Abstract
Aims
Clinical observations in patients with long QT syndrome carrying sodium channel mutations (LQT3) suggest that bradycardia caused by parasympathetic stimulation may provoke torsades de pointes (TdP). β-Adrenoceptor blockers appear less effective in LQT3 than in other forms of the disease.
Methods and results
We studied effects of autonomic modulation on arrhythmias in vivo and in vitro and quantified sympathetic innervation by autoradiography in heterozygous mice with a knock-in deletion (ΔKPQ) in the Scn5a gene coding for the cardiac sodium channel and increased late sodium current (LQT3 mice). Cholinergic stimulation by carbachol provoked bigemini and TdP in freely roaming LQT3 mice. No arrhythmias were provoked by physical stress, mental stress, isoproterenol, or atropine. In isolated, beating hearts, carbachol did not prolong action potentials per se, but caused bradycardia and rate-dependent action potential prolongation. The muscarinic inhibitor AFDX116 prevented effects of carbachol on heart rate and arrhythmias. β-Adrenoceptor stimulation suppressed arrhythmias, shortened rate-corrected action potential duration, increased rate, and minimized difference in late sodium current between genotypes. β-Adrenoceptor density was reduced in LQT3 hearts. Acute β-adrenoceptor blockade by esmolol, propranolol or chronic propranolol in vivo did not suppress arrhythmias. Chronic flecainide pre-treatment prevented arrhythmias (all P < 0.05).
Conclusion
Cholinergic stimulation provokes arrhythmias in this model of LQT3 by triggering bradycardia. β-Adrenoceptor density is reduced, and β-adrenoceptor blockade does not prevent arrhythmias. Sodium channel blockade and β-adrenoceptor stimulation suppress arrhythmias by shortening repolarization and minimizing difference in late sodium current.
Keywords: Long QT syndrome 3, Antiarrhythmic therapy, Basic mechanisms of arrhythmias, Murine model, Telemetry ECG, Stress, Autoradiography
1. Introduction
Gain-of-function mutations in the cardiac sodium channel gene Scn5a can cause long QT syndrome 3 (LQT3) and arrhythmic death by torsades de pointes (TdP).1 In contrast to most patients carrying mutations in potassium channel genes,2 TdP and death occur predominantly during bradycardia, intermittent AV block, or sleep in LQT3 patients.2–8 While these observations may suggest that heightened parasympathetic tone can provoke TdP in LQT3, this has never been systematically studied.
β-Adrenoceptor-blockers, the standard first-line therapy in long QT syndrome patients, seem to be less efficacious in LQT3 in clinical observations.9 Sodium channel blockers are increasingly used as part of antiarrhythmic therapy in LQT3, mainly based on acute effects of such drugs in isolated cells and organs,10–12 and on a QT-shortening effect in LQT3 patients.13 The interaction between chronic sodium channel inhibition and autonomic triggers for arrhythmias in LQT3 has not yet been studied.
We therefore systematically studied effects of acute and chronic autonomic modulation in vivo and in vitro in heterozygous ΔKPQ-Scn5a knock-in mice with LQT3 (ΔKPQ-SCN5A).14,15
2. Methods
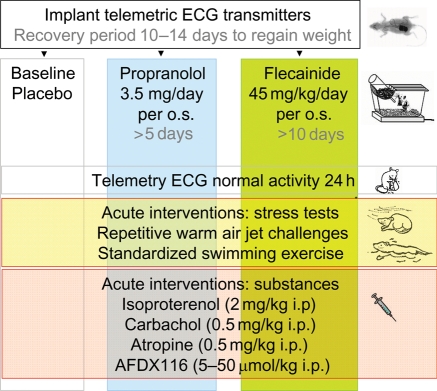
The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and was approved by the local institutional review board (G61/99, G83/2004). Combination of chronic and acute pharmacologic interventions during telemetry is illustrated in Figure 1. Additional experiments were performed in isolated, beating hearts and isolated myocytes. Experiments and analyses performed in adult littermate wild-type and heterozygous ΔKPQ-SCN5A mice aged 22 ± 9 weeks (mean ± SD) were blinded to genotype and type of therapy. Genotyping was performed as described by Nuyens et al.16 and in supplemental methods. Chemicals used were from Sigma-Aldrich, Munich, Germany, if not indicated otherwise.
Figure 1.
In vivo study flow chart. Telemetry at baseline and during chronic pharmacological interventions (columns), baseline and acute in vivo stress tests and pharmacological modulation of the autonomic nervous system (rows). Additional experiments were performed ex vivo (functional studies, cellular electrophysiology, and autoradiography).
2.1. Arrhythmias in freely roaming mice and during in vivo interventions
We implanted telemetric ECG transmitters (DSI, St Paul, MN, USA) and recorded telemetric ECGs during normal activity for 24 h, standardized swimming exercise, and repetitive warm air jet challenges17 at baseline, after 5 days of chronic β1-β2-adrenoceptor-blocker propranolol (propranololhydrochloride) 3.5 mg/day per os (p.o.) resulting in rate reduction and therapeutic plasma levels (77.5 ± 6.5 ng/mL)18 or after 10 days of sodium channel blocker flecainide treatment (50 mg/5 mL, MEDA Pharma, Bad Homburg, Germany), 45 mg/kg/day p.o., resulting in therapeutic plasma levels (509 ± 110 ng/mL), both applied orally via drinking water, mean fluid intake 7.1 ± 0.3 mL SD, or 0.2 mL/kg body weight (BW)/day (Figure 1). The cholinergic agonist carbachol [carbamylcholine chloride, 0.5 mg/kg intraperitoneally (i.p.)], the anticholinergic drug atropine (0.5 mg/kg i.p.), the antimuscarinic agent AFDX116 (Tocris, Ellisville, MO, USA) at 5, 12.5, 25, 50 μg/kg BW i.p., and the β-adrenergic catecholamine isoproterenol (isoproterenol hydrochloride 2 mg/kg i.p.) were applied separately.
Heart rate variability was calculated as the standard deviation of RR-intervals during periods of rest and exercise of at least 5 min.19,20
2.2. Electrophysiological study in isolated, beating hearts
A tissue bath ECG, and right and left ventricular monophasic action potentials (MAP) were simultaneously recorded from isolated, beating Langendorff-perfused hearts with mechanically induced AV block during spontaneous rhythm and pacing15,21 at baseline, after adding β1-β2-adrenoceptor-stimulator orciprenaline (1.7 × 10−6 M) with and without β1-β2-adrenoceptor-blocker esmolol 10−7–10−5 M (100 mg /10 mL, Baxter, Munich, Germany), or propranolol 1.7 × 10−6 M and 1.7 × 10−7 M applied in random order. Carbachol 10−7–10−5 M was applied in hearts without AV block.
2.3. Microautoradiography for cardiac β-adrenoceptors
After perfusion with modified Krebs–Henseleit solution,15,21 hearts were embedded in Tissue-Tek® (Sekura, Heppenheim, Germany) and frozen. Cryofixated hearts were sliced in short axis orientation (20 μm). For each heart, a pair of adjacent tissue sections was prepared. Microautoradiography was performed as described.22 Tissues were incubated in a buffer solution [50 nM HEPES, 5 mM MgCl2, 3% BSA, ph 7.4 (Roth, Karlsruhe, Germany)] for 10 min and dried. Tissues were then coated with solutions of radioactively labelled β-adrenoceptor antagonist 125I-ICYP (Perkin Elmer, Rodgau-Jügesheim, Germany). A uniform radioligand concentration of 80 pM (approximately five times dissociation constant) was chosen to ensure a plateau in specific binding after 2 h of incubation.22,23 Tissues were washed in PBS (Invitrogen, Karlsruhe, Germany) and distilled water afterwards. To differentiate specific from unspecific binding, one of two slices was additionally incubated with an excess of unlabelled l-propranolol (10 μM). Microautoradiography was performed for 8 h using a high-resolution digital autoradiography system (Micro Imager, Biospace Lab, France). Short axis sections were analysed by manual segmentation of left ventricle using ImageJ (Rasband, National Institutes of Health, Bethesda, USA). Specific binding representing β-adrenoceptor density was calculated by subtraction of unspecific binding (125I-ICYP + Propranolol) from total tracer binding (125I-ICYP).
