Abstract
There is current interest in identifying drugs that facilitate fear extinction, as this form of learning is the basis of certain cognitive therapies for anxiety disorders. Following an initial report several years ago that the α2-adrenoreceptor antagonist yohimbine facilitated extinction in mice, more recent studies have shown mixed effects or even impairment. It has become clear that the effect of yohimbine on extinction depends on a number of factors, including genetic background, contextual variables and the presence of competing behaviors. To what extent theses effects of yohimbine are mediated through the α2-adrenoreceptor, as opposed to other sites of action, is also uncertain. More work is needed before this drug can be approved as a pharmacological adjunct for extinction-based therapies. More generally, the case of yohimbine may serve as a model for the development of other extinction facilitators.
Background
The prevalence and burden of anxiety disorders such as posttraumatic stress disorder (PTSD) is rapidly increasing, but effective treatments for these disorders remain inadequate. Therefore, there has been recent interest in identifying novel therapeutic targets. Fear extinction is a form of learning that occurs in response to repeated exposure to a conditioned stimulus and inhibits the expression of a traumatic memory [1]. This behavior is readily measured in animal models and humans and is mediated by a homologous and increasingly well-defined neural circuit comprising the prefrontal cortex (PFC), amygdala and hippocampus [2]. For these reasons, there has been a resurgence of interest in fear extinction as a particularly tractable experimental model for translating basic research into clinical leads for anxiety disorders [3,4]. There is now an intensive research effort directed at identifying drugs that facilitate fear extinction in rodents and that may, by extension, have efficacy as treatments in anxiety disorders (see Box 1). Here, we discuss an example of one compound, yohimbine, that, with the exception of D-cycloserine, has probably been the most well studied drug for effects on fear extinction.
Introduction: Noradrenergic modulation of fear extinction and yohimbine as a facilitator of extinction
A wealth of research supports a major role for the noradrenergic system in the formation and maintenance of emotional memories [5]. Early work demonstrated that neurochemical lesions of the locus coeruleus, the site of a major group of noradrenergic cells innervating the fore-brain, impaired fear extinction in rats [6,7]. Extending these findings, work from one of our groups demonstrated that microinjection of propranolol, an antagonist of noradrenergic β-receptors, into the infralimbic region of the rat PFC prior to extinction training impaired later retrieval of the extinction memory [8]. On the basis of the finding that injection of norepinephrine increased the excitability of neurons in this region, the extinction impairing effects of propranolol were attributed to a depression of infralimbic excitability [8]. By extension, these findings suggest that drugs targeting the noradrenergic system could facilitate fear extinction and be of therapeutic value for treating anxiety disorders.
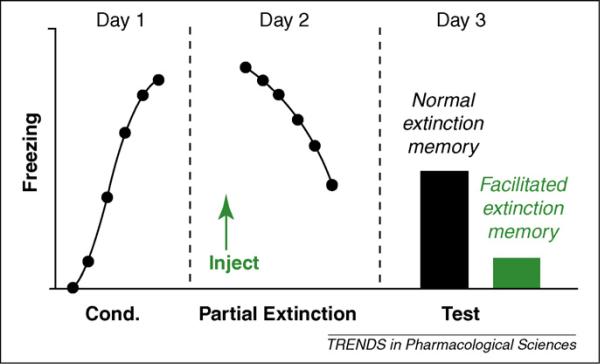
Yohimbine is a competitive antagonist at both postsynaptic and presynaptic α2-adrenoreceptors. By blocking autoreceptor inhibition of norepinephrine release, yohimbine increases extracellular levels of norepinephrine in forebrain regions known to mediate fear and fear extinction including the hippocampus, amygdala and prefrontal cortex [9–12] Cain et al. first reported that systemic treatment with yohimbine facilitated fear extinction. They found that mice treated with 5 mg/kg yohimbine prior to (but not immediately after) extinction training showed improved retrieval of both cued and contextual extinction memories, as measured by reduced fear to the conditioned stimulus or context, respectively, when tested drug-free the following day [13]. The extinction-facilitating effect of yohimbine occurred in mice given partial extinction training (i.e. five extinction trials or 20 minutes context exposure) (see Figure 1) but not more extensive extinction training (i.e. ten or more extinction trials or 40 minutes context exposure). Improved extinction retrieval was also evident in mice that were treated with yohimbine prior to temporally spaced extinction training [13], a procedure that impairs long-term fear extinction [14].
Figure 1.
A commonly employed procedure for testing the effects of putative extinction-facilitating drugs such as yohimbine in rodents. On Day 1, subjects undergo Pavlovian fear conditioning in which a previous innocuous stimulus, for example auditory tone, (conditioned stimulus, CS) is repeatedly paired with an aversive stimulus, for example footshock (unconditioned stimulus, US). The fear response to the tone is often measured by quantifying freezing. On Day 2, subjects are treated with the drug (or an appropriate vehicle control) and then undergo partial fear extinction training in which CS is repeatedly presented in the absence of the US. On Day 3, extinction retrieval is tested. If drug treatment was effective in facilitating fear extinction, the fear response to the CS in drug-treated subjects will be significantly less than in vehicle-treated controls.
Box 1. Prolonged exposure as a therapy for anxiety disorders.
