Abstract
Many traditional and nontraditional risk factors contribute to vascular calcification among maintenance hemodialysis (MHD) patients. It is not clear whether coronary artery calcification (CAC) delineates a higher mortality risk independent of known risk factors. We examined 6-year (10/2001–9/2007) survival of 166 MHD patients, aged 53 ± 13 years, with baseline CAC scores. Patients were grouped into four CAC groups: 0, 1–100, 101–400, and 400+. The 101–400 and 400+ groups were associated with a significantly higher adjusted risk of death than CAC 0 with hazard ratios (HR) 8.5 (95% CI: 1.1–48.1, p = 0.02) and 13.3 (95% CI: 1.3–65.1, p = 0.01), respectively, independent of demographics, comorbidity, lipids and other cardiovascular risks, surrogates of bone disease, nutritional and inflammatory markers and dialysis dose. Total CAC [HR 6.7 (1.1–21.5, p = 0.03)] followed by the presence of CAC in the left main [4.6 (2.2–9.8, p = 0.001)] and left anterior descending artery [4.3 (2.1–14.2, p = 0.001)] were strong independent predictors of mortality even after adjusting for above covariates. Total and vessel-specific CAC predict mortality in MHD patients independent of traditional and nontraditional risk factors.
Key Words: Chronic kidney disease, Coronary artery calcium, Dialysis, Inflammation, Phosphorus binder, Sevelamer, Death risk
Introduction
Chronic kidney disease patients carry a greater overall mortality when compared to the general population [1,2]. Cardiovascular disease accounts for approximately 45% of all-cause mortality among chronic kidney disease stage 5 subjects, also known as end-stage renal disease [3,4]. Potential contributing factors to this elevated incidence include traditional risk factors such as age, gender, dyslipidemia, diabetes mellitus (DM) and several nontraditional risk factors such as hyperparathyroidism, malnutrition, inflammation, coronary artery calcium (CAC), and mineral disarrays, specifically elevated levels of phosphorus and calcium-phosphorus product (Ca × P) [5,6,7]. Limited data is available regarding CAC effect on mortality in subjects with end-stage renal disease.
Nonenhanced computed tomography (CT) enables the quantification of vascular calcification including the coronary arteries. A recent study in some 25,000 asymptomatic persons in the general population showed that CAC provided independent prediction of all-cause mortality beyond traditional risk factors [8]. CAC has proven to be a strong predictor of coronary heart disease among major racial and ethnic groups [9,10]. In maintenance hemodialysis (MHD) patients, extent and prevalence of vascular calcification may be predictors of cardiovascular disease and all-cause mortality [11,12,13,14]. Block et al. [14] specifically showed, among 127 new to MHD patients, that baseline CAC was a significant predictor of mortality after adjustment for age, gender, race, and DM (p = 0.002). In the Treat to Goal study by Chertow et al. [15], in which 200 MHD patients were randomized to either sevelamer or a calcium-based phosphate binder (CBPB), median CAC increased significantly in the CBPB group. Furthermore, Block et al. [14] showed that patients on CBPB entailed a greater risk of death, suggesting that treatment with a CBPB may contribute to the vascular calcification burden.
Considering the above-mentioned associations, we hypothesized that both total and potentially vessel-specific CAC scores proportionally predict all-cause mortality independent of both traditional and nontraditional risk factors, including mineral metabolism, inflammatory markers, and CBPB use, in MHD patients. We analyzed a cohort of MHD patients from the prospective Nutritional and Inflammatory Evaluation of Dialysis Patients (NIED) Study who underwent cardiac CT and assessed all-cause mortality in relation to baseline cardiac CT scan, comparing four groups defined as CAC 0, CAC 1–100, CAC 101–400, and CAC 400+ and further comparing CAC scores of all epicardial coronary arteries.