2.4. Whole-cell voltage clamp experiments in isolated ventricular cardiomyocytes
Peak and late sodium current was recorded at room temperature (22°C) at baseline and during β-adrenoceptor-stimulation with isoproterenol from rod-shaped, striated, and Ca2+-tolerant cells within 8 h of isolation using standard solutions and techniques.12,14,24 Currents were recorded with an HEKA-EPC 9 amplifier, and data were collected and analysed with PULSE (HEKA Elektronik, Lambrecht/Pfalz, Germany), Kaleidagraph 4.0 (Synergy, Reading, PA), and IGOR (WaveMetrics, Lake Oswego, OR) software. Isoproterenol (1.6 μM) diluted in external solution was applied with a motorstepper-controlled perfusion system. The holding potential was −100 mV, test pulse to 0 mV. Whole cell currents were recorded without pharmacological testing.
2.5. Statistical analyses
Paired and unpaired t-tests, univariate analysis of variance (ANOVA), and ANOVA analyses for multiple comparisons and repetitive measurements were used to compare continuous parameters (SPSS, version 12.0G for Windows). Fisher's exact test was used to compare arrhythmia occurrence. Values are reported as mean ± SEM unless indicated otherwise. Differences were considered significant at a two-tailed alpha level of P < 0.05 and marked by an asterisk (*) unless indicated otherwise.
3. Results
3.1. ΔKPQ-SCN5A mice show bradycardia and TdP-like arrhythmias during sleep
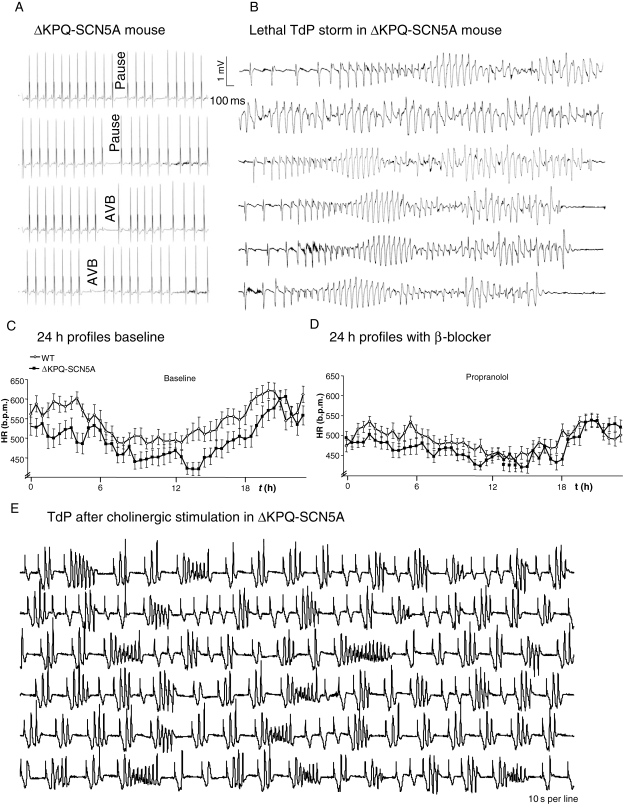
ΔKPQ-SCN5A mice had more spontaneous pauses than WT littermate due to asystole or intermittent AV block during sleep (Figure 2A). 3/21 ΔKPQ-SCN5A mice died spontaneously during long-term telemetric ECG monitoring showing extreme bradycardia (3/3) and TdP-like polymorphic ventricular arrhythmias (1/3) prior to death (Figure 2B). None of 17 WT died spontaneously during monitoring. Heart rate was lower in freely roaming ΔKPQ-SCN5A mice compared with WT at rest and during stress (Table 1, Figure 2C).
Figure 2.
Illustrations of intrinsic bradycardia and arrhythmias in ΔKPQ-SCN5A mice. (A) Telemetric ECG of pauses and atrioventricular block in unrestrained ΔKPQ-SCN5A mice. (B) Bradycardia-induced lethal torsades de pointes storm in an unrestrained ΔKPQ-SCN5A mouse. Within 30 min, 21 torsades de pointes episodes (duration 10 s–min) occurred. (C) Heart rate in unrestrained ΔKPQ-SCN5A and littermate WT mice during 24 h monitoring (mean ± SEM). ΔKPQ-SCN5A were bradycardic. (D) Propranolol decreased heart rates and blunted changes between rest during the day and activity at night and between genotypes. (E) Carbachol-induced episode of bradycardia with intermittent bigemini and torsades de pointes like arrhythmias in a ΔKPQ-SCN5A mouse.
Table 1.
Heart rate, sinus pauses, and AV block in unrestrained mice at baseline and effect of acute and chronic interventions
| Baseline |
Propranolol |
|||
|---|---|---|---|---|
| WT | ΔKPQ-SCN5A | WT | ΔKPQ-SCN5A | |
| Mean heart rate (HR) 24 h | 548 ± 47(16) | 498 ± 52*(18) | 489 ± 33***(13) | 474 ± 40*,***(15) |
| HR variability SD-HR | 76 ± 15(16) | 86 ± 15(18) | 57 ± 13***(13) | 64 ± 14***(15) |
| HR 10 am - 6 pm (at rest) | 505 ± 20**(16) | 451 ± 24*,**(18) | 464 ± 20**,***(13) | 446 ± 22*,**(15) |
| HR swim stress | 705 ± 35**(10) | 677 ± 22*,**(13) | 555 ± 54**,***(10) | 539 ± 32**,***(13) |
| HR air jet stress | 678 ± 35**(10) | 640 ± 44*,**(13) | 583 ± 37**,***(10) | 550 ± 57**,***(13) |
| HR isoproterenol i.p. | 725 ± 41**(11) | 694 ± 35*,**(14) | 699 ± 71**(4) | 710 ± 26**(4) |
| HR atropine i.p. | 641 ± 42**(3) | 612 ± 68**(6) | no value | no value |
| HR carbachol i.p. | 223 ± 38**(7) | 230 ± 51**(9) | 190 ± 21**(4) | 240 ± 46**(4) |
| HR carbachol i.p. prior ES | 207 ± 40**(4) | 210 ± 54**(8) | 180 ± 24**(4) | 207 ± 43**(6) |
| AVB or pauses/5 min/24 h | 1.4 ± 1.3(6) | 3.1 ± 1.8*(6) | 1.7 ± 1.4(6) | 2.5 ± 1.4*(6) |
| Max pause (ms cycle length) | 227(6) | 601(6) | 489(5) | 993(6) |
Mean ± SD of heart rates (n of mice in brackets) in unrestrained ΔKPQ-SCN5A and littermate WT mice at baseline and during chronic oral propranolol treatment. Pauses or AV block numbers were determined as mean of 24 5-min-time periods over 24 consecutive hours per animal.
b.p.m., beats per minute; HR, variability SD-HR standard deviation of heart rate; i.p., intraperitoneal (time 2–12 min after i.p. injection was analysed, only sinus beats counted); ES, extrasystole (10 beats prior to first ES or arrhythmia analysed); AVB, atrioventricular block.
*P < 0.05 ΔKPQ-SCN5A vs. WT.
**P < 0.05 heart rate vs. respective 24 h heart rate.
***P < 0.05 baseline vs. propranolol.
3.2. Stress in freely roaming ΔKPQ-SCN5A mice does not provoke arrhythmias
Mental stress, exercise, β-adrenoceptor-stimulation (isoproterenol 2 mg/kg i.p.), or parasympathetic block by atropine (0.5 mg/kg i.p.) increased heart rate irrespective of genotype in freely roaming mice (Table 1). We never observed arrhythmias under these conditions. At high heart rate, QT intervals did not differ between genotypes, indicating alleviation of the long QT phenotype in vivo (Table 2). ΔKPQ-SCN5A mice showed relative bradycardia compared with WT during physiological stress tests (Table 1).
Table 2.
