Abstract
In this article, marking the 65th anniversary of the Journal of Gerontology, we offer a broad-brush overview of the new synthesis between neuroscientific and psychological approaches to cognitive aging. We provide a selective review of brain imaging studies and their relevance to mechanisms of cognitive aging first identified primarily from behavioral measurements. We also examine some new key discoveries, including evidence favoring plasticity and compensation that have emerged specifically from using cognitive neuroscience methods to study healthy aging. We then summarize several recent neurocognitive theories of aging, including our own model—the Scaffolding Theory of Aging and Cognition. We close by discussing some newly emerging trends and future research trajectories for investigating the aging mind and brain.
Keywords: Aging, Plasticity, Cognitive Neuroscience, Imaging
THE use of brain imaging technologies to study the neural underpinnings of human behavior is changing the field of psychology. New visions of the human mind are emerging from the integration of psychological science with brain science, and this is especially true for the field of cognitive aging. Indeed the past 15 years have brought a 10-fold increase in annual publications using brain-based measures to study the healthy aging mind. In this article, marking the 65th anniversary of the Journal of Gerontology, we offer a broad-brush overview of how this growing corpus of human neuroscience work has advanced cognitive aging research.
We recognize three major ways that brain-based approaches are having an impact on the study of cognitive aging. First, converging neuroscience evidence can inform and specify psychological constructs derived from behavioral work that have figured prominently in the cognitive aging literature. The first section of our review provides several key examples. Second, discoveries from neuroimaging are generating testable new hypotheses about the aging mind and brain, and we review several of these in the second section of this article. Third, cognitive neuroscience is stimulating the development of new constructs and theories about aging, and these are discussed in Section 3. We close by mentioning new developments and neuroscience frontiers in human aging, including affective processes, cross-cultural comparisons, and interventions.
CONVERGING EVIDENCE FOR COGNITIVE CONSTRUCTS
To date, cognitive neuroscience studies of aging have been primarily cross-sectional, focusing on differences between younger adults, aged 18–30, and older adults, aged 60–80. Functional magnetic resonance imaging (fMRI), which measures changes in blood flow to index localized changes in neural activity, has largely replaced positron emission tomography (PET) as the dominant brain imaging methodology. In the 1980s and 1990s, behavioral approaches to cognitive aging implicated four major domains of age-related decline—working memory, inhibitory control, processing speed, and long-term memory. Here, we consider how imaging research provides converging support and mechanistic insight into age effects in each domain. Critically, neuroscience evidence reveals that each domain is associated with age-related alterations indicating that no single cognitive mechanism or unitary account proposed to date will suffice to explain cognitive aging.
Working Memory
Working memory, the cognitive system that actively maintains several pieces of information in mind for immediate use in higher-order processing tasks, has figured prominently in cognitive theories of aging (e.g., Park et al., 2002; Salthouse, 1996), and is a central focus of neuroscience research on aging as well (see Rajah & D’Esposito, 2005 and Reuter-Lorenz & Sylvester, 2005, for reviews).
Performance differences between younger and older adults are generally minimal on simple span tasks (i.e., digit span) or item recognition that require only rote rehearsal, whereas differences are pronounced when executive functions such as memory updating, reordering, or inhibition are added to the task (e.g., word or operation span, N-back tasks; Craik & Jennings, 1992; Babcock & Salthouse, 1990). Imaging studies of item recognition, however, reveal pronounced activation differences, despite age-equivalent recognition accuracy, especially in regions of prefrontal cortex (dorsolateral, inferior frontal gyrus; e.g., Cabeza et al., 2004; Nagel et al., 2009; Park et al., 2003; Reuter-Lorenz et al., 2000; Rypma, Prabhakaran, Desmond, & Gabrieli, 2001). Varying memory load parametrically reveals that both age groups generally recruit the same brain regions but older adults recruit more (stronger activation, more sites of activation or both) at lower objective memory loads compared with younger adults (Cappell, Gmeindl, & Reuter-Lorenz, 2010; Schneider-Garces et al., 2010). This pattern of prefrontal overactivation suggests that older adults are engaging additional “executive” resources to support short-term memory maintenance. This interpretation gains support from the finding that younger adults also recruit these additional prefrontal sites, but at higher objective memory loads. Indeed, as load increases (e.g., greater than four items), older adults typically perform more poorly than younger adults, and this behavioral decline is accompanied by less prefrontal activation for older compared with younger adults—a pattern referred to as underactivation—suggesting a resource ceiling has been reached (Cappell et al., 2010; Rypma, Eldreth, & Rebbechi, 2007; Rypma et al., 2001).
For complex working memory tasks like operation span and N-back, older adults also show overactivation in dorsolateral prefrontal regions under conditions of age-equivalent performance (Emery, Heaven, Paxton, & Braver, 2008; Mattay et al., 2006; Smith et al., 2001). With increasing demand, older adults shift to underactivation coupled with performance that falls below their younger counterparts (Mattay et al., 2006; Smith et al., 2001). Taken together, these results suggest frontally mediated executive processes may be flexibly recruited to support working memory performance in older adults but are more resource dependent and resource limited (Craik & Byrd, 1982; Reuter-Lorenz & Cappell, 2008).
Inhibitory Control
Hasher and Zacks (1988) proposed that excitatory or activational aspects of attention are preserved in older adults, whereas inhibitory processes are deficient. Their view focused on the role of inhibition in controlling the contents of working memory by keeping out irrelevant information, and deleting no-longer relevant contents. Although the ubiquity of inhibitory dysfunction has been challenged, brain imaging has documented specific inhibitory impairment in older adults. For example, during item recognition, they are more vulnerable than young adults to interference from distractors appearing in the retention interval. Gazzaley, Cooney, Rissman, and D’Esposito (2005) demonstrated these effects using faces as the memoranda, and natural scenes such as distractors, or vice versa. These classes of stimuli are advantageous because they activate distinct localized regions making it possible to separate the brain’s response to the memoranda and the distractors. Older and younger adults show equivalent activation for the to-be-remembered items; however, older adults showed more distractor-related activity than younger adults and distractor-related activation was correlated inversely with memory performance.
Other work suggests that activational aspects of attentional selection may also be compromised with age. Johnson, Mitchell, Raye, and Greene (2004) have shown that the simple instruction to selectively activate or refresh a representation in working memory can improve subsequent memory for that item and activates a region of left dorsolateral prefrontal cortex. Older adults show neither the behavioral benefit nor the neural correlate of the refresh operation suggesting that this activational process is deficient.
Inhibitory deficit theory also proposes that older adults are deficient in the deletion of no-longer relevant information, as evident in greater proactive interference (PI) for older than younger adults (Lustig, May, & Hasher, 2001). Greater PI in older adults is associated with impaired activation in a region of left inferior frontal gyrus that has been linked specifically to interference control (Jonides et al., 2000). Thus, brain-based measures indicate several correlates of inhibitory dysfunction with age that involve altered engagement of prefrontal control processes and impaired top-down control at earlier stages of input processing.