It may seem paradoxical, but an effective treatment for people with anxiety disorders is to repeatedly expose them to the triggers of their anxiety. This can include objects of simple phobias (e.g. snakes or heights), sounds or pictures associated with trauma (e.g. war-related stimuli), or even a patient-generated script of the traumatic event. Performed within a therapeutic context, ‘prolonged exposure’ (PE) uses a process of extinction to inhibit fear responses to anxiety triggers. In effect, the exposure disconfirms the hypothesis in the patient's brain that the stimuli signal danger. PE is carried out in approximately 15 sessions with a trained therapist, and this includes therapeutic ‘homework’ in which the patient continues the exposure at home [60]. A course of PE has been found to reduce symptoms in 85% of patients and is significantly more effective than other treatments or control conditions [61].
However, there is room for improvement. Not all patients respond favorably to PE and others are unable to comply with what is quite a stressful procedure. For this reason, neuroscientists have been looking for pharmacological agents that could act as adjuncts to PE; that is, drugs that can be given during PE and facilitate the learning or, ideally, long term retention of extinction. The first such drug was D-cycloserine, a partial agonist of the NMDA receptor. Building on basic research demonstrating that extinction learning was dependent upon NMDA receptor function, D-cycloserine has been shown to facilitate PE for several types of anxiety disorders [62]. Along similar lines of reasoning, various drugs that facilitate extinction in rodent studies could be tested for their efficacy as adjuncts to PE in clinical trials. These include yohimbine, as well as compounds targeting endocannabinoids, growth factors, corticosterone, dopa-mine, and various cognitive enhancers.
The discovery by Cain et al. sparked interest in the field [15] because it suggested a new avenue for treating anxiety disorders by treating patients with yohimbine (or a drug with a similar mechanism of action) as they underwent extinction-like clinical procedures such as exposure therapy. The finding also spoke to a more general issue of how emotional state during extinction determines the long-term strength of the extinction memory and, thereby, the efficacy of the treatment. This is because yohimbine has potent arousal and anxiety-provoking effects in rodents [16–18] and humans [19]. While it may at first seem paradoxical that an anxiogenic drug would facilitate extinction of fear, such an effect would be consistent with the hypothesis that extinction learning will be more effective if it occurs when the patient is in an emotionally excited state similar to that experienced when the fear memory was initially formed [20]. Cain et al. concluded that ‘yohimbine may be a useful adjunct to such behavior therapy of human anxiety disorders, despite being anxiogenic’ and ‘may not only accelerate treatment when given with otherwise effective behavioral exposure protocols, but also, in some cases, convert ineffective exposures into effective treatments.’ Given that yohimbine is already in clinical use for other indications, such as erectile dysfunction [21], its application as a therapy for anxiety disorders would circumvent much of the lengthy process (e.g. toxicology evaluation) that a novel compound would have to undergo.
Are yohimbine's extinction-facilitating effects dependent upon genetic factors?
Since Cain et al.'s initial finding, a number of studies have now reported on yohimbine's effects on extinction. The findings paint a more complex picture of the drug's actions (summarized in Table 1). Cain and colleagues demonstrated pro-extinction activity of yohimbine in C57BL/6J mice. This is an inbred strain commonly used in behavioral neuroscience which exhibits relatively low levels of anxiety-like behavior and stress-reactivity in various tasks [22]. The C57BL/6J strain also displays good fear extinction relative to certain other inbred strains, notably 129S1/ SvImJ [23,24]. Davis et al. recently reported that systemic (2.5 or 5 mg) yohimbine treatment of C57BL/6J mice prior to extinction training reduced ‘fear’ (note: dissociating fear-reducing from locomotor hyperactivity-inducing effects of yohimbine can be difficult) but failed to affect extinction retrieval the following day [25]. By contrast, when tested under the same conditioning and extinction procedures, Hefner et al. demonstrated that the same doses of yohimbine significantly improved extinction retrieval in the 129S1/SvImJ inbred strain [24]. Importantly, the two strains differed markedly in their baseline levels of fear extinction. The extensive (fifty-trial) extinction training protocol used in these studies produced strong extinction retrieval in C57BL/6J mice but failed to produce any demonstrable extinction in 129S1/SvImJ mice (see Box 2). One reasonable conclusion, therefore, is that fear extinction was already at asymptote in C57BL/6J mice and there was little left to be augmented by yohimbine. This would seem consistent with Cain et al. data showing that extinction-facilitation was only seen in C57BL/6J mice that were partially extinguished. These findings also raise the intriguing alternative possibility that the drug's effects on extinction may be specific to genetic backgrounds in which extinction is deficient. This warrants further study given the potential clinical implication that yohimbine may be preferentially effective in treating anxiety disorders in subpopulations of patients with a certain genetic profile causing a particularly intractable extinction deficit.
Table 1.
