Abstract
Background/Aims
In this study we tested the hypothesis that H2S regulates collagen deposition, matrix metalloproteinases (MMP) and inflammatory molecules during hyperhomocysteinemia (HHcy) resulting in attenuation of glomerulosclerosis and improved renal function.
Materials and Methods
A genetic model of HHcy, cystathionine β-synthase heterozygous (CBS+/−) and wild-type (WT) 2-kidney (2K) mice were used in this study and supplemented with or without NaHS (30 μmol/l, H2S donor) in drinking water for 8 weeks. To expedite the renal damage associated with HHcy, uninephrectomized (1K) mice of similar groups were also used.
Results
Results demonstrated that NAD(P)H oxidase (p47phox subunit) and blood pressure were upregulated in WT 1K, CBS+/− 2K and CBS+/− 1K mice with downregulation of H2S production and reduced glomerular filtration rate. These changes were normalized with H2S supplementation. Both pro- and active MMP-2 and -9 and collagen protein expressions and glomerular depositions were also upregulated in WT 1K, CBS+/− 2K and CBS+/− 1K mice. Increased expressions of inflammatory molecules, intercellular cell adhesion molecule-1 and vascular cell adhesion molecule-1, as well as increased macrophage infiltration, were detected in WT 1K, CBS+/− 2K and CBS+/− 1K mice. These changes were ameliorated with H2S supplementation.
Conclusion
Together, these results suggest that increased oxidative stress and decreased H2S in HHcy causes matrix remodeling and inflammation resulting in glomerulosclerosis and reduced renal function.
Key Words: Collagen, Matrix metalloproteinase, Inflammation, Fibrosis, Hypertension, Renal dysfunction
Introduction
It is now well established that hyperhomocysteinemia (HHcy), an increased plasma homocysteine (Hcy) level, is a potent inducer of endothelial dysfunction, particularly in small vessels [1]. HHcy promotes atherosclerosis and thrombosis in susceptible animals, such as cystathionine β-synthase heterozygous (CBS+/−) mice fed with a high methionine diet [2]. The pathophysiological mechanisms of these effects, however, are less understood in the kidney, particularly in the process of glomerulosclerosis.
An increase in the Hcy level has been shown to induce oxidative stress through reactive oxygen species in the kidney [3,4] and has been reported to induce local oxidative stress, mesangial expansion and podocyte dysfunction resulting in renal fibrosis [5]. In rat mesangial cell culture, Hcy promotes collagen accumulation and is associated with increased NAD(P)H activity [5]. This suggests that Hcy induces local oxidative stress, cellular dysfunction and extracellular matrix metabolism in the glomerulus, all of which are associated with increased NAD(P)H activity.
The three enzymes CBS, cystathionine γ-lyase (CSE) and 3-mercaptopyruvate sulfurtransferase (3MST) metabolize Hcy to produce H2S in the body [6,7,8,9]. The physiological function of endogenous H2S is not clear and could be multifaceted; however, it is involved in the regulation of vascular tone [10] and protects neuronal cells from oxidative stress by increasing the intracellular concentration of antioxidant (glutathione) [11]. Increasing evidence suggests the potential antioxidant properties of H2S in normal and pathophysiological conditions [12,13,14]. In addition, recent reports have demonstrated that H2S is a potential anti-inflammatory substance [10,15,16]. However, the physiological role of H2S in HHcy-associated renal remodeling is incompletely defined.
Matrix metalloproteinases (MMPs) degrade both the collagenous and noncollagenous components of the extracellular matrix, and are thereby actively involved in matrix turnover. Gelatinases, members of the family of MMPs, digest these products into smaller peptides. Among gelatinases, MMP-2 and MMP-9 have gained potential interest because of their capability to disrupt the kidney architecture [17,18,19]. Similarly, experimental evidence implicated sustained elevation of cell adhesion molecules, such as intercellular cell adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1), in chronic inflammatory disorder, which leads to sclerosis [20]. HHcy has been reported to increase the expression of ICAM-1 [19] and VCAM-1 [19,21] in experimental models by independent laboratories, including our own. In addition, macrophage infiltration in the kidney is one of the most important events for progression of nephropathy [22]. Despite these facts, however, the major contributing factors of HHcy-associated inflammation in the process of glomerulosclerosis still need to be defined comprehensively.
In this study we tested the hypothesis that HHcy-induced oxidative stress upregulates collagen deposition in the glomerulus leading to glomerulosclerosis through modulation of MMPs and inflammatory molecules. In addition, the regulatory role of H2S to modulate this renal remodeling process was determined in an HHcy kidney.
Materials and Methods
Animals
Wild-type (WT, C57BL/6J) and CBS+/− mice aged 8 weeks were obtained from Jackson Laboratories (Bar Harbor, Me., USA) and bred at the animal care facility at the University of Louisville. Genotypes of these mice were determined and 10-week-old male mice were used for this study. Mice were divided into 2 sets. The first set of mice had 2 normal kidneys (2K) and were divided into the following groups: WT, CBS+/−, WT + H2S [30 μmol/l for 8 weeks, NaHS (sodium hydrosulfide) was used as a donor of H2S] and CBS+/− + H2S (30 μmol/l for 8 weeks). The second set of mice were uninephrectomized (1K) and separated as the 4 groups above. At the end of the experiments, mice were deeply anesthetized and sacrificed to harvest the tissues. All animal procedures were in accordance with the National Institute of Health Guidelines for Animal Research and were approved by the Institutional Animal Care and Use Committee of the University of Louisville School of Medicine.
H2S Treatment
H2S was supplemented to mice through drinking water in the form of NaHS, which in an aqueous solution produces an equal concentration of H2S. Typically, physiological ranges of H2S vary from 10–100 μmol/l [12,23] and can rise as high as 160 μmol/l in the human plasma during septic shock [24]. To keep H2S supplementation within the physiological range, we supplemented animals with 30 μmol/l of H2S. The density of H2S is about 18% higher than the air; therefore, the evaporation rate of H2S from the solutions is normally low. Moreover, the water bottle had a drinking tube with a small aperture, which minimized evaporation. In order to make sure animals took the appropriate amount of H2S through drinking water, approximately every 12 h we changed the water with fresh H2S in the appropriate groups. Within this period we observed a negligible amount of H2S loss (approx. 2–3%). Also, water consumption in treated versus nontreated groups was recorded, but no differences were found. No apparent toxicities of H2S were noticed other than its effect on blood pressure (reported in ‘Results’).
Cell Culture
Mouse kidney mesangial cells were purchased from ATTC, and were cultured and maintained in T-25 flasks. The culture medium was DMEM/F-12 (50/50) containing 10% fetal calf serum and antibiotics (Cellgro; Mediatech Inc., Herndon, Va., USA). Cells were trypsinized as described earlier [19] and plated onto 8-well chamber slides. After experiments, the cells were processed for immunostaining and visualized under a laser scanning confocal microscope (FluoView 1000, Olympus) using an appropriate filter.
Antibodies and Reagents
Rat monoclonal antibody F4/80 was purchased from Abcam (Cambridge, Mass., USA). Anti-p47phox, MMP-2 and -9, collagen IV, ICAM-1, VCAM-1 and HRP-linked secondary antibodies were from Santa Cruz Biotechnology (Santa Cruz, Calif., USA). Anti-β-actin antibody, NaHS and other analytical reagents were from Sigma-Aldrich (St. Louis, Mo., USA). Polyvinylidene difluoride membrane was from Bio-Rad (Hercules, Calif., USA).
Cryosectioning
At the end of the experiments, kidneys were excised and cryopreserved in Peel-A-Way disposable plastic tissue embedding molds (Polysciences Inc., Warrington, Pa., USA) containing tissue freezing media (Triangle Biomedical Sciences, Durham, N.C., USA). These molds were kept frozen (−70°C) until serial 5-μm (for histological staining) or 2-μm (for immunostaining) tissue sections were made in cryocut (Leica CM 1850). Cryosections were placed on Superfrost Plus microscope slides, air-dried and processed for staining.
