Abstract
In vitro bioaccessibility (IVBA) studies were carried out on samples of mercury (Hg) mine-waste calcine (roasted Hg ore) by leaching with simulated human body fluids. The objective was to estimate potential human exposure to Hg due to inhalation of airborne calcine particulates and hand-to-mouth ingestion of Hg-bearing calcines. Mine waste calcines collected from Hg mines at Almadén, Spain, and Terlingua, Texas, contain Hg sulfide, elemental Hg, and soluble Hg compounds, which constitute primary ore or compounds formed during Hg retorting. Elevated leachate Hg concentrations were found during calcine leaching using a simulated gastric fluid (as much as 6200 μg of Hg leached/g sample). Elevated Hg concentrations were also found in calcine leachates using a simulated lung fluid (as much as 9200 μg of Hg leached/g), serum-based fluid (as much as 1600 μg of Hg leached/g), and water of pH 5 (as much as 880 μg of Hg leached/g). The leaching capacity of Hg is controlled by calcine mineralogy; thus, calcines containing soluble Hg compounds contain higher leachate Hg concentrations. Results indicate that ingestion or inhalation of Hg mine-waste calcine may lead to increased Hg concentrations in the human body, especially through the ingestion pathway.
Short abstract
In vitro studies were performed to estimate potential human exposure to mercury by leaching of mercury mine waste with simulated human body fluids.
Introduction
Mercury is a heavy metal of environmental concern because elevated concentrations can be toxic to all living organisms. Mercury has no known biological function, and human exposure to Hg is considered undesirable and potentially hazardous (1,2). Generally, humans and organisms do not easily eliminate Hg, and as a result, Hg tends to concentrate in their tissues (bioaccumulation). When ecosystems are exposed to high Hg concentrations, the highest Hg concentrations are generally found in the highest trophic levels of the food chain, a process called biomagnification. High concentrations of Hg in humans adversely affect the central nervous system, especially the sensory, visual, and auditory parts that affect coordination (3). In extreme cases, Hg poisoning can lead to death (1,2,4). For all organisms, the early stages of development (especially embryos) are the most sensitive to Hg (4,5).
Consumption of fish and fish products is the primary pathway of Hg uptake in humans (3). However, human exposure to Hg through inhalation and ingestion of particulates originating from Hg mines has not been well studied, although Hg contamination originating from gold mine tailings has been evaluated (6−8). Mine wastes in areas of past Hg mining are especially problematic because these wastes contain highly elevated concentrations of Hg (9−11). The dominant form of Hg ore worldwide is cinnabar (HgS, hexagonal); however, during retorting, cinnabar ore is converted to elemental Hg (Hg0), which is the final product of Hg mining (10). Mine waste calcines found at Hg mines are known to contain unconverted cinnabar, but in addition, calcines contain minor metacinnabar (HgS, isometric, metastable relative to cinnabar), elemental Hg°, and highly reactive Hg compounds such as Hg chlorides (mercurous and mercuric), oxides, oxychlorides, and sulfates, which are formed during ore retorting (11−14).
Various studies of mine runoff water and laboratory experiments indicate that Hg mine wastes have the capacity to release (leach) significant concentrations of Hg into watersheds downstream from Hg mines (9,11,15−18). Leaching experiments of Hg mine wastes using water to simulate storm runoff conditions have shown a high potential to release Hg into aquatic systems surrounding Hg mines (15). Previous research has reported bioaccessibility of Hg using in vitro studies of soil (6,19,20), but little is known about the release of Hg into human body fluids during ingestion or inhalation of Hg-bearing mine-waste calcines that are highly enriched in Hg. Throughout the world, mine-waste calcine has been used in road construction for many years, and in addition, recreational areas, homes, farms, and gardens are built on top of calcines or abandoned Hg mines, and towns are on, or are in near proximity to, abandoned Hg mines (9,10,14,15)—all of these practices and situations potentially lead to human ingestion and inhalation of calcine particulates.
The objective of this study was to evaluate leaching of Hg and Hg bioaccessibility when samples of Hg mine-waste calcine were leached with (1) a simulated human gastric fluid, (2) a simulated human lung fluid, (3) a protein-enriched serum-based fluid (RPMI-1640 with fetal bovine serum, developed by Roswell Park Memorial Institute, used for the culture of human normal and neoplastic leukocytes), and (4) deionized water acidified to pH 5.0. Resultant leachates were analyzed for concentrations of Hg. In addition, in vitro cell line experiments were carried out to evaluate effects on cultures of living cells (cell lines) when they are exposed to contaminants, such as high concentrations of Hg. Calcines used in this study were collected from two sites (a) Almadén, Spain, (38° 47′, 4° 51′), and (b) Terlingua, Texas (29° 19′, 103° 37′).
Study Areas
Samples of mine-waste calcine were collected from Almadén, Spain, the world’s largest Hg mining district, which has produced over 286 000 000 kg of Hg during more than 2000 years of mining (21). Production from Almadén represents more than 30% of the total known Hg produced worldwide (22,23). Mining activity at the Almadén mine ceased in May 2002, but significant piles of mine-waste calcine remain throughout the Almadén district.