Effects of yohimbine on fear extinction in rodents as a function of strain and extinction training and testing protocol. Note - all studies administered yohimbine systemically, via the intra-peritoneal route, prior to extinction training
| Species (strain) sex | Dose (mg/kg) | Key features of fear extinction | Contextual design | Effect on long-term fear extinction | Reference |
|---|---|---|---|---|---|
| Mouse (C57BL/6) male | 5.0 | Partial training | ABB | Facilitated | [13] |
| Mouse (C57BL/6) male | 5.0 | Extensive training | ABB | No effect | [13] |
| Mouse (C57BL/6) male | 5.0 | Spaced training | ABB | Facilitated | [13] |
| Mouse (C57BL/6) male | 2.5, 5.0 | Extensive training | ABB | No effect | [25] |
| Mouse (129/SvImJ) male | 5.0 | Extensive training but impaired learning | ABB | Facilitated | [24] |
| Rat (Wistar) female | 0.1, 5.0 | Extensive training | ABB | No effect | [26] |
| Rat (Wistar) female | 1.0 | Extensive training | ABB | Facilitated | [26] |
| Rat (Wistar) female | 1.0 | Extensive training | ABCC | Facilitated | [26] |
| Rat (Wistar) female | 1.0 | Extensive training | ABA | No effect | [26] |
| Rat (Wistar) female | 1.0 | Extensive training | ABC | Impaired | [26] |
| Rat (Sprague-Dawley) male | 1.0, 2.0, 5.0 | Partial training | AAA | No effect | [29] |
| Rat (Sprague-Dawley) male | 1.0 | Partial training | ABB | No effect | [29] |
Are yohimbine's extinction-facilitating effects dependent upon contextual variables?
A recent study by Morris and Bouton found that rats systemically administered 0.1, 1 or 5 mg/kg yohimbine prior to extinction training reduced initial fear expression, while only those rats treated with 1 mg/kg dose also showed showed improved fear extinction when tested drug-free the following day [26]. It is well established that the expression of extinction memory is contextually gated, such that fear ‘renews’ after extinction if tested in a context other than that in which extinction learning occurred [27]. Morris and Bouton found that the extinction-facilitating effect of (1 mg/kg) yohimbine was lost when retrieval was tested in the original (fear-renewing) conditioning context; similar observations have been made with another pro-extinction drug, D-cycloserine [28]. They then went on to demonstrate that when retrieval was tested in a novel context, rats treated with this dose of yohimbine prior to extinction learning failed to express the extinction memory, and actually showed stronger renewal than vehicle-treated controls [27]. Moreover, they found yohimbine given prior to exposure to a context was sufficient to subsequently reduce fear to a conditioned stimulus presented in that same context (although only when the stimulus had been at least partially extinguished). To account for this pattern of results Morris and Bouton propose that during extinction training, the extinction context acquires the capacity to inhibit later fear in that context, regardless of whether there was also exposure to the conditioned stimulus, and that yohimbine's primary mode of action is to strengthen this inhibitory effect.
Box 2. A mouse model of impaired fear extinction.
Individuals differ widely in being at risk for anxiety disorders, such as PTSD and phobias [63]. Susceptibility is probably due to genetic predisposition interacting with exposure to environmental traumas [63–66]. However, although there are now a number of examples of stress-induced fear extinction deficits [67–69], there still remain relatively few ‘genetic’ preclinical models of impaired extinction [70]. By assessing a panel of inbred mouse strains that are commonly used in anxiety research [22], recent work identified the 129S1/SvImJ strain as exhibiting a profound deficit in fear extinction, as compared to other strains, such as C57BL/6J [24]. This trait appears to be a specific fear-related trait, but a general characteristic of the 129 family of inbred strains, of which there are many substrains [23].
A mouse with a phenotype of impaired extinction, such as the 129S1/SvImJ strain, provides a valuable approach to modeling the pathology anxiety disorders. Such a model offers a tool for delineating neural, molecular and ultimately genetic mechanisms underlying impaired extinction and perhaps the symptoms of PTSD more broadly. It also provides a novel approach to screening for extinction facilitating drugs. 129S1/SvImJ mice do not respond to the pro-extinction effects of D-cycloserine treatment [24] - most probably due to the necessity of at least some level of extinction learning for this drug to bolster [62]. Yohimbine treatment, however, produces significant extinction facilitation in these mice [25], indicating that this drug is capable of producing pro-extinction effects against an extinction resistant baseline. These findings augur well for the future utility of this model as a valuable addition to the researcher's toolbox for discovering new drugs that promote fear extinction.
Recently, Mueller and colleagues found that rats systemically treated with either 1, 2 or 5 mg/kg yohimbine showed reduced fear behavior but did not show evidence of improved fear extinction retrieval when tested the next day [29]. Negative effects on extinction were found regardless of whether rats were trained and extinguished in the same or different contexts. This study also found that yohimbine produced non-specific decreases in locomotor activity, demonstrating a potential confound of acute reductions in ‘fear.’ The reason for the discrepancy of these findings and those of Morris and Bouton is not yet fully clear but may relate to differences in the experimental methods employed. For example, in the task employed by Mueller et al., fear was measured by suppression of bar-pressing as well as freezing. It is possible that factors that support the bar-pressing, such as appetitive motivation and food deprivation, somehow mitigate the effects of yohimbine. Another possibility, given the conclusion of Morris and Bouton that yohimbine is particularly effective in increasing the learning of contextual inhibition of responding to a fear-conditioned stimulus, is that the incorporation of bar-pressing renders the context more ambiguous, which serves to weaken yohimbine's effects. A third potentially critical factor is that Mueller et al. employed Sprague-Dawley rats, while Morris and Bouton tested in female Wistar rats. Differences in subjects’ sex and/or strain could potentially account for the differing findings.