Western Blots
The kidney cortical tissues were minced into fine particles with scissors and incubated with protein extraction buffer (0.01 mol/l cacodylic acid pH 5.0, 0.15 mol/l NaCl, 1 μmol/l ZnCl2, 0.02 mol/l CaCl2, 0.0015 mol/l NaN3 and 0.01% v/v Triton X-100) overnight at 4°C with gentle agitation. The extracted protein was collected and pH rose to 7.4 by adding 1.5 mol/l Tris (pH 8.8.). An equal amount of protein was analyzed by 10% SDS-PAGE, transferred to a polyvinylidene difluoride membrane and probed with appropriate antibodies followed by reprobing with β-actin as described earlier [19].
Immunostaining
Cryosectioned (2-μm) renal tissue samples and cells in the 8-well chamber slides were washed with PBS (pH 7.4) and blocked with 1% BSA for 15 min followed by 2 washes with PBS (5 min each). Slides were then fixed with 4% paraformaldehyde containing 0.25% L-α-lysophosphatidylcholine for 30 min. After washing with PBS (3×, 5 min each), samples were blocked with 1% BSA for 1 h. Slides were then washed twice, primary antibody (p47phox, 1:100 dilutions in 1% BSA) was added, and incubated overnight at 4°C with gentle agitation. Excess antibody was washed with PBS (3×, 5 min each) and secondary fluorescence-conjugated antibody (1:500 dilutions in 1% BSA) was added and incubated for 2 h. Unbound secondary antibody was removed by PBS wash (3×, 5 min each), mounted on slides and visualized with fluorescence in a laser scanning confocal microscope (Olympus FluoView 1000) with an appropriate filter.
Masson's Trichrome Staining
Cryosectioned kidney tissue samples (5-μm) were processed for Masson's trichrome (Richard-Allan Scientific, Kalamazoo, Mich., USA) staining according to the manufacturer's instructions for detecting collagen. Collagen appeared as a blue color.
RT-PCR
Total RNA was extracted from renal cortical tissue using Trizol Reagent (Gibco BRL) according to the manufacturer's instructions. The cDNA template was synthesized using a Promega kit. Primer sequences for mouse collagen IV were used as described elsewhere [25].
Peroxidase-Based DAB Immunohistochemical Staining
Kidney tissues were cryosectioned (2-μm thickness; Leica, CM 1850)and placed on slides, with the slides brought to room temperature before being washed with PBS (pH 7.4, 2×, 5 min each). The sections were then blocked with 1% BSA (in PBS) for 15 min, washed with PBS (2×, 5 min each) and fixed with 4% paraformaldehyde containing L-α-lysophosphatidylcholine (0.25% w/v) for 1 h. Following fixation, sections were washed with PBS (3×, 5 min each) and then blocked again with 1% BSA for 1 h. The blocking solution was discarded and the slides were washed with PBS (2×, 5 min each) followed by incubation with primary antibody (1:100 in 1% BSA) overnight at 4°C. Slides were washed with PBS (3×, 5 min each) to remove excess antibody, followed by incubation with HRP-conjugated secondary antibody (1:250 in 1% BSA) for 2 h at room temperature. Slides were then washed with PBS (3×, 5 min each) and DAB substrate-chromogen was applied, incubated at 37°C for 30 min, rinsed with distilled water and mounted in mounting medium. DAB staining was visualized under a microscope with dark brown end-product at the site of the target antigen.
Measurement of Glomerular Filtration Rate
Alzet Mini-Osmotic Pumps (Durect Corp., Cupertino, Calif., USA) with a constant flow rate (8 μl/h) filled with 200 μl of 5% FITC-inulin (dialyzed) were inserted into the peritoneal cavity through a small midline incision under light anesthesia. Animals were then placed in metabolic cages. Both collected plasma and urine samples were buffered at pH 7.4 with 500 mmol/l HEPES before taking the FITC fluorescence value to minimize the effect of pH [26]. The titrated 50-μl samples were then loaded onto each well of a 96-well plate. Fluorescence was determined using Spectra Max (Molecular Device, Sunnyvale, Calif., USA) with 485-nm excitation and read at 538-nm emission. Glomerular filtration rate (GFR) was calculated based on inulin clearance using the 24-hour urinary FITC-inulin excretion rate (urinary fluorescence counts/24 h) divided by the concentration of plasma FITC-inulin (fluorescence counts/μl).
Statistical Analysis
Values are given as means ± SEM from ‘n’ numbers of animals in each group as mentioned in each of the figure legends. The difference between mean values of multiple groups were analyzed by one-way analysis of variance (ANOVA) followed by Scheffe's post hoc analysis. Comparisons between groups were made with the use of Student's independent t test. Significance was accepted at p < 0.05.
Results
Endogenous Production of Tissue H2S and CSE Expression Was Attenuated in HHcy Mice
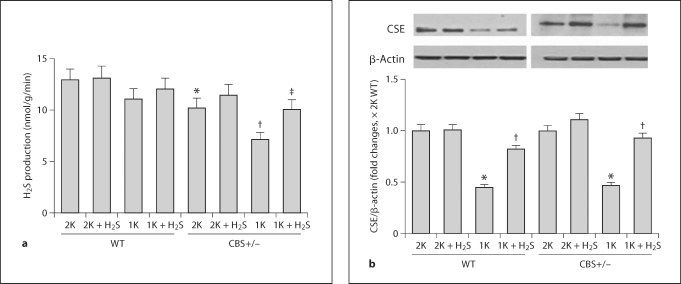
CBS+/− mice are hyperhomocysteinemic, and previously we reported that 1K CBS+/− mice further developed HHcy [18]. Also, 1K WT exhibited a moderate increase in plasma Hcy compared to their 2K littermates [18]. Here, we sought to determine whether the increased plasma level of Hcy had any causative effect on endogenous production of H2S in the renal cortical tissue. Our results showed that H2S production was marginally low in 1K WT and was further downregulated in 2K CBS+/− mice compared to 2K WT mice (fig. 1a). However, in 1K CBS+/− mice, H2S production was significantly attenuated compared to both 2K littermates and 2K WT mice. Interestingly, supplementation of H2S (30 μmol/l) improved endogenous generation of H2S in these animal groups (fig. 1a). These results clearly suggest that HHcy attenuates endogenous H2S production and that H2S supplementation normalizes H2S production.
Fig. 1.
Attenuated renal cortical tissue H2S production and CSE expressions were normalized by H2S treatment. a In the presence of 10 mmol/l L-cysteine and 2 mmol/l pyridoxol 5′-phosphate, H2S production in the kidney cortex tissue homogenates was measured following the procedure as described in the ‘Materials and Methods’. ∗ p < 0.01 vs. 2K WT; † p < 0.05 vs. 2K CBS+/−; ‡ p < 0.05 vs. 1K CBS+/−. b Representative Western blot showed CSE expression in the kidney cortex tissue in different experimental animal groups. The bar diagram showed densitometric analyses of CSE expression against β-actin loading control. Data represents the mean ± SEM, n = 4–7. ∗ p < 0.01 vs. respective 2K mice and † p < 0.01 vs. respective 1K mice.
CSE is one of the enzymes involved in the metabolism of Hcy and production of H2S. In order to assess whether HHcy has any modulatory role in the expression of CSE, we measured renal cortical CSE expression by Western blot. Our results showed that CSE expression was downregulated in 1K WT and 1K CBS+/− mice (fig. 1b). However, supplementation of H2S ameliorated CSE expression, suggesting that H2S plays a role in CSE expression during HHcy.