Patients and Methods
Patients
Patients who participated in the cardiac arm (those who underwent cardiac CT) of the NIED study from October 1, 2001 to September 30, 2006, and whose mortality was followed up to September 30, 2007 were included in this analysis. The NIED study (www.NIEDstudy.org) was a prospective cohort study designed to determine whether nutritional and inflammatory states in a MHD patient population affect mortality, morbidity, and other clinical outcomes. Patients who were included in the NIED study were 18 years or older and signed a written consent [16]. Patients with malignancy or other terminal diseases with less than a 6-month life expectancy were excluded. The subjects were all receiving thrice weekly MHD via high-flux dialyzers, and their dialysis membranes were routinely reused. New subjects were recruited semiannually and followed with repeated measures for up to 5 years or 10 semiannual rounds, with each round consisting of a semiannual assessment of malnutrition and inflammatory variables including biochemical markers, anthropometric measurements, hospitalization rates, and mortality. Medical chart review, including comorbidities, traditional cardiac risk factors, and outpatient medication usage were performed on all new recruits and then on a yearly basis thereafter. A modified version of the Charlson Comorbidity Index, without the age and kidney disease components, was used to assess the severity of comorbidities [17,18].
Assessment of the subject's use of phosphate binders, HMG-CoA reductase inhibitors (statins), and typical demographics were also obtained. All subjects underwent medical chart review by a physician. CBPB included calcium carbonate (Tums®, GlaxoSmithKline, Waltham, Mass., USA), calcium acetate (PhosLo® 667-mg tablets; Braintree Pharmaceuticals, Inc., Braintree, Mass., USA) and other forms of calcium-based binders. The non-calcium-based binder included only sevelamer hydrochloride (Renagel® 400- and 800-mg tablets; GelTex Pharmaceuticals, Waltham, Mass., USA).
Dialysis and Laboratory Data
The Kt/V was used to represent the weekly dialysis dose of each subject. Dialysis vintage time was calculated based on the time between first dialysis date and CT scan date. Serum levels of calcium, phosphorus, intact parathyroid hormone, and albumin were obtained through routine laboratory measurements performed by DaVita Laboratories (Deland, Fla., USA) using automated methods. Averaged values for each of the noted tests within a 13-week period were used to define the laboratory values of a particular study round. The averaged laboratory value for the particular study round that coincided closest to each subject's CT date was used for the data analysis in this study.
Study-Specific Laboratory Tests
Serum high-sensitivity C-reactive protein, two proinflammatory cytokines, interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α), serum total cholesterol, LDL-C, HDL-C, as well as homocysteine levels were measured from fasting samples in all patients. The high-sensitivity C-reactive protein was measured by a turbidometric immunoassay where a serum sample is mixed with latex beads coated with anti-human CRP antibodies forming an insoluble aggregate (WPCI, Osaka, Japan; mg/l, normal range <3.0 mg/l) [19]. High sensitivity IL-6 and TNF-α immunoassay kits based on a solid-phase sandwich enzyme-linked immunosorbent assay (ELISA) using recombinant human IL-6 and TNF-α were used to measure the serum proinflammatory cytokines (R&D Systems, Minneapolis, Minn., USA; normal range IL-6, <9.9 pg/ml; TNF-α, <4.7 pg/ml) [20]. Single lab measures were obtained on a semiannual basis. For this particular study, the single time laboratory value that coincided closest with each subject's CT date was used for data analysis.
Computed Tomography
The nonenhanced CT studies were performed with an E-Speed electron beam scanner (GE-Imatron, South San Francisco, Calif., USA). Coronary arteries were imaged with rapid acquisition of approximately 30–40 contiguous images of 3 mm slice thickness without gap during mid-diastole using ECG triggering during a single 15-second breath hold. CAC was quantified using the scoring method previously described by Agatston et al. [21]. Calcium was considered present in a coronary artery when a density of >130 Hounsfield units was detected in >3 contiguous pixels (>1 mm2) overlying that coronary artery. CAC was computed from the product of the attenuation factor and the area of calcification (mm2), with the total CAC of each coronary artery being equal to the sum CAC of all the lesions from that artery. The total calcium score was calculated by summing CAC from the left main, left anterior descending, left circumflex, and right coronary arteries.