ECG parameters in unrestrained ΔKPQ-SCN5A and WT mice
| PQ duration (ms) |
QT interval (ms) |
||||
|---|---|---|---|---|---|
| WT | ΔKPQ-SCN5A | WT | ΔKPQ-SCN5A | ||
| Baseline | 600 b.p.m. | 32.5 ± 0.7 | 32.6 ± 0.6 | 48.9 ± 0.9 | 53.1 ± 0.7* |
| 450 b.p.m. | 34.7 ± 0.6 | 35.8 ± 0.6 | 55.6 ± 1.4 | 62.5 ± 1.3* | |
| n | 13(13) | 23(23) | 13(13) | 23(23) | |
| Propranolol | 600 b.p.m. | 29.7 ± 1.0** | 32.6 ± 0.5* | 50.0 ± 2.0 | 54.8 ± 1.0 |
| 450 b.p.m. | 32.1 ± 1.2 | 35.1 ± 0.7* | 55.6 ± 1.2 | 63.0 ± 1.7* | |
| n | 5(5) | 11(11) | 5(5) | 11(11) | |
| Carbachol i.p. mono | 200 b.p.m. | 33.4 ± 0.9 | 38.3 ± 0.9* | 104.0 ± 3.7** | 129.7 ± 4.2*,** |
| n | 14(10) | 18(16) | 14(10) | 18(16) | |
| Carbachol i.p. +propranolol | 200 b.p.m. | 36.3 ± 3.1 | 37.4 ± 1.2 | 108.8 ± 3.4** | 121.7 ± 4.9** |
| n | 5(4) | 7(5) | 5(4) | 7(5) | |
| Carbachol i.p. + flecainide | 200 b.p.m. | 38.9 ± 1.5** | 44.2 ± 2.7** | 127.9 ± 4.0** | 133.8 ± 3.9** |
| n | 7(4) | 7(4) | 7(4) | 7(4) | |
| Isoproterenol i.p. | 700 b.p.m. | 29.9 ± 0.5 | 31.5 ± 0.7 | 51.9 ± 0.8 | 51.9 ± 2.3 |
| n | 9(7) | 14(12) | 9(7) | 14(12) | |
| Swim stress | 700 b.p.m. | 30.2 ± 1.8 | 26.5 ± 2.3 | 52.0 ± 5 | 40.4 ± 3.5 |
| n | 3(3) | 5(5) | 3(3) | 5(5) | |
| Air jet stress | 700 b.p.m. | 29.7 ± 0.5 | 29.3 ± 1.7 | 53.5 ± 2.0 | 51.1 ± 2.4 |
| n | 3(3) | 5(5) | 3(3) | 5(5) | |
Stable frequencies of at least 3 × 20 beats for generation of summary ECG potentials were selected (200–700 b.p.m.); n given as episodes analysed (n animals in brackets); mean ± SEM.
*P < 0.05 vs. WT.
**P<0.05 vs. baseline at 450 and 600 b.p.m.
3.3. Cholinergic stimulation in vivo provokes arrhythmias in ΔKPQ-SCN5A
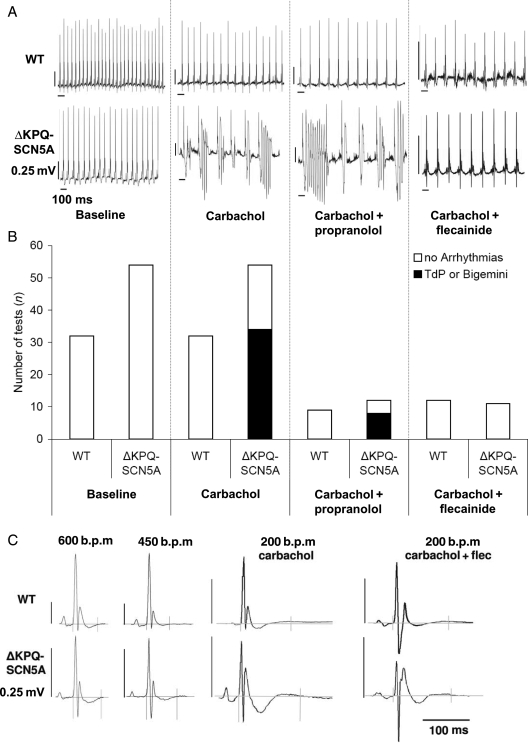
Cholinergic stimulation with carbachol, a clinically used non-specific muscarinic and nicotinic agonist, aggravated bradycardia [0.5 mg/kg i.p. (approximately 0.275 μmol/kg), Table 1, Figures 2E, 3A and D], prolonged QT interval (Table 2, Figure 3C), and provoked bigemini or runs of TdP in ΔKPQ-SCN5A mice (Figures 2E and 3). WT mice showed similar bradycardia after carbachol challenge, but only sporadic ventricular extrasystoles and neither bigemini nor TdP (Figure 3, Table 1). Atropine (0.5 mg/kg i.p.) prevented carbachol-induced bradycardia and related arrhythmias (n = 3/3 ΔKPQ-SCN5A mice). The muscarinic receptor blocker AFDX116, 12.5, 25, or 50 μmol/kg BW, applied 15 min before carbachol, abolished the effect of carbachol and prevented arrhythmias in n = 3/3 ΔKPQ-SCN5A mice (Figure 3D, see Supplementary material online, Figure S2).
Figure 3.
Effect of carbachol on ECG morphology and arrhythmias in unrestrained ΔKPQ-SCN5A mice with and without chronic propranolol or flecainide treatment. (A) Representative telemetry ECG recordings at baseline and during carbachol challenge with and without chronic propranolol or flecainide treatment in unrestrained ΔKPQ-SCN5A mice. (B) Bar graphs give numbers of experiments with arrhythmias (filled bars, one to three challenges per animal) and without arrhythmias (open bars) in both genotypes. Carbachol induced arrhythmias in ΔKPQ-SCN5A mice only. Chronic flecainide prevented arrhythmias, chronic propranolol did not (all P < 0.05). (C) QT interval in unrestrained ΔKPQ-SCN5A and littermate WT mice. Tele-electrocardiographic summary potentials selected from spontaneous frequencies during heart rates at baseline (600 b.p.m, 450 b.p.m.), and low (200 b.p.m.) heart rates after carbachol challenge with or without chronic flecainide therapy. Carbachol-induced bradycardia exacerbated QT prolongation in ΔKPQ-SCN5A, (values and animal numbers in Table 2). (D) Telemetry heart rate during drug exposures. Alternating heart rate indicates bigemini and arrhythmias. Arrows indicate intraperitoneal injection of carbachol 0.5 mg/kg BW.
3.4. Chronic β-adrenoceptor-blockade does not prevent carbachol-induced arrhythmias in vivo
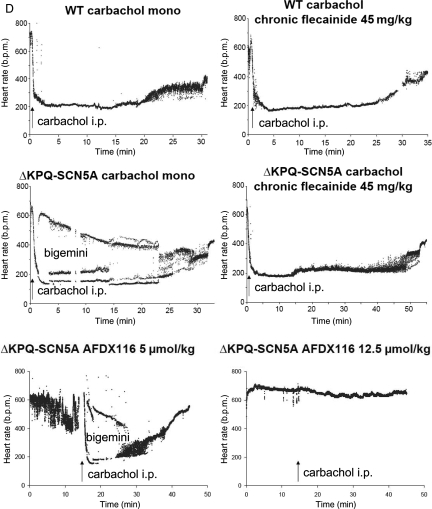
Chronic propranolol therapy reduced heart rate, heart rate variability, and heart rate increase during 24 h monitoring, swimming, and mental stress in freely roaming WT and ΔKPQ-SCN5A mice (Figure 2D, Table 1). Chronic propranolol therapy (3.5 mg/day) did not prevent carbachol-provoked arrhythmias (8/12 TdP or bigemini in ΔKPQ-SCN5A with propranolol *vs. 0/9 WT, Figure 3, Table 1). However, care had to be taken to keep animals in a stressless environment during cholinergic stimulation because physical or mental activation of animals resulted in cessation of arrhythmias.