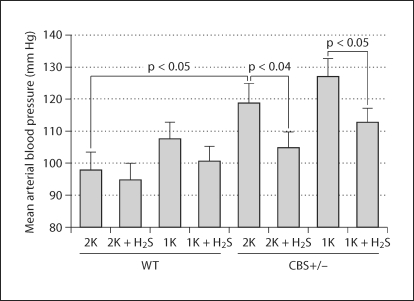
H2S Reduced Increased Blood Pressure in CBS+/− Mice
We used a 1K model to expedite the glomerular damage; however, the 1K animal often develops hypertension and, consequently, renal damage. In order to assess the degree of hypertension and the role of H2S treatment in regulating blood pressure, we measured blood pressure; the summarized data is shown in figure 2. 1K WT animals developed a small increase of blood pressure compared to 2K WT, which was not significant. There was, however, a significant increase of blood pressure in 2K CBS+/− mice compared to 2K WT animals. 1K CBS+/− mice showed a further increase of blood pressure (6 to 7 mm Hg), but this was not significant compared to 2K CBS+/− animals. Interestingly, H2S supplementation normalized this increase of blood pressure in 1K WT and 2K and 1K CBS+/− mice. The heart rate, however, did not change between groups with or without H2S supplementation (data not shown). These results specifically suggest that H2S acts at least through two different mechanisms: (1) through reducing blood pressure, which has immense impact on glomerular damage; and (2) through its antioxidant properties, which reduces oxidative damages induced by HHcy.
Fig. 2.
Increased blood pressure was normalized with H2S supplementation. Blood pressure was measured using radiotelemetry devices (model TA11PA-C10; DSI, St. Paul, Minn., USA). A miniature intra-arterial catheter was surgically implanted in the ventral subcutaneous space with the catheter inserted into the left carotid artery. Mice were allowed to recover for 1 week before they were placed on telemetric matrix while they remained in their cages, and devices were turned on for hemodynamics measurement. Summarized data (n = 4–6 mice/group).
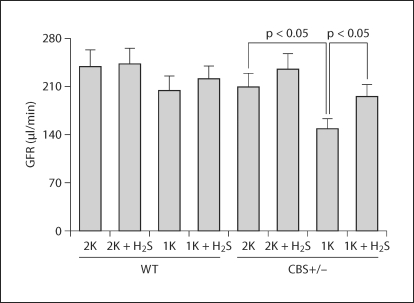
H2S Supplementation Improved GFR
To assess the impact of HHcy on renal function and any regulatory role of H2S on it, GFR was measured using the gold standard method (FITC-inulin clearance) as described in ‘Materials and Methods’. 2K CBS+/− mice showed a marginally low GFR compared to their age-matched 2K WT littermates (fig. 3). Although GFR in 1K WT mice was low, it was not significant compared to 2K WT mice. However, GFR of 1K CBS+/− mice was significantly lower compared to 2K CBS+/− littermates. Interestingly, exogenous supplementation of H2S dramatically improved GFR in 1K CBS+/− mice (fig. 3), suggesting the beneficial effect of H2S on Hcy-induced kidney function.
Fig. 3.
Impaired GFR in HHcy mice was ameliorated by H2S supplementation. NaHS (H2S donor, 30 μmol/l) was supplemented with drinking water for 8 weeks in the appropriate groups as shown. GFR was measured using the gold standard method (FITC-inulin) as described in ‘Materials and Methods’. Data represents the mean ± SEM, n = 7 in each group.
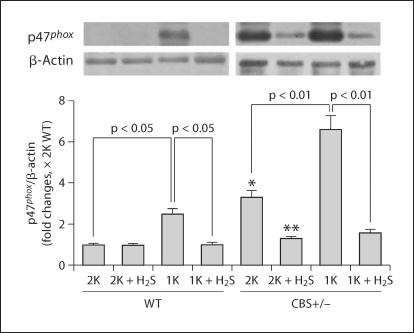
Expression of the NAD(P)H Oxidase p47phox Subunit in the Renal Cortical Tissue
Previously, we reported that plasma Hcy increased with a declining trend of plasma H2S level, followed by an increase of renal injury in CBS+/− mice, especially in 1K animals [18]. This was evidenced with an increase in superoxide (O2–·) production, urinary protein loss and apoptotic cell death in the glomerulus. Here we determined the NAD(P)H oxidase p47phox subunit in the different experimental groups used in this study because it is involved in the oxidative redox signal. Also, to explore whether or not H2S has a regulatory role on this molecule, we examined its expression in the cortical tissue extract following H2S supplementation. A representative blot showed increased p47phox expressions in the renal cortical tissue extract of 1K WT, 2K CBS+/− and 1K CBS+/− mice compared to 2K WT animals (fig. 4). These increased expressions of p47phox were almost completely abolished in a separate group of 1K WT mice, and significantly diminished in 2K CBS+/− and 1K CBS+/− mice supplemented with H2S (NaHS, 30 μmol/l; fig. 4). Thus, these results suggest that high Hcy induces the NAD(P)H oxidase p47phox subunit, causing oxidative stress. This oxidative stress induced by Hcy plays a pivotal role in glomerular injury and H2S triggers this mechanism, partly through its antioxidant properties.
Fig. 4.
H2S mitigated NAD(P)H oxidase (p47phox subunit) in the HHcy kidney. Western blot was performed to measure the p47phox subunit of NAD(P)H oxidase in the kidney cortex tissue extract using anti-p47phox antibody. Data represents the mean ± SEM, n = 5–7. ∗ Significant difference (p < 0.05) compared to 2K WT, and ∗∗ significant difference (p < 0.05) compared to 2K CBS+/−.
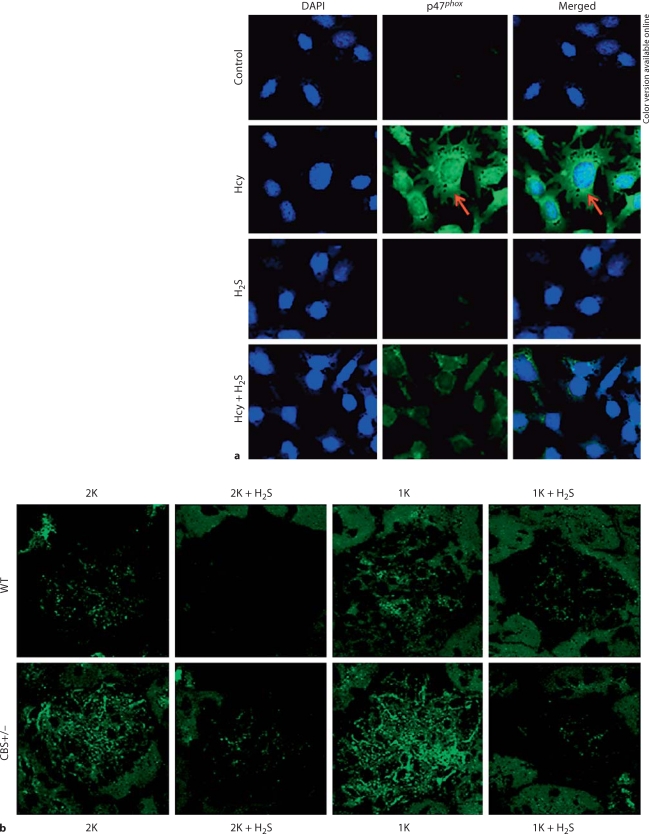
In vitro H2S Attenuated Hcy-Induced p47phox Upregulation
To determine the biological impact of H2S in HHcy-associated p47phox expression, both in vitro and in vivo experiments were performed using kidney mesangial cells and renal tissue sections. Figure 5a shows that in in vitro cell culture, Hcy induced upregulation of p47phox. When cells were pretreated and incubated with H2S along with Hcy, this upregulation was ameliorated. Figure 5b shows the expression of p47phox in the glomerulus. The fluorescence intensity was higher in 1K WT and 2K CBS+/− mice compared to 2K WT littermates. However, this intensity was robustly increased in 1K CBS+/− glomerulus, indicating higher expression of p47phox compared to other nontreated groups. When a similar group of mice was treated with H2S, this induction of p47phox was attenuated (fig. 5b). Together, these results suggest that Hcy induces p47phox and H2S downregulates p47phox during HHcy.
Fig. 5.