Statistics
Mean ± SD and proportions were used to summarize the characteristics of the study sample. Continuous variables were compared by ANOVA, and categorical variables were compared by Kruskal-Wallis nonparametric ANOVA. Conventional Student t test, and χ2 test were used, as appropriate, to detect significant differences among groups defined as CAC 0, CAC 1–100, CAC 101–400, and CAC 400+. Kaplan-Meier survival curves were generated, and all outcomes other than death, including subjects who underwent kidney transplant, subjects lost to follow-up, subjects still alive at the end-point of outcome adjudication, i.e. 30 September 2007, were censored and compared with the log-rank test. Multivariate regression analysis and analysis of covariance were performed to obtain adjusted p valuescontrolled for case-mix and other covariates. Case-mix covariates included age, gender, ethnicity, DM (yes/no), race (black versus other), dialysis vintage (number of months on MHD treatment), HMG-CoA reductase inhibitor use (yes/no), smoking, family history of premature cardiac disease, body mass index (BMI), and CBPB use. Laboratory covariates in fully adjusted multivariate models included IL-6, albumin concentrations, LDL-C, iPTH, and mineral metabolism measures including calcium, phosphorus, and Ca × P. Hazard ratios (HRs) include 95% confidence intervals (CI). A p valueless than 0.05 or a 95% CI that does not span 1.0 is considered statistically significant; p values between 0.05 and 0.20 are listed with two decimals for consideration of potential type II errors. HRs of death were calculated by means of stepwise Cox proportional hazard regression analysis to compare four groups defined as CAC 0, CAC 1–100, CAC 101–400, and CAC 400+, adjusting for covariants that potentially contribute to mortality using the forward stepwise model to determine independent predictors for all-cause mortality. The effect of left main, left anterior descending, left circumflex and right coronary artery calcification on all-cause mortality was further determined by using stepwise Cox regression analysis. Descriptive and multivariate statistics were carried out using the statistical software SAS, version 9.13 (SAS Institute Inc., Cary, N.C., USA).
Results
Table 1 summarizes the baseline characteristics of the subjects. Of the 166 subjects, the mean age ± SD was 53 ± 13 years, the majority were male (59%), diabetic (51%), and hypertensive (74%). A large proportion of the subjects were Hispanic (39%), Black (43%), had a family history of premature heart disease (25%), smokers (22%), and on statin therapy (34%). The majority of patients at the time of CT scan had been on dialysis for >2 years (64%).
Table 1.
Baseline characteristics
| All subjects (n = 166) | |
|---|---|
| Mean age ± SD, years | 53 ±13 |
| Male gender, % | 59 |
| Race black, % | 42.5 |
| Ethnicity Hispanic, % | 38.6 |
| DM, % | 50.9 |
| Hypertension, % | 73.5 |
| Statin therapy, % | 33.7 |
| History of smoking, % | 22.3 |
| Family history of premature CVD, % | 24.7 |
| Dialysis vintage, % | |
| <6 months | 7.3 |
| 6–24 months | 28.7 |
| >24 months | 64 |
CVD = Cardiovascular disease.
Table 2 summarizes the characteristics of the subjects differentiated into the four defined groups of CAC 0, CAC 1–100, CAC 101–400, and CAC 400+. There was a significant difference among age (p = 0.0001), gender (p = 0.03), DM (p = 0.0001), Charlson Comorbidity Index (p = 0.0001), and intact parathyroid hormone (iPTH, p = 0.01). There notably was no statistically significant (p > 0.05) difference among vintage time, BMI, serum albumin, statin therapy, hypertension status, family history of heart disease, calcium phosphate product, inflammatory markers, and use of CBPB among the four groups. There was a trend towards increased age, greater proportion of diabetics, increased use of CBPB, and higher Charlson Comorbidity Index as CAC increased across the four groups.
Table 2.
