Abstract
Autophagy functions as an important catabolic mechanism by mediating the turnover of intracellular organelles and protein complexes. Although the induction of autophagy by starvation has been extensively studied, we still understand very little about how autophagy is regulated under normal nutritional conditions. Here we describe a study using a small molecule autophagy inducer, fluspirilene, as a tool to explore the mechanism of autophagy induction in normal living cells. We confirm the activity of fluspirilene in inhibiting Ca2+ flux. Furthermore, we show that reducing intracellular Ca2+ prevents the cleavage of ATG5, which in turn increases the levels of full length ATG5 and ATG12-ATG5 conjugate. Using siRNA mediated gene silencing, we demonstrate that inhibiting calpain1 is sufficient to induce autophagy in living cells. We conclude that calpain1 plays an important role in controlling the levels of autophagy in normal living cells by regulating the levels of a key signaling molecule, ATG12-ATG5 conjugate.
Keywords: autophagy, Fluspirilene, calpain, ATG5, Ca2+, cleavage, ATG12-ATG5
INTRODUCTION
Autophagy is a cellular catabolic mechanism mediating the turnover of intracellular organelles and proteins through a lysosome-dependent degradative pathway.1, 2 An autophagosome sequesters cytoplasmic constituents, such as mitochondria, endoplasmic reticulum, and ribosomes, by forming a double-membrane vesicle. The outer membrane of the autophagosome then fuses with a lysosome to deliver the sequestered content into the lumen of lysosome for degradation. Autophagy functions as an essential survival mechanism for unicellular organisms such as yeast under starvation conditions by recycling intracellular material for macromolecular synthesis and energy production.3
Regulation of autophagy under starvation has been extensively studied. In mammalian cells, mTOR kinase, the target of rapamycin, mediates a major inhibitory signal that shuts off autophagy under nutrient-rich conditions.3 In this regard, rapamycin is the most commonly used tool to mimic starvation-induced autophagy. However, starvation is uncommon for mammalian cells under physiological conditions. In mammalian cells, autophagy functions as an important host defense mechanism and can be rapidly up-regulated within minutes upon stress or invasion by pathogens.1–4 Thus, we need tools to study the regulation of autophagy in mammalian cells under normal nutritional conditions. We have previously carried out a high-throughput image-based screen and identified 7 FDA-approved drugs which can induce autophagy without inducing cell death.5 Interestingly, five of the seven compounds were previously known to have activity in inhibiting intracellular Ca2+.6–10
A number of previous studies have proposed the possible involvement of intracellular Ca2+ in regulating autophagy. William et al. reported that L-type Ca2+ channel antagonists, the K+ATP channel opener minoxidil, and the Gi signaling activator clonidine could induce autophagy in mTOR-independent manner.11 Furthermore, William et al. demonstrated that inhibition of calpains induces autophagy and reduces the accumulation of misfolded polyglutamine. However, the mechanism by which inhibition of calpain in living cells leads to the induction of autophagy has not been fully demonstrated.
In this study, we explored the mechanism of one of the autophagy inducers, fluspirilene, in inducing autophagy. We confirm the activity of fluspirilene in inhibiting Ca2+ flux. We show that calpains play an important role in controlling the levels of autophagy in normal living cells by regulating the levels of ATG5. Reducing intracellular Ca2+ prevents the cleavage of ATG5, which in turn increases the levels of full length ATG5 and ATG12-ATG5 conjugate. Using siRNA mediated gene silencing, we demonstrate that calpain1 plays an important role in controlling the levels of autophagy in living cells under nutrient-rich conditions.
MATERIALS AND METHODS
Cell-Culture assays
H4 cells were maintained in Dulbecco’s modified Eagle’s medium (GIBCO, 12100-061) supplemented with 10% FBS (GIBCO, 16000-044), 100 units/ml penicillin/streptomycin, and 2 mM L-glutamine (Invitrogen, 21051-024) at 37°C, 5%CO2.
Antibody Sources
The sources of antibodies used: anti-LC3 antibody (Sigma, L7543), anti-tubulin antibody (Sigma, T4026), anti-ATG12 antibody (CST, 2010 for human cells and 2011 for mouse cells) for ATG12-ATG5 complex (53KD), anti-ATG5 antibodies (Abgent, AP1812a for 24KD tATG5 and AP1812b for 32KD full length ATG5), anti-MARCKs antibody (Abcam, ab52616), anti-calpain1 antibody (CST, 2556), anti-calpain2 antibody (CST, 2539) and anti-calpain4 antibody (Abnova, H00000826-M01).