H2S diminished Hcy-induced p47phox upregulation in mesangial cells as well as in the glomerulus. a Kidney mesangial cells were cultured in 8-well chamber slides. Cells were pretreated with H2S (NaHS, 30 μmol/l) 15 min before cells were exposed to Hcy (50 μmol/l) for 48 h (NaHS and Hcy were freshly added after every 12 h). Cells were fixed, permeabilized, blocked with 1% BSA in PBS, and immunostained with anti-p47phox antibody secondarily conjugated with FITC. Also, cells were counterstained with DAPI. Fluorescence images were taken under the laser scanning confocal microscope (FluoView 1000, Olympus). Green fluorescence, as indicated by arrows, indicated p47phox expression. Representative images from independent experiments (n = 5). b Representative images of p47phox immunostained glomerulus (n = 5–7 animals/group) from different experimental animal groups.
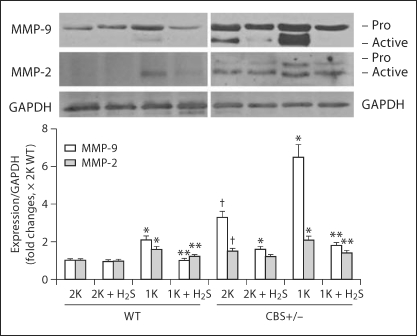
Induction of MMP-2 and -9 in the Renal Cortex
Oxidative stress regulates MMPs, which play a role in collagen remodeling in the matrix during HHcy [18,27,28]. Therefore, we measured the expressions of MMP-2 and -9 in the renal cortex tissue-extracted protein by Western blot. Summarized data in figure 6 showed that both pro- and active forms of MMP-9 were increased significantly in 1K WT, 2K CBS+/− and 1K CBS+/− mice. Interestingly, H2S inhibited expression of both these forms in the cortex. The expression of the active form of MMP-2 was higher in 1K WT animals compared to 2K WT, whereas both pro- and active forms of this proteinase were found upregulated in 2K and 1K CBS+/− animals (fig. 6). Like MMP-9, upregulation of MMP-2 was also attenuated, at least in 1K WT and 1K CBS+/− mice, with the supplementation of H2S (fig. 6). These results suggest that H2S plays a role in mitigating MMP-2 and -9 inductions during HHcy.
Fig. 6.
H2S attenuated MMP-2 and -9 expressions in the kidney. Western blot analyses were performed to measure MMP-2 and -9 in the kidney cortical tissue extract using anti-MMP-2 and anti-MMP-9 antibodies, respectively. The bar diagram indicated densitometric analyses of expressed protein. Data represents the mean ± SEM; n = 5–7 per group. ∗ p < 0.05 vs. respective 2K; ∗∗ significant differences (p <0.05) vs. respective 1K; † p < 0.05 vs. 2K WT.
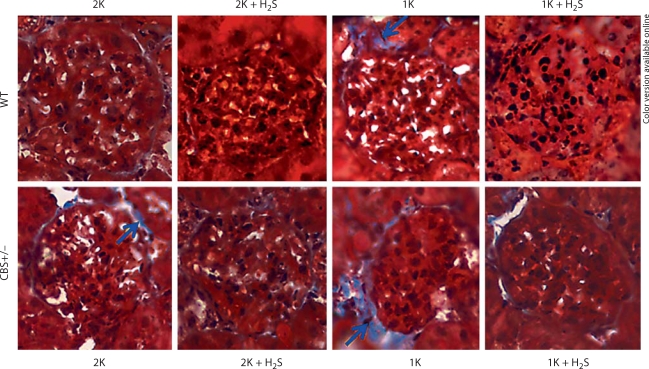
Exogenous H2S Modulated Collagen Remodeling in the Glomerulus
Collagen is one of the major components of extracellular matrix, and excessive collagen accumulation is related to the pathophysiology of tissue remodeling. Here, we sought to determine collagen deposition in the glomerulus of WT and CBS+/− mice in different experimental groups. The results, as shown in figure 7, suggest that exogenous H2S ameliorates Hcy-induced collagen deposition in the glomerulus.
Fig. 7.
Excessive collagen deposition in HHcy mice was ameliorated by H2S supplementation. Histological kidney tissue sections were stained with Masson trichrome (collagen appears as blue, and indicated by arrows). 2K CBS+/− mice showed increased collagen deposition in the glomerular basement membrane, compared to 2K WT. This collagen deposition in the glomerulus of 1K CBS+/− mice was even higher than in 2K CBS+/− mice. When a separate group of 1K CBS+/− mice was supplemented with H2S (NaHS, 30 μmol/l in drinking water) for 8 weeks postsurgery, the collagen appeared much less in the glomerulus. Notably, their 1K WT littermates had very little collagen appearance and this disappeared with NaHS supplementation (n = 7/group; ×200).
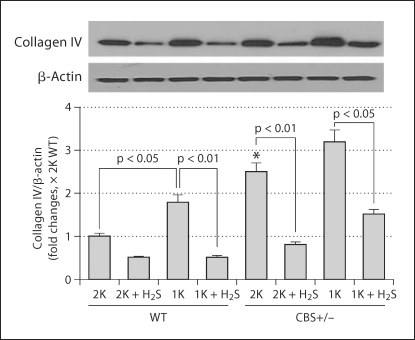
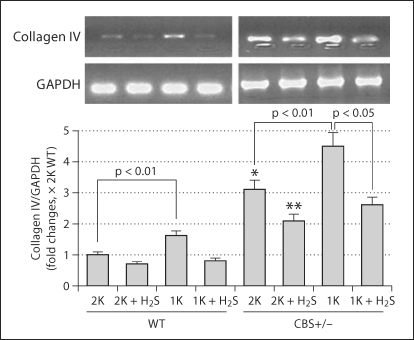
Collagen IV is a structural component of glomerular basal lamina, and excessive accumulation of collagen IV in the lamina adversely affects glomerular function. Therefore, we measured collagen IV expression in the renal cortical tissue-extracted protein. In accordance with histological collagen distribution in the kidney tissues, Western blot analyses of cortical tissues demonstrated that, although 2K CBS+/− mice had higher amounts of collagen IV expression compared to 2K WT, the presence of collagen protein in 1K CBS+/− was much higher than in 1K WT and 2K CBS+/− mice (fig. 8). H2S, however, normalized this protein expression in the renal cortical tissue (fig. 8). Previously, we reported increased renal injury in 1K CBS+/− mice as a result of elevated plasma Hcy level and Hcy-induced oxidative stress [18]. The present results confirm that this injury leads to excessive accumulation of collagen leading to glomeruloslerosis. H2S, however, ameliorates collagen expression, probably through its anti-oxidant properties.
Fig. 8.
H2S-attenuated collagen IV protein expression. Kidney cortex tissues were analyzed by Western blot using anticollagen IV antibody. While collagen expression was high in 1K WT and both 2K and 1K CBS+/− mice, this increased expression was ameliorated with H2S supplementation (NaHS, 30 μmol/l) for 8 weeks postsurgery. The bar diagram indicated densitometric analyses of the blots. Data represents the mean ± SEM; n = 5–7/group. ∗ Significant difference (p <0.05) compared to 2K WT.
Additionally, the collagen IV gene expression level was measured and normalized with internal control GAPDH (fig. 9). This suggests that expression of protein level was due to transcriptional regulation of the collagen IV gene.
Fig. 9.
Expression of collagen IV gene in the kidney cortical tissues. Renal cortical collagen protein was analyzed by Western blot. The bar diagram showed densitometric analyses of blots. Data represents the mean ± SEM, n = 5–7/group. ∗ p < 0.01 compared to WT 2K; ∗∗ p < 0.05 compared to CBS+/− 2K.