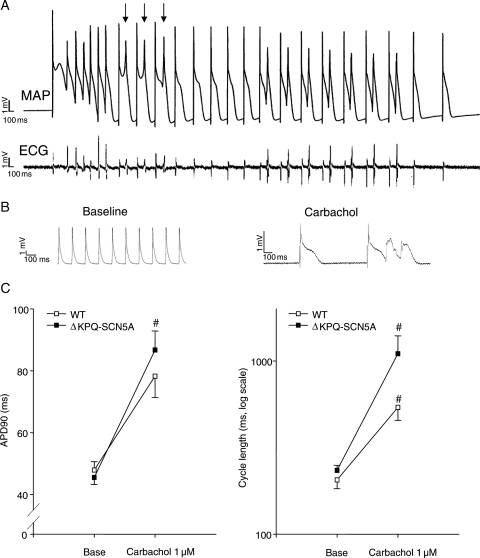
3.5. Carbachol provokes bigemini and TdP in isolated ΔKPQ-SCN5A hearts
Carbachol perfusion caused similar effects on the isolated heart as in vivo, i.e. bradycardia, bigemini, and TdP: Cycle length was 739 ± 171 ms during carbachol 10−7 M; 1128 ± 310 ms during carbachol 10−6 M (*vs. 234 ± 17 ms baseline). Bigemini occurred in 33/42 and TdP in 13/42 ΔKPQ-SCN5A hearts during carbachol (*vs. 22/42 bigemini and 4/42 TdP at baseline, Figure 4A). Carbachol neither induced arrhythmias nor prolonged APD during controlled rates (Supplementary material online, Table), but increased spontaneous APD by decreasing heart rate in ΔKPQ-SCN5A hearts (APD90: baseline 45 ± 2 ms, carbachol 10−7 M 64 ± 7 ms, carbachol 10−6 M 87 ± 7 ms, *vs. baseline and *vs. WT, Figure 4B and C).
Figure 4.
Carbachol (10−7–10−5 M) provoked marked bradycardia, potential early afterdepolarizations (EADs), and ventricular arrhythmias in isolated, beating ΔKPQ-SCN5A mouse hearts. (A) Representative monophasic action potential recording of carbachol-induced TdP-like arrhythmias in an isolated, beating ΔKPQ-SCN5A mouse heart. Arrows indicate potentially conducted EADs. (B) Representative action potentials during spontaneous rhythm at baseline and after carbachol perfusion in a ΔKPQ-SCN5A mouse heart. (C) Mean action potential duration (left) and cycle length (right) during spontaneous rhythm in ΔKPQ-SCN5A and WT littermate mouse hearts. Carbachol provoked bradycardia and bradycardia-dependent APD prolongation in ΔKPQ-SCN5A mouse hearts (WT n = 24, ΔKPQ-SCN5A n = 28 hearts; P < 0.05 vs. WT and vs. baseline. Results depicted for carbachol 10−6 M; #P < 0.05 for carbachol vs. baseline).
3.6. β-Adrenoceptor-blockade impairs antiarrhythmic effects of catecholamines and can prolong ventricular action potentials in isolated hearts from ΔKPQ-SCN5A mice
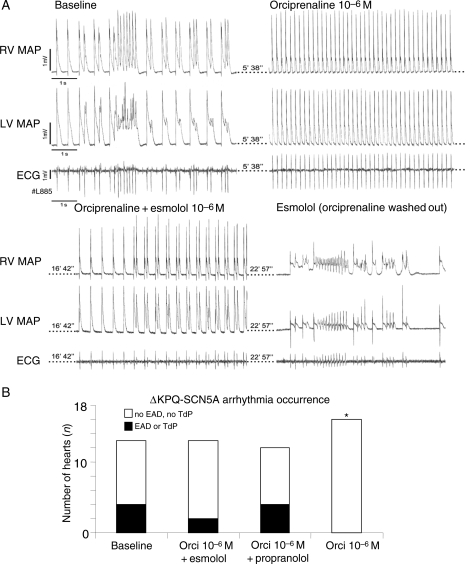
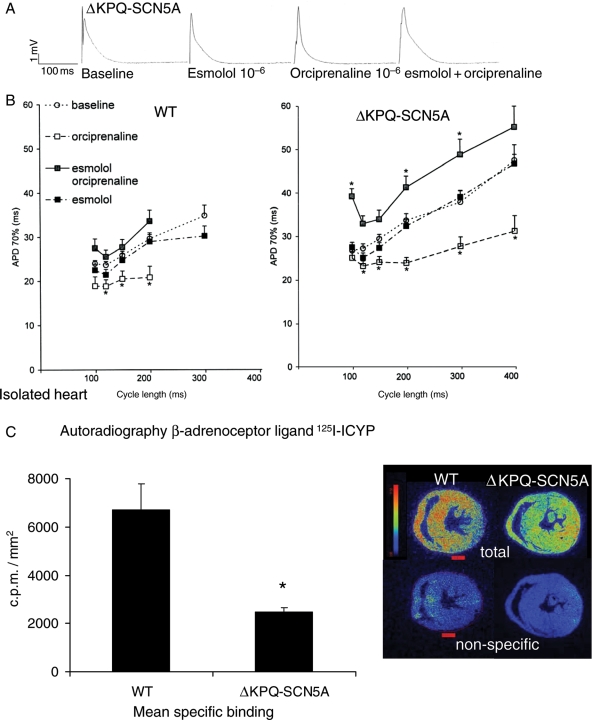
β-Adrenoceptor-stimulation by orciprenaline (1.7 μM) completely prevented and suppressed EADs and TdP in isolated hearts with mechanical AV block (0/16 ΔKPQ-SCN5A hearts *vs. baseline 4/13, Figure 5A and B). Orciprenaline shortened ventricular APD (Figure 6A and B) and decreased APD dispersion at paced cycle lengths of 200 and 300 ms (orciprenaline 9 ± 1 *vs. baseline 19 ± 2 ms). Propranolol (1.7 × 10−6 M and 1.7 × 10−7 M) did not prolong APD in ΔKPQ-SCN5A hearts (data not shown). Co-infusion of esmolol and orciprenaline rendered APD similar to baseline values in WT (+9% APD), but caused prolongation of APD and dispersion of APD up to 25% above baseline values in ΔKPQ-SCN5A hearts (P < 0.05, Figure 6B). Orciprenaline lost its antiarrhythmic effect in the presence of propranolol or esmolol (Figure 5B). Propranolol slowed intrinsic ventricular rhythm after AV block even in presence of equimolar orciprenaline, and complete asystole was more frequent (5/12 ΔKPQ-SCN5A hearts, *vs. baseline 1/27). Propranolol alone (1.7 × 10−6 M) suppressed EADs and TdP in hearts with mechanical AV block, but caused bradycardia with temporary loss of ventricular rhythm in 4/4 ΔKPQ-SCN5A isolated hearts. Orciprenaline caused accelerated regular ventricular rhythms in all isolated hearts.
Figure 5.
Antiarrhythmic effect of β-adrenoceptor stimulation in isolated beating hearts. (A) Representative recording of two simultaneous monophasic action potentials (MAP) and ECG during afterdepolarizations and short torsades de pointes (TdP) episodes at baseline after AV block (top left). TdP are suppressed by β-adrenoceptor stimulation (orciprenaline, top right). Ectopy reappears during co-infusion of orciprenaline and β-adrenoceptor-blocker esmolol, and pause-triggered TdP occurs during infusion of esmolol alone. (B) Number of ΔKPQ-SCN5A hearts with arrhythmias at baseline, with β-adrenoceptor-blockers, during co-infusion of β-adrenoceptor-blockers and orciprenaline, and with orciprenaline alone; *P < 0.05 vs. baseline (number of hearts depicted as columns).
Figure 6.