RT-PCR
Total RNA was isolated from H4 cells. First-strand cDNA was prepared from total RNA and oligo dT using First Strand cDNA Synthesis Kit, ReverTra Ace-a-™ (TOYOBO, FSK-100). Real-time PCR was conducted with RT-PCR Kit, ReverTra Dash™ (TOYOBO, PCR-400). The primers for human Atg5 were TGGATTTCGTTATATCC CCTTTAG and CCTAGTGTGTGCAACTGTCCA. Human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was utilized to calibrate the original concentration of mRNA.
Ca2+ Flux assays
Calcium flux assays were carried out according to the protocol provided by the BD Biosciences using Fura-2 (Invitrogen, F-6774). The cells were stimulated with 10 μM (final concentration) ATP (Sigma, A2383) and Ca2+ flux was measured with the BD pathway 855™ using its Calcium flux program.
RNA interference assays
The siRNAs (Shanghai GenePharma) were transfected into cells with Lipofectamine™ 2000 using protocol provided by the manufacture (Invitrogen, 11668-019). siRNA sequence: 5′ to 3′:
Calpain1-a: CCACGGAACUGCUGUCAAATT/UUUGACAGCAGUUCCGUGGGA;
Calpain1-b: GGGUGAACCCUCAGUUCAATT/UUGAACUGAGGGUUCACCCAG;
Calpain1-c: GGAAGUUUGACCUGGACAATT/UUGUCCAGGUCAAACUUCCGG;
Calpain2-a: CGGAUGCUAUGAAGCGCUATT/UAGCGCUUCAUAGCAUCCGTT;
Calpain2-b: CCAGCGAUACCUACAAGAATT/UUCUUGUAGGUAUCGCUGGTG;
Calpain2-c: CGCUAUUCAAGAUAUUUAATT/UUAAAUAUCUUGAAUAGCGTT;
Calpain4-a: GGUGGCAGGCCAUAUACAATT/UUGUAUAUGGCCUGCCACCTT;
Calpain4-b: CCUGAAUGAGCAUCUCUAUTT/AUAGAGAUGCUCAUUCAGGTG;
Calpain4-c: CGACGCUACUCAGAUGAAATT/UUUCAUCUGAGUAGCGUCGGA.
RESULTS
Fluspirilene increases the flux of autophagy in H4 cells
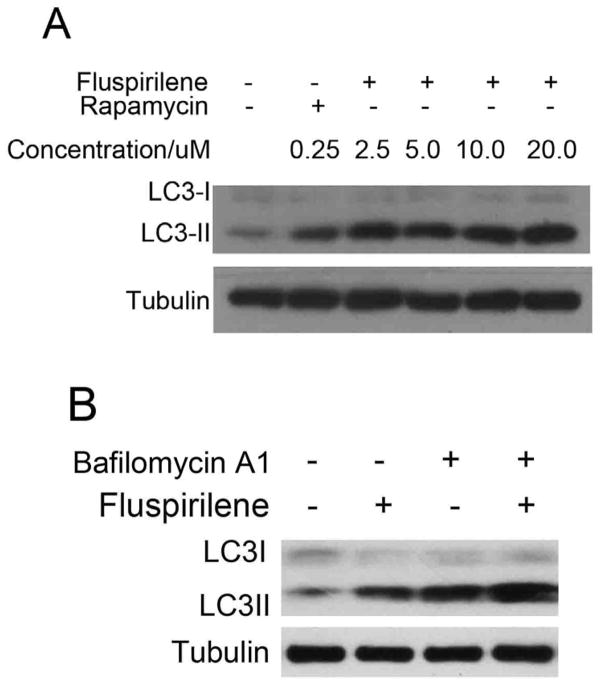
We further characterized the effects of fluspirilene on autophagy using LC3II, the lipidated product of mammalian LC3 and an established marker for autophagosomes.1, 12 We first determined the dose-response of fluspirilene on LC3II levels in H4 cells, a human neuroblastoma cell line. H4 cells were chosen because of their high basal mTORC1 activity due to the loss of PTEN tumor suppressor, allowing for the identification of mTORC1 independent regulators of autophagy. As shown in Fig. 1A, the levels of endogenous LC3II increased in a dose dependent manner in response to fluspirilene treatment (Fig. 1A).
Figure 1.