Mine waste calcine was also collected from Hg mines of the Terlingua district, Texas. Mercury was mined in this area from 1888−1973 (24,25), but these mines are now inactive. Total production from this region was >5 000 000 kg of Hg (24). Consequently, the Terlingua district ranks as the third largest Hg producing region in the United States, and only Hg mines in the California Coast Ranges (120 000 000 kg of Hg) and McDermitt, Nevada (10 000 000 kg of Hg) are larger (10,26). The Terlingua district includes over 30 separate mines (27), but our work focused on the Mariscal, Study Butte, Mariposa, and Terlingua (also known as the Chisos mine) mines.
Similar to most Hg mines worldwide, Hg ore in the Almadén and Terlingua districts was dominantly cinnabar, but minor Hg minerals including metacinnabar, elemental Hg0, and Hg chlorides and oxychlorides were identified at some mines (24,27). Elemental Hg0 is locally abundant in ore at some localities, especially in the Almadén district (28). As a result of the Hg ore retorting process, most Hg-bearing ore was converted to Hg0. In addition, highly reactive, water-soluble ionic Hg compounds such as calomel (Hg2Cl2) and Hg oxychlorides (i.e., Hg2ClO or Hg4Cl2O), formed during Hg retorting, have been observed in calcine at various Hg mines worldwide (12,18).
Experimental Section
Sample Collection and Preparation
Samples of mine-waste calcine were collected from various mined sites in the Almadén and Terlingua Hg mining districts. The calcine samples used in this study were collected and archived from previous studies (10,28). All calcine samples were stored in glass vessels with Teflon-lined lids and frozen until analysis. Prior to geochemical analysis, the calcine samples were air-dried at room temperature and sieved to minus-100-mesh (0.150 μm).
Simulated Gastric Solution
A simulated gastric solution was made containing 0.4 M glycine (free base, reagent grade glycine in deionized water), adjusted to pH 1.5 ± 0.05 using HCl (trace metal grade concentrated HCl) following the procedure developed for IVBA studies of Pb in soil (29). A solid-to-fluid ratio of 1:100 (w/v) was used for leaching by weighing 0.2 ± 0.0005 g of mine-waste calcine into a 20-mL acid-washed glass scintillation vial and adding 20 mL of the gastric solution. The samples were then placed into a Vitron dynamic organ culture incubator for 1 h at 37 °C, which rotated the samples slowly at 2.5 rpm. Following incubation, the samples were allowed to settle briefly, and then the leachate was filtered with a 0.45 μm nitrocellulose syringe filter to obtain samples for Hg analysis.
Simulated Lung, Serum-Based, and Deionized Water Solutions
The simulated lung fluid used in leaching of samples of mine-waste calcine was a modified Gamble’s solution adapted from previous studies (30−33). This solution was designed to model interactions of particles with extracellular lung fluids. The simulated lung fluid contained NaCl (6800 mg/L), NH4Cl (5300 mg/L), NaHCO3 (2300 mg/L), H3PO4 (1200 mg/L), NaH2PO4·H20 (1700 mg/L), Na2CO3 (630 mg/L), Na-acetate (580 mg/L), K-acid-phthalate (200 mg/L), glycine (450 mg/L), H2SO4 (510 mg/L), Na3-citrate·2H2O (590 mg/L), CaCl2·2H2O (290 mg/L), and citric acid·H2O (420 mg/L), which was adjusted to pH 7.4 using HCl.
A modified serum-based (RPMI-1640) fluid was also used in this study to provide an indication of the interaction of the mine-waste calcines with the protein-enriched solution (used as a dosing medium for the cell line analysis discussed in the next section). The modified RPMI-1640 solution contained glucose (4500 mg/L), HEPES (2400 mg/L), NaHCO3 (1500 g/L), Na-pyruvate (110 mg/L), l-glutamine (290 mg/L), penicillin/streptomycin (5 mL), and fetal bovine serum (50 mL).
Deionized water leaching studies were conducted on samples of mine-waste calcine using a procedure modified from EPA Method 1312 (34). Deionized water was adjusted to pH 5.0 using ultrapure H2SO4/HNO3, which is the pH recommended to simulate rainwater in the western United States (34).
All solutions were made fresh daily before leaching and analysis. A solid-to-fluid ratio of 1:20 (w/v) was used for the simulated lung and serum-based fluids and deionized water leach studies by weighing 1.00 ± 0.05 g of mine-waste calcine into a 30 mL scintillation vial (prewashed in 10% HCl) equipped with a polyethylene mesh insert, and 20 mL of solution was added. The vials were then placed into a Vitron Dynamic Organ Culture Incubator for 24 h at 37 °C, which rotated the samples at 2.5 rpm. Following incubation, the vials were centrifuged for 5 min at 15 000 rpm and the leachate was filtered with a 0.45 μm nitrocellulose syringe filter. The final leachate pH was measured using an Orion pH meter on a small aliquot of all samples. In addition to the 1-h gastric fluid and 24-h water leach experiments, timed experiments were carried out at 1/2, 1, 4, and 24 h using water and gastric fluids.