β-Adrenoceptors and autonomic modulation. (A) Representative monophasic action potential recordings at a pacing cycle length of 200 ms in isolated, beating hearts after mechanical AV block. (B) Mean action potential durations during fixfrequent pacing at baseline, during infusion of esmolol 1.7 × 10−6 M, orciprenaline 1.6 × 10−6 M; and orciprenaline 1.6 × 10−6 M plus esmolol 1.7 × 10−6 M. Mean of 19–33 action potentials from 10 to 11 WT hearts and 11 to 31 action potentials from 10 to 12 ΔKPQ-SCN5A hearts. At long cycle lengths, number of action potentials was reduced to five WT, seven ΔKPQ-SCN5A, due to fast intrinsic heart rates. (C) β-Adrenoceptor binding was decreased in ΔKPQ-SCN5A mouse hearts. Specific binding of 125I-ICYP to β-adrenoceptors on cryofixated myocardial short axis sections in n = 4–5 per genotype. Binding is expressed as counts/min/mm2. Inset shows examples of total ICYP and non-specific binding in two representative hearts.
3.7. Decreased β-adrenoceptor density in ΔKPQ-SCN5A
β-Adrenoceptor density as measured by quantitative microautoradiography was markedly decreased in ΔKPQ-SCN5A vs. WT hearts (Figure 6C).
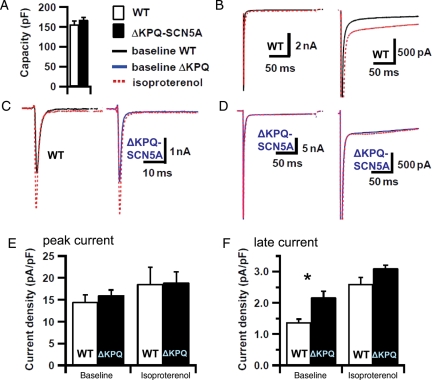
3.8. β-Adrenoceptor-stimulation blunts the difference in INa, late between ΔKPQ-SCN5A and WT cardiomyocytes
Late sodium current (INa, late) was increased in ΔKPQ-SCN5A cardiomyocytes (Figure 7). Peak (early) sodium current densities did not differ between groups under both conditions (P = ns, Figure 7). Isoproterenol (10−6 M superfusion) increased INa, late in both genotypes. INa, late was not different between genotypes during adrenergic stimulation (*vs. baseline; P = ns for ΔKPQ-SCN5A vs. WT) due to a higher relative increase of INa, late in WT cells compared with ΔKPQ-SCN5A (increase in INa, late in ΔKPQ-SCN5A 49 ± 10%, WT 90 ± 24%, Figure 7).
Figure 7.
Effect of isoproterenol on sodium current in isolated ventricular cardiomyocytes. (A) Capacity, (B) late current example for WT, (C) peak current for WT and ΔKPQ-SCN5A (blue), (D) late current example for ΔKPQ-SCN5A. (E) refers to peak current and (F) refers to late current. Mean values shown are derived from seven to 16 cells from nine WT hearts and seven to 18 cells from six ΔKPQ-SCN5A hearts. Peak sodium current (E) was unchanged between genotypes. Late sodium current (F) was increased in ΔKPQ-SCN5A at baseline but no longer different from WT during β-adrenergic stimulation, *P < 0.05 vs. WT.
3.9. Chronic flecainide therapy prevents arrhythmias induced by cholinergic stimulation in freely roaming ΔKPQ-SCN5A mice
Chronic flecainide (45 mg/kg BW/day) did not prevent carbachol-induced bradycardia (WT 223 ± 33 b.p.m., vs. ΔKPQ-SCN5A 203 ± 17 b.p.m., P = ns vs. WT, P < 0.05 vs. baseline), but prevented carbachol-induced arrhythmias in unrestrained ΔKPQ-SCN5A mice (TdP or bigemini in 0/11 carbachol challenges, P < 0.05 vs. in 7/11 in the same mice without flecainide, Figure 3).
4. Discussion
This systematic study of interactions of chronic and acute autonomic modulation and sodium channel blockade identified several new findings in this model of LQT3:
Cholinergic stimulation provokes bradycardia and arrhythmias in vivo in this model of LQT3.
Stress and catecholamines suppress arrhythmias in vivo and shorten ventricular action potentials in this model. INa, late in ΔKPQ-SCN5A myocytes is relatively insensitive to β-adrenergic stimulation.
β-Adrenoceptor-blockade does not prevent arrhythmias, but blunts the antiarrhythmic effects of catecholamines, and prolongs repolarization in vivo and in vitro in this model.
Cardiac β-adrenoceptor expression is markedly reduced in this model of LQT3.
Chronic pharmacological inhibition of sodium currents by flecainide prevents arrhythmias in vivo in this model of LQT3.
Within the limitations of a preclinical study, and taken together with other studies in isolated hearts or in cellular preparations,12,25–29 our data confirm the clinical observation that patients carrying sodium channel mutations do not benefit from β-adrenoceptor-blocker therapy. Cholinergic stimulation appears proarrhythmic in patients carrying this mutation or SCN5A mutations with similar biophysical characteristics. Finally, our data show that chronic sodium channel inhibition by flecainide can prevent arrhythmias in freely roaming animals, thereby extending the existing data on sodium channel blockade in cells and isolated organs.12,25,26,29
4.1. Cholinergic stimulation is highly arrhythmogenic in this model of LQT3
Cholinergic stimulation had a marked proarrhythmic effect showing TdP after bradycardia and QT prolongation in LQT3 in vivo. This is consistent with observations in patients with LQT330 and may be mediated by bradycardia2,5,6,8 and by the unique kinetics of the increased INa, late in the ΔKPQ mutant. INa, late is more pronounced at low heart rates in ΔKPQ-SCN5A.29,31–33 These biophysical properties of the mutated sodium channel can also explain excessive prolongation of QT interval in response to bradycardia at rest, and a shortening of repolarization during sinus tachycardia in freely roaming ΔKPQ-SCN5A mice in this study. Effects of carbachol on cardiac electrophysiology are predominantly mediated by muscarinic receptors, most of them of the M2 subtype,34,35 and muscarinic stimulation does no longer cause bradycardia in M22y2 mutant mice.36 Carbachol-induced arrhythmias were no longer observed if either atropine or the muscarinic antagonist AFDX116 was co-applied, suggesting that muscarinic and preferential M2 activation mediated the proarrhythmic effect of carbachol in this model.
4.2. Sympathetic stimulation suppresses and prevents arrhythmias in ΔKPQ-SCN5A
Endogenous catecholamines shortened QT interval and prevented arrhythmias in unrestrained ΔKPQ-SCN5A mice, and even interrupted arrhythmias induced by cholinergic stimulation in vivo. β-Adrenoceptor-stimulation completely suppressed TdP in the intact, beating heart, consistent with reports in transgenic14,37 and pharmacological38 models of LQT3, and reduced the difference of late sodium current between WT and ΔKPQ-SCN5A cardiomyocytes.
β-Adrenoceptor-stimulation can affect sodium currents by PKA-dependent and PKA-independent mechanisms: PKA phosphorylates cardiac sodium channels, and sodium channels can directly bind to β-adrenoceptors in conjunction with G-protein α-units.39 Activation of α1-adrenoceptors can activate protein kinase C and alter sodium current.40,41 α1-Adrenoceptor activation can inhibit sodium channel bursting via protein kinase C in ΔKPQ-SCN5A mutant cardiomyocytes.28,42 Multiple effects have been seen in expression systems, e.g. in another model of LQT3 using PKA stimulation in transfected HEK293 cells.43 The known effects of β-adrenoceptor stimulation on repolarizing channels may be responsible for catecholamine-induced action potential shortening in WT and ΔKPQ-SCN5A hearts, as their expression is not altered in ΔKPQ-SCN5A hearts (RNA of KCNQ1 and KCNE1 encoding IKs, MERG1B encoding IKr, Kv1.4, Kv4.2, and Kv4.3 encoding Ito, and Kv1.5 encoding IK,slow14).
4.3. β-Adrenoceptor-blockade inhibits beneficial catecholamine-effects in ΔKPQ-SCN5A
Chronic β-adrenoceptor-blocker therapy prevented antiarrhythmic effects of physical stress or catecholamines in freely roaming ΔKPQ mice and β-adrenoceptor-blocker treatment did not prevent arrhythmias, consistent with observations in LQT3 patients (Table 1 and 2, Figure 3 and 525, Supplementary material online, Figure S3).4,44–46
β-Adrenoceptor-blockade by esmolol caused lengthening of ventricular action potentials (Figure 6). These observations suggest that β-adrenoceptor-blockers may not only blunt the beneficial effects of catecholamines on ventricular action potential duration and QT interval in vivo, but inhibit beneficial effects of ‘endogenous’ catecholamines in the heart, e.g. stemming from spontaneous release and incomplete re-uptake of sympathetic granules from sympathetic nerve endings.