Relevant demographic, clinical, and laboratory values according to CAC score categories in 166 MHD patients
| CACO (n=18) | CAC 1–100 (n = 48) | CAC 101–400 (n = 28) | CAC 400+ (n = 72) | p value | |
|---|---|---|---|---|---|
| Age, years | 47±11 | 45 ±15 | 58 ±10 | 59 ±10 | 0.0001 |
| Male gender, % | 33.3 | 52.1 | 71.4 | 65.3 | 0.03 |
| Statin therapy, % | 29.4 | 20.5 | 42.3 | 39.7 | 0.14 |
| Hypertension, % | 82.4 | 64.4 | 71.4 | 78.6 | 0.32 |
| DM, % | 33.3 | 33.3 | 64.3 | 73.6 | 0.0001 |
| Family history of CVD, % | 23.5 | 23.3 | 22.2 | 26.1 | 0.9 |
| History of smoking, % | 25.0 | 20.5 | 14.3 | 25.7 | 0.65 |
| CBPB therapy, % | 38.9 | 43.8 | 60.7 | 63.4 | 0.08 |
| Charlson Comorbidity Index | 0.94±1.16 | 1.06± 1.19 | 2.07±1.15 | 2.63 ±1.8 | 0.0001 |
| hs-CRP, mg/1 | 4.6 ±5.6 | 4.8 ±5.0 | 4.3 ±4.2 | 4.2 ±3.9 | 0.98 |
| Homocysteine, (xmol/1 | 24.8 ±7.4 | 25.8 ±8.1 | 27.1 ±10.7 | 26.8 ±8.2 | 0.76 |
| IL-6, pg/1 | 7.3 ±5.8 | 8.6 ±10.0 | 9.1 ±11.6 | 10.7±10.2 | 0.51 |
| TNF-α, pg/dl | 8.1 ±8.6 | 6.4 ±9.6 | 6.0 ±9.6 | 6.2 ±5.4 | 0.78 |
| Calcium (adjusted), mg/dl | 9.7 ±0.3 | 9.7 ±0.6 | 9.6 ±0.6 | 9.7 ±0.5 | 0.83 |
| Phosphorus, mg/dl | 4.9 ±1.0 | 5.8 ±1.4 | 5.4±1.1 | 5.5 ±1.3 | 0.09 |
| iPTH, pg/ml | 255 ±169 | 340 ±414 | 254 ±199 | 236 ±158 | 0.01 |
| Albumin, g/dl | 4.1 ±0.3 | 4.0 ±0.3 | 3.9 ±0.3 | 3.9 ±0.3 | 0.43 |
| Ca × P, mg2/dl2 | 48.2 ±10.5 | 57.3 ±15.1 | 52.4 ±10.6 | 53.6± 11.6 | 0.08 |
| BMI | 24.1 ±3.7 | 28.1 ±4.4 | 27.6 ±4.8 | 27.3 ±4.9 | 0.16 |
| Follow-up (CT-event) | 48 ±20 | 40 ±19 | 33 ±19 | 30 ±15 | 0.001 |
| Vintage, % | |||||
| >6 months | 1.2 | 1.8 | 2.4 | 1.8 | 0.29 |
| 6–24 months | 4.9 | 10.4 | 3.7 | 9.8 | |
| >24 months | 4.3 | 17.1 | 11 | 31.6 |
Values expressed as mean ± SD for continuous variables. hs-CRP = High-sensitivity C-reactive protein.