Cell Line Analysis
In vitro cell analysis was used to evaluate the potential toxicity effects of the Hg-rich calcines on cultures of living cells, with counts of viable cells following exposure compared to control used as the indicator of toxicity. A transformed rat RLE-6TN cell line (35) (American Tissue Cell Company, Manassas, VA) was used for the analysis. Rat cell lines are widely used in cell viability studies due to their relatively low cost, their similarity to human lung cells, and their successful use in various applications (36). Cells were cultured in 12-well plates (Fisher Scientific, Pittsburgh, PA) at a density of 105 cells/mL. The cells were maintained in a specialty medium for rat lung epithelial cells (BRFF-RLuE culture media, BRFF, Ijamsville, MD) containing 10% fetal bovine serum and 1× penicillin/streptomycin antibiotics (Sigma, St. Louis, MO). Cell media were replenished every 24−30 h until 95% confidence was achieved. Cells were then incubated with 40 μg particle weight/mL media (following methods used in asbestos research) (37) or controls for 24 h at which time the media were removed. Cell viability was determined by the trypan blue exclusion assay (38). Each sample was quantified in triplicate and averaged to obtain the final cell viability number (Supporting Information, Table S2). These studies were conducted at the University of Arizona Steele Memorial Children’s Research Center (Tucson, AZ).
Mercury Analysis
The concentration of Hg was determined in the calcine samples using an aqua-regia digestion and cold-vapor atomic fluorescence spectrometry (CVAFS) following EPA method 1631 (39,40). Quality control for Hg analysis was established using method blanks, blank spikes, matrix spikes, standard reference materials (SRMs), and sample duplicates. Recoveries for Hg on blank and matrix spikes were 93−121%. The relative percent difference for Hg in calcine sample duplicates was ≤18%. For SRMs, NIST 2704 and PACS-2 analyzed in this study, recoveries ranged from 96% to 107% of certified concentrations for Hg. Method blanks were below the limits of determination for Hg. The lower limit of determination for Hg in the calcines was 0.002 μg/g.
Concentrations of Hg were determined in the simulated body fluid leachates of calcines using cold-vapor atomic fluorescence spectrometry (CVAFS) following EPA method 1631e (41). Recoveries for Hg on blank and matrix spikes were 95−121%. The relative percent difference of Hg in sample duplicates varied from 4% to 19% in the gastric fluid, 4% to 12% in the lung fluid, 1% to 21% in the serum-based fluid, and 1% to 16% in water leachates. Concentrations of Hg in blanks of the simulated gastric fluid, lung fluid, serum-based fluid, and water leachates were below the lower limit of determination. The lower limit of determination for Hg was 0.01 μg/L in the simulated gastric fluid and 0.05 μg/L in the lung fluid, serum-based fluid, and water leachates.
Mineralogical Studies
Scanning electron microscopy (SEM), electron microprobe (EMP), and X-ray diffraction (XRD) analysis were used.
The SEM analysis was performed using a JEOL 5800LV instrument at the U.S. Geological Survey (Denver, CO), operating in high-vacuum mode and equipped with an energy-dispersive X-ray spectroscopy. Data reduction was performed using the Oxford ISIS standardless analysis package using automated correction procedures known as ZAF corrections. Analyses were normalized to 100%. Analysis using SEM is qualitative, and thus only the presence of certain minerals were identified in this study (Supporting Information, Table S1).
The EMP was carried out on various Hg-mineral grains in epoxy-impregnated, polished thin sections using an automated JEOL 8900 electron microprobe with five scannable wavelength-dispersive crystal spectrometers. Calibration was performed using well-characterized Hg-mineral standards. Analytical precision based on replicate analysis of standards was better than ±2% relative concentrations for major and minor elements and equal to counting statistics for trace (<1 wt %) elements.
The dominant mineralogy was determined by XRD on powdered, hand-ground samples of mine-waste calcine using a Philips APD 3720 automated X-ray diffractometer. The collected data were evaluated and minerals identified using JADE+ software (Materials Data Inc.). Semiquantitative mineralogy was determined for each sample as using general % ranges (>25%, 5−25%, <5%, and <1%).