These data again underscore the complex actions of this drug on extinction. This complexity has important implications for how generalizable the drug's effects would be in clinical settings, where variables including context are likely to be critical to determining the efficacy of treatment [30]. Thus, if yohimbine's pro-extinction effects do not transfer beyond the clinician's office, or as Morris and Bouton's data suggest, could even increase the chances of relapse in such settings, this would severely bring into question the drug's potential.
Are yohimbine's effects on extinction mediated via α2-adrenoreceptors?
Yohimbine's effects on increasing levels of extracellular norepinephrine are thought to be the mechanism by which the drug produces it's pro-anxiety effects [17,19]. Increased extracellular norepinephrine levels with yohimbine would also be consistent with the fact that antidepressant drugs that block norepinephrine reuptake (amitriptyline, imipramine, desipramine) can have similar effects on extinction in rats [31].The pharmacology of yohimbine is, however, complex, with actions on dopamine D2 receptors and multiple 5-HT receptor subtypes (5-HT1A, 5-HT1B, 5-HT1D) [32–40] (Table 2). The potential contribution of these actions on the behavioral effects of yohimbine, including fear and extinction, has largely been ignored. Some recent findings do shed some light on this issue, however. Work in (C57BL/6J strain) mice has shown that systemic treatment with atipamezole, a more selective α2-adrenoreceptor antagonist than yohimbine, failed to improve extinction retrieval and actually trended towards impairing extinction [25]. The same study also examined the effects of both yohimbine and atipamezole on another form of extinction in mice: extinction of a conditioned place preference for cocaine. Results showed that yohimbine impaired, rather than facilitated, extinction of cocaine CPP, while atipamezole had no effect. Moreover, when mice with a targeted null mutation of the αa2-adrenoreceptor were employed to test whether these effects of yohimbine were dependent upon the receptor, extinction was not only present but actually enhanced in the mice.
Table 2.
Pharmacological effects of yohimbine at dopamine and 5-HT receptors. Data are radioligand binding affinity (pKi) measurements in human hippocampal, CHO and cells originally reported in Millan et al. [33]
| Receptor targets |
|||||||
|---|---|---|---|---|---|---|---|
| D2 | D3 | 5-HT1A | 5-HT1B | 5-HT1D | 5-HT2A | 5-HT2C | |
| Known action | Antagonist | - | Partial agonist | Partial agonist | Partial agonist | - | - |
| pKi | 6.4 | 5.4 | 7.3 | 6.8 | 7.6 | <6.0 | <6.0 |
Collectively, these data make two important points. First, the impairing effects of yohimbine on an appetitive form of extinction contrast with the putative extinction-facilitating effects on fear extinction. Second, for cocaine CPP extinction at least, the effects of yohimbine do not appear to be mediated by the α2-adrenoreceptor, which is the putative primary site of the drug's behavioral effects. A key question for future studies is whether facilitating effects of yohimbine on fear extinction (under the specific experimental conditions and genetic backgrounds in which they occur) are also mediated by a mechanism that is independent of the α2-adrenoreceptor. Indeed, a recent report showed that systemic blockade of noradrenergic beta receptors with propranolol treatment had no effect on fear extinction [41], further calling into question the extent to which extinction requires noradrenergic stimulation.
If not the α2-adrenoreceptor, the question then becomes, what is the target? One obvious candidate is the 5-HT1A receptor. Yohimbine has high affinity for the 5-HT1A receptor (pKi=7.32) [33] and acts as a 5-HT1A receptor partial agonist [42]. The selectivity of yohimbine for α2-adrenoreceptors over 5-HT1A receptors is relatively poor, as estimated by receptor-binding [34,43]; ~10-fold in humans and even less (~4-fold) in rats. In mouse drug discrimination studies, yohimbine effectively generalizes to drugs such as 8-OH-DPAT that are 5-HT1A receptor agonists with little affinity for α2-adrenoreceptors [43,44]. Moreover, a variety of yohimbine effects, including disruption of prepulse inhibition of startle [45], hypothermia [33], increased alcohol seeking [46], and anxiogenic-like effects [47,48], have been attributed to actions at 5-HT1A receptors rather than α2-adrenoreceptors. Finally in vivo microdialysis in rats showed that systemically administered (2.5-20 mg/kg) yohimbine reduced firing of 5-HT dorsal raphe neurons and decreased extracellular levels of 5-HT in cortex (in addition to increasing norepinephrine and dopamine levels) [33]. Clearly, yohimbine treatments shown to alter extinction likely had potent effects on the 5-HT1A receptor and the 5-HT system more generally. However, the potential involvement of 5-HT actions to yohimbine's effects is far from clear - in part, because the contribution of the 5-HT system to fear extinction is itself uncertain. For example, while certain genetic perturbations of the 5-HT system impair fear extinction in mice [49], pharmacological treatments (e.g. anti-depressant medications) that boost 5-HT levels alter fear but not extinction in rats and mice [50,51]. In terms of the 5-HT1A receptor per se, we are unaware of any reports on the effects of 5-HT1A receptor drugs on fear extinction. As such, there are not yet solid grounds for suggesting that actions at the 5-HT1A receptor account for the pro-extinction effects of yohimbine.
