Smith DC. The effect of mannitol on renal function following cardio-pulmonary bypass in patients with normal pre-operative creatinine. Anaesthesia. 2008;63:576–582. doi: 10.1111/j.1365-2044.2008.05540.x. [DOI] [PubMed] [Google Scholar]
- 85.Yavuz S, Ayabakan N, Dilek K, Ozdemir A. Renal dose dopamine in open heart surgery. Does it protect renal tubular function? J Cardiovasc Surg. 2002;43:25–30. [PubMed] [Google Scholar]
- 86.Yavuz S, Ayabakan N, Goncu MT, Ozdemir IA. Effect of combined dopamine and diltiazem on renal function after cardiac surgery. Med Sci Monit. 2002;8:P45–P50. [PubMed] [Google Scholar]
- 87.Parikh CR, Garg AX. Acute kidney injury: Better biomarkers and beyond. Kidney Int. 2008;73:801–803. doi: 10.1038/ki.2008.17. [DOI] [PubMed] [Google Scholar]
- 88.Furnary AP, Wu Y, Hiratzka LF, Grunkemeier GL, Page US., 3rd Aprotinin does not increase the risk of renal failure in cardiac surgery patients. Circulation. 2007;116:127–133. doi: 10.1161/CIRCULATIONAHA.106.681395. [DOI] [PubMed] [Google Scholar]
- 89.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve acute kidney injury outcomes. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]