H2S Acted as an Anti-Inflammatory Substance
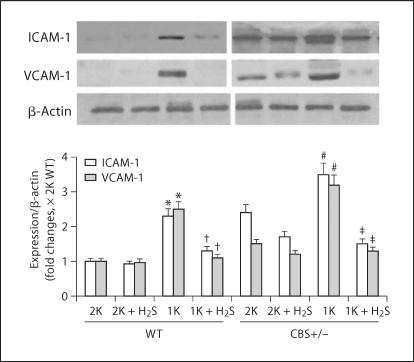
Inflammation causes upregulation of inflammatory molecules, such as ICAM-1 and VCAM-1, leading to inflammatory disorder and sclerosis. Therefore, we measured these molecules in our next experiment. As shown in the representative figure (fig. 10), increased expressions of the inflammatory molecules ICAM-1 and VCAM-1 were observed in 1K WT as well as in 2K and 1K CBS+/− mice, and were significantly higher compared to 2K WT control animals. We reported earlier that these mice were hyperhomocysteinemic with low plasma H2S levels [18]. Interestingly, exogenous supplementation of H2S in the similar groups of animals attenuated these adhesion molecule expressions (fig. 10). These results indicate that Hcy induces the inflammatory molecules ICAM-1 and VCAM-1 in the renal cortex, and H2S acts as an anti-inflammatory substance which downregulates these molecules in Hcy-induced renal cortex.
Fig. 10.
Inflammatory molecule ICAM-1 and VCAM-1 were mitigated by H2S. Representative Western blot showed ICAM-1 and VCAM-1 expressions in the kidney cortex tissue in different experimental animal groups. The bar diagram indicated densitometric analyses of expressed protein in the respective blots. Data represents the mean ± SEM, n = 5–7/group. ∗ Significant difference (p < 0.01) compared to 2K WT littermates; † p < 0.01 vs. 1K WT; # p < 0.01 vs. 2K CBS+/−; ‡ p < 0.01 vs. 1K CBS+/−.
H2S Supplementation Inhibited Macrophage Infiltration
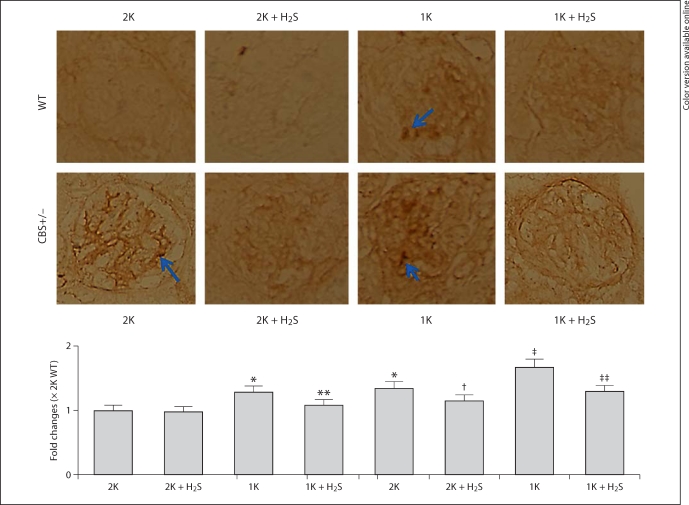
Macrophage infiltration and accumulation in the kidney is one of the most important events for the progression of glomerulosclerosis [22]. In our next experiment, we determined macrophage infiltration in the glomerulus. To do this, we immunostained kidney sections (2-μm thickness) with murine F4/80 antibody, one of the markers of macrophages. As shown in the representative images (fig. 11), increased expression of F4/80 antigen was observed in the glomerulus of 1K WT and both 2K and 1K CBS+/− mice compared to 2K WT control animals. The abundance of F4/80 antigen expression in the glomerulus was attenuated with H2S supplementation in similar groups of animals (fig. 11). These results indicate that increased plasma Hcy and a subsequent decrease in plasma H2S level induces macrophage infiltration in the glomerulus, whereas supplementation of H2S limits this infiltration associated with HHcy, thus preventing glomerulosclerosis.
Fig. 11.
Increased macrophage infiltration was mitigated by H2S. a Kidney tissue cryosections (2-μm) were immunostained with macrophage F4/80 marker and visualized with DAB (3,3′-diaminobenzidine) staining. Increased brown staining, as indicated by arrows, in 1K WT, 2K CBS+/− and 1K CBS+/− mice indicated more macrophage infiltration in the glomerulus. In the similar groups, H2S supplementation prevented this infiltration. Representative images (n = 5/group); data represent the mean ± SEM. b Bar diagram showed intensity changes of DAB staining against 2K WT glomerulus. ∗ p < 0.05 vs. 2K WT; ∗∗ p < 0.05 vs. 1K WT; †,‡ p < 0.05 vs. 2K CBS+/− and ‡‡ p < 0.05 vs. 1K CBS+/−.
Discussion
We previously reported that the decreased plasma level of H2S in WT 1K, CBS+/− 2K and CBS+/− 1K animals was strongly associated with increased plasma Hcy and that supplementation of H2S ameliorated HHcy-associated chronic renal failure [18]. Results from the present study demonstrated that abilities of renal cortical tissue to generate endogenous H2S were impaired in 1K WT mice with further disabilities in both 2K and 1K CBS+/− mice (fig. 1a). This may be due to downregulation of one of the Hcy-metabolizing enzymes, CSE (fig. 1b), in addition to one copy of mutant CBS gene in CBS+/− mice. Hcy is one of the precursors of endogenous H2S production. Therefore, an increased Hcy level and downregulation of its metabolizing enzyme will essentially decrease plasma H2S level, a potent vasorelaxant gas. This will consequently result in hypertension, as we have observed in our study (fig. 2).
Our results also suggested that supplementation of H2S resulted in normalization of blood pressure associated with HHcy (fig. 2). In addition to that, NAD(P)H oxidase (p47phoxsubunit) was upregulated in WT 1K and CBS+/− 2K and 1K mice. The increased expression of this protein was normalized with H2S supplementation, suggesting the anti-oxidant properties of H2S. Increased MMP-2 and -9, collagen protein expression, and histological collagen depositions were at least in part due to an increased oxidative-redox state. During HHcy, supplementation of H2S normalized MMPs expressions and collagen accumulation in the glomerulus, suggesting the antifibrotic properties of H2S. Increased expressions of inflammatory molecules, ICAM-1 and VCAM-1, along with increased macrophage marker protein F4/80, were augmented with the decline of H2S generation in the mouse-exhibited HHcy, whereas H2S supplementation prevented inflammation. Together, these results suggest that HHcy causes a decrease in endogenous H2S generation, which eventually leads to hypertension, oxidative stress, matrix remodeling and chronic inflammation resulting in glomerulosclerosis and reduced glomerular function (GFR; fig. 3).
Renal dysfunction is recognized as a risk factor for increased cardiovascular morbidity and mortality [29], and chronic kidney disease is a graded cardiovascular risk factor that leads from renal impairment to end-stage renal failure [30]. The kidney also plays a major role in Hcy metabolism [31] and plasma total Hcy increases with the declines of renal function [29]. Hcy, however, in the mammalian tissue can be metabolized further using three endogenous enzymes (CBS, CSE and 3MST) to produce H2S. Although H2S has been known for decades as a toxic gas with intoxication effects on the central nervous system and respiratory system, recently it has been documented as an important physiological gaseous molecule with antioxidant [13,18], antihypertensive [32], anti-inflammatory [10,16] and antifibrotic [33,34] properties.
It is important to point out that both Hcy and cysteine are substrates for H2S generation, and that the CSE enzyme mainly uses cysteine to produce H2S endogenously. Biochemically during HHcy, Hcy competes for binding to CSE with cysteine; therefore, increases in Hcy will decrease H2S production from cysteine through substrate inhibition [35,36]. The reaction occurs as follows:
Previously we reported a decreased occurrence of plasma H2S levels during HHcy [18]. In the present study we observed decreased renal production of H2S in HHcy that resulted in hypertension (fig. 2). This result indicates that above substrate inhibition, a mechanism may be playing a role in producing H2S during HHcy. Furthermore, protein homocysteinylation is a major reaction in the presence of thiolactone and homocysteinylation led to protein damage. This manifested as multimerization and precipitation of extremely modified proteins [37]. Therefore, it may also be possible that Hcy at elevated levels homocysteinylates its metabolizing enzymes, including CSE, resulting in damage and precipitation of modified CSE. Therefore, we detected low expression of CSE protein in the renal tissue (fig. 1b). Although this was not within the scope of the present study, this potential mechanism does require further experimentation.