Perhaps a more likely ‘off-target’ candidate for these effects is the dopamine D2 receptor. As noted, yohimbine has high affinity (pKi=6.4) for the dopamine D2 receptor and acts as an antagonist at the receptor [33]. Blockade of D2 autoreceptors may contribute to yohimbine-induced increases in cortical levels of dopamine [52,53] and striatal dopamine synthesis [39] -although α2-adrenoreceptors on dopamine terminals are probably involved too. Systemic pre-extinction administration of the D2-like receptor antagonist sulpiride facilitated fear extinction in mice [54] while administration of the D2-like agonist quinpirole impaired fear extinction in mice [54] and rats [55].Although more studies are needed to substantiate the contribution of the D2 receptor to fear extinction, we can speculate that blockade of these receptors could contribute to the pro-extinction effects of yohimbine. It will be important to test this hypothesis because if actions at D2 receptors do account for these effects, then the focus of further drug development could be more mechanistically focused. On the other hand, if the D2 receptor (or any other specific pharmacological target of yohimbine), is alone sufficient to account for the drug's pro-extinction effects, then this would suggest these effects in fact result from the combined, multi-target (‘dirty’) pharmacological profile of the drug.
Conclusions and future directions
The preclinical work on yohimbine's effects on extinction still needs to be followed-up with well designed clinical studies. At the time of writing, the first clinical study of yohimbine was published. Powers and colleagues treated 12 claustrophobic patients with 10.8 mg yohimbine (and 12 with placebo) prior to exposure to an enclosed space [56]. This is a higher single dose than recommended for other indications, such as erectile dysfunction (e.g. 5.4 mg). Yohimbine has acute anxiogenic and panicogenic effects in patients with anxiety disorders [57,58]. However, such strong fear responses are typically seen with higher doses in the range of 20 mg [59], and patients in this study reported no significant anxiety or arousal. On follow-up one week later, yohimbine-treated patients self-reported reduced fear scores on claustrophobia questionnaires and during enclosed space exposure, as compared to those receiving placebo. This is an encouraging first step supporting the efficacy of yohimbine as an adjunct to exposure therapy. It is worth noting that, given the nature of the phobia (i.e. of enclosed spaces), the fear-inducing stimulus was itself a context. This may be important in light of the aforementioned preclinical data suggesting that yohimbine might work through a context-dependent mechanism. As such, it will be necessary to determine if reduced fear after yohimbine is maintained in other enclosed spaces, including the real world where relapse occurs. It will also be important to test yohimbine's efficacy as an adjunct to exposure therapies involving specific phobic stimuli rather than contexts.
Is yohimbine likely to prove an effective tool for treating anxiety disorders? As is often the case with a potential new breakthrough in translational research the subsequent accrual of data points to a more complex and uncertain road ahead. The preclinical literature indicate that a better understanding of the contextual variables and genetic factors modulating yohimbine's effects on extinction in preclinical models will be needed to evaluate the potential clinical applicability of this drug for anxiety disorders. More generally, the case of yohimbine serves as a valuable test case for the development of extinction facilitators, and underscores the promise but also the complexities and caveats of this translational approach to discovering new anti-anxiety medications.
Acknowledgments
AH is supported by the NIAAA intramural research program. GJQ is supported by NIH grants MH058883 and MH081975.
References
- 1.Pavlov IP. Conditioned reflexes. Oxford University Press; 1927. [Google Scholar]
- 2.Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacol. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Curr. Opin. Neurobiol. 2006 doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Myers KM, Davis M. Mechanisms of fear extinction. Mol. Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- 5.McGaugh JL. Memory - a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 6.Mason ST, Fibiger H. Noradrenaline, fear and extinction. Brain Res. 1979;165:47–56. doi: 10.1016/0006-8993(79)90043-x. [DOI] [PubMed] [Google Scholar]
- 7.McCormick DA, Thompson RF. Locus coeruleus lesions and resistance to extinction of a classically conditioned response: involvement of the neocortex and hippocampus. Brain Res. 1982;245:239. doi: 10.1016/0006-8993(82)90806-x. [DOI] [PubMed] [Google Scholar]
