Abstract
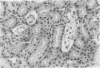
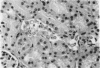
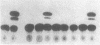
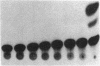
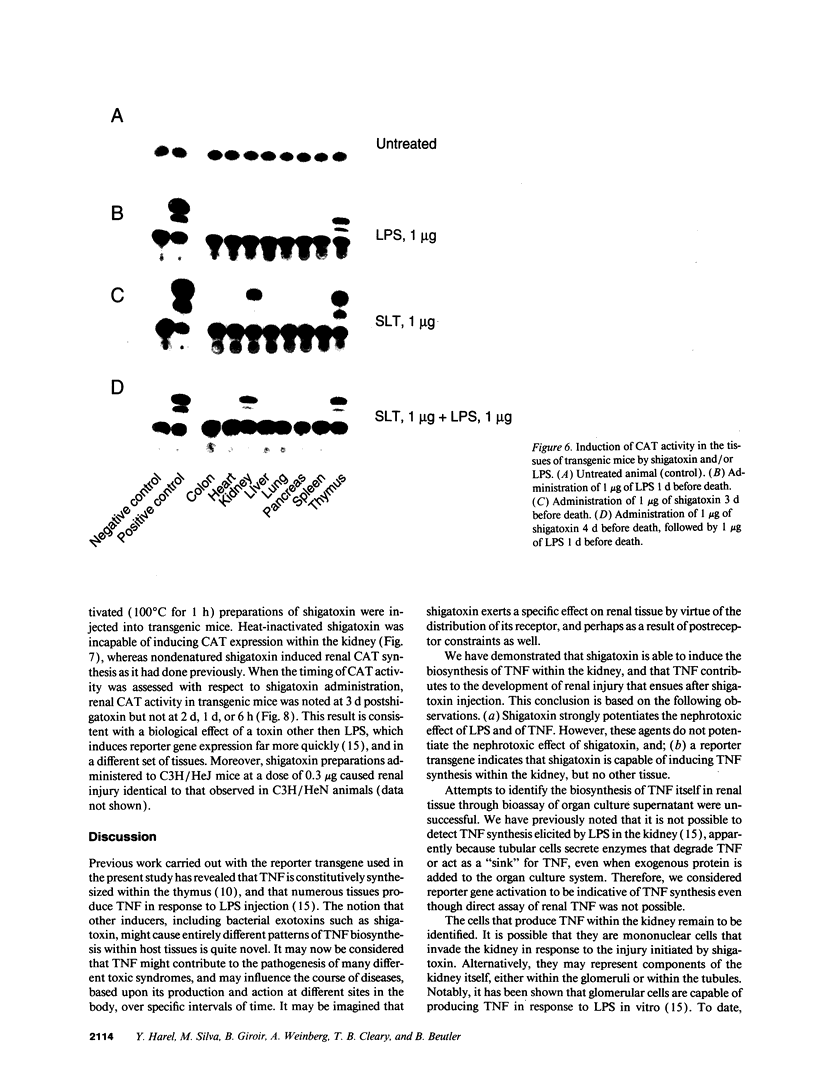
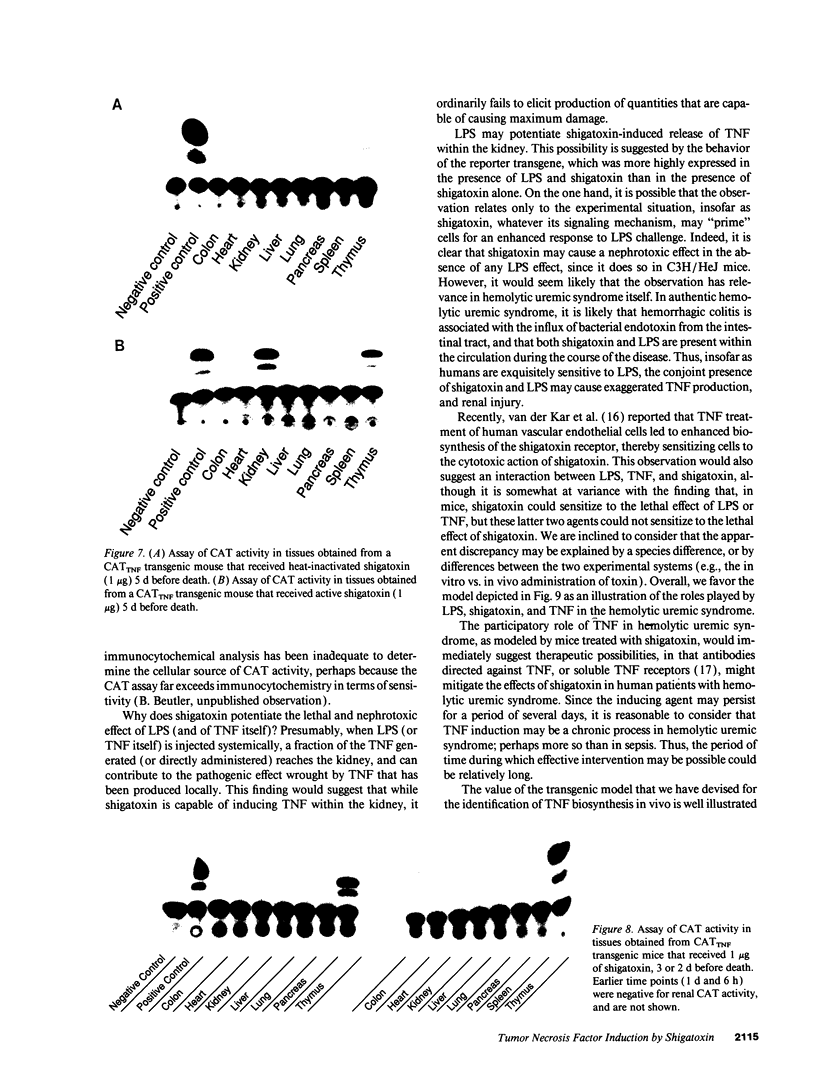
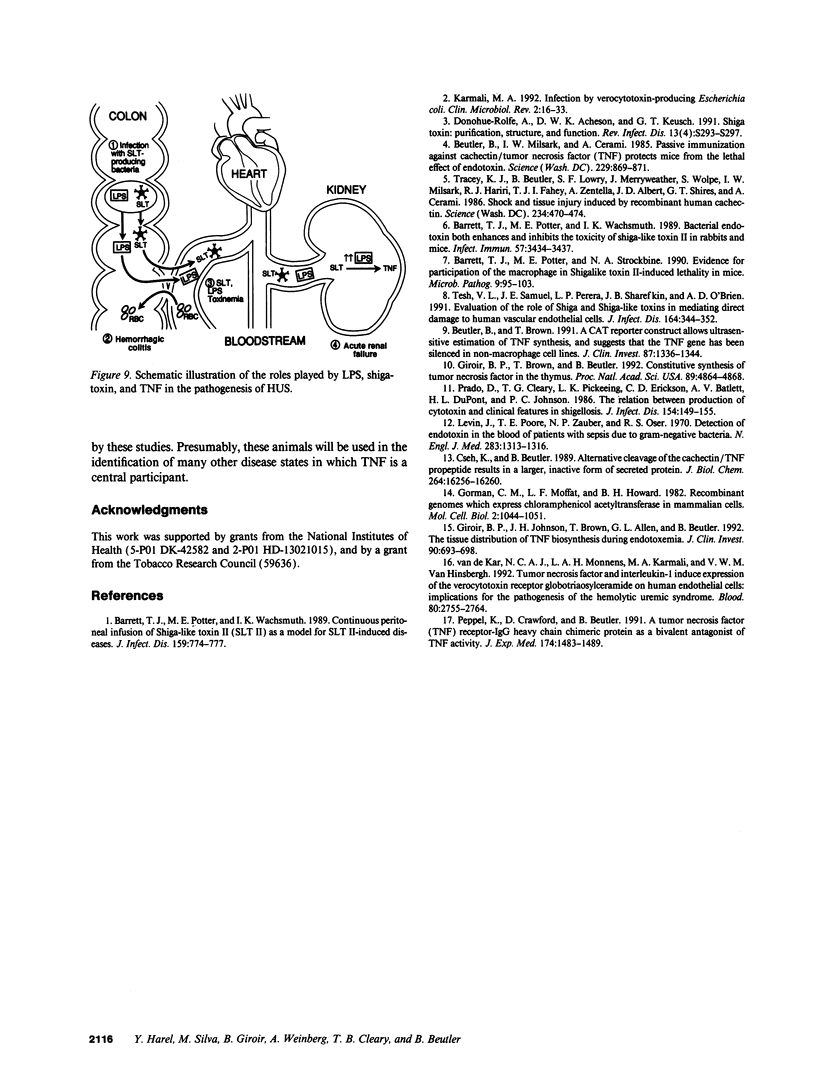
We have examined the hypothesis that TNF may play a pathogenetically important role in the hemolytic uremic syndrome. Specifically, we considered the possibility that shigatoxin, which eventuates this syndrome, might induce TNF biosynthesis, and/or that TNF and shigatoxin might sensitize animals, each to the toxic effects of the other agent. Shigatoxin was found to sensitize mice to the lethal effect of LPS and to the lethal effect of TNF. On the other hand, pretreatment of animals with either TNF or LPS did not noticeably sensitize mice to the lethal effect of shigatoxin. Intraperitoneal injections of shigatoxin did not induce the production of detectable quantities of TNF in the plasma of mice. When shigatoxin was injected into transgenic mice bearing a chloramphenicol acetyltransferase (CAT) reporter gene that indicates TNF synthesis, CAT activity was induced within the kidney, but not in other tissues. We therefore conclude that shigatoxin acts to induce TNF synthesis within the kidney, and at the same time increases renal sensitivity to the toxic effects of TNF. While this mouse model does not reproduce the hemolytic uremic syndrome as it occurs in humans, it does suggest that local synthesis of TNF within the kidney may contribute to renal injury induced by shigatoxin.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett T. J., Potter M. E., Strockbine N. A. Evidence for participation of the macrophage in Shiga-like toxin II-induced lethality in mice. Microb Pathog. 1990 Aug;9(2):95–103. doi: 10.1016/0882-4010(90)90083-3. [DOI] [PubMed] [Google Scholar]