4.4. Reduced β-adrenoceptor density
Reduced cardiac β-adrenoceptor density was found in this model (Figure 6C). Although up to date, little is known about β-adrenoceptor density in LQT3 patients, reduced β-adrenoceptor density was found in symptomatic patients with arrhythmogenic right ventricular cardiomyopathy.47 As there may be phenotypic variations in patients with SCN5A mutations (‘overlap syndromes’), our observations warrant studies of β-adrenoceptor density in LQT3 patients. Reduction of β-adrenoceptor binding may add another explanation why β-adrenoceptor-blockers did not prevent ventricular arrhythmias in ΔKPQ-SCN5A mice. Reduced β-adrenoceptor expression in structurally and functionally normal LQT3 hearts invites further study.
4.5. Antiarrhythmic effects of chronic flecainide therapy in vivo
Marked β-adrenoceptor-blocker-induced action potential prolongation was blunted when propranolol, a β-adrenoceptor-blocker that is also a weak sodium channel blocker,48 was used. Long-term sodium channel blockade by flecainide completely prevented spontaneous and carbachol-induced arrhythmias in this study, thereby extending prior reports of acute effects of sodium channel blockade in vitro in this model11,14,15 and in another model of LQT349 to beneficial effects of chronic therapy. Our observation of beneficial effects of chronic sodium channel inhibition in vivo is consistent with clinical observation.2 Prevention of arrhythmias by sodium channel block confirms that abnormal late sodium current is required for arrhythmias in this LQT3 model in vivo, and supports the notion that inhibition of late sodium current may be effective in LQT3, in contrast to β-adrenoceptor-blockade. In addition to sodium current blockade, flecainide has parasympatholytic effects. This might be considered as an additional potential antiarrhythmic effect warranting future study.
4.6. Limitations and potential clinical implications
Afterdepolarizations and triggered beats can be difficult to verify in MAP even if recording quality is high and standardized. In vivo or in-organ mapping techniques may confirm the role of afterdepolarizations and triggered beats in this model in the future. Our findings on sodium currents are in accordance with experiments from Fredj et al.12 which showed a difference in late sodium current while peak currents where not altered. Nuyens et al.14 reported a higher peak sodium current in the LQT3 model at high frequencies (10 Hz).
Cholinergic stimulation had arrhythmogenic effects in vivo and in the isolated heart in ΔKPQ-SCN5A, suggesting that parasympatholytic agents such as atropine may be antiarrhythmic in LQT3. Comparable to the findings reported here, sinus bradycardia, spontaneous pauses, and atrioventricular block (see Supplementary material online, Figure S3) were observed in patients with LQTS3.4,50 Bradycardia in LQT3 may be due to altered inactivation kinetics in the sinus node,51 and/or to electrotonic modulation of sinus nodal firing by surrounding cells. This warrants further study.
Electrical storm in patients with Brugada syndrome has been successfully treated with β-mimetic agents (e.g. isoproterenol) or quinidine, whereas parasympathetic stimulation and β-adrenoceptor-blockade may provoke arrhythmias in Brugada syndrome. It is conceivable that some of the pathophysiological mechanisms observed here may also apply to selected patients with a Brugada ECG pattern, sodium channel mutation, and ‘overlap syndromes’ although biophysical properties of SCN5A mutations found in Brugada syndrome usually cause loss-of-function.
Chronic sodium channel blockade suppressed spontaneous and carbachol-induced arrhythmias in vivo and in the beating heart in this model of LQT3. Our findings may apply to other forms of LQT3.50 Within the limitations of a preclinical study, our data provide another rationale for genetic testing and clinical alertness5 in LQT patients to identify those with sodium channel mutations, in order to start or maintain sodium channel blocker therapy.13,52 Conduction slowing and aggravation of bradycardia by sodium channel blocking drugs must be considered.53–55 More selective inhibitors of the INa, late like mexiletine or ranolazine may be considered in LQT3 patients at risk of arrhythmias due to conduction slowing.
These preclinical findings cannot be directly transferred to clinical situations noting differences between mice and men. Nonetheless, reduced β-adrenoceptor density, prolongation of ventricular action potentials by esmolol, and lack of antiarrhythmic effects of β-adrenoceptor-blockers in vivo caution use of β-adrenoceptor-blockers in LQT3, especially when published reports in this model are considered, and suggest that catecholamines may have acute antiarrhythmic effects in this model, if only by increasing rate.11,12,14,25,29,32,37
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by: Medical Faculty, University of Münster, ‘Innovative Medical Research’ [IMF FA120431 to L.F., P.K.]; Deutsche Forschungsgemeinschaft DFG [FA 413-3/1 to L.F., Ki/731/1-1 to P.K., Ma 2252 to S.K.G.M., SFB 656 MoBil A5 to B.R., P.K.; A8 to L.F., P.K.; C1 to G.B., Z5 to B.R.]; Interdisciplinary Centre for Clinical Research IZKF Münster [Kih1/020/07 U.K., L.F.; core units CarTel to P.K., SmAP to M.S.], and Fondation Leducq [ENAFRA 07CVD03 to P.K.]. Funding to pay the open access publication charge was provided by DFG FA 413/3-1 and SFBMoBil.
Supplementary Material
Acknowledgements
We thank Nina Kreienkamp, Marcel Tekook, Peter Kahr, Christoph Waldeyer, Ilaria Piccini, Jenny Muck, and Sandra Schröer for excellent technical assistance, and Tonja Fabritz for artwork.
Conflict of interest: none declared except for the fact that flecainide plasma levels were measured free of charge by MEDA Pharma.