Processing Speed
Evidence for age-related slowing is pervasive in the cognitive aging literature. A variety of analytic behavioral approaches demonstrate a prominent (Salthouse, 1996), although not exclusive, role of processing speed in age-related declines (Charlton et al., 2008; Park et al., 2002). Nevertheless, speed deficits with age are clearly associated with age differences in brain-based measures that provide a reasonable proxy of neural transmission. These include measures of white matter volume (e.g., Sullivan & Pfefferbaum, 2006) and microstructure (as assessed with diffusion tensor imaging [DTI] a measure of white matter integrity; see Kennedy & Raz, 2009 for a recent review). Localized white matter deficits mediate the relationship between speed and memory retrieval (Bucur et al., 2008; however, see Charlton et al., 2008). However, different cognitive measures (e.g., processing speed, episodic memory, working memory, inhibition, and attention) have been associated with different localized regions or networks of white matter decline (Kennedy & Raz, 2009). Such regional specificity suggests that age-related effects on different cognitive processes depend on the integrity of different neural substrates, supporting the conclusion that one unitary cause of cognitive decline with age is unlikely (Kennedy & Raz, 2009).
Long-Term Memory
Aging is thought to have its greatest effect on long-term episodic memory. Accordingly, the Web of Science indicates that the majority of publications on imaging and cognitive aging focus on memory, posing a considerable challenge for a succinct summary of this area. We focus here on studies illuminating two major questions: What might be going wrong in the older brain when memory is poor? And likewise, what neural processes can be linked to successful memory performance in the older brain?
Poor memory in older adults stems, in part, from deficient encoding, which is unlikely to be effortful or strategic unless specific encoding instructions are provided. Environmental support can improve memory and minimize age differences (Craik, 1994; Craik & Byrd, 1982). Brain imaging supports these statements. With minimal instruction, older adults show less activation than young adults in left inferior prefrontal regions that mediate semantic encoding operations (e.g., Logan, Sanders, Snyder, Morris, & Buckner, 2002; see Grady, 2008, for a review). With the help of cues or encoding strategies, older adults show improved performance and a corresponding “increase” in left prefrontal activation (e.g., Logan et al., 2002).
The neural correlates of memory success have been productively investigated by classifying trials from the encoding phase of an experiment based on their memory outcome. These “subsequent memory” effects permit comparisons between encoding activity that leads to remembering versus forgetting (see Park & Gutchess, 2005, for a review). Based on this approach, older adults often show weaker activation in regions of the medial temporal lobe ([MTL] including hippocampus and parahippocampus), but “increased” activation in prefrontal regions compared with younger adults (Gutchess et al., 2005; Morcom, Li, & Rugg, 2007). It is tempting to interpret prefrontal overactivations as compensatory because they are associated with successful memory.
Episodic memory decline with age has also been attributed to impaired binding—the association of an item or event and its context (e.g., Naveh-Benjamin, 2000). Associative memory decline is related to volumetric decreases in MTL regions (see Mitchell & Johnson, 2009, for a review), which are particularly vulnerable to age-related shrinkage (Raz, 2008). Moreover, binding deficits are associated with MTL and prefrontal activity reductions, along with weaker correlations between these regions, indicative of reduced functional connectivity (e.g., Chee et al., 2006; Dennis et al., 2008). Older adults who perform well on tasks requiring memory for context show additional involvement of prefrontal regions that may compensate for binding deficiencies (e.g., Morcom et al., 2007).
The proposal that aging spares automatic processing but not effortful processing (e.g., Hasher & Zacks, 1979, Jennings & Jacoby, 1993) also receives neuroscientific support. First, preventing younger adults from allocating full attention to memory encoding creates activation patterns resembling older brains under full attention conditions (e.g., Anderson et al., 2000). Second, familiarity-based memory, which is preserved in older adults, can be accompanied by prefrontal overactivation and increased connectivity with certain MTL regions (Daselaar, Fleck, Dobbins, Madden, & Cabeza, 2006), suggesting compensatory circuitry. In contrast, recollective memory, which is especially poor in older adults, leads to pronounced underactivation in MTL consistent with processing deficiency (see Park & Gutchess, 2005, for a review). Third, implicit memory, as reflected in priming, is relatively preserved (Light, 1988) and associated with age-equivalent activation patterns, particularly involving left inferior prefrontal regions associated with semantic processing (e.g., Lustig & Buckner, 2004; Bergerbest et al., 2009). In short, brain imaging supports a number of hypothesized mechanisms for memory decline in older age.
NEUROIMAGING DISCOVERIES ABOUT AGING
This section examines four discoveries about aging brain function that have emerged from brain imaging research. We begin with the finding of more widespread activation in older than younger adults. Although underactivation in older adults is also a common pattern (Persson & Nyberg, 2006), overactivation has been a particularly unexpected and intriguing result making it the topic of other recent reviews and debate (Grady, 2008; Greenwood, 2007; Park & Reuter-Lorenz, 2009; Reuter-Lorenz & Lustig, 2005). Here, we present some highlights of these issues.
Overactivation
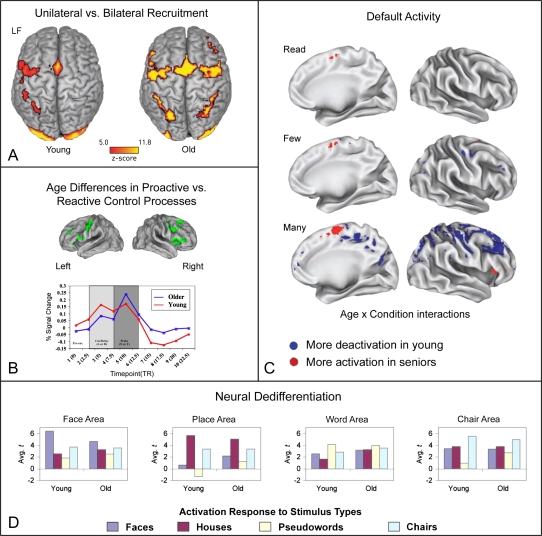
Early PET studies first reported that older adults activated more and different brain areas than younger adults (e.g., Backman et al., 1997; Grady et al., 1992; see Park & Reuter-Lorenz, 2009, for a review). The tasks used were either perceptual or memory based, and overactivity emerged in posterior and anterior brain regions. These results challenged the traditional doctrine of “localization of function”—the idea that brain functions were mediated by and localized to specific brain regions. Further challenge arose when left hemisphere dominance for verbal processing and right hemisphere dominance for spatial processing were found lacking in the brain activation patterns of older adults (Reuter-Lorenz et al., 2000). So, like age-related overactivation generally, this age-related alteration in hemispheric specialization, a pattern dubbed “hemispheric asymmetry reduction in older adults” or HAROLD (Cabeza, 2002), means that cognitive neuroscience studies of brain aging are raising new questions with broad neuroscientific implications (Figure 1A).