- 8.Mueller D, et al. Noradrenergic signaling in infralimbic cortex increases cell excitability and strengthens memory for fear extinction. J. Neurosci. 2008;28:369–375. doi: 10.1523/JNEUROSCI.3248-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khoshbouei H, et al. Behavioral reactivity to stress: amplification of stress-induced noradrenergic activation elicits a galanin-mediated anxiolytic effect in central amygdala. Pharmacol. Biochem. Behav. 2002;71:407–417. doi: 10.1016/s0091-3057(01)00683-9. [DOI] [PubMed] [Google Scholar]
- 10.Abercrombie ED, et al. Characterization of hippocampal norepinephrine release as measured by microdialysis perfusion: pharmacological and behavioral studies. Neuroscience. 1988;27:897–904. doi: 10.1016/0306-4522(88)90192-3. [DOI] [PubMed] [Google Scholar]
- 11.Aghajanian GK, VanderMaelen CP. alpha 2-adrenoceptor-mediated hyperpolarization of locus coeruleus neurons: intracellular studies in vivo. Science. 1982;215:1394–1396. doi: 10.1126/science.6278591. [DOI] [PubMed] [Google Scholar]
- 12.Becker C, et al. Prevention by 5-HT1A receptor agonists of restraint stress- and yohimbine-induced release of cholecystokinin in the frontal cortex of the freely moving rat. Neuropharmacol. 1999;38:525–532. doi: 10.1016/s0028-3908(98)00209-3. [DOI] [PubMed] [Google Scholar]
- 13.Cain CK, et al. Adrenergic transmission facilitates extinction of conditional fear in mice. Learn Mem. 2004;11:179–187. doi: 10.1101/lm.71504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cain CK, et al. Temporally massed CS presentations generate more fear extinction than spaced presentations. J. Exp. Psychol. Anim. Behav. Process. 2003;29:323–333. doi: 10.1037/0097-7403.29.4.323. [DOI] [PubMed] [Google Scholar]
- 15.Quirk GJ. Learning not to fear, faster. Learn Mem. 2004;11:125–126. doi: 10.1101/lm.75404. [DOI] [PubMed] [Google Scholar]
- 16.Davis M, et al. Noradrenergic agonists and antagonists: effects on conditioned fear as measured by the potentiated startle paradigm. Psychopharmacology (Berl) 1979;65:111–118. doi: 10.1007/BF00433036. [DOI] [PubMed] [Google Scholar]
- 17.Holmes A, et al. Evaluation of an anxiety-related phenotype in galanin overexpressing transgenic mice. J. Mol. Neurosci. 2002;18:151–165. doi: 10.1385/JMN:18:1-2:151. [DOI] [PubMed] [Google Scholar]
- 18.Singewald N, et al. Induction of c-Fos expression in specific areas of the fear circuitry in rat forebrain by anxiogenic drugs. Biol. Psychiatry. 2003;53:275–283. doi: 10.1016/s0006-3223(02)01574-3. [DOI] [PubMed] [Google Scholar]
- 19.Bremner JD, et al. Noradrenergic mechanisms in stress and anxiety: II. Clinical studies. Synapse. 1996;23:39–51. doi: 10.1002/(SICI)1098-2396(199605)23:1<39::AID-SYN5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 20.Foa EB, Kozak MJ. Emotional processing of fear: exposure to corrective information. Psychol. Bull. 1986;99:20–35. [PubMed] [Google Scholar]
- 21.Ernst E, Pittler MH. Yohimbine for erectile dysfunction: a systematic review and meta-analysis of randomized clinical trials. J. Urol. 1998;159:433–436. doi: 10.1016/s0022-5347(01)63942-9. [DOI] [PubMed] [Google Scholar]
- 22.Millstein RA, Holmes A. Effects of repeated maternal separation on anxiety- and depression-related phenotypes in different mouse strains. Neurosci. Biobehav. Rev. 2007;31:3–17. doi: 10.1016/j.neubiorev.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Camp MC, et al. Impaired Pavlovian fear extinction is common across genetic lineages of the 129 inbred mouse strain. Genes Brain. Behav. doi: 10.1111/j.1601-183X.2009.00519.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hefner K, et al. Impaired fear extinction learning and corticoamygdala circuit abnormalities in a common genetic mouse strain. J. Neurosci. 2008;28:8074–8085. doi: 10.1523/JNEUROSCI.4904-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis AR, et al. Yohimbine impairs extinction of cocaine-conditioned place preference in an alpha2-adrenergic receptor independent process. Learn Mem. 2008;15:667–676. doi: 10.1101/lm.1079308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morris RW, Bouton ME. The effect of yohimbine on the extinction of conditioned fear: a role for context. Behav. Neurosci. 2007;121:501–514. doi: 10.1037/0735-7044.121.3.501. [DOI] [PubMed] [Google Scholar]
- 27.Bouton ME, King DA. Contextual control of the extinction of conditioned fear: tests for the associative value of the context. J. Exp. Psychol. Anim. Behav. Process. 1983;9:248–265. [PubMed] [Google Scholar]
- 28.Woods AM, Bouton ME. D-cycloserine facilitates extinction but does not eliminate renewal of the conditioned emotional response. Behav. Neurosci. 2006;120:1159–1162. doi: 10.1037/0735-7044.120.5.1159. [DOI] [PubMed] [Google Scholar]