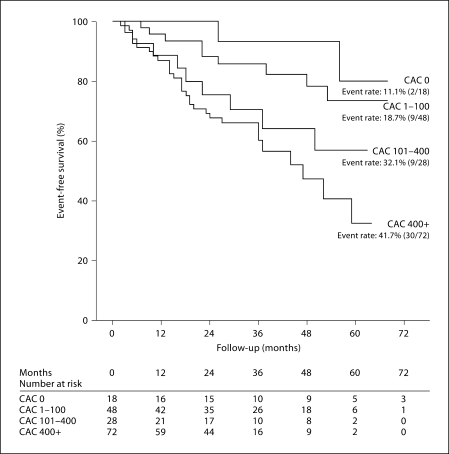
At the end of follow-up there were 50 deaths among the 166 participants including 30 deaths in the CAC 400+ group and 2 deaths among the CAC 0 group. Subjects with no evidence of CAC had higher event-free survival rates (88.9%) vs. CAC 400+ (58.3%). Baseline CAC scores was a significant predictor of all-cause mortality as shown in figure 1. Using stepwise Cox proportional hazard regression analysis with adjustment for case-mix variables, mineral metabolism including albumin, iPTH and Ca × P, Kt/V, LDL, and inflammatory marker IL-6, the HR of death across the three groups was 2.9 (CI 0.9–21.9, p = 0.1), 8.5 (CI 1.1–48.1, p = 0.02), and 13.3 (CI 1.3–65.1, p = 0.01) for CAC 1–100, CAC 101–400, and CAC 400+, respectively, when compared to CAC 0 (table 3).
Fig. 1.
Event-free survival among CAC subsets – association between CAC scores and event-free survival across the four groups (CAC 0, CAC 1–100, CAC 101–400, and CAC 400+). Event rates increased from 11.1 to 41.7% as CAC increased across the groups.
Table 3.
HR across CAC groups by means of Cox proportional hazard regression analysis models (n = 166)
| CACO | CAC 1–100 | p value | CAC 101–400 | p value | CAC 400+ | p value | |
|---|---|---|---|---|---|---|---|
| Unadjusted CAC | 1 | 2.1 (0.7–9.4) | 0.3 | 4.1 (1.2–18.6) | 0.009 | 6.3 (1.5–26.5) | 0.006 |
| Case-mix | 1 | 2.6 (0.9–15.8) | 0.1 | 6.3 (1.3–31.2) | 0.01 | 7.7 (1.2–29.8) | 0.009 |
| Kt/V, albumin, Ca, phosphorus, Ca × P, minerals, iPTH | 1 | 2.8 (0.9–20.7) | 0.1 | 7.4 (1.2–47.3) | 0.03 | 12.1 (1.2–52.9) | 0.01 |
| LDL, IL-6 | 1 | 2.9 (0.9–21.9) | 0.1 | 8.5 (1.1–48.1) | 0.02 | 13.3 (1.3–65.1) | 0.01 |
95% CI expressed in parentheses. LDL = Low-density lipoprotein.
Case-mix: CAC, age, gender, vintage, DM, hypertension, statin therapy, smoking, family history of premature CVD, ethnicity (Hispanic vs. other), race (black vs. other), BMI, CBPB.
The HR of death when comparing the presence (vs. absence) of total and individual coronary artery calcification (all coronaries, left main, left anterior descending, left circumflex, and right coronary artery) was 6.7 (CI 1.1–21.5, p = 0.03), 4.6 (CI 2.2–9.8, p = 0.001), 4.3 (CI 2.1–14.2, p = 0.001), 2.3 (CI 1.1–8.7, p = 0.03), and 1.9 (CI 1.3–4.6, p = 0.04), respectively, after adjusting for the same covariates (table 4).
Table 4.
HR across total and individual coronary artery groups by means of Cox proportional hazard regression analysis models (n = 166)
| CACO | Total CAC>0 | p value | LM CAC>0 | p value | LAD CAC>0 | p value | LCx CAC>0 | p value | RCA CAC>0 | p value | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted CAC | 1 | 4.4 (1.3–11.2) | 0.001 | 3.7 (1.8–5.8) | 0.001 | 3.5 (1.7–6.2) | 0.001 | 2.1 (1.3–4.1) | 0.001 | 1.7 (1.1–3.0) | 0.02 |
| Case-mix | 1 | 5.2 (1.2–14.3) | 0.01 | 4.1 (1.8–5.9) | 0.001 | 4.0 (1.7–12.2) | 0.005 | 2.1 (1.2–5.5) | 0.009 | 1.7 (1.1–3.2) | 0.03 |
| Kt/V, albumin, Ca, Phos, Ca × P, minerals, iPTH | 1 | 6.6 (1.1–15.7) | 0.02 | 4.4 (1.9–6.2) | 0.001 | 4.1 (1.8–13.5) | 0.007 | 2.2 (1.1–6.4) | 0.01 | 1.8 (1.1–4.2) | 0.04 |
| LDL, IL-6 | 1 | 6.7 (1.1–21.5) | 0.03 | 4.6 (2.2–9.8) | 0.001 | 4.3 (2.1–14.2) | 0.001 | 2.3 (1.1–8.7) | 0.03 | 1.9 (1.3–4.6) | 0.04 |
95% CI expressed in parentheses. CVD = Cardiovascular disease; LAD = left anterior descending; LCx = left circumflex; LM = left main; LDL = low-density lipoprotein; Phos = phosphorus; RCA = right coronary artery.