Induction of autophagy by fluspirilene. (A) Dose-dependent induction of LC3II by fluspirilene in H4 cells. H4 cells were treated with indicated concentrations of fluspirilene for 4 hours. The cell lysates were harvested and analyzed by western blotting using anti-LC3. Anti-tubulin was used as a loading control. (B) H4 cells were treated with fluspirilene (10 μM), bafilomycin A1 (100 nM), or fluspirilene and bafilomycin A1 together for 4 hours. 0.1% DMSO is used as negative control. The cell lysates were harvested and analyzed by western blotting using anti-LC3 antibody. Anti-tubulin was used as a loading control.
Increases in the levels of LC3II could be due to either an increase in the flux of autophagosome formation or a reduction in the turnover of autophagosomes due to a block in the lysosomal pathway. To distinguish these two possibilities, we compared the effect of fluspirilene alone and that together with bafilomycin A1. Bafilomycin A1 is a specific inhibitor of the vacuolar type H (+)-ATPase (V-ATPase) in cells, and inhibits the acidification of organelles containing this enzyme, such as lysosomes and endosomes. In this way, bafilomycin A1 will block the turnover of autophagosomes.13 Consistent with an effect of fluspirilene in promoting the flux of autophagosomes, the presence of bafilomycin A1 led to a further increase in the levels of LC3II compared to that of fluspirilene alone (Fig. 1B). Thus, we conclude that fluspirilene most likely induces an increase in the flux of autophagy, rather than a block in the turnover of autophagosomes. This is also consistent with our previous finding that fluspirilene increases the turnover of long-lived proteins.5
Using quantitative image analysis of LC3-GFP, we quantified the time-course and dose-response of fluspirilene in H4-LC3 cells (Supplementary Fig. S1). The effect of fluspirilene on LC3-GFP peaks at 4 hours and its EC50 in inducing autophagy of H4 cells is ~2.5 μM. We have shown previously that the treatment of fluspirilene increases the levels of FYVE-RFP, a marker for PtdIns3P.5 However, we could not detect any obvious effect of fluspirilene on the interactions of Vps34/Beclin1 or Atg14/Beclin1, the class III PI3 kinase complexes that regulate the production of PtdIns3P (data not shown). Although this does not definitively rule out an effect of fluspirilene on the class III I3 kinase, we turned our attention to the other key signaling complex of autophagy, ATG12-ATG5 conjugate.14
Fluspirilene increases the levels of endogenous ATG12-ATG5 conjugate in H4 cells
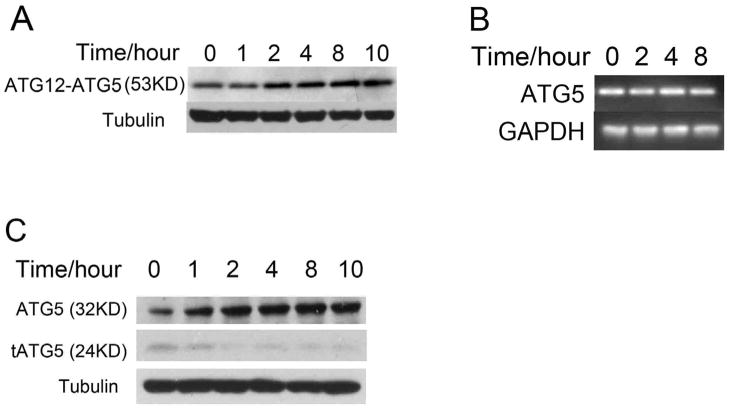
The two ubiquitin-like conjugation systems, ATG12-ATG5 and Atg8 (LC3), are required for the initiation and expansion of autophagosomal membrane.12, 15 We determined the effect of fluspirilene on endogenous ATG12-ATG5 conjugate in H4 cells. Interestingly, we found that the levels of ATG12-ATG5 conjugate increased significantly as a function of time with the treatment of fluspirilene (Fig. 2A).
Figure 2.
The effects of fluspirilene on the levels of ATG12-ATG5 in H4 cells. (A) H4 cells were treated with 10μM fluspirilene for indicated length of time. The cell lysates were harvested and analyzed by western blotting using anti-ATG12 antibody. Anti-tubulin was used as a loading control. (B) H4 cells were treated with 10μM fluspirilene for indicated length of time. The mRNA levels of ATG5 are analyzed by RT-PCR as described in the methods. (C) H4 cells were treated with 10μM fluspirilene for indicated length of time. The cell lysates were harvested and analyzed by western blotting with anti-ATG5 antibodies. Anti-tubulin was used as a loading control.
