Abstract
Aims
Our aim was to test the hypothesis that the repeated, binge administration of methamphetamine would produce oxidative stress in the myocardium leading to structural remodeling and impaired left ventricular function.
Methods and results
Echocardiography and Millar pressure–volume catheters were used to monitor left ventricular structure and function in rats subjected to four methamphetamine binges (3 mg/kg, iv for 4 days, separated by a 10-day drug-free period). Hearts from treated and control rats were used for histological or proteomic analysis. When compared with saline treatment, four methamphetamine binges produced eccentric left ventricular hypertrophy. The drug also significantly impaired systolic function (decreased fractional shortening, ejection fraction, and adjusted maximal power) and produced significant diastolic dysfunction (increased −dP/dt and tau). Dihydroethedium staining showed that methamphetamine significantly increased (285%) the levels of reactive oxygen species in the left ventricle. Treatment with methamphetamine also resulted in the tyrosine nitration of myofilament (desmin, myosin light chain) and mitochondrial (ATP synthase, NADH dehydrogenase, cytochrome c oxidase, prohibitin) proteins. Treatment with the superoxide dismutase mimetic, tempol in the drinking water prevented methamphetamine-induced left ventricular dilation and systolic dysfunction; however, tempol (2.5 mM) did not prevent the diastolic dysfunction. Tempol significantly reduced, but did not eliminate dihydroethedium staining in the left ventricle, nor did it prevent the tyrosine nitration of mitochondrial and contractile proteins.
Conclusion
This study shows that oxidative stress plays a significant role in mediating methamphetamine-induced eccentric left ventricular dilation and systolic dysfunction.
Keywords: Dilated myopathy, Heart, Pressure–volume relationship, Cardiac toxicity, Drug abuse
1. Introduction
Methamphetamine is a highly addictive sympathomimetic stimulant whose illicit use is a growing problem in the USA. In 2006, methamphetamine accounted for roughly 8% of illicit drug-related emergency room visits nationwide, the majority of which were for cardiac- and cardiovascular-related incidents.1 A recent retrospective study of hospital discharges in the state of Texas for patients aged 18–44 years revealed a significant association between amphetamine use and acute myocardial infarction.2 An increasing number of clinical and autopsy reports also link methamphetamine use with angina, tachycardia, hypertension, myocarditis, dilated cardiomyopathy, arrhythmia, and sudden death.3–4 The cardiovascular toxicity documented in these clinical reports is often difficult to evaluate due to the lack of data regarding the quantity and frequency of drug administration, the co-administration of other drugs, and the presence of other risk factors such as smoking and diabetes.
Most experimental studies of methamphetamine's actions on the heart have focused on its histopathological effects. Daily dosing of rats with methamphetamine (3–5 mg/kg, ip) for 1 week produced contraction bands and myocyte degeneration.5 After 2 weeks of daily dosing, the hearts showed mitochondrial degeneration with disrupted cristae, myofibrillar hypercontraction, enlargement of the sarcoplasmic reticulum, and loss of myofilaments.5–6
In spite of methamphetamine's ability to produce severe cardiac toxicity, surprisingly few controlled studies have systematically documented the changes in cardiac structure and function produced by this drug. A single study in mice showed that daily administration of escalating large doses of methamphetamine (15–40 mg/kg, ip) for 12 weeks decreased cardiac contractile function and produced myocardial fibrosis and concentric left ventricular hypertrophy.7 Given the paucity of information regarding the effects of long-term methamphetamine administration on the heart, the first goal of this study was to systematically characterize the drug's effects on left ventricular structure and function in rats using an established binge model.8
The mechanisms underlying methamphetamine-induced cardiac toxicity are largely unknown;8–9 however, several lines of evidence suggest that oxidative stress may play an important role. The repeated binge administration of 3,4-methlyeledioxymethamphetamine (ecstasy), a structurally similar amphetamine analogue, produces oxidative stress, eccentric left ventricular dilation, and diastolic dysfunction in the rat heart.10 Oxidative stress has also been shown to play an important role in the neurotoxic actions of methamphetamine.11–12 In mice and rats, administration of methamphetamine increases the levels of reactive oxygen species (ROS) and reactive nitrogen species in the brain.13–14 Moreover, methamphetamine-induced neurotoxicity can be prevented by the inhibition of nNOS, the over-expression of superoxide dismutase, or the administration of antioxidants.15 Therefore, the second goal of this study was to test the hypothesis that oxidative stress is an important mediator of methamphetamine-induced cardiac dysfunction.