Case-mix: CAC, age, gender, vintage, diabetes mellitus, hypertension, statin therapy, smoking, family history of premature CVD, ethnicity (Hispanic vs. other), race (black vs. other), BMI, CBPB.
Discussion
Evidence indicates that general vascular calcification serves as a marker of increased cardiovascular and noncardiovascular complications, but to our knowledge only two previous studies address mortality risk among MHD subjects undergoing nonenhanced CT for CAC assessment. Matsuoka et al. [13] showed that CAC scores were an independent predictor of death in 104 MHD patients. In the said study CAC was adjusted for age, sex, vintage time, DM, hypertension, albumin, and dyslipidemia [13]. In a follow-up study of the Renagel in New Dialysis (RIND) trial, Blocket al. [14] demonstrated, in 127 subjects randomized to sevelamer or a CBPB, that a baseline CAC 400+ was significantly associated with increased mortality (HR = 4.5, p = 0.016, CI 1.3–15.1). The study controlled for age, race, gender, and DM. Our current larger study supports the previous trials and further contributes by additionally controlling for other key cofactors and nontraditional risk factors such as iPTH levels, inflammatory cytokines, and mineral metabolism. This becomes particularly important because serum elevated calcium, phosphorus, Ca × P, and secondary hyperparathyroidism are common complications of chronic kidney disease that, when untreated, may contribute to increased morbidity and mortality [7,22]. Other risk factors known to contribute to increased mortality, including age, diabetes, smoking status [23] and vintage time were all adjusted for in this analysis.
Our study shows elevated CAC scores are a significant independent predictor of all-cause mortality among MHD patients even after extensive adjustment for both these traditional and nontraditional risk factors. The CAC 400+ group showed a tendency towards increased age, large proportion of males, increase use of CBPB, and greater prevalence of DM, but the significant prediction for all-cause mortality persisted and was even a stronger predictor of death after adjustment for these factors and other covariates. When comparing across adjusted groups, HR increased dramatically from 2.9 (CI 0.9–21.9, p = 0.1) in the CAC 1–100 group to 13.3 (CI 1.3–65.1, p = 0.01) in the CAC 400+ group. Also noted, HR increased from 6.3 (CI 1.5–26.5, p = 0.006) to 13.3 (CI 1.3–65.1, p = 0.01) in the CAC 400+ group with each model. This suggests CAC is in itself an overwhelmingly important determinant of the increased mortality.
In the general population, therapies targeting CRP are proving to be effective at reducing cardiovascular events as demonstrated by the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) trial [24]. Inflammation may prove to play an important role in predicting the increased prevalence of cardiovascular disease and mortality in chronic kidney disease patients [25]. In our study high-sensitivity C-reactive protein levels were not significantly different among the CAC groups. The addition of CRP to the models did not have any significant effect.
Our study uniquely demonstrated that the presence of CAC in major epicardial coronary arteries may also play a role in influencing mortality. Presence of CAC in the left main followed by LAD are strong independent predictors of mortality even after adjusting for multiple variables, although total CAC still served as the strongest predictor (table 4).