Results and Discussion
Analysis using XRD and SEM indicated that the calcine samples are predominantly composed (5−25%) of quartz (SiO2), calcite (CaCO3), hematite (Fe2O3), gypsum (CaSO4·2H2O), muscovite (KAl2(AlSi3)O10(OH)2), kaolinite (Al2Si2O5(OH)4), jarosite (KFe3(OH)6(SO4)2), and potassium feldspar (KAlSi3O8) (Supporting Information, Table S1). These data are consistent with field observations of calcines at various mines in the Almadén and Terlingua districts (28,42). However, more important are various Hg compounds found in the calcines because such compounds largely determine leaching of Hg and Hg bioaccessibility in water and the simulated human body fluids used in this study. The XRD, EMP, and SEM analyses identified several Hg compounds in Almadén calcine samples including up to 1% cinnabar (HgS, hexagonal), metacinnabar (HgS, isometric), calomel (Hg2Cl2), and corderoite (Hg3S2Cl2), as well as the presence of some montroydite (HgO), kleinite (Hg2N(Cl,SO4)·n(H2O)), terlinguaite (Hg2ClO), and gianellaite (Hg4(SO4)N2 (Supporting Information, Table S1). Calcines collected from the Terlingua district generally contained fewer identified Hg minerals in lower abundance (compared to Almadén samples), and only cinnabar was found to be present up to 1% in four of eight samples, whereas the presence of metacinnabar, elemental Hg0, corderoite, kleinite, and terlinguaite was observed by SEM in a few samples (Supporting Information, Table S1). The presence of these Hg compounds in calcines from Almadén and Terlingua is consistent with previous studies using edge-extended X-ray absorption fine structure analyses that have reported the presence of several Hg oxides, chlorides, oxychlorides, and sulfates (43,44). The presence of such Hg compounds in mine-waste calcine collected in this study is important even in low abundance because many Hg-bearing chlorides, oxychlorides, and sulfates are highly toxic to humans and are soluble in circum-neutral and weakly acidic water, whereas cinnabar, metacinnabar, and elemental Hg0 are not.
Several IVBA studies have evaluated bioaccessibility of Hg in soil, which has been reported to vary from 0.3% to 46% (6,19,20,45). However, there are no known IVBA studies that have attempted to evaluate Hg bioaccessibility or leaching in calcine collected from Hg mines such as those shown here. The percent of Hg bioacessibility is calculated similar to that as previously reported (6):
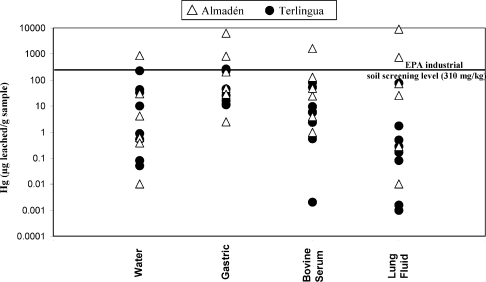
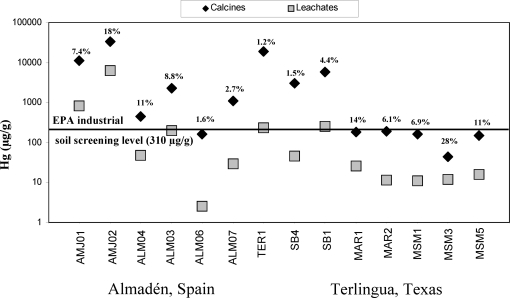
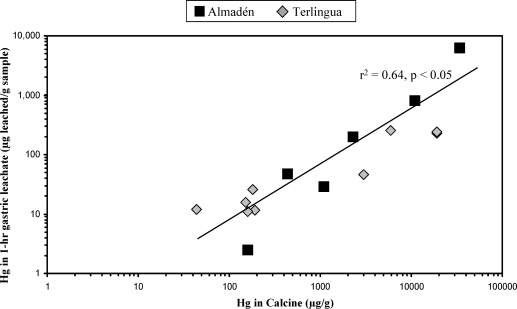
Of the leaching fluids used in these in vitro studies (water, gastric fluid, serum-based fluid, and lung fluid), simulated gastric fluids generally leached the most Hg from the calcines (Figure 1). Results indicate that bioaccessibility in gastric fluid varies from 1.2% to 28% during leaching of 1 h (Figure 2). These gastric results are generally consistent with previously published Hg bioaccessibility in soil, which has been reported to range from 0.3 to 46% (6,19,20). Whereas the % of Hg bioaccessibility is significant when calcine samples are leached with simulated gastric fluids, a more important measurement from a potential human exposure perspective is the amount of Hg leached (in μg of Hg leached/g of calcine sample). Concentrations of Hg varied from 2.5 to 6200 μg/g in the 1-h gastric leachate of the calcine samples (Table 1, Figure 2). Concentrations of Hg for the majority of the calcine leachates obtained during the 1-h gastric leaching experiments exceeded both the residential (23 μg/g) and industrial (310 μg/g) screening level for inorganic Hg in soil established by the USEPA (46), but the industrial screening level is more applicable here, as this study was of industrial (mine) wastes, not residential soil. All gastric leachate Hg concentrations exceeded the probable effect Hg concentration of 1.06 μg/g, above which harmful effects are likely to be observed in sediment-dwelling organisms (47). In addition, there is a significant correlation of Hg in calcines with Hg in 1-h gastric fluid leachates (Figure 3, r2 = 0.64). Highly elevated Hg concentrations in the in vitro gastric experiments are due to the presence of soluble Hg compounds, which were identified in the calcines by SEM and XRD analysis (Supporting Information, Table S1, Figure 4). Such soluble byproduct Hg compounds, which are known to have formed during ore retorting, have been shown by previous studies to be an important source of leachable Hg in calcines (12,15,48). The leachability of Hg from the calcines (Figures 1−3) by gastric fluids of pH 1.5 suggests that ingestion of calcines into the human gastric system, either via hand-to-mouth activity or by swallowing of inhaled particles cleared from the upper respiratory tract by mucociliary action, is undesirable and potentially harmful.