2. Methods
2.1. General methods
Experiments were performed using male Sprague–Dawley rats (270–350 g, Harlan, Indianapolis, IN, USA). All procedures were in accordance with National Institutes of Health guidelines for the Care and Use of Experimental Animals (NIH Publication No. 85-23, revised 1996) and were approved by the Institutional Animal Care and Use Committee at Louisiana State University Health Sciences Center. The rats were individually housed in a temperature- and humidity-controlled room with a 12-h light/dark cycle. Standard rat chow and tap water were available ad libitum. A polyurethane catheter (Micro-renathane, 0.033 in od × 0.014 in id) was placed into the femoral vein under ketamine/xylazine (90/10 mg/kg, ip) anaesthesia.8 Following surgery, the rats were allowed to recover under a heat lamp and were administered penicillin G (60 000 U, im), buprenorphine (0.1 mg/kg), and fluids. Rats were allowed to recover for 2 days before starting the binge protocol.
2.2. Binge dosing protocol
Binge dosing is a common pattern of chronic methamphetamine use characterized by frequent (several times a day) drug administration for a short period (‘binge’) followed by a period of abstinence. This cyclic pattern of frequent, short-term use, and abstinence is usually repeated many times. The 3 mg/kg dose of methamphetamine was chosen, because it was within the range of doses producing neurotoxicity in rodents and primates.8,16 We previously showed that binge administration of this dose also alters vascular responsiveness and produces cardiac inflammation and necrosis in rat myocardium.8 Three experimental groups were used in these studies. The first group was given twice daily injections of methamphetamine (3 mg/kg, iv) for 4 days. Each dose of methamphetamine (27–35 µL) was followed by a 0.1 mL saline flush. This binge was followed by 10-day drug-free period. Each rat received a total of four methamphetamine binges. The second group received iv injections of saline according to the same schedule. The third group received methamphetamine as described above and was given the superoxide dismutase (SOD)-mimetic tempol (2.5 mM) in their drinking water beginning 1 day before, until 1 day after the conclusion of each binge.17–19 The addition of tempol to the drinking water did not affect water consumption (data not shown).
2.3. Echocardiographic studies
Echocardiograms were performed in all rats 1 day before the first binge and 1 day after the fourth binge as described previously.10 In some rats, echocardiograms were also performed 1 day after the second binge. During the echocardiographic examination, the rats were anaesthetized with isoflurane (1–2% with 2 L/min O2). ECG electrodes were placed in a standard limb configuration to monitor heart rate. Ultrasound images were obtained at 8.5 MHz (Toshiba Aplio). Two-dimensional and M-mode echocardiographic measurements of posterior wall thickness during diastole (PWd) and during systole (PWs), left ventricular end-diastolic diameter (EDD), and left ventricular end-systolic diameter (ESD) were recorded in the parasternal long-axis view of the left ventricle, as well as the parasternal short-axis view at the level of the papillary muscles. The relative wall thickness ratio 2 × PWd/EDD was used to evaluate eccentric vs. concentric structural changes of the left ventricle. Left ventricular systolic function was measured by fractional shortening (EDD−ESD/EDD). All measurements were performed on three different cardiac cycles and the values averaged at each time point.