A strength of the study was that specific markers of inflammation and nutritional status were extensively characterized and subjects were randomly selected without any prior knowledge of CAC scores or risk factors. The average vintage time for the 166 patients was 3.58 years from start of dialysis to CT date. Earlier dates could have served to further strengthen the notion that early elevated CAC scores predict increased mortality, but this does not negate the notion that an increased CAC score entails greater risk.
In conclusion, total CAC scores and vessel-specific coronary artery calcium scores were independent and incremental predictors of all-cause mortality. CAC scores of 100+ were a strong predictor of mortality. Coronary artery calcium scores may have a role in monitoring and predicting survival in the MHD population. This potentially may be used to help delineate those in need of more aggressive therapy and risk management by such means as phosphate binder choice, calcium-phosphate control, lipid lowering medications, and diligent monitoring. Maintaining a lower CAC burden may have positive implications on the patient's mortality in this particular population.
Disclosure
K.K.-Z., C.P.K. and/or M.J.B. have received grants and/or honoraria from Abbott (manufacturer of Calcijex™ and Zemplar™), Amgen (manufacturer of Sensipar™), Fresenius (distributor of PhosLo), Genzyme (manufacturer of Hectoral™, Renagel and Renvela™), and/or Shire (manufacturer of Fosrenol™). Other authors have not declared conflicts of interest.
Acknowledgements
The authors are grateful to Ms. Stephanie Griffith and Dr. Victor Goh, at Harbor-UCLA GCRC Core Laboratories for the management of blood samples and measuring inflammatory markers. The authors also are grateful to Jima Tiano for her help in scheduling subjects and collecting data.
This study was supported by National Institutes of Health, National Institute of Diabetes, Digestive and Kidney Disease grants R21-DK61162 and K23-DK061162 (for K.K.-Z.). Additional sources of funding include research grants from DaVita Clinical Research, a research grant from Genzyme, a philanthropist grant by Mr. Harold Simmons (for K.K.-Z.), and a General Clinical Research Center grant No. M01-RR00425 from the National Centers for Research Resources, National Institutes of Health.
Footnotes
The abstract of this article was presented during the National Kidney Foundation (NKF) annual conference, April 2–6, 2008, in Dallas, Tex., USA.
References
- 1.US Renal Data System, USRDS 2008 Annual Data Report . Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2008. [Google Scholar]
- 2.National Kidney Foundation K/DOQI clinical practice guidelines for management of dyslipidemias in patients with kidney. Am J Kidney Dis. 2003;41(suppl 3):S1–S91. [PubMed] [Google Scholar]
- 3.Hage FG, Venkataraman R, Zoghbi GJ, Perry GJ, DeMattos AM, Iskandrian AE. The scope of coronary heart disease in patients with chronic kidney disease. J Am Coll Cardiol. 2009;53:2129–2140. doi: 10.1016/j.jacc.2009.02.047. [DOI] [PubMed] [Google Scholar]
- 4.Foley RN, Parfrey PS, Sarnak MJ. Epidemiology of cardiovascular disease in chronic renal disease. J Am Soc Nephrol. 1998;9:S16–S23. [PubMed] [Google Scholar]
- 5.Zoccali C. Cardiovascular risk in uraemic patients – is it fully explained by classical risk factors? Nephrol Dial Transplant. 2000;15:454–457. doi: 10.1093/ndt/15.4.454. [DOI] [PubMed] [Google Scholar]
- 6.Block GA, Hulbert-Shearon TE, Levin NW, Port FK. Association of serum phosphorus and calcium × phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis. 1998;31:601–617. doi: 10.1053/ajkd.1998.v31.pm9531176. [DOI] [PubMed] [Google Scholar]