To determine if treatment of fluspirilene might have an effect on the expression of ATG5, we measured the mRNA levels of ATG5 in control and fluspirilene treated H4 cells by RT-PCR but no difference was found (Fig. 2B). This result led us to examine an alternative possibility, namely fluspirilene affects the levels of ATG5 proteins. ATG5 protein is known to be present in three forms, full-length ATG5 (32 KD) and truncated ATG5 (24 KD) and ATG12-ATG5 conjugate (53KD).16 Interestingly, we found a significant increase in the levels of full length ATG5 and a corresponding reduction of truncated ATG5 in fluspirilene treated cells (Fig. 2C). We also observed similar changes in the expression pattern of ATG5 proteins in MEF cells (mouse embryonic fibroblasts) (Supplementary Fig. S2). Since ATG5 may be cleaved by calpains,16 this result suggests that fluspirilene may prevent the cleavage of ATG5 and thus reduce the levels of truncated ATG5 to lead to a corresponding increase in the levels of full length ATG5. As a result of increased supplies of full-length ATG5, the levels of ATG12-ATG5 conjugate also increase correspondingly which in turn functions to increase the levels of LC3II and induce autophagy.14 This possibility was further tested by experiments described below.
Fluspirilene regulates autophagy by inhibiting Ca2+ channels
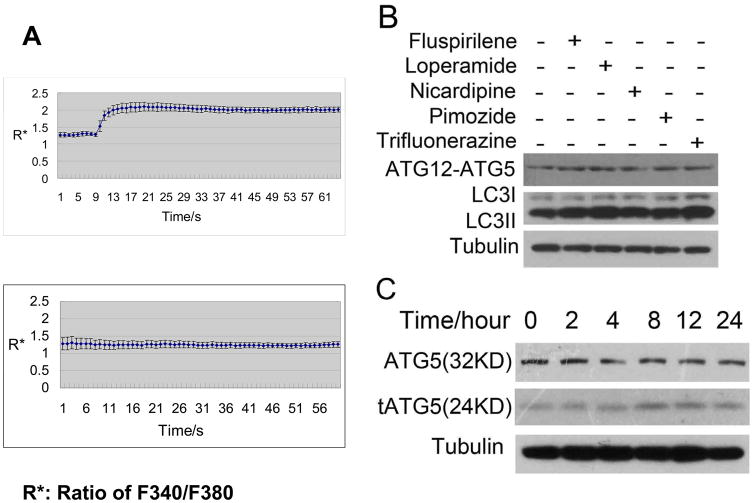
Since fluspirilene has been shown to block both P-type and N-type Ca2+ channels in neurons,6 we consider the possibility that fluspirilene reduces intracellular Ca2+ concentration by blocking Ca2+ channels. To verify this hypothesis, we first checked the effects of autophagy inducers on intracellular Ca2+ by Ca2+ flux assay. Ca2+ influx was induced by stimulating with ATP, which activates purinergic receptors to promote IP3 formation and IP3-induced Ca2+ release (IICR).17 The rise of intracellular Ca2+ concentration was measured (Fig. 3A). Indeed, fluspirilene (Fig. 3A) as well as 4 other autophagy inducers identified by Zhang et al,5 including loperamide, pimozide, trifluoperazine, and nicardipine, could inhibit the Ca2+ influx induced by ATP (Supplementary Fig. S3B). Consistent with a role of Ca2+ on the levels of ATG12-ATG5, all 5 compounds could induce increases in the levels of ATG12-ATG5 in H4 cells (Fig. 3B). This result is consistent with our proposal for a role of intracellular Ca2+ in regulating the levels of ATG12-ATG5 conjugate under normal nutritional conditions. Furthermore, Bay K-8644, an L-type Ca2+ channel agonist, could induce the level of intracellular Ca2+, and has been reported to inhibit autophagy and impair the clearance of A53T α-synuclein.11 Consistent with a role Ca2+ in mediating autophagy, we found that the tAtg5 levels in H4 cells under the treatment of Bay K-8644 increased with time, while that of full length Atg5 reduced (Fig. 3C).
Figure 3.