- Barrett T. J., Potter M. E., Wachsmuth I. K. Bacterial endotoxin both enhances and inhibits the toxicity of Shiga-like toxin II in rabbits and mice. Infect Immun. 1989 Nov;57(11):3434–3437. doi: 10.1128/iai.57.11.3434-3437.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett T. J., Potter M. E., Wachsmuth I. K. Continuous peritoneal infusion of Shiga-like toxin II (SLT II) as a model for SLT II-induced diseases. J Infect Dis. 1989 Apr;159(4):774–777. doi: 10.1093/infdis/159.4.774. [DOI] [PubMed] [Google Scholar]
- Beutler B., Brown T. A CAT reporter construct allows ultrasensitive estimation of TNF synthesis, and suggests that the TNF gene has been silenced in non-macrophage cell lines. J Clin Invest. 1991 Apr;87(4):1336–1344. doi: 10.1172/JCI115137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B., Milsark I. W., Cerami A. C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985 Aug 30;229(4716):869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- Cseh K., Beutler B. Alternative cleavage of the cachectin/tumor necrosis factor propeptide results in a larger, inactive form of secreted protein. J Biol Chem. 1989 Sep 25;264(27):16256–16260. [PubMed] [Google Scholar]
- Donohue-Rolfe A., Acheson D. W., Keusch G. T. Shiga toxin: purification, structure, and function. Rev Infect Dis. 1991 Mar-Apr;13 (Suppl 4):S293–S297. doi: 10.1093/clinids/13.supplement_4.s293. [DOI] [PubMed] [Google Scholar]
- Giroir B. P., Brown T., Beutler B. Constitutive synthesis of tumor necrosis factor in the thymus. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4864–4868. doi: 10.1073/pnas.89.11.4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giroir B. P., Johnson J. H., Brown T., Allen G. L., Beutler B. The tissue distribution of tumor necrosis factor biosynthesis during endotoxemia. J Clin Invest. 