2.4. Measurement of left ventricular function
Left ventricular function was quantified after the fourth binge in saline, methamphetamine, and methamphetamine plus tempol-treated rats using pressure–volume loop analysis.10 Rats were anaesthetized and ventilated using isoflurane (1–2% with 2 L/min O2). The right jugular vein was cannulated. A microtip pressure–volume catheter (SPR-838; Millar Instruments) was introduced into the right carotid artery and advanced into the left ventricle. Following a 15 min equilibration period, the pressure and volume signals were continuously recorded (sampling rate 1000 Hz) using the MPVS-400 pressure–volume conductance system (Millar Instruments). Baseline measures of heart rate, peak left ventricular systolic pressure, left ventricular end-diastolic pressure (EDP), end-diastolic volume (EDV), maximal slope of the diastolic pressure increment (−dP/dt), tau, ejection fraction (EF), stroke volume (SV), cardiac output (CO), and adjusted maximal power were computed (Millar PVAN). Load-independent measures of systolic (end-systolic pressure–volume relationship, ESPVR) and diastolic (end-diastolic pressure–volume relationship, EDPVR) functions were determined while varying preload. Preload was altered by partial occlusion of the subdiaphragmatic inferior vena cava.10 Volume calibration of the pressure–volume catheter was performed as described previously.10
2.5. Proteomic analysis
After completing the ventricular function studies, the hearts were quickly removed and rinsed in ice-cold PBS. Portions of the mid-wall of the left ventricles were flash frozen in liquid nitrogen and stored at −80°C. Left ventricle lysates from saline, methamphetamine, and methamphetamine plus tempol-treated rats were homogenized using tissue lysis buffer (0.1% Triton X-100, 0.1% deoxycholate, 25 mM HEPES, Ph 7.4, 50 mM NaCl, 1 mM MgCl2, 2 mm EGTA, 10 mM pyrophosphate, 10 µg/mL aprotinin, 10 µg/mL leupeptin, 0.5 mM PMSF, and 500 µM Na3VO4) and sonicated. Samples were centrifuged at 10 600 g for 10 min at 4°C. Protein concentrations were assessed using a bicinchonic acid assay (Pierce). Two-dimensional PAGE analysis was performed as previously described.10 Briefly, 600 µg of pooled left ventricle lysates from three hearts per group were immunoprecipitated with anti-nTyr antibodies coupled to agarose beads (Upstate). Immunoprecipitates were resuspended in 150 µL of isoelectric focusing (IEF) buffer. Precast immobilized pH gradient strips (pH 3–10, Invitrogen) were allowed to rehydrate overnight. IEF was performed using an Invitrogen Zoom runner IEF cell. For the second dimension, strips were placed above standard format mini-gels (5–15%, Invitrogen). SDS–PAGE gels were stained with Colloidal Blue and visualized via PDQuest image analysis software to identify spots of interest. Gel spots were excised, destained, dried in vacuo, and hydrolyzed ‘in-gel’ with trypsin at 37°C overnight. The trypsin digests were then adjusted with alphahydroxy-cinnamic acid. One microlitre of sample was spotted onto a matrix-assisted laser-desorption ionization time-of-flight (MALDI-TOF) sample plate, and analysed by MALDI-TOF mass spectrometry (MALDI-TOF-MS). The parent polypeptides were identified by comparing the profile of tryptic peptide masses generated by the mass spectrometer with predicted tryptic peptides from all known polypeptides using the MASCOT program.
2.6. Dihydroethidium staining for ROS
Dihydroethidium (DHE) staining was used as an indicator of ROS generation. In the presence of superoxide, hydrogen peroxide, or hydroxyl radical, DHE is oxidized to ethidium, which intercalates into nuclear DNA and fluoresces under ultraviolet light (excitation, 500–530 nm; emission, 590–620 nm). After the fourth binge, saline, methamphetamine, and methamphetamine plus tempol-treated rats were anaesthetized; DHE (5 µM, 0.3 mL) was injected into left ventricle through the apex of the heart. The DHE was allowed to circulate for 15 min before the hearts were removed. Excised hearts were rinsed in PBS (4°C), imbedded in optimum temperature cutting compound and frozen. Four sections were taken from the mid-left ventricular region of each heart (5 µm) and thaw-mounted on glass slides. The sections were examined using a fluorescence microscope and digitally photographed.
Fluorescent DHE staining was quantified from the digital images of the left ventricular sections (four sections/heart) using an automated image processing technique. A section of each image was divided into approximately 1000 image blocks (256 × 256 pixels). To identify fluorescent nuclei in each image block, a local-maximum detection procedure was employed. A low-pass filter (15 × 15 pixels) was applied over the image to find the background fluorescence. The background was then subtracted from the original image and all the resulting negative pixel values were then replaced with zeros to obtain a background-free image. Pixels in the background-free image corresponding to locally maximum fluorescent intensity were counted as one labelled nucleus. Using this procedure, the number of nuclei was counted within each 256 × 256 image block. The median value of nuclei counts from all 256 × 256 blocks was used to determine the nuclei density within each section.
2.7. Statistics
Data are expressed as mean ± SEM. The echocardiographic, DHE fluorescence, and left ventricular pressure–volume measurements were analysed by one-way ANOVA and difference between means determined by Holm–Sidak non-parametric tests. When comparison among the groups failed the normality test, one-way ANOVA on ranks were performed followed by Dunn's non-parametric tests. P < 0.05 was considered statistically significant.