Figure 1.
(A) With permission from Schneider-Garces et al. (2010; Figure 4). This image illustrates the greater bilateral activity in older adults compared with younger adults. Statistical brain maps (axial surface projection) of the working memory task minus rest contrast for younger and older adults, collapsed across set sizes. LF = left front. (B) With permission from Braver et al., (2009; Figure 1). This image illustrates the prominence of reactive control in older adults compared with younger adults for whom proactive control is more prominent. The upper image in this panel displays the set of 17 age-related ROIs that show a crossover in the temporal profile resembling that illustrated in the lower panel. These regions were originally reported in Paxton, Barch, Racine, & Braver (2008). The lower image displays the activation dynamics during trials for younger and older adults (averaged across all ROIs). Older adults show reduced cue-related but increased probe-related activity, associated with a reactive control mode. (C) With permission from Persson et al., (2007, Figure 7). This image illustrates default network dysregulation in older adults, as a function of increasing task demand. The map displays statistically significant group differences across the brain for each of the conditions (i.e., baseline > read/few/many from top to bottom illustrating increasing deactivation in younger adults relative to older adults). Each interaction was masked by an overall contrast to indicate the direction of effects. (D) With permission from Park et al. (2004, Figure 2). This figure illustrates results supporting dedifferentiation. Mean t values as a function of age and stimulus category. The 15 voxels are most activated by faces, places, pseudowords, and chairs. As can be seen in this figure, younger adults demonstrate more category specificity than seniors. Consider the face voxels in the left bar graph, for example. The dark blue bar represents the response (mean t value) of the top 15 face voxels to the face stimuli. For young adults, these 15 voxels were significantly less active to pictures of houses, pseudowords, and chairs. In contrast, in the older adults, the top voxels that responded to faces were also active to houses, pseudowords, and chairs, indicating shared activation to these different categories.
But could such effects be due to age differences in neurovascular coupling on which both PET and fMRI depend (i.e., the blood-oxygenation level dependent or BOLD response)? Indeed, cerebrovascular aging probably underlies the decreased signal-to-noise ratio, amplitude reduction, and increased lag of the hemodynamic response in healthy older adults (D’Esposito, Deouell, & Gazzaley, 2003). Yet, despite these changes, the shape, summation, and refractory properties of the hemodynamic response do not differ due to age (see D’Esposito et al., 2003, for a review), nor are the findings of “greater” activation in older adults readily explained by alterations in neurovascular coupling.
So, if frank age-related confounds are not at play, what is the functional significance of age-related overactivation? One plausible interpretation is that overactivation is a neural correlate of cognitive decline. This possibility is consistent with results from simple motor tasks, and some measures of verbal fluency where greater activation of the nondominant hemisphere predicts poorer performance (see e.g., Meinzer et al., 2009; Seidler et al., 2010). However, evidence to the contrary from complex motor tasks and other cognitive domains, especially memory, finds overactivation in higher performing seniors (e.g., Bergerbest et al., 2009; Cabeza, Anderson, Locantore, & McIntosh, 2002; Emery et al., 2008; Heuninckx, Wenderoth, & Swinnen, 2008; Reuter-Lorenz et al., 2000), and in association with processes that lead to successful performance (Morcom et al., 2007; Gutchess et al., 2005).
These latter brain-behavior relations lead instead to the alternative popular view, which is that age-related overactivation indicates compensatory processing. By compensation, we mean that compared with younger adults, older adults are performing the same task using different neural circuitry, which in turn optimizes their performance. Older adults could be using additional brain regions to implement the same cognitive strategies as young adults or they could be using these brain regions to implement different strategies. Current evidence is insufficient to decide which interpretation is correct, and most likely a single account will not suffice for all tasks and all performance levels (Nagel et al., 2009). Nevertheless, a compensation account is favored for at least some overactivation patterns in some cognitive domains (see e.g., Reuter-Lorenz & Cappell, 2008). Also, overactivation could mediate “successful compensation” in that it supports performance equivalent to younger adults or in the upper range of an age-matched cohort, or “attempted compensation” that is insufficient to bring performance to such high levels (Rajah & D’Esposito, 2005; Duverne, Motamedinia, & Rugg, 2009). Moreover, as we discuss below, compensatory recruitment may have a cost.
Dedifferentiation
The concept of dedifferentiation originated in behavioral research with older adults (Lindenberger & Baltes, 1994; Reinert, 1970) but has gained prominence through neuroimaging. In the neural context, dedifferentiation refers to the loss of regional specialization or specificity. Dedifferentiation connotes negative plasticity in older adults resulting in a breakdown of functional specificity, and therefore needs to be distinguished from beneficial or compensatory overrecruitment.
As noted earlier, ventral visual cortex has functionally distinct subregions that respond selectively to categories or domains of visual input, such as faces, places/landmarks, and alphanumeric characters. Park and colleagues (2004; Figure 1D) have documented declining specificity in older adults for whom face regions are also more responsive to places than in young adults where regions respond discriminatively to one category (see also Chee et al., 2006; Voss et al., 2008). Ventral visual dedifferentiation in older adults during working memory encoding together with prefrontal overactivation (Payer et al., 2006) raises the possibility that frontal activity may compensate for lost perceptual specificity. Although much work remains to be done concerning the circuit changes and ubiquity of dedifferentiation, there are initial indications that it is greater in individuals with greater general reduction in cortical gray matter (Voss et al., 2008).
Frontal Compensation
Prefrontal overactivation is a paradoxical result in the cognitive neuroscience of aging. The paradox is because prefrontal regions are susceptible to considerable age-related atrophy (Raz, 2008) and have been implicated in an array of age-related deficits (West, 1996). Nevertheless, medial, lateral, and anterior prefrontal overactivation are prevalent in older adults across a range of tasks (Davis, Dennis, Daselaar, Fleck, & Cabeza, 2007; Gutchess et al., 2005; Heuninckx et al., 2008). Importantly, prefrontal overactivation is often associated with underactivation in more posterior regions, including MTL and ventral visual cortex, as well as with improved performance (e.g., Davis et al., 2007; Gutchess et al., 2005; Heuninckx et al.). The two findings, referred to as posterior–anterior shift in aging (Davis et al.), jointly support the interpretation that these regions are compensating for processing deficiencies elsewhere.
Default Network Alterations
The default network includes medial prefrontal, medial, and lateral parietal brain regions that have highly correlated activity and tend to be more active at rest than during task-related cognition (Raichle et al., 2001). The prevailing view is that this network mediates cognitions associated with self-focus, reflective memories, and ambient attention to the environment (Buckner, Andrews-Hanna, & Schacter, 2008). The default network was discovered first in healthy younger adults, but was soon found to be age sensitive with older adults showing greater default activation during cognitive tasks (Lustig et al., 2003) and less default suppression with greater task demand (Persson, Lustig, Nelson, & Reuter-Lorenz, 2007; Figure 1C). Older adults also show less connectivity among default regions (e.g., Damoiseaux et al., 2008). Heightened frontal activation is associated with inefficient default activity in older adults during semantic retrieval (Persson et al., 2007) and measures of subsequent memory (Miller et al., 2008). These results support the proposal that age-associated dysregulation of default activity may promote compensatory overactivation elsewhere (Park & Reuter-Lorenz, 2009; Reuter-Lorenz & Lustig, 2005).