1992 Sep;90(3):693–698. doi: 10.1172/JCI115939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin J., Poore T. E., Zauber N. P., Oser R. S. Detection of endotoxin in the blood of patients with sepsis due to gran-negative bacteria. N Engl J Med. 1970 Dec 10;283(24):1313–1316. doi: 10.1056/NEJM197012102832404. [DOI] [PubMed] [Google Scholar]
- Peppel K., Crawford D., Beutler B. A tumor necrosis factor (TNF) receptor-IgG heavy chain chimeric protein as a bivalent antagonist of TNF activity. J Exp Med. 1991 Dec 1;174(6):1483–1489. doi: 10.1084/jem.174.6.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado D., Cleary T. G., Pickering L. K., Ericsson C. D., Bartlett A. V., 3rd, DuPont H. L., Johnson P. C. The relation between production of cytotoxin and clinical features in shigellosis. J Infect Dis. 1986 Jul;154(1):149–155. doi: 10.1093/infdis/154.1.149. [DOI] [PubMed] [Google Scholar]
- Tesh V. L., Samuel J. E., Perera L. P., Sharefkin J. B., O'Brien A. D. Evaluation of the role of Shiga and Shiga-like toxins in mediating direct damage to human vascular endothelial cells. J Infect Dis. 1991 Aug;164(2):344–352. doi: 10.1093/infdis/164.2.344. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Beutler B., Lowry S. F., Merryweather J., Wolpe S., Milsark I. W., Hariri R. J., Fahey T. J., 3rd, Zentella A., Albert J. D. Shock and tissue injury induced by recombinant human cachectin. Science. 1986 Oct 24;234(4775):470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- van de Kar N. C., Monnens L. A., Karmali M. A., van Hinsbergh V. W. Tumor necrosis factor and interleukin-1 induce expression of the verocytotoxin receptor globotriaosylceramide on human endothelial cells: implications for the pathogenesis of the hemolytic uremic syndrome. Blood. 1992 Dec 1;80(11):2755–2764. [PubMed] [Google Scholar]