- 29.Mueller D, et al. The effects of yohimbine and amphetamine on fear expression and extinction in rats. Psychopharmacology (Berl) 2009;204:599–606. doi: 10.1007/s00213-009-1491-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bouton ME. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol. Psychiatry. 2002;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- 31.Telegdy G, et al. Effects of a new antidepressant drug on active avoidance behavior in rats. Comparative study with tricyclic antidepressants. Arch. Int. Pharmacodyn. Ther. 1983;266:50–59. [PubMed] [Google Scholar]
- 32.Thomas DR, et al. Pharmacological characterisation of [35S]-GTPgammaS binding to Chinese hamster ovary cell membranes stably expressing cloned human 5-HT1D receptor subtypes. J. Recept. Signal Transduct. Res. 1995;15:199–211. doi: 10.3109/10799899509045217. [DOI] [PubMed] [Google Scholar]
- 33.Millan MJ, et al. Agonist and antagonist actions of yohimbine as compared to fluparoxan at alpha(2)-adrenergic receptors (AR)s, serotonin (5-HT)(1A), 5-HT(1B), 5-HT(1D) and dopamine D(2) and D(3) receptors. Significance for the modulation of frontocortical monoaminergic transmission and depressive states. Synapse. 2000;35:79–95. doi: 10.1002/(SICI)1098-2396(200002)35:2<79::AID-SYN1>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 34.Newman-Tancredi A, et al. Actions of alpha2 adrenoceptor ligands at alpha2A and 5-HT1A receptors: the antagonist, atipamezole, and the agonist, dexmedetomidine, are highly selective for alpha2A adrenoceptors. Naunyn Schmiedebergs Arch. Pharmacol. 1998;358:197–206. doi: 10.1007/pl00005243. [DOI] [PubMed] [Google Scholar]
- 35.Winter JC, Rabin RA. Antagonism of the stimulus effects of yohimbine and 8-hydroxydipropylaminotetralin. Pharmacol. Biochem. Behav. 1993;44:851–855. doi: 10.1016/0091-3057(93)90016-m. [DOI] [PubMed] [Google Scholar]
- 36.Llado J, et al. The alpha 2-adrenoceptor antagonist idazoxan is an agonist at 5-HT1A autoreceptors modulating serotonin synthesis in the rat brain in vivo. Neurosci. Lett. 1996;218:111–114. doi: 10.1016/s0304-3940(96)13132-3. [DOI] [PubMed] [Google Scholar]
- 37.Herrick-Davis K, et al. Serotonin 5-HT1D receptors in human prefrontal cortex and caudate: interaction with a GTP binding protein. J. Neurochem. 1988;51:1906–1912. doi: 10.1111/j.1471-4159.1988.tb01176.x. [DOI] [PubMed] [Google Scholar]
- 38.Weinshank RL, et al. Human serotonin 1D receptor is encoded by a subfamily of two distinct genes: 5-HT1D alpha and 5-HT1D beta. Proc. Natl. Acad. Sci. U. S. A. 1992;89:3630–3634. doi: 10.1073/pnas.89.8.3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scatton B, et al. Antidopaminergic properties of yohimbine. J. Pharmacol. Exp. Ther. 1980;215:494–499. [PubMed] [Google Scholar]
- 40.Waeber C, Palacios JM. 5-HT1 receptor binding sites in the guinea pig superior colliculus are predominantly of the 5-HT1D class and are presynaptically located on primary retinal afferents. Brain Res. 1990;528:207–211. doi: 10.1016/0006-8993(90)91659-5. [DOI] [PubMed] [Google Scholar]
- 41.Rodriguez-Romaguera J, et al. Systemic propranolol acts centrally to reduce conditioned fear in rats without impairing extinction. Biol. Psychiatry. 2009;65:887–892. doi: 10.1016/j.biopsych.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arthur JM, et al. Partial agonist properties of rauwolscine and yohimbine for the inhibition of adenylyl cyclase by recombinant human 5-HT1A receptors. Biochem. Pharmacol. 1993;45:2337–2341. doi: 10.1016/0006-2952(93)90208-e. [DOI] [PubMed] [Google Scholar]
- 43.Winter JC, Rabin RA. Yohimbine as a serotonergic agent: evidence from receptor binding and drug discrimination. J. Pharmacol. Exp. Ther. 1992;263:682–689. [PubMed] [Google Scholar]
- 44.Sanger DJ, Schoemaker H. Discriminative stimulus properties of 8-OH-DPAT: relationship to affinity for 5HT1A receptors. Psychopharmacology (Berl) 1992;108:85–92. doi: 10.1007/BF02245290. [DOI] [PubMed] [Google Scholar]
- 45.Powell SB, et al. Yohimbine disrupts prepulse inhibition in rats via action at 5-HT1A receptors, not alpha2-adrenoceptors. Psychopharmacology (Berl) 2005;180:491–500. doi: 10.1007/s00213-005-2193-7. [DOI] [PubMed] [Google Scholar]
- 46.Dzung Le A, et al. The role of noradrenaline and 5-hydroxytryptamine in yohimbine-induced increases in alcohol-seeking in rats. Psychopharmacology (Berl) 2009;204:477–488. doi: 10.1007/s00213-009-1481-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cole JC, et al. Anxiolytic-like effects of yohimbine in the murine plus-maze: strain independence and evidence against alpha 2-adrenoceptor mediation. Psychopharmacology (Berl) 1995;118:425–436. doi: 10.1007/BF02245943. [DOI] [PubMed] [Google Scholar]
- 48.De Vry J, et al. Shock-induced ultrasonic vocalization in young adult rats: a model for testing putative anti-anxiety drugs. Eur. J. Pharmacol. 1993;249:331–339. doi: 10.1016/0014-2999(93)90530-u. [DOI] [PubMed] [Google Scholar]