NEURALLY BASED THEORIES OF COGNITIVE AGING
Cognitive neuroscience has given rise to new theories of cognitive aging that focus on functional or structural mechanisms to explain both deficient and preserved cognitive performance in older adults. Here, we briefly review a few of these new theoretical accounts that integrate brain imaging data with “cognitive behavioral effects” that accompany aging. Moving away from the “lesion models” of aging (e.g., West, 1996), the new generation of cognitive neuroscience theories incorporate constructs of plasticity and compensation as critical factors in understanding the aging mind.
Increased Noise
Li and her colleagues (Backman, Nyberg, Lindenberger, Li, & Farde, 2006; Li, Lindenberger, & Sikstrom, 2001) have proposed a model of aging that is based on age-related alterations in neural transmission, particularly related to dopaminergic decline. This theory and its computational implementation propose that aging is associated with decreased signal-to-noise ratio, which leads to less distinct neural representations; these effects may be exacerbated in pathological aging (Backman et al., 2006). The proposed mechanism is one that fits well with observations suggesting that some processes in the aging brain such as representational operations in ventral visual cortex can be characterized by dedifferentiation.
Proactive Versus Reactive Control
This theoretical proposal advanced by Braver, Paxton, Locke, and Barch (2009) focuses on the temporal dynamics of executive control of information processing operations. Proactive control is preparatory, taking advantage of cuing and other contextual information that permits selective processing prior to the imperative events, whereas reactive control occurs later in the processing sequence, typically in response to or succeeding the imperative events. This dual mechanism view was proposed to account for imaging and behavioral results across a variety of tasks that permit different forms of control. Measures of memory (e.g., Velanova, Lustig, Jacoby, & Buckner, 2007) and executive function (Braver et al., 2009) indicate that altered prefrontal function in older adults is associated with poor proactive control, causing greater reliance on reactive control than in younger adults (see Figure 1B). These theoretical proposals are also reminiscent of the idea discussed previously that older adults are impaired at effortful, self-initiated processes (Craik & Byrd, 1982).
CRUNCH
The compensation-related utilization of neural circuits hypothesis (CRUNCH) was proposed to account for patterns of overactivation and underactivation in older adults (Reuter-Lorenz & Cappell, 2008). The CRUNCH model posits that declining neural efficiency leads older adults to engage more neural circuits than young adults to meet task demands. Therefore, older adults are likely to show overactivation, including frontal or bilateral recruitment, at lower levels of cognitive demand where younger adults show more focal activations. However, as load increases, younger adults may shift to an overactive or bilateral pattern to address task demands, whereas older adults, who may have already maxed out their neural resources at the lower load, show underactivation and performance decline. The predictions of the CRUNCH model have been upheld in several studies of working memory (Cappell et al., 2010; Mattay et al., 2006; Schneider-Garces et al., 2010). This model meshes well with the notion of “reserve” in that individuals with more reserve may reach their resource limit at higher load levels (Stern 2009), making overactivation less likely at lower loads (e.g., Nagel et al., 2009; Smith et al., 2001; Rypma et al., 2007). This may be why longitudinal work has found that overactivation predicted subsequent cognitive decline (Persson et al., 2006). CRUNCH is also compatible with Scaffolding Theory of Aging and Cognition (STAC; see below) because compensatory activations may provide scaffolding to support aging cognition. Furthermore, CRUNCH is reminiscent of resource accounts of cognitive aging, which propose resource deficits as a basis for poorer performance of older adults (see Cappell et al., 2010; Reuter-Lorenz, Stanczak, & Miller, 1999).
Scaffolding Theory of Aging and Cognition
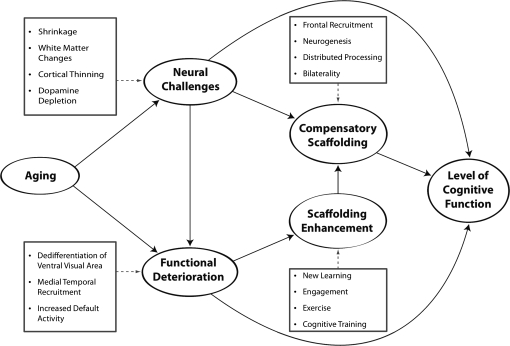
The STAC model (Park & Reuter-Lorenz, 2009) depicted in Figure 2 provides the most integrative account of the theories reviewed here. The STAC model integrates the major findings from the cognitive neuroscience of aging and addresses both neurocognitive decline as well as neuroplasticity of the aging mind. STAC posits that the aging brain is subject to a range of neural challenges to which it must adapt. These challenges include amyloid deposition, atrophy, white matter deterioration, and dopamine receptor depletion. Such “hardware” changes are accompanied by the kinds of functional alterations reviewed above including dedifferentiation, default network dysregulation, and decreased activation of MTL structures. According to STAC, the brain responds to this corpus of neural burden by forging alternative neural circuitry (scaffolds) that may operate less efficiently than the focal, well-honed networks of young adulthood. The scaffolding process permits individuals to maintain a high level of cognitive function at advanced ages and is represented by patterns of overactivation, primarily in the frontal cortex, but may include areas of parietal, mediotemporal, and occipital regions. Although the capacity for neurogenesis, synaptogenesis, and angiogensis decline with aging, the model posits that all mechanisms remain functional and provide plausible means for forging alternative neural circuitry. Importantly, compensatory scaffolding is affected by experiences, so that new learning, enhanced cardiovascular health, sustained engagement in a mentally challenging activity, and cognitive training might all operate to enhance the brain’s ability to build effective new scaffolding to maintain a high level of cognitive function that can no longer be supported by deteriorating structures and older functional networks. As Figure 2 demonstrates, the magnitude of the neural deterioration combined with the effectiveness of new compensatory scaffolds operates to predict overall level of cognitive function.
Figure 2.
A conceptual model of the Scaffolding Theory of Aging and Cognition. Reprinted with permission from Park and Reuter-Lorenz (2009).