The effects of Ca2+ channel inhibitors or agonist on ATG5. (A) Fluspirilene inhibits ATP induced Ca2+ flux. HeLa cells were treated with 0.1% DMSO (above) or 10 μM fluspirilene (below) for 10 min before adding ATP. The Ca2+ flux was measured using dye fura-2. (B) The effects of Ca2+ channel inhibitors on the levels of ATG12-ATG5 and LC3II in H4 cells. H4 cells were treated with indicated compounds (10 μM) for 4 hours. 0.1% DMSO was used as a negative control. The cell lysates were harvested and analyzed by western blotting using anti-ATG12 and anti-LC3. Anti-tubulin was used as a loading control. (C) The effects of Bay K-8644 on the levels of ATG5 and tATG5 in H4 cells. H4 cells were treated with 10 μM Bay K-8644 for indicated length of time. The cell lysates were harvested and analyzed by western blotting using anti-ATG5. Anti-tubulin was used as a loading control.
To further characterize the relationship of intracellular Ca2+ and the levels of ATG12-ATG5, we determined the structure-activity relationships (SAR) of fluspirilene. We synthesized 83 chemical derivatives of fluspirilene and characterized their activities on autophagy and Ca2+ channels. A strong correlation between these two activities was observed. Essentially, all derivatives that are active as Ca2+ channel inhibitors also induce autophagy, while the compounds that are inactive in inhibiting Ca2+ channels have no effects on autophagy (data not shown). Interestingly two compounds with chemical structures that differ from that of fluspirilene in minor ways have no effects on autophagy or Ca2+ channel (Supplementary Fig. S3). These data demonstrate a close structural and functional relationship of fluspirilene series between their activities of autophagy induction and Ca2+ channel inhibition and suggest a critical role of Ca2+ in regulating the basal levels of autophagy under normal nutritional conditions.
Calpains regulate the levels of ATG12-ATG5 in living cells
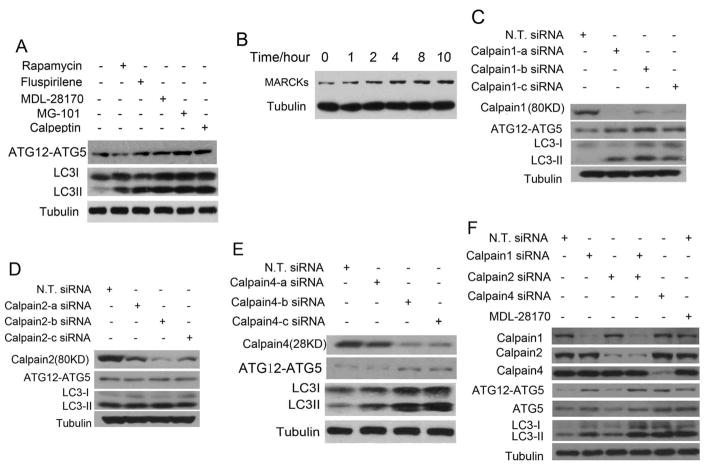
Calpain, a family of Ca2+ dependent ubiquitous non-lysosomal cysteine proteases, has been proposed to cleave full length ATG5 in apoptosis.16 The ability of fluspirilene to reduce intracellular Ca2+ and to reduce tATG5 while increase full length ATG5 and ATG12-ATG5 complex led us to postulate that calpain might play a role in regulating autophagy in normal living cells. We considered the possibility that in living cells, low levels of calpain activity might inhibit autophagy by cleaving ATG5 and reducing the levels of ATG12-ATG5 complex. To test our hypothesis, we examined the effects of known calpain inhibitors on endogenous ATG12-ATG5 complex and LC3II levels. We found that all calpain inhibitors tested, including MDL-28170, MG-101, Calpeptin, could increase the levels of LC3II and ATG12-ATG5 in H4 cells (Fig. 4A). In contrast, rapamycin has no effects on ATG12-ATG5 complex (Fig. 4A), providing a control for the specificity. MARCKs (myristoylated alanine-rich protein kinase C substrate) is a well-established substrate and marker of calpain activity, its level increases when calpain activity is inhibited.18 To further characterize the relationship of fluspirilene and calpain activity, we analyzed the levels of MARCKs in H4 cells treated with fluspirilene. Consistent with our proposal that fluspirilene impairs calpain activity by inhibiting intracellular Ca2+ flux, we found that MARCKs levels increase steadily with fluspirilene treatment (Fig. 4B).
Figure 4.