- 7.Young EW. Mineral metabolism and mortality in patients with chronic kidney disease. Adv Chronic Kidney Dis. 2007;14:13–21. doi: 10.1053/j.ackd.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Budoff MJ, Shaw LJ, Liu ST, et al. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol. 2007;49:1860–1870. doi: 10.1016/j.jacc.2006.10.079. [DOI] [PubMed] [Google Scholar]
- 9.Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 10.Nasir N, Shaw LJ, Liu ST, et al. Ethnic differences in the prognostic value of coronary artery calcification for all-cause mortality. J Am Coll Cardiol. 2007;50:953–960. doi: 10.1016/j.jacc.2007.03.066. [DOI] [PubMed] [Google Scholar]
- 11.Raggi P, Boula A, Chasan-Taber S, et al. Cardiac calcification in adult hemodialysis patients a link between end-stage renal disease and cardiovascular disease? J Am Coll Cardiol. 2002;39:695–701. doi: 10.1016/s0735-1097(01)01781-8. [DOI] [PubMed] [Google Scholar]
- 12.Blacher J, Guerin AP, Pannier B, et al. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension. 2001;38:938–942. doi: 10.1161/hy1001.096358. [DOI] [PubMed] [Google Scholar]
- 13.Matsuoka M, Iseki K, Tamashiro M, et al. Impact of high coronary artery calcification score (CACS) on survival in patients on chronic hemodialysis. Clin Exp Nephrol. 2004;8:54–58. doi: 10.1007/s10157-003-0260-0. [DOI] [PubMed] [Google Scholar]
- 14.Block GA, Raggi P, Bellasi A, Kooienga L, Spiegel DM. Mortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patients. Kidney Int. 2007;71:438–441. doi: 10.1038/sj.ki.5002059. [DOI] [PubMed] [Google Scholar]
- 15.Chertow GM, Burke SK, Raggi P, Treat to Goal Working Group Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int. 2002;62:245–252. doi: 10.1046/j.1523-1755.2002.00434.x. [DOI] [PubMed] [Google Scholar]
- 16.Colman S, Bross R, Benner D, et al. The Nutritional and Inflammatory Evaluation in Dialysis patients (NIED) study: overview of the NIED study and the role of dietitians. J Ren Nutr. 2005;15:231–243. doi: 10.1053/j.jrn.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Mehrotra R, Kermah D, Fried L, et al. Chronic peritoneal dialysis in the United States: declining utilization despite improving outcomes. J Am Soc Nephrol. 2007;18:2781–2788. doi: 10.1681/ASN.2006101130. [DOI] [PubMed] [Google Scholar]
- 18.Beddhu S, Bruns FJ, Saul M, Seddon P, Zeidel ML. A simple comorbidity scale predicts clinical outcomes and costs in dialysis patients. Am J Med. 2000;108:609–613. doi: 10.1016/s0002-9343(00)00371-5. [DOI] [PubMed] [Google Scholar]
- 19.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 20.Kalantar-Zadeh K, McAllister CJ, Lehn RS, Lee GH, Nissenson AR, Kopple JD. Effect of malnutrition-inflammation complex syndrome on EPO hyporesponsiveness in maintenance hemodialysis patients. Am J Kidney Dis. 2003;42:761–773. doi: 10.1016/s0272-6386(03)00915-6. [DOI] [PubMed] [Google Scholar]
- 21.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 22.Chertow GM. Slowing the progression of vascular calcification in hemodialysis. J Am Soc Nephrol. 2003;14(suppl 4):S310–S314. doi: 10.1097/01.asn.0000081666.10967.05. [DOI] [PubMed] [Google Scholar]
- 23.Goodkin DA, Bragg-Gresham JL, Koenig KG, et al. Association of comorbid conditions and mortality in hemodialysis patients in Europe, Japan, and the United States: the Dialysis Outcomes and Practice Patterns Study (DOPPS) J Am Soc Nephrol. 2003;14:3270–3277. doi: 10.1097/01.asn.0000100127.54107.57. [DOI] [PubMed] [Google Scholar]
- 24.Ridker PM, Danielson E, Fonseca FA, et al. JUPITER Study Group Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 25.Kalantar-Zadeh K, Ikizler A, Block G, Avram MM, Kopple JD. Malnutrition-inflammation complex syndrome in dialysis patients: causes and consequences. Am J Kidney Dis. 2003;42:864–881. doi: 10.1016/j.ajkd.2003.07.016. [DOI] [PubMed] [Google Scholar]