One key idea in STAC is that scaffolding is not a process that simply begins in older age. Rather, across the life span, the individual is confronted with cognitive challenges to which the brain must adapt. Throughout childhood, existing circuitry must be harnessed and used to scaffold the forging of new circuitry to support the acquisition of new cognitive skills. Likewise, scaffolding supports new skill acquisition in adulthood (Petersen, van Mier, Fiez, & Raichle, 1998). Early phases of new learning are often accompanied by high levels of activation and increased reliance on prefrontal circuitry. The proposed continuity of scaffolding across the life span corresponds with the parallel between the sequence of neural development in childhood and age-related neuroanatomical decline, which follows a “last in, first out” progression: the regions that are later to myelinate developmentally are the first to decline in older age (Raz, 2008; Kennedy & Raz, 2009). Thus, aspects of neural decline may recapitulate earlier developmental states, and thereby revisit scaffolding solutions that originally aided in the acquisition of cognitive abilities in early development. So, scaffolding is a lifelong process that is relied upon continually and increasingly in the later stages of life.
NEW DIRECTIONS IN THE PSYCHOLOGICAL NEUROSCIENCE OF AGING
A number of rapidly developing domains of neuroscientific inquiry are beginning to influence understanding of the aging mind and are likely to constitute new frontiers in the field.
Continued research on emotional regulation, affect, and aging is fundamental to understanding the aging mind. Abundant behavioral evidence shows decreased negative affect and a bias to process and remember positive information in older adults (Carstensen & Mikels, 2005). These biases are reflected in electrophysiological indices (Langeslag & Van Strien, 2009), greater amygdala response for positive stimuli (Mather et al., 2004), and increased fronto-limbic connectivity in older compared with younger adults (St. Jacques, Dolcos, & Cabeza, 2009). Likewise, aging may positively impact decision making (Mohr et al., 2010) along with representations of self and other, pointing to important frontiers for a social neuroscience of aging (e.g., Gutchess, Kensinger, & Schacter, 2007; Mitchell et al., 2009). Affective regulation may also play an important role in the development of neural scaffolds in that poor emotional regulation could have deleterious effects on the ability to develop compensatory scaffolding.
Another topic of increasing interest is the universality of patterns of neurocognitive aging, given evidence that cultural environments sculpt cognitive processes (Park & Gutchess, 2006). Cross-cultural studies of the aging brain have the potential to reveal what aspects of the aging brain are biologically programmed and what aspects are more affected by the environment (Park & Gutchess, 2006).
Evidence favoring continued neural plasticity into older age has energized the investigation of the effects of exercise, engagement, and cognitive training on neurocognitive function in older adults (see Hertzog, Kramer, Wilson, & Lindenberger, 2009 and Lustig, Seidler, Shah & Reuter-Lorenz, 2009 for reviews). Although the potential for cognitive plasticity in older adults has been recognized for decades (e.g., Baltes & Schaie, 1976), the use of neural measures to identify mechanisms and correlates of improved performance is stimulating innovative and promising research. Current evidence is fairly conclusive that enhanced cardiovascular health is associated with better neurocognitive function (Hertzog et al., 2009). Moreover, there are strong correlations between an engagement in intellectual pursuits and later onset of Alzheimer’s disease (Hall, Lipton, Sliwinski, Derby, & Verghese, 2009), although the causal relationship has not been determined. Critically, certain forms of cognitive training appear to change cognitive and even neural function, which can sometimes be sustained, but the demonstration of generalized improvement in cognitive function has proven more elusive (Hertzog et al.; Lustig et al., 2009). Finally, over the next decade, the availability of new biological methods for genotyping (Jagust, 2009) and imaging destructive brain proteins such as beta amyloid which figures prominently in Alzheimer’s disease (Buckner et al., 2009) will transform our understanding of the relationship between healthy and pathological life span trajectories, and help to harness the full potential for successful aging.
CONCLUSIONS
This review highlights three core contributions that have emerged from the cognitive neuroscience approaches to aging. The first pertains to the benefits of converging evidence: decades of behavioral aging research identified fundamental mechanisms that have garnered support from brain-based methods. Neuroimaging methods are promoting new neurally informed mechanistic accounts of cognitive decline and the realization that adverse effects of aging are unlikely to stem from a unitary or dominant cognitive mechanism.
The second contribution is evidence favoring enduring plasticity. From imaging, to animal models, to interventions, brain-based discoveries are revealing that psychological aging results from the combined effects of both negative and positive plasticity. The capacity for positive plasticity and compensation are proving more prevalent and integral to the aging mind than previously recognized. Human neuroscience and genetic approaches hold great promise for discovering the bases for successful aging and new ways to promote positive plasticity.
Third, brain-based studies of cognitive aging have broader human neuroscientific implications. Evidence for altered lateralization, changing specificity of visual cortex, and the pervasive recruitment of prefrontal areas indicate the dynamic nature of functional brain organization that can be relevant to compensatory and recovery processes across the life span. Moreover, brain-based approaches to aging suggest continuities across the life span whereby investments made earlier in life, in the form of intellectual, social, and physical enrichment, may increase neural reserve and potential for effective scaffolding as people meet increasing challenges in later life (Park & Reuter-Lorenz, 2009; Stern, 2009).
In conclusion, the combined use of behavioral and biologically-based approaches to studying the aging mind has proven to be far more fruitful than either alone. We hope this review will further disseminate information about the new synthesis of psychological and brain science to study human aging and thereby energize new research that will be celebrated in future anniversary issues of this and other journals in the field.
FUNDING
This work was supported by a grant from the National Institute on Aging (5R37AG006265-24) whose support is gratefully acknowledged.
Acknowledgments
The authors thank Blair Flicker, Mary Mohrbach, and members of the Reuter-Lorenz lab for their assistance with the preparation of this manuscript preparation.