Hcy contains a reactive sulfhydryl group and, like most thiols (RSH), can undergo oxidation to the disulfide (RSSR) at physiological pH in the presence of O2[38]. A variety of reactive oxygen species can be produced upon Hcy oxidation, including superoxide (O2–·) and hydrogen peroxide (H2O2) [39,40]. Increasing evidence suggests that reactive oxygen species play an important role in the pathophysiology of glomerular dysfunction, interstitial fibrosis and glomerulosclerosis [5,41]. Multiple enzymes contribute to exacerbating oxidative stress in different tissues and cells; however, studies have demonstrated that NAD(P)H oxidase is a major enzyme to produce superoxide (O2–·) in the kidney under physiological conditions [42]. Initially, NAD(P)H was characterized in neutrophils including membrane subunits p22phox and gp91phox and cytoplasmic subunits p47phox, p40phox, p67phox, and Rac GTPase [43,44]. It is generally accepted that in the event of NAD(P)H oxidase activation, the p47phox subunit plays a vital role [45,46]. Therefore, we tested the expression of p47phox both in in vivo and in vitro experimental conditions. Our results suggested that p47phox was upregulated in the animal groups exhibiting HHcy (fig. 4, and 5b) and associated with a decrease in renal production of H2S (fig. 1a). In an in vitro experiment using mesangial cells, we observed a similar increase of p47phox expression during HHcy, and this increased expression was normalized in the presence of H2S (fig. 5a). Thus, from our study it is very clear that in the event of HHcy, p47phox-mediated oxidative stress induces glomerular injury and H2S plays a key role in this mechanism.
It is very interesting that the current report showed p47phox was upregulated in HHcy (fig. 4), which is inconsistent with a previous report showing that p47phox was not changed in a different HHcy model [47]. This discrepancy could be due to the difference in H2S level between these two HHcy animal models. However, the discrepancy in p47phox levels between the present study and a previous report using the same type of cultured cells in vitro is puzzling [48]. The other report did not show a change in p47phox levels by Hcy in cultured renal mesangial cells [48]. One possible reason may be due to Hcy stimulation for a prolonged period of time (48 h) in the present study versus the earlier report (16 h). However, the in vivo result of the present study suggests an upregulation of p47phox in the glomerulus which is diminished by H2S treatment.
MMP-2 and -9 are collagenases with more affinity for collagen IV and have been shown to have a renoprotective role by promoting turnover and, therefore, preventing matrix accumulation [49,50]. On the contrary, elevated expressions and activities of MMP-2 and -9 have been observed in the fibrotic renal cortex; therefore, they are documented as playing an important role in matrix accumulation associated with progressive renal scarring (22, 95). MMP-2 and -9 also degrade elastin more efficiently than other MMPs [51]. Because the turnover of collagen is faster than elastin, oxidatively modified collagen (glycated collagen) is deposited faster than elastin or any other ultra-structural matrix proteins [52].
We have previously reported that an increase of MMP-2 and -9 activity was observed in the renal tissue from the animals exhibiting HHcy and lower levels of plasma H2S. In the present study we measured increased levels of MMP-2 and -9, collagen IV mRNA, and protein with the overall increase of deposition of collagen in the glomerulus of WT 1K, CBS+/− 2K and CBS+/− 1K mice. These results support our previous data [18] and suggest that MMP-2 and -9 as well as collagen remodeling are associated with HHcy-related chronic renal failure. Supplementation of H2S can prevent these remodeling processes by regulating MMPs and collagen during HHcy-associated pathogenesis in the kidney (fig. 6, 7, 8, 9).
Typically, glomerulosclerosis refers to a hardening of the glomerulus which frequently occurs due to collagen deposition. Glomerulosclerosis may be total where the entire glomerulus becomes hard and nonfunctional. Whereas partial glomerulosclerosis is often known as focal segmental glomerulosclerosis where part of the glomerulus becomes scar tissue. Early stages of glomerulosclerosis may produce little symptoms of scar tissue, which is collagen accumulation in our study (fig. 7); however, the most important warning sign is proteinuria. We have previously reported that HHcy animal groups developed proteinuria, whereas H2S treatment partially prevented urinary protein loss [18]. Our present study further confirms our previous findings that the protein loss was primarily due to focal segmental glomerulosclerosis.
The excessive production of reactive oxygen species mediates renal fibrotic injury through activation of proinflammatory molecules. Recent studies of cytokines, chemokines and adhesion molecules have enhanced our understanding of molecular mechanisms of leukocyte trafficking and their activation in the inflammatory phase of various renovascular diseases [53]. Increased expressions of ICAM-1 and VCAM-1 have been shown as a potential mechanism facilitating lymphocytic infiltration and organ dysfunction [54]. More importantly, experimental evidence has implicated sustained elevation of cell adhesion molecules, such as ICAM-1 and VCAM-1, in chronic inflammatory disorder, which leads to sclerosis [20]. HHcy has now been recognized as a pathophysiological stimulus of vascular endothelial dysfunction and has been shown to increase expression of ICAM-1 [19] and VCAM-1 [19,21] in experimental models. However, an Hcy-induced inflammatory reaction in the glomerulus during renal diseases was clearly not defined. The present study further supports our previous findings [18] and demonstrates that macrophage infiltration and increased expression of ICAM-1 and VCAM-1 (fig. 10, 11) are associated with HHcy. These events are partially, if not completely, preventable by H2S treatment, suggesting the possible protective role of H2S through its antioxidant properties during HHcy-associated renal inflammation and remodeling. However, the relationship between oxidant stress and inflammation, i.e. which is the upstream effector in HHcy, was not adequately addressed in the present study because inflammation has also been shown to cause oxidant stress. Blocking either pathway may clarify this question. Nevertheless, the present results reveal that oxidative stress is one of the, if not only, upstream effectors of HHcy.
In summary, we demonstrated that increased oxidative stress in HHcy-associated renal cortical tissue is due to, in part, reduced renal H2S production and upregulation of the p47phox subunit of NAD(P)H oxidase. These lead to MMPs and collagen remodeling in the glomerulus along with the increase in inflammatory response resulting in glomerulosclerosis, reduced glomerular function and hypertension. These structural and functional changes are associated with HHcy and are preventable by H2S supplementation.
Acknowledgement
This study was supported, in part, by NIH grants HL-71010, HL-74185, HL-88012 and NS-51568.