3. Results
3.1. Methamphetamine produces left ventricular dilation and dysfunction
To characterize the effects of binge methamphetamine on cardiac structure and function, rats were treated with saline (n = 7), methamphetamine (n = 7), or methamphetamine plus tempol (n = 6). Prior to the first binge, the body weights of the saline (311 ± 3 g), methamphetamine (313 ± 3 g), and methamphetamine plus tempol (310 ± 4 g) treated rats were not significantly different. After the fourth binge, the body weights of the methamphetamine-treated rats were significantly less (348 ± 7 g; P < 0.001) than the controls (388 ± 3 g) or methamphetamine plus tempol-treated rats (373 ± 6 g).
3.2. Echocardiography
Echocardiographic parameters of left ventricular structure and function were similar between the three treatment groups before the start of the first binge (Table 1). After the second binge, these parameters were again similar except for EDD and ESD which were significantly smaller in the methamphetamine plus tempol-treated group than in saline-treated rats (Table 1). One day after the fourth binge, EDD and ESD were significantly larger in methamphetamine than in saline-treated rats (Table 1). In addition, PWd and PWs were significantly thinner in methamphetamine-treated than in saline rats (Table 1). Fractional shortening and the ratio of 2 × PWd/EDD were both significantly reduced in the methamphetamine compared with saline-treated rats (Table 1). Treatment with tempol in the drinking water prevented methamphetamine-induced increases in left ventricular diameter and the decreases in PWd and PWs (Table 1). Treatment with tempol also prevented the methamphetamine-induced reduction in fractional shortening (Table 1). As significant increases in left ventricular diameter and decreases in systolic function were observed after exposure to four methamphetamine binges, we chose this time point to assess the left ventricular pressure–volume relationships in these rats.
Table 1.
Echocardiographic parameters in saline (n = 7), methamphetamine (n= 7), and methamphetamine plus tempol (n = 6) treated rats 1 day before the start of the first and second binge and 1 day after the fourth binge
| Before binge 1 |
1 Day after binge 2 |
1 Day after binge 4 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Saline | Meth | Meth + tempol | Saline | Meth | Meth + tempol | Saline | Meth | Meth + tempol | |
| EDD (mm) | 7.25 ± 0.03 | 7.28 ± 0.12 | 7.06 ± 0.05 | 7.72 ± 0.05 | 7.62 ± 0.07 | 7.23 ± 0.04* | 7.88 ± 0.07 | 9.04 ± 0.05* | 7.77 ± 0.05 |
| ESD (mm) | 4.4 ± 0.08 | 4.45 ± 0.1 | 4.19 ± 0.08 | 4.82 ± 0.09 | 5.0 ± 0.15 | 4.41 ± 0.12 * | 4.75 ± 0.12 | 6.59 ± 0.06* | 4.57 ± 0.16 |
| PWd (mm) | 1.01 ± 0.04 | 1.08 ± 0.04 | 1.09 ± 0.05 | 1.07 ± 0.12 | 1.04 ± 0.08 | 1.11 ± 0.04 | 1.15 ± 0.05 | 0.84 ± 0.04* | 1.18 ± 0.03 |
| PWs (mm) | 1.88 ± 0.06 | 1.88 ± 0.04 | 1.77 ± 0.12 | 1.93 ± 0.07 | 1.76 ± 0.03* | 2.0 ± 0.12 | 1.91 ± 0.09 | 1.45 ± 0.05* | 2.09 ± 0.07 |
| 2 × PWd/EDD | 0.28 ± 0.01 | 0.30 ± 0.01 | 0.31 ± 0.01 | 0.28 ± 0.01 | 0.27 ± 0.01 | 0.33 ± 0.01 | 0.29 ± 0.01 | 0.19 ± 0.01* | 0.30 ± 0.01 |
| FS (%) | 39 ± 2 | 39 ± 1 | 41 ± 1 | 38 ± 1 | 34 ± 2 | 39 ± 2 | 40 ± 1 | 27 ± 1* | 41 ± 2 |
EDD, end-diastolic diameter; ESD, end-systolic diameter; PWd, posterior wall thickness during diastole; PWs, posterior wall thickness during systole; FS, fractional shortening.
*P < 0.05 from saline treatment groups.
3.3. Analysis of pressure–volume relationships
Left ventricular function was assessed using a high-fidelity pressure–volume catheter in the saline (n = 5), methamphetamine (n = 5), and methamphetamine plus tempol (n = 6) treated rats under baseline conditions and at different EDVs. Owing to technical problems, pressure–volume data could not be obtained from two of the saline and two of the methamphetamine-treated rats.