References
- Anderson ND, Iidaka T, Cabeza R, Kapur S, McIntosh AR, Craik FIM. The effects of divided attention on encoding- and retrieval-relted brain activity: A PET study of younger and older adults. Journal of Cognitive Neuroscience. 2000;12:775–792. doi: 10.1162/089892900562598. [DOI] [PubMed] [Google Scholar]
- Babcock RL, Salthouse TA. Effects of increased processing demands on age differences in working memory. Psychology and Aging. 1990;5:421–428. doi: 10.1037//0882-7974.5.3.421. [DOI] [PubMed] [Google Scholar]
- Backman L, Almkvist O, Andersson J, Nordberg A, Winblad B, Reineck R, Langstrom B. Brain activation in young and older adults during implicit and explicit retrieval. Journal of Cognitive Neuroscience. 1997;9:378–391. doi: 10.1162/jocn.1997.9.3.378. [DOI] [PubMed] [Google Scholar]
- Backman L, Nyberg L, Lindenberger U, Li S-C, Farde L. The correlative triad among aging, dopamine, and cognition: Current status and future prospects. Neuroscience & Biobehavioral Reviews. 2006;30:791–807. doi: 10.1016/j.neubiorev.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Baltes PB, Schaie W. On the plasticity of intelligence in adulthood and old age: Where Horn and Donaldson fail. American Psychologist. 1976;13:720–725. doi: 10.1037//0003-066x.31.10.720. [DOI] [PubMed] [Google Scholar]
- Bergerbest D, Gabrieli JDE, Whitfield-Gabrieli S, Kim H, Stebbins GT, Bennett DA, Fleischman DA. Age-associated reduction of asymmetry in prefrontal function and preservation of conceptual repetition priming. NeuroImage. 2009;45:237–246. doi: 10.1016/j.neuroimage.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Paxton JL, Locke HS, Barch DM. Flexible neural mechanisms of cognitive control within human prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:7351–7356. doi: 10.1073/pnas.0808187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Annals of the New York Academy of Science. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, Andrews-Hanna JR, Sperling RA, Johnson KA. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. Journal of Neuroscience. 2009;29:1860–73. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucur B, Madden DJ, Spaniol J, Provenzale JM, Cabeza R, White LE, Huettel SA. Age-related slowing of memory retrieval: Contributions of perceptual speed and cerebral white matter integrity. Neurobiology of Aging. 2008;7:1070–1079. doi: 10.1016/j.neurobiolaging.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychology and Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: Compensatory brain activity in high-performing older adults. Neuroimage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Daselaar SM, Dolcos F, Prince SE, Budde M, Nyberg L. Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cerebral Cortex. 2004;14:364–375. doi: 10.1093/cercor/bhg133. [DOI] [PubMed] [Google Scholar]
- Cappell KA, Gmeindl L, Reuter-Lorenz PA. Age differences in prefrontal recruitment during verbal working memory maintenance depend on memory load. Cortex. 2010;46:462–473. doi: 10.1016/j.cortex.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstensen LL, Mikels JA. At the intersection of emotion and cognition: Aging and the positivity effect. Current Directions in Psychological Science. 2005;14:117–121. [Google Scholar]
- Charlton RA, Landau S, Schiavone F, Barrick TR, Clark CA, Markus HS, Morris RG. A structural equation modeling investigation of age-related variance in executive function and DTI measured white matter damage. Neurobiology of Aging. 2008;29:1547–1555. doi: 10.1016/j.neurobiolaging.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Chee MW, Goh JO, Venkatraman V, Tan JC, Gutchess A, Sutton B. Hebrank A. Leshikar E. Park D. Age-related changes in object processing and contextual binding revealed using fMR adaptation. Journal of Cognitive Neuroscience. 2006;18:495–507. doi: 10.1162/jocn.2006.18.4.495. [DOI] [PubMed] [Google Scholar]
- Craik FIM. Memory changes in normal aging. Current Directions in Psychological Science. 1994;3:155–158. [Google Scholar]
- Craik FIM, Byrd M. Aging and cognitive deficits: The role of attentional resources. In: Craik FIM, Trehub S, editors. Aging and cognitive processes. New York: Plenum Press; 1982. pp. 191–211. [Google Scholar]
- Craik FIM, Jennings JM. Human memory. In: Craik FIM, Salthouse TA, editors. Handbook of aging and cognition. Hillsdale, MI: Erlbaum; 1992. pp. 51–109. [Google Scholar]
- Damoiseaux JS, Beckmann CF, Arigita E, Barkhof F, Scheltens P, Stam CJ, Rombouts SB. Reduced resting-state brain activity in the “default network” in normal aging. Cerebral Cortex. 2008;18:1856–1864. doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Dobbins IG, Madden DJ, Cabeza R. Effects of healthy aging on hippocampal and rhinal memory functions: An event-related fMRI study. Cerebral Cortex. 2006;16:1771–1782. doi: 10.1093/cercor/bhj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Que PASA? The posterior anterior shift in aging. Cerebral Cortex. 2007;18:1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis NA, Hayes SM, Prince SE, Madden DJ, Huettel SA, Cabeza R. Effects of aging on the neural correlates of successful item and source memory encoding. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2008;34:791–808. doi: 10.1037/0278-7393.34.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Esposito M, Deouell LY, Gazzaley A. Alterations in the bold fMRI signal with ageing and disease: A challenge for neuroimaging. Nature Reviews Neuroscience. 2003;4:863–872. doi: 10.1038/nrn1246. [DOI] [PubMed] [Google Scholar]
- Duverne S, Motamedinia S, Rugg MD. The relationship between aging, performance, and the neural correlates of successful memory encoding. Cerebral Cortex. 2009;19:733–744. doi: 10.1093/cercor/bhn122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery L, Heaven TJ, Paxton JL, Braver TS. Age-related changes in neural activity during performance matched working memory manipulation. NeuroImage. 2008;42:1577–1586. doi: 10.1016/j.neuroimage.2008.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, Rissman J, D’Esposito M. Top-down suppression deficit underlies working memory impairment in normal aging. Nature Neuroscience. 2005;8:1298. doi: 10.1038/nn1543. [DOI] [PubMed] [Google Scholar]
- Grady CL. Cognitive neuroscience of aging. Annals of the New York Academy of Sciences. 2008;1124:127–144. doi: 10.1196/annals.1440.009. [DOI] [PubMed] [Google Scholar]
- Grady CL, Haxby JV, Horwitz B, Schapiro MB, Rapoport S, Ungerleider LG, Herscovitch P. Dissociation of object and spatial vision in human extrastriate cortex: Age- related changes in activation of regional cerebral blood flow measured with [15O] water and positron emission tomography. Journal of Cognitive Neuroscience. 1992;4:23–34. doi: 10.1162/jocn.1992.4.1.23. [DOI] [PubMed] [Google Scholar]
- Greenwood P. Functional plasticity in cognitive aging: Review and hypothesis. Neuropsychology. 2007;21:657–673. doi: 10.1037/0894-4105.21.6.657. [DOI] [PubMed] [Google Scholar]
- Gutchess AH, Kensinger EA, Schacter DL. Aging, self referencing, and medial prefrontal cortex. Social Neuroscience. 2007;2:117–133. doi: 10.1080/17470910701399029. [DOI] [PubMed] [Google Scholar]