Figure 1.
Concentrations of Hg determined in water, gastric fluid, serum-based fluid, and lung fluid leachates of calcine samples collected in the Almadén and Terlingua Hg mining districts. The EPA screening level for inorganic Hg in residential soil (23 μg/g) is shown for reference.
Figure 2.
Comparison of concentrations of Hg determined in calcine samples and 1-h gastric fluid leachates of calcine samples from the Almadén and Terlingua Hg mining districts. Bioaccessibility (in %) is also shown for reference. Symbol size represents analytical error. Almadén samples are those with the abreviation AMJ (Almadenejos mine) and ALM (Almadén mine), and Terlingua samples are TER (Terlingua mine, also known as Chisos), SB (Study Butte mine), MAR (Mariposa mine), and MSM (Mariscal mine).
Table 1. Summary of Hg Concentrations for Calcines and Leachates of Calcines from the Almadén and Terlingua Hg Mining Districtsa.
| sample no. | location | calcine (μg/g) | water leach (μg/g) | water leach, final pH | gastric leach, 1 h (μg/g) | gastric leach, final pH | serum (μg/g) | serum, final pH | lung fluid (μg/g) | lung fluid, final pH |
|---|---|---|---|---|---|---|---|---|---|---|
| AMJ01 | Almadenejos mine | 11000 | 30 | 8.2 | 820 | 1.7 | 128 | 7.4 | 760 | 7.7 |
| AMJ02 | Almadenejos mine | 34000 | 880 | 9.8 | 6200 | 1.7 | 1620 | 7.6 | 9200 | 7.8 |
| ALM04 | Almadén mine | 440 | 0.60 | 8.4 | 47 | 1.8 | 24 | 7.1 | 0.28 | 7.6 |
| ALM03 | Almadén mine | 2300 | 4.2 | 4.0 | 200 | 1.7 | 48 | 6.8 | 26 | 7.0 |
| ALM06 | Almadén mine | 160 | 0.01 | 7.7 | 2.5 | 1.8 | 0.98 | 7.2 | 0.01 | 7.4 |
| ALM07 | Almadén mine | 1100 | 0.38 | 5.2 | 29 | 1.8 | 4.0 | 7.0 | 72 | 7.5 |
| TER1 | Terlingua | 19000 | 4.5 | 6.1 | 230 | 1.8 | 80 | 7.2 | 76 | 7.0 |
| SB4 | Study Butte mine | 3000 | 0.011 | 3.8 | 46 | 1.7 | 6.0 | 7.1 | 0.28 | 7.0 |
| SB1 | Study Butte mine | 5900 | 0.84 | 7.4 | 260 | 1.8 | 52 | 7.3 | 1.7 | 7.5 |
| MAR1 | Mariposa mine | 170 | 0.20 | 7.7 | 26 | 2.0 | 5.8 | 7.5 | 0.17 | 7.6 |
| MAR2 | Mariposa mine | 190 | 0.68 | 7.4 | 12 | 2.0 | 9.6 | 7.4 | 0.5 | 7.6 |
| MSM1 | Mariscal mine | 160 | 0.001 | 7.3 | 11 | 2.2 | 0.002 | 7.4 | 0.001 | 7.4 |
| MSM3 | Mariscal mine | 44 | 0.0016 | 7.1 | 12 | 2.2 | 0.54 | 7.4 | 0.0016 | 7.4 |
| MSM5 | Mariscal mine | 150 | 0.017 | 7.3 | 16 | 2.1 | 2.4 | 7.3 | 0.08 | 7.5 |
Data given for pH is that found at the end of the leachate experiments.
Figure 3.
Hg in calcine versus Hg in 1-h gastric leachates of calcines from the Almadén and Terlingua Hg mining districts. Regression line (r2 = 0.64) is for all samples. Symbol size represents analytical error.
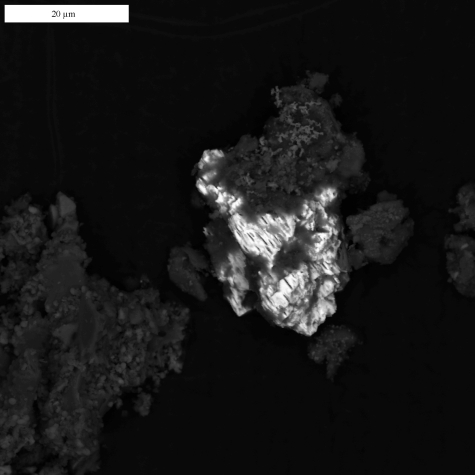
Figure 4.
Backscatter SEM image of calomel identified in calcine collected in the Almadén district.
Leaching of the calcine samples with water, serum-based fluid, and lung fluids also produced elevated leachate Hg concentrations, especially in samples from Almadén (Figure 1). However, Hg concentrations found in leachates of the water, serum-based fluid, and lung fluids were generally lower than those found during gastric fluid leaching. The Hg concentrations for the water, serum-based fluid, and lung fluids leachates are generally lower than the 310 μg/g industrial screening level for inorganic Hg in soil established by the USEPA (Figure 1).