References
- 1.Lentz SR. Mechanisms of homocysteine-induced atherothrombosis. J Thromb Haemost. 2005;3:1646–1654. doi: 10.1111/j.1538-7836.2005.01364.x. [DOI] [PubMed] [Google Scholar]
- 2.Dayal S, Bottiglieri T, Arning E, Maeda N, Malinow MR, Sigmund CD, Heistad DD, Faraci FM, Lentz SR. Endothelial dysfunction and elevation of S-adenosylhomocysteine in cystathionine beta-synthase-deficient mice. Circ Res. 2001;88:1203–1209. doi: 10.1161/hh1101.092180. [DOI] [PubMed] [Google Scholar]
- 3.Diez N, Perez R, Hurtado V, Santidrian S. Hyperhomocysteinaemia induced by dietary folate restriction causes kidney oxidative stress in rats. Br J Nutr. 2005;94:204–210. doi: 10.1079/bjn20051468. [DOI] [PubMed] [Google Scholar]
- 4.Zhang F, Siow YL, O K. Hyperhomocysteinemia activates NF-kappaB and inducible nitric oxide synthase in the kidney. Kidney Int. 2004;65:1327–1338. doi: 10.1111/j.1523-1755.2004.00510.x. [DOI] [PubMed] [Google Scholar]
- 5.Yi F, Li PL. Mechanisms of homocysteine-induced glomerular injury and sclerosis. Am J Nephrol. 2008;28:254–264. doi: 10.1159/000110876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shibuya N, Mikami Y, Kimura Y, Nagahara N, Kimura H. Vascular endothelium expresses 3-mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. J Biochem. 2009;146:623–626. doi: 10.1093/jb/mvp111. [DOI] [PubMed] [Google Scholar]
- 7.Shibuya N, Tanaka M, Yoshida M, Ogasawara Y, Togawa T, Ishii K, Kimura H. 3-mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid Redox Signal. 2009;11:703–714. doi: 10.1089/ars.2008.2253. [DOI] [PubMed] [Google Scholar]
- 8.Geng B, Yang J, Qi Y, Zhao J, Pang Y, Du J, Tang C. H2S generated by heart in rat and its effects on cardiac function. Biochem Biophys Res Commun. 2004;313:362–368. doi: 10.1016/j.bbrc.2003.11.130. [DOI] [PubMed] [Google Scholar]
- 9.Swaroop M, Bradley K, Ohura T, Tahara T, Roper MD, Rosenberg LE, Kraus JP. Rat cystathionine beta-synthase. Gene organization and alternative splicing. J Biol Chem. 1992;267:11455–11461. [PubMed] [Google Scholar]
- 10.Lowicka E, Beltowski J. Hydrogen sulfide (H2S) – the third gas of interest for pharmacologists. Pharmacol Rep. 2007;59:4–24. [PubMed] [Google Scholar]
- 11.Kimura Y, Dargusch R, Schubert D, Kimura H. Hydrogen sulfide protects HT22 neuronal cells from oxidative stress. Antioxid Redox Signal. 2006;8:661–670. doi: 10.1089/ars.2006.8.661. [DOI] [PubMed] [Google Scholar]
- 12.Yonezawa D, Sekiguchi F, Miyamoto M, Taniguchi E, Honjo M, Masuko T, Nishikawa H, Kawabata A. A protective role of hydrogen sulfide against oxidative stress in rat gastric mucosal epithelium. Toxicology. 2007;241:11–18. doi: 10.1016/j.tox.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 13.Wei HL, Zhang CY, Jin HF, Tang CS, Du JB. Hydrogen sulfide regulates lung tissue-oxidized glutathione and total antioxidant capacity in hypoxic pulmonary hypertensive rats. Acta Pharmacol Sin. 2008;29:670–679. doi: 10.1111/j.1745-7254.2008.00796.x. [DOI] [PubMed] [Google Scholar]
- 14.Fu Z, Liu X, Geng B, Fang L, Tang C. Hydrogen sulfide protects rat lung from ischemia-reperfusion injury. Life Sci. 2008;82:1196–1202. doi: 10.1016/j.lfs.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Zanardo RC, Brancaleone V, Distrutti E, Fiorucci S, Cirino G, Wallace JL. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J. 2006;20:2118–2120. doi: 10.1096/fj.06-6270fje. [DOI] [PubMed] [Google Scholar]
- 16.Hu LF, Wong PT, Moore PK, Bian JS. Hydrogen sulfide attenuates lipopolysaccharide-induced inflammation by inhibition of p38 mitogen-activated protein kinase in microglia. J Neurochem. 2007;100:1121–1128. doi: 10.1111/j.1471-4159.2006.04283.x. [DOI] [PubMed] [Google Scholar]
- 17.Rao VH, Lees GE, Kashtan CE, Nemori R, Singh RK, Meehan DT, Rodgers K, Berridge BR, Bhattacharya G, Cosgrove D. Increased expression of MMP-2, MMP-9 (type IV collagenases/gelatinases), and MT1-MMP in canine X-linked Alport syndrome (XLAS) Kidney Int. 2003;63:1736–1748. doi: 10.1046/j.1523-1755.2003.00939.x. [DOI] [PubMed] [Google Scholar]
- 18.Sen U, Basu P, Abe OA, Givvimani S, Tyagi N, Metreveli N, Shah KS, Passmore JC, Tyagi SC. Hydrogen sulfide ameliorates hyperhomocysteinemia-associated chronic renal failure. Am J Physiol Renal Physiol. 2009;297:F410–F419. doi: 10.1152/ajprenal.00145.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sen U, Tyagi N, Kumar M, Moshal KS, Rodriguez WE, Tyagi SC. Cystathionine-beta-synthase gene transfer and 3-deazaadenosine ameliorate inflammatory response in endothelial cells. Am J Physiol Cell Physiol. 2007;293:C1779–C1787. doi: 10.1152/ajpcell.00207.2007. [DOI] [PubMed] [Google Scholar]
- 20.Silverman MD, Tumuluri RJ, Davis M, Lopez G, Rosenbaum JT, Lelkes PI. Homocysteine upregulates vascular cell adhesion molecule-1 expression in cultured human aortic endothelial cells and enhances monocyte adhesion. Arterioscler Thromb Vasc Biol. 2002;22:587–592. doi: 10.1161/01.atv.0000014221.30108.08. [DOI] [PubMed] [Google Scholar]
- 21.Hofmann MA, Lalla E, Lu Y, Gleason MR, Wolf BM, Tanji N, Ferran LJ, Jr, Kohl B, Rao V, Kisiel W, Stern DM, Schmidt AM. Hyperhomocysteinemia enhances vascular inflammation and accelerates atherosclerosis in a murine model. J Clin Invest. 2001;107:675–683. doi: 10.1172/JCI10588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kikuchi Y, Imakiire T, Yamada M, Saigusa T, Hyodo T, Hyodo N, Suzuki S, Miura S. Mizoribine reduces renal injury and macrophage infiltration in non-insulin-dependent diabetic rats. Nephrol Dial Transplant. 2005;20:1573–1581. doi: 10.1093/ndt/gfh888. [DOI] [PubMed] [Google Scholar]
- 23.Richardson CJ, Magee EA, Cummings JH. A new method for the determination of sulphide in gastrointestinal contents and whole blood by microdistillation and ion chromatography. Clin Chim Acta. 2000;293:115–125. doi: 10.1016/s0009-8981(99)00245-4. [DOI] [PubMed] [Google Scholar]
- 24.Li L, Bhatia M, Zhu YZ, Zhu YC, Ramnath RD, Wang ZJ, Anuar FB, Whiteman M, Salto-Tellez M, Moore PK. Hydrogen sulfide is a novel mediator of lipopolysaccharide-induced inflammation in the mouse. FASEB J. 2005;19:1196–1198. doi: 10.1096/fj.04-3583fje. [DOI] [PubMed] [Google Scholar]
- 25.Maezawa Y, Yokote K, Sonezaki K, Fujimoto M, Kobayashi K, Kawamura H, Tokuyama T, Takemoto M, Ueda S, Kuwaki T, Mori S, Wahren J, Saito Y. Influence of C-peptide on early glomerular changes in diabetic mice. Diabetes Metab Res Rev. 2006;22:313–322. doi: 10.1002/dmrr.612. [DOI] [PubMed] [Google Scholar]
- 26.Lorenz JN, Gruenstein E. A simple, nonradioactive method for evaluating single-nephron filtration rate using FITC-inulin. Am J Physiol. 1999;276:F172–F177. doi: 10.1152/ajprenal.1999.276.1.F172. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez WE, Tyagi N, Joshua IG, Passmore JC, Fleming JT, Falcone JC, Tyagi SC. Pioglitazone mitigates renal glomerular vascular changes in high-fat, high-calorie-induced type 2 diabetes mellitus. Am J Physiol Renal Physiol. 2006;291:F694–F701. doi: 10.1152/ajprenal.00398.2005. [DOI] [PubMed] [Google Scholar]