The effects of inhibiting calpains on the levels of ATG12-ATG5 and LC3II in H4 cells. (A) H4 cells were treated with indicated concentrations of rapamycin (0.25 μM), fluspirilene (10 μM), MDL-28170 (10 μM), MG-101 (10 μM) and Calpeptin (10 μM), respectively, for 4 hours. 0.1% DMSO was used as a negative control. The cell lysates were harvested and analyzed by western blotting using anti-ATG12 and anti-LC3. Anti-tubulin was used as a loading control. (B) H4 cells were treated with 10μM fluspirilene for indicated length of time. The cell lysates were harvested and analyzed by western blotting with anti-MARCKs antibodies. Anti-tubulin was used as a loading control. (C–E) H4 cells were transfected with indicated siRNAs for 72 hrs and no targets siRNA (N. T. siRNA) was used as negative control. The cell lysates were harvested and analyzed by western blotting using anti-calpain1 (C), anti-calpain2 (D), anti-calpain4 (E), anti-ATG12 and anti-LC3 (C–E). Anti-tubulin was used as loading controls. (F) H4 cells were transfected with siRNAs indicated for 72 hrs or treated with MDL-28170 (10 μM) for 4 hrs and the cell lysates were harvested and analyzed by western blotting using anti-Calpain1, anti-Calpain2, anti-Calpain4, anti-ATG12, anti-ATG5 and anti-LC3 antibody. Anti-tubulin was used as a loading control.
Calpain1 regulates autophagy in nutrient sufficient condition
Since fluspirilene can induce autophagy in multiple cell lines with different tissue origins (Supplementary Fig. S4) including neural cells (H4), lung epithelium (A549), breast epithelium (MDA-MB-231 cells), cervical epithelium (HeLa), liver cells (HepG2 cells), and kidney fibroblasts (293T), we postulate that the calpains that are targeted by fluspirilene should be ubiquitously expressed. Calpain1, also known as μ-calpain, and calpain2, also known as m-calpain are the two most widely expressed calpains. Calpain1 is activated by micromolar concentrations of Ca2+ whereas calpain2 requires millimolar concentrations of Ca2+ for activation. Both calpain1 and calpain2 are heterodimers of a large catalytic subunit (80 KD) and a small regulatory subunit (28 KD). The small subunit, known as calpain4 (calpain S1), is shared by both calpain1 and calpain2. Using siRNA mediated knockdown, we found that inhibiting the expression of calpain1 and calpain4, but not calpain2 led to a significant induction of autophagy with increases in the levels of LC3II and ATG12-ATG5 complex (Fig. 4C–F). Taken together, our results suggest that the activity of calpain1 controlled by physiological concentrations of intracellular Ca2+ plays an important role in mediating homeostatic suppression of autophagy under normal nutritional conditions by regulating the levels of ATG12-ATG5 conjugate.
DISCUSSION
Although autophagy is maintained at very low levels in normal living cells, it can be rapidly induced within minutes upon starvation or invasion by intracellular pathogens.1–4 Thus, the basic components of the autophagy machinery are fully synthesized in cells under normal nutritional condition when the levels of autophagy are very low and ready to be rapidly assembled into autophagosomes upon stimulation. Currently, we know very little about how autophagy inducing signals, such as stress or invasion of pathogens, can be rapidly translated into an assembly order to lead to rapid increases in the levels of autophagy. Our results here suggest that calpain1 might play an important role under normal nutritional condition to keep autophagy under a tight control by down-regulating the levels of ATG12-ATG5 conjugate. Inhibition of calpain as a result of reductions in intracellular Ca2+ and calpain1 activity might serve as such a signal under selected conditions to lead to the activation of autophagy machinery by increasing the levels of a key autophagy signaling molecule, ATG5 and ATG12-ATG5 conjugate.
Calpains have been proposed to mediate multiple cellular processes under physiological conditions. For example, leukocyte extravasation, the process of leukocytes infiltrating inflamed tissues, involves the expansion of plasma membrane to lead to “spreading”, the molecular pathway for the leukocyte plasma membrane spreading may involve Ca2+ activation of calpain1 and cleavage of cytoskeletal linkage molecules.19 Since cytosolic concentrations of Ca2+ in normal living cells are at nM, it has been proposed that localized spatial and temporal localized changes in Ca2+ concentrations in living cell might account for restricted activation of calpains in living cells.20 It is possible, therefore, that the levels of full length ATG5 are under the control of such localized activated calpain1. Such controls might provide a means to rapidly up-regulate autophagy upon a reduction of intracellular Ca2+.