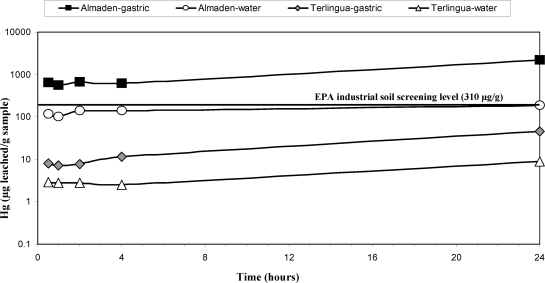
A series of timed experiments (1/2, 1, 4, and 24 h) were carried out to evaluate leaching of Hg from calcines in water and gastric fluids for a 24-h duration (Figure 5). Results of these experiments indicated that 32−61% of the leachable Hg is obtained in the first 1/2 h of leaching using water (comparison of 1/2-h leachate Hg relative to 24-h leachate Hg) and 17−29% using gastric fluids. For all four of the timed experiments, concentrations of Hg in both the water and gastric leachates are higher in the Almadén calcine samples compared to the Terlingua calcines (Figure 5), a result of a higher abundance of soluble Hg compounds in Almadén calcine samples (Supporting Information, Table S1). It is unlikely that ingested material remains in the human gastric system for as long as 24 h, and the 1/2- and 1-h experiments are probably a realistic estimation of Hg that might be obtained during leaching in the human gastric system. Conversely, the 24-h experiments using water are likely a reasonable estimation of potential Hg leaching during longer-term precipitation events, whereas the shorter timed experiments (1/2 and 1 h) are likely a reasonable estimation of Hg leaching during storm events that produce high volume runoff in short durations.
Figure 5.
Time versus Hg in water and 1-h gastric leachates of calcines from the Almadén and Terlingua Hg mining districts. Symbol size represents analytical error.
A rat type II alveolar epithelial cell line (RLE-6TN cells) was used for the in vitro cellular toxicity experiments. The cell line results indicate that cell counts for nearly all samples analyzed are within analytical error (±20%) using the trypan blue technique (Supporting Information, Table S2). Only three samples (AMJ02, ALM04, and MAR1) indicated cell loss outside of 20%, but these are not significant. These cell viability data indicate that there was no significant cell response resulting from exposure to high concentrations of Hg in the calcines, suggesting that the cells did not have an adverse response when exposed to Hg-bearing calcines.
Data shown here indicate that long-term human exposure to airborne dust containing Hg mine-waste calcine particulates or continual hand-to-mouth exposure to Hg-bearing calcines should be limited as much as possible to avoid unnecessary ingestion of Hg. The early stages of human development are the most sensitive to Hg, and thus leachate data presented here indicate that pregnant women and young children should avoid exposure to Hg mine wastes. Abandoned Hg mines are found worldwide, and human activities in the vicinity of these mines are generally unrestricted. Of particular concern are areas near Hg mines where (1) residences have been built, (2) local road construction has made use of Hg mine wastes, (3) towns are located in near proximity to Hg mines, and (4) where farming activities take place on land contaminated by such wastes. These practices and situations may lead to undesired human exposure through inhalation or ingestion of mine-waste particulates containing high concentrations of Hg.
Acknowledgments
This research was funded by the U.S. Geological Survey, Mineral Resources Program, and by the Spanish Science and Technology Ministry (Projects CTM2006-13091- C02) and the Castilla-La Mancha Regional Vice Ministry for Universities and Research (Projects PII1I09-0142-4389). We thank Thomas Ziegler for adapting methods that allowed us to study leaching using simulated human body fluids. We thank David Smith and Ruth Wolf (USGS, Denver) and four reviewers for ES&T for review comments that improved the paper. Use of trade names is for descriptive purposes and does not imply an endorsement by the U.S. Geological Survey.
There were errors in Table S2 of the Supporting Information published ASAP May 21, 2010; the corrected version published ASAP May 26, 2010.
Supporting Information Available
Mineralogical results and cell line data for samples analyzed in this study. This material is available free of charge via the Internet at http://pubs.acs.org.
Supplementary Material
References
- An Assessment of Mercury in the Environment; National Academy of Sciences, National Research Council: Washington, D.C., 1978; p 185. [Google Scholar]
- Eisler R. Mercury hazards to fish, wildlife, and invertebrates: A synoptic review.. U.S. Fish Wild. Serv., Biol. Rep. 1987, 85 (1.10), 90. [Google Scholar]
- Fitzgerald W. F.; Clarkson T. W. Mercury and monomethylmercury: Present and future concerns. Environ. Health Perspect. 1991, 96, 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USEPA, Mercury study report to Congress. In U.S. Environmental Protection Agency, I-VIII, EPA-452/R-97−003:1997.