- 28.Li N, Chen YF, Zou AP. Implications of hyperhomocysteinemia in glomerular sclerosis in hypertension. Hypertension. 2002;39:443–448. doi: 10.1161/hy02t2.102992. [DOI] [PubMed] [Google Scholar]
- 29.Rodionov RN, Lentz SR. The homocysteine paradox. Arterioscler Thromb Vasc Biol. 2008;28:1031–1033. doi: 10.1161/ATVBAHA.108.164830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schiffrin EL, Lipman ML, Mann JF. Chronic kidney disease: effects on the cardiovascular system. Circulation. 2007;116:85–97. doi: 10.1161/CIRCULATIONAHA.106.678342. [DOI] [PubMed] [Google Scholar]
- 31.Friedman AN, Bostom AG, Selhub J, Levey AS, Rosenberg IH. The kidney and homocysteine metabolism. J Am Soc Nephrol. 2001;12:2181–2189. doi: 10.1681/ASN.V12102181. [DOI] [PubMed] [Google Scholar]
- 32.Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, Snyder SH, Wang R. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fang L, Li H, Tang C, Geng B, Qi Y, Liu X. Hydrogen sulfide attenuates the pathogenesis of pulmonary fibrosis induced by bleomycin in rats. Can J Physiol Pharmacol. 2009;87:531–538. doi: 10.1139/y09-039. [DOI] [PubMed] [Google Scholar]
- 34.Mishra PK, Tyagi N, Sen U, Givvimani S, Tyagi SC. H2S ameliorates oxidative and proteolytic stresses and protects the heart against adverse remodeling in chronic heart failure. Am J Physiol Heart Circ Physiol. 2010;298:H451–H456. doi: 10.1152/ajpheart.00682.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stabler SP, Steegborn C, Wahl MC, Oliveriusova J, Kraus JP, Allen RH, Wagner C, Mudd SH. Elevated plasma total homocysteine in severe methionine adenosyltransferase I/III deficiency. Metabolism. 2002;51:981–988. doi: 10.1053/meta.2002.34017. [DOI] [PubMed] [Google Scholar]
- 36.Chang L, Geng B, Yu F, Zhao J, Jiang H, Du J, Tang C. Hydrogen sulfide inhibits myocardial injury induced by homocysteine in rats. Amino Acids. 2008;34:573–585. doi: 10.1007/s00726-007-0011-8. [DOI] [PubMed] [Google Scholar]
- 37.Jakubowski H. Protein homocysteinylation: possible mechanism underlying pathological consequences of elevated homocysteine levels. FASEB J. 1999;13:2277–2283. [PubMed] [Google Scholar]
- 38.Jacobsen DW. Hyperhomocysteinemia and oxidative stress: Time for a reality check? Arterioscler Thromb Vasc Biol. 2000;20:1182–1184. doi: 10.1161/01.atv.20.5.1182. [DOI] [PubMed] [Google Scholar]
- 39.Jacobsen DW, Troxell LS, Brown KL. Catalysis of thiol oxidation by cobalamins and cobinamides – reaction-products and kinetics. Biochemistry. 1984;23:2017–2025. [Google Scholar]
- 40.Misra HP. Generation of superoxide free radical during the autoxidation of thiols. J Biol Chem. 1974;249:2151–2155. [PubMed] [Google Scholar]
- 41.Brezniceanu ML, Liu F, Wei CC, Chenier I, Godin N, Zhang SL, Filep JG, Ingelfinger JR, Chan JS. Attenuation of interstitial fibrosis and tubular apoptosis in db/db transgenic mice overexpressing catalase in renal proximal tubular cells. Diabetes. 2008;57:451–459. doi: 10.2337/db07-0013. [DOI] [PubMed] [Google Scholar]
- 42.Griendling KK, Sorescu D, Lassegue B, Ushio-Fukai M. Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology. Arterioscler Thromb Vasc Biol. 2000;20:2175–2183. doi: 10.1161/01.atv.20.10.2175. [DOI] [PubMed] [Google Scholar]
- 43.Brandes RP, Kreuzer J. Vascular NADPH oxidases: molecular mechanisms of activation. Cardiovasc Res. 2005;65:16–27. doi: 10.1016/j.cardiores.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 44.Takeya R, Ueno N, Kami K, Taura M, Kohjima M, Izaki T, Nunoi H, Sumimoto H. Novel human homologues of p47phox and p67phox participate in activation of superoxide-producing NADPH oxidases. J Biol Chem. 2003;278:25234–25246. doi: 10.1074/jbc.M212856200. [DOI] [PubMed] [Google Scholar]
- 45.Madamanchi NR, Vendrov A, Runge MS. Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol. 2005;25:29–38. doi: 10.1161/01.ATV.0000150649.39934.13. [DOI] [PubMed] [Google Scholar]
- 46.Sellmayer A, Obermeier H, Danesch U, Aepfelbacher M, Weber PC. Arachidonic acid increases activation of NADPH oxidase in monocytic U937 cells by accelerated translocation of p47-phox and co-stimulation of protein kinase C. Cell Signal. 1996;8:397–402. doi: 10.1016/0898-6568(96)00077-0. [DOI] [PubMed] [Google Scholar]
- 47.Ungvari Z, Csiszar A, Edwards JG, Kaminski PM, Wolin MS, Kaley G, Koller A. Increased superoxide production in coronary arteries in hyperhomocysteinemia: role of tumor necrosis factor-alpha, NAD(P)H oxidase, and inducible nitric oxide synthase. Arterioscler Thromb Vasc Biol. 2003;23:418–424. doi: 10.1161/01.ATV.0000061735.85377.40. [DOI] [PubMed] [Google Scholar]
- 48.Yi F, Zhang AY, Janscha JL, Li PL, Zou AP. Homocysteine activates NADH/NADPH oxidase through ceramide-stimulated Rac GTPase activity in rat mesangial cells. Kidney Int. 2004;66:1977–1987. doi: 10.1111/j.1523-1755.2004.00968.x. [DOI] [PubMed] [Google Scholar]
- 49.Endo T, Nakabayashi K, Sekiuchi M, Kuroda T, Soejima A, Yamada A. Matrix metalloproteinase-2, matrix metalloproteinase-9, and tissue inhibitor of metalloproteinase-1 in the peripheral blood of patients with various glomerular diseases and their implication in pathogenetic lesions: study based on an enzyme-linked assay and immunohistochemical staining. Clin Exp Nephrol. 2006;10:253–261. doi: 10.1007/s10157-006-0438-3. [DOI] [PubMed] [Google Scholar]
- 50.Rysz J, Banach M, Stolarek RA, Pasnik J, Cialkowska-Rysz A, Koktysz R, Piechota M, Baj Z. Serum matrix metalloproteinases MMP-2 and MMP-9 and metalloproteinase tissue inhibitors TIMP-1 and TIMP-2 in diabetic nephropathy. J Nephrol. 2007;20:444–452. [PubMed] [Google Scholar]
- 51.Senior RM, Griffin GL, Fliszar CJ, Shapiro SD, Goldberg GI, Welgus HG. Human 92- and 72-kilodalton type IV collagenases are elastases. J Biol Chem. 1991;266:7870–7875. [PubMed] [Google Scholar]
- 52.Camp TM, Tyagi SC, Senior RM, Hayden MR. Gelatinase B(MMP-9) an apoptotic factor in diabetic transgenic mice. Diabetologia. 2003;46:1438–1445. doi: 10.1007/s00125-003-1200-y. [DOI] [PubMed] [Google Scholar]
- 53.Bonventre JV. Pathophysiology of acute kidney injury: roles of potential inhibitors of inflammation. Contrib Nephrol. 2007;156:39–46. doi: 10.1159/000102069. [DOI] [PubMed] [Google Scholar]
- 54.Anderson JA, Lentsch AB, Hadjiminas DJ, Miller FN, Martin AW, Nakagawa K, Edwards MJ. The role of cytokines, adhesion molecules, and chemokines in interleukin-2-induced lymphocytic infiltration in C57BL/6 mice. J Clin Invest. 1996;97:1952–1959. doi: 10.1172/JCI118627. [DOI] [PMC free article] [PubMed] [Google Scholar]