- Gutchess AH, Welsh RC, Hedden T, Bangert A, Minear M, Liu LL, Park DC. Aging and the neural correlates of successful picture encoding: Frontal activations compensate for decreased medial-temporal activity. Journal of Cognitive Neuroscience. 2005;17:84–96. doi: 10.1162/0898929052880048. [DOI] [PubMed] [Google Scholar]
- Hall CB, Lipton RB, Sliwinski M, Derby CA, Verghese J. Cognitive activities delay onset of memory decline in persons who develop dementia. Neurology. 2009;73:356–361. doi: 10.1212/WNL.0b013e3181b04ae3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasher L, Zacks RT. Automatic and effortful processes in memory. Journal of Experimental Psychology: General. 1979;108:356–388. [Google Scholar]
- Hasher L, Zacks RT. Working memory, comprehension, and aging: A review and a new view. In: Bower GH, editor. The psychology of learning and motivation. Vol. 22. New York: Academic Press; 1988. pp. 193–225. [Google Scholar]
- Hertzog C, Kramer AF, Wilson RS, Lindenberger U. Enrichment effects on adult cognitive development. Psychological Science in the Public Interest. 2009;9:1–65. doi: 10.1111/j.1539-6053.2009.01034.x. [DOI] [PubMed] [Google Scholar]
- Heuninckx S, Wenderoth N, Swinnen SP. Systems neuroplasticity in the aging brain: recruiting additional neural resources for successful motor performance in elderly persons. Journal of Neuroscience. 2008;28:91–99. doi: 10.1523/JNEUROSCI.3300-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagust W. Mapping brain beta-amyloid. Current Opinion in Neurobiology. 2009;22:356–361. doi: 10.1097/WCO.0b013e32832d93c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JM, Jacoby LL. Automatic versus intentional uses of memory: Aging, attention, and control. Psychology & Aging. 1993;8:283–293. doi: 10.1037//0882-7974.8.2.283. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Mitchell KJ, Raye CL, Greene EJ. An age-related deficit in prefrontal cortical function associated with refreshing information. Psychological Science. 2004;15:127–132. doi: 10.1111/j.0963-7214.2004.01502009.x. [DOI] [PubMed] [Google Scholar]
- Jonides J, Marshuetz C, Smith E, Reuter-Lorenz PA, Keoppe R, Hartley A. Age differences in behavior and PET activation reveal differences in interference resolution in verbal working memory. Journal of Cognitive Neuroscience. 2000;12:188–196. doi: 10.1162/089892900561823. [DOI] [PubMed] [Google Scholar]
- Kennedy KM, Raz N. Aging white matter and cognition: Differential effects of regional variations in diffusion properties on memory, executive functions, and speed. Neuropsychologia. 2009;47:916–927. doi: 10.1016/j.neuropsychologia.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langeslag SJ, van Strien JW. Aging and emotional memory: The co-occurrence of neurophysiological and behavioral positivity effects. Emotion. 2009;9:369–377. doi: 10.1037/a0015356. [DOI] [PubMed] [Google Scholar]
- Li SC, Lindenberger U, Sikstrom S. Aging cognition: From neuromodulation to representation. Trends in Cognitive Science. 2001;5:479–486. doi: 10.1016/s1364-6613(00)01769-1. [DOI] [PubMed] [Google Scholar]
- Light LL. Preserved implicit memory in old age. In: Gruneberg MM, Morris PE, Sykes RN, editors. Practical aspects of memory: Current research and issues. Vol. 2. Chichester: Wiley; 1988. pp. 90–95. [Google Scholar]
- Lindenberger U, Baltes PB. Sensory functioning and intelligence in old age: A strong connection. Psychology and Aging. 1994;9:339–355. doi: 10.1037//0882-7974.9.3.339. [DOI] [PubMed] [Google Scholar]
- Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL. Under-recruitment and nonselective recruitment: Dissociable neural mechanisms associated with aging. Neuron. 2002;33:827–840. doi: 10.1016/s0896-6273(02)00612-8. [DOI] [PubMed] [Google Scholar]
- Lustig C, Buckner RL. Preserved neural correlates of priming in old age and dementia. Neuron. 2004;42:865–875. doi: 10.1016/j.neuron.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Lustig C, May CP, Hasher L. Working memory span and the role of proactive interference. Journal of Experimental Psychology: General. 2001;130:199–207. doi: 10.1037//0096-3445.130.2.199. [DOI] [PubMed] [Google Scholar]
- Lustig C, Seidler RS, Shah P, Reuter-Lorenz PA. Aging, training and the brain: A review and future directions. Special Issue on Aging: Compensation and Decline. Neuropsychology Review. 2009;19:504–222. doi: 10.1007/s11065-009-9119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig C, Snyder AZ, Bhakta M, O’Brien KC, McAvoy M, Raichle ME, Buckner RL. Functional deactivations: Change with age and dementia of the Alzheimer type. Proceedings of the National Academy of Science of the United States of America. 2003;100:14504–14509. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Canli T, English T, Whitfield S, Wais P, Ochsner K, Carstensen LL. Amygdala responses to emotionally valenced stimuli in older and younger adults. Psychological Science. 2004;15:259–263. doi: 10.1111/j.0956-7976.2004.00662.x. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Fera F, Tessitore A, Hariri AR, Berman KF, Das S, Weinberger DR. Neurophysiological correlates of age-related changes in working memory capacity. Neuroscience Letters. 2006;392:32–37. doi: 10.1016/j.neulet.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Meinzer M, Flaisch T, Wilser L, Eulitz C, Rockstroh B, Conway T, Crosson B. Neural signatures of semantic and phonemic fluency in young and old adults. Journal of Cognitive Neuroscience. 2009;10:2007–2018. doi: 10.1162/jocn.2009.21219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SL, Celone K, DePeau K, Diamond E, Dickerson BC, Rentz D, Sperling RA. Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proceedings of the National Academy of Science of the United States of America. 2008;105:2181–2186. doi: 10.1073/pnas.0706818105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell KJ, Johnson MK. Source monitoring 15 years later: What have we learned from fMRI about the neural mechanisms of source memory? Psychological Bulletin. 2009;135:638–677. doi: 10.1037/a0015849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell KJ, Raye CL, Ebner NC, Tubridy SM, Frankel H, Johnson MK. Age-group differences in medial cortex activity associated with thinking about self-relevant agendas. Psychology and Aging. 2009;24:438–449. doi: 10.1037/a0015181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr PNC, Li S-C, Heekeren HR. Neuroeconomics and aging: Neuromodulation of economic decision making in old age. Neuroscience and Biobehavioral Reviews. 2010;34:678–688. doi: 10.1016/j.neubiorev.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Morcom AM, Li J, Rugg MD. Age effects on the neural correlates of episodic retrieval: Increased cortical recruitment with matched performance. Cerebral Cortex. 2007;17:2491–2506. doi: 10.1093/cercor/bhl155. [DOI] [PubMed] [Google Scholar]
- Nagel I, Preuschhof C, Li SC, Nyberg L, Bäckman L, Lindenberger U, Heekeren H. Performance level modulates adult age differences in brain activation during spatial working memory. Proceedings of the National Academy Sciences of the United States of America. 2009;106:22552–22557. doi: 10.1073/pnas.0908238106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naveh-Benjamin M. Adult-age differences in memory performance: Tests of an associative deficit hypothesis. Journal of Experimental Psychology: Learning, Memory and Cognition. 2000;26:1170–1187. doi: 10.1037//0278-7393.26.5.1170. [DOI] [PubMed] [Google Scholar]