- Clarkson T. W. Human health risks from methylmercury in fish. Environ. Toxicol. Chem. 1990, 9, 957–961. [Google Scholar]
- Zagury G. J.; Bedeaux C.; Welfringer B. Influence of mercury speciation and fractionation on bioaccessibility in soils. Arch. Environ. Contam. Toxicol. 2009, 56, 371–379. [DOI] [PubMed] [Google Scholar]
- Welfringer B.; Zagury G. J. Evaluation of two in vitro protocols for determination of mercury bioaccessibility: Influence of mercury fractionation and soil properties. J. Environ. Qual. 2009, 38, 2237–2244. [DOI] [PubMed] [Google Scholar]
- Shaw S. A.; Al T. A.; MacQuarrie K. T. B. Mercury mobility in unsaturated gold mine tailings, Murray Brook mine, New Brunswick, Canada. Appl. Geochem. 2006, 21, 1986–1998. [Google Scholar]
- Gray J. E.; Greaves I. A.; Bustos D. M.; Krabbenhoft D. P. Mercury and methylmercury contents in mine-waste calcine, water, and sediment collected from the Palawan Quicksilver Mine, Philippines. Environ. Geol. 2003, 43, 298–307. [Google Scholar]
- Gray J. E.; Hines M. E.; Biester H. Mercury methylation influenced by areas of past mercury mining in the Terlingua district, Southwest Texas, USA. Appl. Geochem. 2006, 21, 1940–1954. [Google Scholar]
- Navarro A. Review of characteristics of mercury speciation and mobility from areas of mercury mining in semi-arid environments. Rev. Environ. Sci. Biotechnol. 2008, 287–306. [Google Scholar]
- Kim C. S.; Bloom N. S.; Rytuba J. J.; Brown G. E. Jr. Mercury Speciation by X-ray Absorption Fine Structure Spectroscopy and Sequential Chemical Extractions: A Comparison of Speciation Methods. Environ. Sci. Technol. 2003, 37, 5102–5108. [DOI] [PubMed] [Google Scholar]
- Biester H.; Gosar M.; Covelli S. Mercury speciation in sediments affected by dumped mining residues in the drainage area of the Idrija mercury mine, Slovenia. Environ. Sci. Technol. 2000, 34, 3330–3336. [Google Scholar]
- Higueras P.; Oyarzun R.; Biester H.; Lillo J.; Lorenzo S. A first insight into mercury distribution and speciation in soils from the Almadén mining district, Spain. J. Geochem. Explor. 2003, 80, 95–104. [Google Scholar]
- Gray J. E. Leaching, transport, and methylation of mercury in and around abandoned mercury mines in the Humboldt River Basin and surrounding areas, Nevada.. U.S. Geol. Surv. Bull. 2003, B2210-C, 15. [Google Scholar]
- Rytuba J. J. Mercury from mineral deposits and potential environmental impact. Environ. Geol. 2003, 43, 326–338. [Google Scholar]
- Navarro A.; Biester H.; Mendoza J. L.; Cardellach E. Mercury speciation and mobilization in contaminated soils of the Valle del Azogue Hg mine (SE, Spain). Environ. Geol. 2006, 49, 1089–1101. [Google Scholar]
- Navarro A.; Cardellach E.; Corbella M. Mercury mobility in mine waste from Hg-mining areas in Almería, Andalusia (SE Spain). J. Geochem. Explor. 2009, 101, 236–246. [Google Scholar]
- Barnett M. O.; Turner R. R. Bioaccessibility of mercury in soils. Soil Sediment Contam. 2001, 10, 301–316. [Google Scholar]
- Davis A.; Bloom N. S.; Que Hee S. S. The environmental geochemistry and bioaccessibility of mercury in soils and sediments: A review. Risk Anal. 1997, 17, 557–569. [DOI] [PubMed] [Google Scholar]
- Moreno T.; Higueras P.; Jones T.; McDonald I.; Gibbons W. Size fractionation in mercury-bearing airborne particles (HgPM10) at Almadén Spain: Implications for inhalation hazards around old mines. Atmos. Environ. 2005, 39, 6409–6419. [Google Scholar]
- Peabody C. E., The association of cinnabar and bitumen in mercury deposits of the California Coast Ranges. In Bitumens in Ore Deposits; Parnell J.; Kucha J.; Landais P., Eds.; Springer-Verlag: Berlin, 1993; pp 179−209. [Google Scholar]
- Hernandez A.; Jebrak M.; Higueras P.; Oyarzun R.; Morata D.; Munha J. The Almadén mercury mining district, Spain. Mineralium Deposita 1999, 34, 539–548. [Google Scholar]
- Sharp R. D.Development of the mercury mining industry Trans-Pecos Texas. In Texas Bureau of Economic Geology, Mineral Resource Circular 64:1980.
- Avery D. W.; Moyle P. R.; Reisenburg R. M.; Fay J. M.. Preliminary assessment and site inspection, the Mariscal mine and mill site, Big Bend National Park, Brewster County, Texas. In U.S. Bureau of Mines, Site Characterization Section:1996.