Different levels of intracellular Ca2+ may have opposite effects on autophagy. Our study is consistent with Williams et al. that normal physiological concentrations of cytosolic Ca2+ may inhibit autophagy in a mTOR-independent manner.11 On the other hand, excess levels of intracellular Ca2+ may lead to induction of autophagy through a mTOR dependent mechanism. Høyer-Hansen et al. reported that treatment of various Ca2+ mobilizing agents including vitamin D(3) compounds, ionomycin, ATP, and thapsigargin inhibit the activity of mTOR and induce massive accumulation of autophagosomes.21 Since the compounds used by Høyer-Hansen et al. such as vitamin D(3) compounds, ionomycin, ATP, and thapsigargin, are likely to lead to massive increases of intracellular Ca2+ beyond micromolar range which can be tolerated by living cells, high levels of Ca2+ may activate autophagy through pathways associated with cell death. Consistent with this proposal, activation of calpains had been reported to induce autophagy in apoptotic cells.22, 23
Hanada et al. have suggested that the ATG12-ATG5 conjugate, but not unconjugated ATG12 or ATG5, strongly enhances the formation of the other conjugate, Atg8-PE (LC3II), by promoting the transfer of Atg8 from Atg3 to the substrate, phosphatidylethanolamine (PE).14 On the other hand, Mizushima and colleagues suggest that the presence of ATG5 alone may be sufficient to initiate the formation of autophagosomes 24. Since our study demonstrates that the treatment of fluspirilene induces increases in the levels of both ATG5 and ATG12-ATG5 conjugates, our result here is consistent with the conclusions of both studies. Taken together, we conclude that increased levels of LC3II in fluspirilene-treated cells promote autophagy by increasing levels of ATG5 and ATG12-ATG5 conjugates.
Acknowledgments
This work was supported in part by grants from the National Institute on Aging (US) (PO1 AG027916, to J. Yuan), the Chinese Academy of Science (KGCX2-SW-209, to D. Ma) and the National Natural Science Foundation of China (90813007, to L. Zhang).
References
- 1.Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290:1717–21. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levine B, Yuan J. Autophagy in cell death: an innocent convict? J Clin Invest. 2005;115:2679–88. doi: 10.1172/JCI26390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lum JJ, DeBerardinis RJ, Thompson CB. Autophagy in metazoans: cell survival in the land of plenty. Nat Rev Mol Cell Biol. 2005;6:439–48. doi: 10.1038/nrm1660. [DOI] [PubMed] [Google Scholar]
- 4.Yorimitsu T, Klionsky DJ. Autophagy: Molecular machinery for self-eating. Cell Death Differ. 2005;12:1542–52. doi: 10.1038/sj.cdd.4401765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang L, Yu J, Pan H, Hu P, Hao Y, Cai W, Zhu H, Yu AD, Xie X, Ma D, Yuan J. Small molecule regulators of autophagy identified by an image-based high-throughput screen. Proc Natl Acad Sci U S A. 2007;104:19023–8. doi: 10.1073/pnas.0709695104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grantham CJ, Main MJ, Cannell MB. Fluspirilene block of N-type calcium current in NGF-differentiated PC12 cells. Br J Pharmacol. 1994;111:483–8. doi: 10.1111/j.1476-5381.1994.tb14762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hagiwara K, Nakagawasai O, Murata A, Yamadera F, Miyoshi I, Tan-No K, Tadano T, Yanagisawa T, Iijima T, Murakami M. Analgesic action of loperamide, an opioid agonist, and its blocking action on voltage-dependent Ca2+ channels. Neurosci Res. 2003;46:493–7. doi: 10.1016/s0168-0102(03)00126-3. [DOI] [PubMed] [Google Scholar]
- 8.Ito K, Nakazawa K, Koizumi S, Liu M, Takeuchi K, Hashimoto T, Ohno Y, Inoue K. Inhibition by antipsychotic drugs of L-type Ca2+ channel current in PC12 cells. Eur J Pharmacol. 1996;314:143–50. doi: 10.1016/s0014-2999(96)00500-6. [DOI] [PubMed] [Google Scholar]