- 49.Wellman CL, et al. Impaired stress-coping and fear extinction and abnormal corticolimbic morphology in serotonin transporter knock-out mice. J. Neurosci. 2007;27:684–691. doi: 10.1523/JNEUROSCI.4595-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burghardt NS, et al. Acute selective serotonin reuptake inhibitors increase conditioned fear expression: blockade with a 5-HT(2C) receptor antagonist. Biol. Psychiatry. 2007;62:1111–1118. doi: 10.1016/j.biopsych.2006.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Norcross M, et al. Effects of adolescent fluoxetine treatment on fear-, anxiety- or stress-related behaviors in C57BL/6J or BALB/cJ mice. Psychopharmacology (Berl) 2008;200:413–424. doi: 10.1007/s00213-008-1215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gobert A, et al. Alpha2-adrenergic receptor blockade markedly potentiates duloxetine- and fluoxetine-induced increases in noradrenaline, dopamine, and serotonin levels in the frontal cortex of freely moving rats. J. Neurochem. 1997;69:2616–2619. doi: 10.1046/j.1471-4159.1997.69062616.x. [DOI] [PubMed] [Google Scholar]
- 53.Gobert A, et al. Simultaneous quantification of serotonin, dopamine and noradrenaline levels in single frontal cortex dialysates of freely-moving rats reveals a complex pattern of reciprocal auto- and heteroreceptor-mediated control of release. Neuroscience. 1998;84:413–429. doi: 10.1016/s0306-4522(97)00565-4. [DOI] [PubMed] [Google Scholar]
- 54.Ponnusamy R, et al. Systemic blockade of D2-like dopamine receptors facilitates extinction of conditioned fear in mice. Learn Mem. 2005;12:399–406. doi: 10.1101/lm.96605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nader K, LeDoux J. The dopaminergic modulation of fear: quinpirole impairs the recall of emotional memories in rats. Behav. Neurosci. 1999;113:152–165. doi: 10.1037//0735-7044.113.1.152. [DOI] [PubMed] [Google Scholar]
- 56.Powers MB, et al. Facilitation of fear extinction in phobic participants with a novel cognitive enhancer: a randomized placebo controlled trial of yohimbine augmentation. J. Anxiety Disord. 2009;23:350–356. doi: 10.1016/j.janxdis.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 57.Van Der Kolk BA. The psychobiology and psychopharmacology of PTSD. Hum. Psychopharmacol. 2001;16 doi: 10.1002/hup.270. [DOI] [PubMed] [Google Scholar]
- 58.Southwick SM, et al. Yohimbine use in a natural setting: effects on posttraumatic stress disorder. Biol. Psychiatry. 1999;46:442–444. doi: 10.1016/s0006-3223(99)00107-9. [DOI] [PubMed] [Google Scholar]
- 59.Charney DS, et al. Neurobiological mechanisms of panic anxiety: biochemical and behavioral correlates of yohimbine-induced panic attacks. Am. J. Psychiatry. 1987;144:1030–1036. doi: 10.1176/ajp.144.8.1030. [DOI] [PubMed] [Google Scholar]
- 60.Foa EB. Psychosocial therapy for posttraumatic stress disorder. J. Clin. Psychiatry. 2006;67(Suppl 2):40–45. [PubMed] [Google Scholar]
- 61.Norton PJ, Price EC. A meta-analytic review of adult cognitive-behavioral treatment outcome across the anxiety disorders. J. Nerv. Ment. Dis. 2007;195:521–531. doi: 10.1097/01.nmd.0000253843.70149.9a. [DOI] [PubMed] [Google Scholar]
- 62.Davis M, et al. Effects of D-cycloserine on extinction: translation from preclinical to clinical work. Biol. Psychiatry. 2006;60:369–375. doi: 10.1016/j.biopsych.2006.03.084. [DOI] [PubMed] [Google Scholar]
- 63.Yehuda R, LeDoux J. Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron. 2007;56:19–32. doi: 10.1016/j.neuron.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 64.Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the aetiology of mental illness: review and methodological analysis. Mol. Psychiatry. 2008;13:131–146. doi: 10.1038/sj.mp.4002067. [DOI] [PubMed] [Google Scholar]
- 65.Hariri AR, Holmes A. Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends Cogn. Sci. 2006;10:182–191. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 66.Caspi A, Moffitt TE. Gene-environment interactions in psychiatry: joining forces with neuroscience. Nat. Rev. Neurosci. 2006;7:583–590. doi: 10.1038/nrn1925. [DOI] [PubMed] [Google Scholar]
- 67.Rau V, et al. Stress-induced enhancement of fear learning: an animal model of posttraumatic stress disorder. Neurosci. Biobehav. Rev. 2005;29:1207–1223. doi: 10.1016/j.neubiorev.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 68.Izquierdo A, et al. Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. J. Neurosci. 2006;26:5733–5738. doi: 10.1523/JNEUROSCI.0474-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miracle AD, et al. Chronic stress impairs recall of extinction of conditioned fear. Neurobiol. Learn Mem. 2006;85:213–218. doi: 10.1016/j.nlm.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 70.Muigg P, et al. Impaired extinction of learned fear in rats selectively bred for high anxiety - evidence of altered neuronal processing in prefrontal-amygdala pathways. Eur. J. Neurosci. 2008;28:2299–2309. doi: 10.1111/j.1460-9568.2008.06511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]