- Park D, Reuter-Lorenz PA. The adaptive brain: Aging and neurocognitive scaffolding. Annual Review of Psychology. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Gutchess AH. Long-term memory and aging: A cognitive neuroscience perspective. In: Cabeza R, Nyberg L, Park DC, editors. Cognitive neuroscience of aging: Linking cognitive and cerebral aging. New York: Oxford University Press; 2005. pp. 218–245. [Google Scholar]
- Park DC, Gutchess AH. The cognitive neuroscience of aging and culture. Current Directions in Psychological Science. 2006;15:105–108. [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. Models of visuospatial and verbal memory across the adult life span. Psychology and Aging. 2002;17:299–320. [PubMed] [Google Scholar]
- Park DC, Polk TA, Park R, Minear M, Savage A, Smith MR. Aging reduces neural specialization in ventral visual cortex. Proceedings of the National Academy Sciences of the United States of America. 2004;101:13091–13095. doi: 10.1073/pnas.0405148101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Welsh RC, Marshuetz C, Gutchess AH, Mikels J, Polk TA, Taylor SF. Working memory for complex scenes: Age differences in frontal and hippocampal activations. Journal of Cognitive Neuroscience. 2003;15:1122–1134. doi: 10.1162/089892903322598094. [DOI] [PubMed] [Google Scholar]
- Paxton JL, Barch DM, Racine CA, Braver TS. Cognitive control, goal maintenance, and prefrontal function in healthy aging. Cerebral Cortex. 2008;18:1010–1028. doi: 10.1093/cercor/bhm135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payer D, Marshuetz C, Sutton B, Hebrank A, Welsh RC, Park DC. Decreased neural specialization in old adults on a working memory task. Neuroreport. 2006;17:487–491. doi: 10.1097/01.wnr.0000209005.40481.31. [DOI] [PubMed] [Google Scholar]
- Persson J, Lustig C, Nelson JK, Reuter-Lorenz PA. Age differences in deactivation: A link to cognitive control? Journal of Cognitive Neuroscience. 2007;19:1021–1032. doi: 10.1162/jocn.2007.19.6.1021. [DOI] [PubMed] [Google Scholar]
- Persson J, Nyberg L. Altered brain activity in healthy seniors: what does it mean? In: Møller AR, editor. Progress in brain research. Vol. 157. New York: Elsevier; 2006. pp. 45–56. [DOI] [PubMed] [Google Scholar]
- Persson J, Nyberg L, Lind J, Larsson A, Nilsson LG, Ingvar M, Buckner RL. Structure-function correlates of cognitive decline in aging. Cerebral Cortex. 2006;16:907–915. doi: 10.1093/cercor/bhj036. [DOI] [PubMed] [Google Scholar]
- Petersen SE, van Mier H, Fiez JA, Raichle ME. The effects of practice on the functional anatomy of task performance. Proceedings of the National Academy Sciences of the United States of America. 1998;95:853–860. doi: 10.1073/pnas.95.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy Sciences of the United States of America. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajah MN, D’Esposito M. Region-specific changes in prefrontal function with age: A review of PET and fMRI studies on working and episodic memory. Brain. 2005;128:1964–1983. doi: 10.1093/brain/awh608. [DOI] [PubMed] [Google Scholar]
- Raz N. Aging of the brain and its impact on cognitive performance: Integration of structural and functional findings. In: Craik FIM, Salthouse TA, editors. Handbook of aging and cognition-II. Mahwah, NJ: Erlbaum; 2008. pp. 1–90. [Google Scholar]
- Reinert G. Comparative factor analytic studies of intelligence throughout the life span. In: Goulet LR, Baltes PB, editors. Life-span developmental psychology: Research and theory. New York: Academic Press; 1970. pp. 476–484. [Google Scholar]
- Reuter-Lorenz PA, Cappell K. Neurocognitive aging and the compensation hypothesis. Current Directions in Psychological Science. 2008;18:177–182. [Google Scholar]
- Reuter-Lorenz PA, Jonides J, Smith E, Hartley A, Miller A, Marshuetz C, Koeppe R. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. Journal of Cognitive Neuroscience. 2000;12:174–187. doi: 10.1162/089892900561814. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Lustig C. Brain aging: Reorganizing discoveries about the aging mind. Current Opinion in Neurobiology. 2005;15:245–251. doi: 10.1016/j.conb.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Stanczak L, Miller A. Neural recruitment and cognitive aging: Two hemispheres are better than one especially as you age. Psychological Science. 1999;10:494–500. [Google Scholar]
- Reuter-Lorenz PA, Sylvester CY. The cognitive neuroscience of aging and working memory. In: Cabeza R, Nyberg L, Park D, editors. The cognitive neuroscience of aging. New York: Oxford University Press; 2005. pp. 186–217. [Google Scholar]
- Rypma B, Eldreth DA, Rebbechi D. Age-related differences in activation-performance relations in delayed-response tasks: A multiple component analysis. Cortex. 2007;43:65–76. doi: 10.1016/s0010-9452(08)70446-5. [DOI] [PubMed] [Google Scholar]
- Rypma B, Prabhakaran V, Desmond JE, Gabrieli JD. Age differences in prefrontal cortical activity in working memory. Psychology and Aging. 2001;16:371–384. doi: 10.1037//0882-7974.16.3.371. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychological Review. 1996;103:403–28. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Schneider-Garces NJ, Gordon BA, Brumback-Peltz CR, Shin E, Lee Y, Sutton BP, Fabiani M. Span, CRUNCH and beyond: Working memory capacity and the aging brain. Journal of Cognitive Neuroscience. 2010;15:655–669. doi: 10.1162/jocn.2009.21230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler RD, Bernard JA, Burutolu TB, Fling BW, Gordon MT, Gwin JT, Kwak Y, Lipps DB. Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neuroscience and Biobehavioral Reviews. 2010;34:721–733. doi: 10.1016/j.neubiorev.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Geva A, Jonides J, Miller A, Reuter-Lorenz PA, Koeppe RA. The neural basis of task switching in working memory: Effects of performance and aging. Proceedings of the National Academy of Science of the United States of America. 2001;98:2095–2100. doi: 10.1073/pnas.98.4.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve. Neuropscyhologia. 2009;47:2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Jacques PL, Dolcos F, Cabeza R. Effects of aging on functional connectivity of the amygdala for subsequent memory of negative pictures: A network analysis of fMRI data. Psychological Science. 2009;20:74–84. doi: 10.1111/j.1467-9280.2008.02258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Diffusion tensor imaging and aging. Neuroscience and Biobehavioral Reviews. 2006;30:749–761. doi: 10.1016/j.neubiorev.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Velanova K, Lustig C, Jacoby LL, Buckner RL. Evidence for frontally mediated controlled processing differences in older adults. Cerebral Cortex. 2007;17:1033–1046. doi: 10.1093/cercor/bhl013. [DOI] [PubMed] [Google Scholar]
- Voss MW, Erickson KI, Chaddock L, Prakash RS, Colcombe SJ, Morris KS, Kramer AF. Dedifferentiation in the visual cortex: An fMRI investigation of individual differences in older adults. Brain Research. 2008;1244:121–131. doi: 10.1016/j.brainres.2008.09.051. [DOI] [PubMed] [Google Scholar]
- West RL. An application of prefrontal cortex function theory to cognitive aging. Psychological Bulletin. 1996;120:272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]