References
- 1.Bennett PB, Valenzuela C, Chen LQ, Kallen RG. On the molecular nature of the lidocaine receptor of cardiac Na+ channels. Modification of block by alterations in the alpha-subunit III-IV interdomain. Circ Res. 1995;77:584–592. doi: 10.1161/01.res.77.3.584. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz PJ, Priori SG, Locati EH, Napolitano C, Cantu F, Towbin JA, et al. Long QT syndrome patients with mutations of the SCN5A and HERG genes have differential responses to Na+ channel blockade and to increases in heart rate. Implications for gene-specific therapy. Circulation. 1995;92:3381–3386. doi: 10.1161/01.cir.92.12.3381. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz PJ, Priori SG, Dumaine R, Napolitano C, Antzelevitch C, Stramba-Badiale M, et al. A molecular link between the sudden infant death syndrome and the long-QT syndrome. N Engl J Med. 2000;343:262–267. doi: 10.1056/NEJM200007273430405. [DOI] [PubMed] [Google Scholar]
- 4.Wedekind H, Smits JP, Schulze-Bahr E, Arnold R, Veldkamp MW, Bajanowski T, et al. De novo mutation in the SCN5A gene associated with early onset of sudden infant death. Circulation. 2001;104:1158–1164. doi: 10.1161/hc3501.095361. [DOI] [PubMed] [Google Scholar]
- 5.Moss AJ, Zareba W, Benhorin J, Locati EH, Hall WJ, Robinson JL, et al. ECG T-wave patterns in genetically distinct forms of the hereditary long QT syndrome. Circulation. 1995;92:2929–2934. doi: 10.1161/01.cir.92.10.2929. [DOI] [PubMed] [Google Scholar]
- 6.An RH, Wang XL, Kerem B, Benhorin J, Medina A, Goldmit M, et al. Novel LQT-3 mutation affects Na+ channel activity through interactions between alpha- and beta1-subunits. Circ Res. 1998;83:141–146. doi: 10.1161/01.res.83.2.141. [DOI] [PubMed] [Google Scholar]
- 7.van Hare GF, Franz MR, Roge C, Scheinman MM. Persistent functional atrioventricular block in two patients with prolonged QT intervals: elucidation of the mechanism of block. Pace Pacing Clin Electrophysiol. 1990;13:608–618. doi: 10.1111/j.1540-8159.1990.tb02077.x. [DOI] [PubMed] [Google Scholar]
- 8.Zareba W, Moss AJ, Schwartz PJ, Vincent GM, Robinson JL, Priori SG, et al. Influence of genotype on the clinical course of the long-QT syndrome. International long-QT syndrome registry research group. N Engl J Med. 1998;339:960–965. doi: 10.1056/NEJM199810013391404. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz PJ, Priori SG, Spazzolini C, Moss AJ, Vincent GM, Napolitano C, et al. Genotype–phenotype correlation in the long-QT syndrome: gene-specific triggers for life-threatening arrhythmias. Circulation. 2001;103:89–95. doi: 10.1161/01.cir.103.1.89. [DOI] [PubMed] [Google Scholar]
- 10.Sabir IN, Li LM, Jones VJ, Goddard CA, Grace AA, Huang CL. Criteria for arrhythmogenicity in genetically-modified Langendorff-perfused murine hearts modelling the congenital long QT syndrome type 3 and the Brugada syndrome. Pflugers Arch. 2008;455:637–651. doi: 10.1007/s00424-007-0326-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stokoe KS, Thomas G, Goddard CA, Colledge WH, Grace AA, Huang CL. Effects of flecainide and quinidine on arrhythmogenic properties of Scn5a + /Delta murine hearts modelling long QT syndrome 3. J Physiol. 2007;578:69–84. doi: 10.1113/jphysiol.2006.117945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fredj S, Sampson KJ, Liu H, Kass RS. Molecular basis of ranolazine block of LQT-3 mutant sodium channels: evidence for site of action. Br J Pharmacol. 2006;148:16–24. doi: 10.1038/sj.bjp.0706709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Windle JR, Geletka RC, Moss AJ, Zareba W, Atkins DL. Normalization of ventricular repolarization with flecainide in long QT syndrome patients with SCN5A:DeltaKPQ mutation. Ann Noninvasive Electrocardiol. 2001;6:153–158. doi: 10.1111/j.1542-474X.2001.tb00100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nuyens D, Stengl M, Dugarmaa S, Rossenbacker T, Compernolle V, Rudy Y, et al. Abrupt rate accelerations or premature beats cause life-threatening arrhythmias in mice with long-QT3 syndrome. Nat Med. 2001;7:1021–1027. doi: 10.1038/nm0901-1021. [DOI] [PubMed] [Google Scholar]
- 15.Fabritz L, Kirchhof P, Franz MR, Nuyens D, Rossenbacker T, Ottenhof A, et al. Effect of pacing and mexiletine on dispersion of repolarisation and arrhythmias in hearts of SCN5A Δ-KPQ (LQT3) mice. Cardiovasc Res. 2003;57:1085–1093. doi: 10.1016/s0008-6363(02)00839-8. [DOI] [PubMed] [Google Scholar]
- 16.Nuyens D. Generation and Characterization of a Mouse Model for a Sodium Channel Based Long QT Syndrome (LQT3), an Inherited Arrhythmogenic Disease. Leuven: Katholieke Universiteit Leuven; 2006. [Google Scholar]
- 17.Knollmann BC, Kirchhof P, Sirenko SG, Degen H, Greene AE, Schober T, et al. Familial hypertrophic cardiomyopathy-linked mutant troponin T causes stress-induced ventricular tachycardia and Ca2 + -dependent action potential remodeling. Circ Res. 2003;92:428–436. doi: 10.1161/01.RES.0000059562.91384.1A. [DOI] [PubMed] [Google Scholar]
- 18.Witte K, Engelhardt S, Janssen BJ, Lohse M, Lemmer B. Circadian and short-term regulation of blood pressure and heart rate in transgenic mice with cardiac overexpression of the beta1-adrenoceptor. Chronobiol Int. 2004;21:205–216. doi: 10.1081/cbi-120037801. [DOI] [PubMed] [Google Scholar]
- 19.Kirchhof P, Fabritz L, Fortmüller L, Lankford AR, Matherne G, Baba HA, et al. Decreased chronotropic response to exercise and atrio-ventricular nodal conduction delay in mice overexpressing the A1-adenosine receptor. Am J Physiol. 2003;285:H145–H153. doi: 10.1152/ajpheart.01036.2002. [DOI] [PubMed] [Google Scholar]
- 20.Fabritz L, Kirchhof P, Fortmüller L, Auchampach J, Baba H, Schmitz W, et al. Gene dose-dependent atrial arrhythmias, heart block and atrial brady-cardiomyopathy in mice overexpressing the A3 -adenosine receptor. Cardiovasc Res. 2004;62:500–508. doi: 10.1016/j.cardiores.2004.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fabritz L, Kirchhof P, Franz MR, Eckardt L, Mönnig G, Milberg P, et al. Prolonged action potential durations, increased dispersion of repolarization, and polymorphic ventricular tachycardia in a mouse model of proarrhythmia. Basic Res Cardiol. 2003;98:25–32. doi: 10.1007/s00395-003-0386-y. [DOI] [PubMed] [Google Scholar]
- 22.Murphree SS, Saffitz JE. Quantitative autoradiographic delineation of the distribution of beta-adrenergic receptors in canine and feline left ventricular myocardium. Circ Res. 1987;60:568–579. doi: 10.1161/01.res.60.4.568. [DOI] [PubMed] [Google Scholar]
- 23.Gu XH, Kompa AR, Summers RJ. Regulation of beta-adrenoceptors in a rat model of cardiac failure: effect of perindopril. J Cardiovasc Pharmacol. 1998;32:66–74. doi: 10.1097/00005344-199807000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Maier SK, Westenbroek RE, Schenkman KA, Feigl EO, Scheuer T, Catterall WA. An unexpected role for brain-type sodium channels in coupling of cell surface depolarization to contraction in the heart. Proc Natl Acad Sci USA. 2002;99:4073–4078. doi: 10.1073/pnas.261705699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas G, Killeen MJ, Grace AA, Huang CL. Pharmacological separation of early afterdepolarizations from arrhythmogenic substrate in DeltaKPQ Scn5a murine hearts modelling human long QT 3 syndrome. Acta Physiol (Oxf) 2008;192:505–517. doi: 10.1111/j.1748-1716.2007.01770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hothi SS, Booth SW, Sabir IN, Killeen MJ, Simpson F, Zhang Y, et al. Arrhythmogenic substrate and its modification by nicorandil in a murine model of long QT type 3 syndrome. Prog Biophys Mol Biol. 2008;98:267–280. doi: 10.1016/j.pbiomolbio.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 27.Thomas G, Gurung IS, Killeen MJ, Hakim P, Goddard CA, Mahaut-Smith MP, et al. Effects of L-type Ca2+ channel antagonism on ventricular arrhythmogenesis in murine hearts containing a modification in the Scn5a gene modelling human long QT syndrome 3. J Physiol. 2007;578:85–97. doi: 10.1113/jphysiol.2006.121921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tateyama M, Kurokawa J, Terrenoire C, Rivolta I, Kass RS. Stimulation of protein kinase C inhibits bursting in disease-linked mutant human cardiac sodium channels. Circulation. 2003;107:3216–3222. doi: 10.1161/01.CIR.0000070936.65183.97. [DOI] [PubMed] [Google Scholar]