- 9.Bhatnagar K, Singh VP. Ca2+ dependence and inhibitory effects of trifluoperazine on plasma membrane ATPase of Thermoactinomyces vulgaris. Curr Microbiol. 2004;49:28–31. doi: 10.1007/s00284-003-4261-8. [DOI] [PubMed] [Google Scholar]
- 10.Amenta F, Tomassoni D, Traini E, Mignini F, Veglio F. Nicardipine: a hypotensive dihydropyridine-type calcium antagonist with a peculiar cerebrovascular profile. Clin Exp Hypertens. 2008;30:808–26. doi: 10.1080/10641960802580190. [DOI] [PubMed] [Google Scholar]
- 11.Williams A, Sarkar S, Cuddon P, Ttofi EK, Saiki S, Siddiqi FH, Jahreiss L, Fleming A, Pask D, Goldsmith P, O’Kane CJ, Floto RA, Rubinsztein DC. Novel targets for Huntington’s disease in an mTOR-independent autophagy pathway. Nat Chem Biol. 2008;4:295–305. doi: 10.1038/nchembio.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie Z, Nair U, Klionsky DJ. Atg8 controls phagophore expansion during autophagosome formation. Mol Biol Cell. 2008;19:3290–8. doi: 10.1091/mbc.E07-12-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klionsky DJ, Elazar Z, Seglen PO, Rubinsztein DC. Does bafilomycin A1 block the fusion of autophagosomes with lysosomes? Autophagy. 2008;4:849–50. doi: 10.4161/auto.6845. [DOI] [PubMed] [Google Scholar]
- 14.Hanada T, Noda NN, Satomi Y, Ichimura Y, Fujioka Y, Takao T, Inagaki F, Ohsumi Y. The ATG12-ATG5 conjugate has a novel E3-like activity for protein lipidation in autophagy. J Biol Chem. 2007;282:37298–302. doi: 10.1074/jbc.C700195200. [DOI] [PubMed] [Google Scholar]
- 15.Hanada T, Ohsumi Y. Structure-function relationship of ATG12, a ubiquitin-like modifier essential for autophagy. Autophagy. 2005;1:110–8. doi: 10.4161/auto.1.2.1858. [DOI] [PubMed] [Google Scholar]
- 16.Yousefi S, Perozzo R, Schmid I, Ziemiecki A, Schaffner T, Scapozza L, Brunner T, Simon HU. Calpain-mediated cleavage of ATG5 switches autophagy to apoptosis. Nat Cell Biol. 2006;8:1124–32. doi: 10.1038/ncb1482. [DOI] [PubMed] [Google Scholar]
- 17.Charest R, Prpi V, Exton JH, Blackmore PF. Stimulation of inositol trisphosphate formation in hepatocytes by vasopressin, adrenaline and angiotensin II and its relationship to changes in cytosolic free Ca2+ Biochem J. 1985;227:79–90. doi: 10.1042/bj2270079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dedieu S, Mazères G, Poussard S, Brustis JJ, Cottin P. Myoblast migration is prevented by a calpain-dependent accumulation of MARCKS. Biol Cell. 2003;95:615–23. doi: 10.1016/j.biolcel.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Dewitt S, Hallett M. Leukocyte membrane “expansion”: a central mechanism for leukocyte extravasation. J Leukoc Biol. 2007;81:1160–4. doi: 10.1189/jlb.1106710. [DOI] [PubMed] [Google Scholar]
- 20.Hood JL, Brooks WH, Roszman TL. Subcellular mobility of the calpain/calpastatin network: an organelle transient. Bioessays. 2006;28:850–9. doi: 10.1002/bies.20440. [DOI] [PubMed] [Google Scholar]
- 21.Høyer-Hansen M, Bastholm L, Szyniarowski P, Campanella M, Szabadkai G, Farkas T, Bianchi K, Fehrenbacher N, Elling F, Rizzuto R, Mathiasen IS, Jäättelä M. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol Cell. 2007;25:193–205. doi: 10.1016/j.molcel.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 22.Demarchi F, Bertoli C, Copetti T, Tanida I, Brancolini C, Eskelinen EL, Schneider C. Calpain is required for macroautophagy in mammalian cells. J Cell Biol. 2006;175:595–605. doi: 10.1083/jcb.200601024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng Y, Qiu F, Huang J, Tashiro S, Onodera S, Ikejima T. Apoptosis-suppressing and autophagy-promoting effects of calpain on oridonin-induced L929 cell death. Arch Biochem Biophys. 2008;475:148–55. doi: 10.1016/j.abb.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 24.Hosokawa N, Hara Y, Mizushima N. Generation of cell lines with tetracycline-regulated autophagy and a role for autophagy in controlling cell size. FEBS Lett. 2006;580:2623–9. doi: 10.1016/j.febslet.2006.04.008. [DOI] [PubMed] [Google Scholar]