- Rytuba J. J. Mercury mine drainage and processes that control its environmental impact. Sci. Total Environ. 2000, 260, 57–71. [DOI] [PubMed] [Google Scholar]
- Ross C. P. The quicksilver deposits of the Terlingua region, Texas. Economic Geol. 1941, 35, 115–142. [Google Scholar]
- Gray J. E.; Hines M. E.; Higueras P. L.; Adatto I.; Lasorsa B. K. Mercury speciation and microbial transformations in mine wastes, stream sediments, and surface waters at the Almadén mining district, Spain. Environ. Sci. Technol. 2004, 38, 4285–4292. [DOI] [PubMed] [Google Scholar]
- Drexler J. W.; Brattin W. J. An in vitro procedure for estimation of lead relative bioavailability: with validation. Hum. Ecol. Risk Assess. 2007, 13, 383–401. [Google Scholar]
- Mattson S. M. Glass Fibers in Simulated Lung Fluid: Dissolution Behavior and Analytical Requirements. Ann. Occup. Hyg. 1994, 38, 857–877. [Google Scholar]
- Eastes W.; Hadley J. G. Dissolution of Fibers Inhaled by Rates. Inhalation Toxicol. 1995, 7, 179–196. [Google Scholar]
- Jurinski J. B.; Rimstidt J. D. Biodurability of talc. Am. Mineral. 2001, 86, 392–399. [Google Scholar]
- Gamble J. L.Chemical anatomy, physiology, and pathology of extracellular fluid: A lecture syllabus, 6th ed.; Harvard University Press: Cambridge, MA, 1942; p164. [Google Scholar]
- USEPA, Test methods for evaluating solid waste. In U.S. Environmental Protection Agency: 1986; Vols. I and II (SW-846), 3rd ed.
- Driscoll K. E.; Carter J. M.; Iype P. T.; Kumari H. L.; Crosby L. L.; Aardema M. J.; Isfort R. J.; Cody D.; Chestnut M. H.; Burns J. L. Establishment of immortalized alveolar type II epithelial cell lines from adult rats. In Vitro Cell. Dev. Biol. Anim. 1995, 31, 516–527. [DOI] [PubMed] [Google Scholar]
- Okeson C. D.; Riley M. R.; Fernandez A.; Wendt J. O. L. Impact of the composition of combustion generated fine particles on epithelial cell toxicity: influences of metals on metabolism. Chemosphere 2003, 51, 1121–1128. [DOI] [PubMed] [Google Scholar]
- Suzuki K.; Hei T. K. Induction of heme oxygenase in mammalian cells by mineral fibers: Distinctive effect of reactive oxygen species. Carcinogenesis 1996, 17, 661–667. [DOI] [PubMed] [Google Scholar]
- Freshney R. I., Culture of Animal Cells: A Manual of Basic Techniques, 5th ed.; Wiley-Liss: New York, 2005. [Google Scholar]
- Bloom N. S.; Fitzgerald W. F. Determination of volatile mercury species at the picogram level by low-temperature gas chromatography with cold-vapour atomic fluorescence detection. Anal. Chim. Acta 1988, 208, 151–161. [Google Scholar]
- USEPA, Total mercury in in tissue, sludge, sediment, and soil by acid digestion and BrCl oxidation. In U.S. Environmental Protection Agency, EPA-821-R-01−013:2001.
- USEPA, Method 1631, Revision E: Mercury in water by oxidation, purge and trap, and cold vapor atomic fluorescence spectrometry. In U.S. Environmental Protection Agency, EPA 821-R-02-019:2002.
- Gray J. E.; Theodorakos P. M.; Krabbenhoft D. P.. Evaluation of mercury contamination at inactive mercury mines in and around Big Bend National Park. In U.S. Geological Survey Circular 1327:2008; pp57−64.
- Kim C. S.; Brown G. E. Jr.; Rytuba J. J. Characterization and speciation of mercury-bearing mine wastes using X-ray absorption spectroscopy. Sci. Total Environ. 2000, 261, 157–168. [DOI] [PubMed] [Google Scholar]
- Bernaus A.; Gaona X.; Esbrí J. M.; Higueras P.; Falkenberg G.; Valiente M. Microprobe techniques for speciation analysis and geochemical characterization of mine environments: The mercury district of Almadén in Spain. Environ. Sci. Technol. 2006, 40, 4090–4095. [DOI] [PubMed] [Google Scholar]
- Paustenbach D. J.; Bruce G. M.; Chrostowski P. Current views on the oral bioavailability of inorganic mercury in soil: Implications for health risk assessments. Risk Anal. 1997, 17, 533–544. [DOI] [PubMed] [Google Scholar]
- USEPA, Human health medium specific screening levels. In U.S. Environmental Protection Agency:2008.
- MacDonald D. D.; Ingersoll C. G.; Berger T. A. Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Arch. Environ. Contam. Toxicol. 2000, 39, 20–31. [DOI] [PubMed] [Google Scholar]
- Stetson S. J.; Gray J. E.; Wanty R. B.; Macalady D. L. Isotopic variability of mercury in ore, mine-waste calcine, and leachates of mine-waste calcine from areas mined for mercury. Environ. Sci. Technol. 2009, 43, 7331–7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.