- 29.Nagatomo T, January CT, Ye B, Abe H, Nakashima Y, Makielski JC. Rate-dependent QT shortening mechanism for the LQT3 deltaKPQ mutant. Cardiovasc Res. 2002;54:624–629. doi: 10.1016/s0008-6363(02)00265-1. [DOI] [PubMed] [Google Scholar]
- 30.Noda T, Takaki H, Kurita T, Suyama K, Nagaya N, Taguchi A, et al. Gene-specific response of dynamic ventricular repolarization to sympathetic stimulation in LQT1, LQT2 and LQT3 forms of congenital long QT syndrome. Eur Heart J. 2002;23:975–983. doi: 10.1053/euhj.2001.3079. [DOI] [PubMed] [Google Scholar]
- 31.Oginosawa Y, Nagatomo T, Abe H, Makita N, Makielski JC, Nakashima Y. Intrinsic mechanism of the enhanced rate-dependent QT shortening in the R1623Q mutant of the LQT3 syndrome. Cardiovasc Res. 2005;65:138–147. doi: 10.1016/j.cardiores.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 32.Fredj S, Lindegger N, Sampson KJ, Carmeliet P, Kass RS. Altered Na+ channels promote pause-induced spontaneous diastolic activity in long QT syndrome type 3 myocytes. Circ Res. 2006;99:1225–1232. doi: 10.1161/01.RES.0000251305.25604.b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruan Y, Liu N, Bloise R, Napolitano C, Priori SG. Gating properties of SCN5A mutations and the response to mexiletine in long-QT syndrome type 3 patients. Circulation. 2007;116:1137–1144. doi: 10.1161/CIRCULATIONAHA.107.707877. [DOI] [PubMed] [Google Scholar]
- 34.Hulme EC, Birdsall NJ, Buckley NJ. Muscarinic receptor subtypes. Annu Rev Pharmacol Toxicol. 1990;30:633–673. doi: 10.1146/annurev.pa.30.040190.003221. [DOI] [PubMed] [Google Scholar]
- 35.Caulfield MP. Muscarinic receptors—characterization, coupling and function. Pharmacol Ther. 1993;58:319–379. doi: 10.1016/0163-7258(93)90027-b. [DOI] [PubMed] [Google Scholar]
- 36.Gomeza J, Shannon H, Kostenis E, Felder C, Zhang L, Brodkin J, et al. Pronounced pharmacologic deficits in M2 muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci USA. 1999;96:1692–1697. doi: 10.1073/pnas.96.4.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Head CE, Balasubramaniam R, Thomas G, Goddard CA, Lei M, Colledge WH, et al. Paced electrogram fractionation analysis of arrhythmogenic tendency in DeltaKPQ Scn5a mice. J Cardiovasc Electrophysiol. 2005;16:1329–1340. doi: 10.1111/j.1540-8167.2005.00200.x. [DOI] [PubMed] [Google Scholar]
- 38.Shimizu W, Antzelevitch C. Differential effects of beta-adrenergic agonists and antagonists in LQT1, LQT2 and LQT3 models of the long QT syndrome. J Am Coll Cardiol. 2000;35:778–786. doi: 10.1016/s0735-1097(99)00582-3. [DOI] [PubMed] [Google Scholar]
- 39.Matsuda JJ, Lee H, Shibata EF. Enhancement of rabbit cardiac sodium channels by beta-adrenergic stimulation. Circ Res. 1992;70:199–207. doi: 10.1161/01.res.70.1.199. [DOI] [PubMed] [Google Scholar]
- 40.Murray KT, Hu NN, Daw JR, Shin HG, Watson MT, Mashburn AB, et al. Functional effects of protein kinase C activation on the human cardiac Na+ channel. Circ Res. 1997;80:370–376. doi: 10.1161/01.res.80.3.370. [DOI] [PubMed] [Google Scholar]
- 41.Clancy CE, Tateyama M, Kass RS. Insights into the molecular mechanisms of bradycardia-triggered arrhythmias in long QT-3 syndrome. J Clin Invest. 2002;110:1251–1262. doi: 10.1172/JCI15928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chandra R, Chauhan VS, Starmer CF, Grant AO. beta-Adrenergic action on wild-type and KPQ mutant human cardiac Na+ channels: shift in gating but no change in Ca2 + :Na+ selectivity. Cardiovasc Res. 1999;42:490–502. doi: 10.1016/s0008-6363(99)00042-5. [DOI] [PubMed] [Google Scholar]
- 43.Tsurugi T, Nagatomo T, Abe H, Oginosawa Y, Takemasa H, Kohno R, et al. Differential modulation of late sodium current by protein kinase A in R1623Q mutant of LQT3. Life Sci. 2009;84:380–387. doi: 10.1016/j.lfs.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Priori SG, Napolitano C, Schwartz PJ, Grillo M, Bloise R, Ronchetti E, et al. Association of long QT syndrome loci and cardiac events among patients treated with beta-blockers. Jama. 2004;292:1341–1344. doi: 10.1001/jama.292.11.1341. [DOI] [PubMed] [Google Scholar]
- 45.Lupoglazoff JM, Cheav T, Baroudi G, Berthet M, Denjoy I, Cauchemez B, et al. Homozygous SCN5A mutation in long-QT syndrome with functional two-to-one atrioventricular block. Circ Res. 2001;89:E16–E21. doi: 10.1161/hh1401.095087. [DOI] [PubMed] [Google Scholar]
- 46.Ten Harkel AD, Blom NA, Reimer AG, Tukkie R, Sreeram N, Bink-Boelkens MT. Implantable cardioverter defibrillator implantation in children in The Netherlands. Eur J Pediatr. 2005;164:436–441. doi: 10.1007/s00431-005-1668-1. [DOI] [PubMed] [Google Scholar]
- 47.Wichter T, Schäfers M, Rhodes CG, Borggrefe M, Lerch H, Lammertsma AA, et al. Abnormalities of cardiac sympathetic innervation in arrhythmogenic right ventricular cardiomyopathy: quantitative assessment of presynaptic norepinephrine reuptake and postsynaptic beta-adrenergic receptor density with positron emission tomography. Circulation. 2000;101:1552–1558. doi: 10.1161/01.cir.101.13.1552. [DOI] [PubMed] [Google Scholar]
- 48.Matthews JC, Baker JK. Effects of propranolol and a number of its analogues on sodium channels. Biochem Pharmacol. 1982;31:1681–1685. doi: 10.1016/0006-2952(82)90668-2. [DOI] [PubMed] [Google Scholar]
- 49.Tian XL, Yong SL, Wan X, Wu L, Chung MK, Tchou PJ, et al. Mechanisms by which SCN5A mutation N1325S causes cardiac arrhythmias and sudden death in vivo. Cardiovasc Res. 2004;61:256–267. doi: 10.1016/j.cardiores.2003.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tan HL, Bardai A, Shimizu W, Moss AJ, Schulze-Bahr E, Noda T, et al. Genotype-specific onset of arrhythmias in congenital long-QT syndrome: possible therapy implications. Circulation. 2006;114:2096–2103. doi: 10.1161/CIRCULATIONAHA.106.642694. [DOI] [PubMed] [Google Scholar]
- 51.Veldkamp MW, Wilders R, Baartscheer A, Zegers JG, Bezzina CR, Wilde AA. Contribution of sodium channel mutations to bradycardia and sinus node dysfunction in LQT3 families. Circ Res. 2003;92:976–983. doi: 10.1161/01.RES.0000069689.09869.A8. [DOI] [PubMed] [Google Scholar]
- 52.Chang CC, Acharfi S, Wu MH, Chiang FT, Wang JK, Sung TC, et al. A novel SCN5A mutation manifests as a malignant form of long QT syndrome with perinatal onset of tachycardia/bradycardia. Cardiovasc Res. 2004;64:268–278. doi: 10.1016/j.cardiores.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 53.Kirchhof P, Fabritz L, Franz MR. Post-repolarization refractoriness versus conduction slowing caused by class I antiarrhythmic drugs—antiarrhythmic and proarrhythmic effects. Circulation. 1998;97:2567–2574. doi: 10.1161/01.cir.97.25.2567. [DOI] [PubMed] [Google Scholar]
- 54.Remme CA, Verkerk AO, Nuyens D, van Ginneken AC, van Brunschot S, Belterman CN, et al. Overlap syndrome of cardiac sodium channel disease in mice carrying the equivalent mutation of human SCN5A-1795insD. Circulation. 2006;114:2584–2594. doi: 10.1161/CIRCULATIONAHA.106.653949. [DOI] [PubMed] [Google Scholar]
- 55.Priori SG, Napolitano C, Schwartz PJ, Bloise R, Crotti L, Ronchetti E. The elusive link between LQT3 and Brugada syndrome: the role of flecainide challenge. Circulation. 2000;102:945–947. doi: 10.1161/01.cir.102.9.945. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.