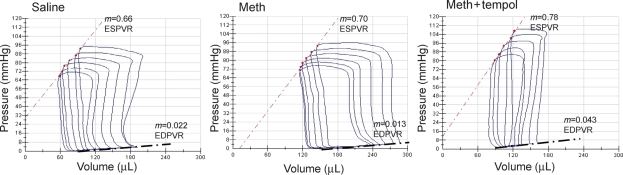
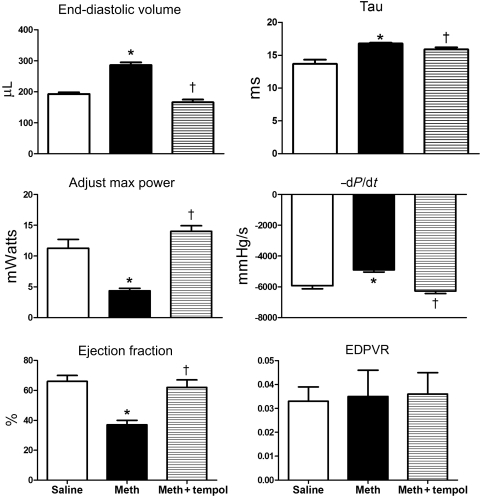
Prior to inserting the pressure–volume catheter, baseline heart rates were the same between the three groups (Table 2). Figure 1 shows representative pressure–volume loops generated in a rat from each of the treatment groups 1 day after the fourth binge. Table 2 summarizes the pressure–volume data for all the animals. When compared with saline, treatment with methamphetamine significantly increased EDV and EDP (Figures 1 and 2, Table 2) and significantly decreased adjusted maximal power and EF (Figure 2). Treatment with methamphetamine alone, also significantly increased tau (isovolumetric relaxation time constant) and −dP/dt indicating impaired ventricular relaxation (Figure 2). EDPVR and ESPVR were not significantly different between saline- and methamphetamine-treated rats (Figure 1 and Table 2). SV, CO, and peak systolic pressure were also not significantly different between saline- and methamphetamine-treated rats (Figure 2).
Table 2.
Parameters of left ventricular function obtained by pressure–volume analysis in rats treated with four binges of saline (n = 5) methamphetamine (n = 5), or methamphetamine plus tempol (n = 6) rats
| Saline | Meth | Meth + tempol | |
|---|---|---|---|
| Heart rate (b.p.m.) | 272 ± 14 | 289 ± 5 | 268 ± 14 |
| SV (µL) | 123 ± 7 | 108 ± 9 | 103 ± 7 |
| CO (µL/min) | 33 327 ± 1547 | 31 663 ± 2800 | 27 716 ± 5971 |
| ESVP (mmHg) | 98 ± 2 | 101 ± 5 | 121.14 ± 4† |
| EDVP(mmHg) | 5.0 ± 0.3 | 7.6 ± 0.3* | 4.54 ± 0.2† |
| ESPVR | 0.792 ± 0.01 | 0.873 ± 0.013 | 0.75 ± 0.015 |
SV, stroke volume; CO, cardiac output; ESVP, end-systolic ventricular pressure; EDVP, end-diastolic ventricular pressure; ESPVR, end-systolic pressure–volume relationship; EDPVR, end-diastolic pressure–volume relationship.
*P < 0.05 between saline and methamphetamine groups.
†P < 0.05 between methamphetamine and methamphetamine plus tempol-treated groups.
Figure 1.
Pressure–volume analysis of systolic and diastolic function. Representative pressure–volume loops generated during changes in preload in a rat treated with four binges of saline, methamphetamine, or methamphetamine plus tempol. Preload was altered by occluding the inferior vena cava. ESPVR, end-systolic pressure–volume relationship; EDPVR, end-diastolic pressure–volume relationship.
Figure 2.
Comparison of systolic and diastolic left ventricular function in rats after four binges of saline (n = 5), methamphetamine (n = 5), or methamphetamine plus tempol (n = 6). *P < 0.05 between saline and methamphetamine groups. †P < 0.05 between methamphetamine and methamphetamine plus tempol groups.
Treatment with tempol prevented methamphetamine-induced increases in EDV and EDP (Figure 2 and Table 2). Adjusted maximal power and EF in rats treated with methamphetamine plus tempol were significantly greater than in rats treated with methamphetamine alone and were similar to those in saline-treated rats (Figure 2). Treatment with tempol also blocked the methamphetamine-induced increases in tau and prevented the methamphetamine-induced decrease in −dP/dt (Figure 2). Except for peak systolic pressure, which was significantly increased after treatment with methamphetamine plus tempol, there were no significant differences in cardiac structure or function between methamphetamine plus tempol- and saline-treated rats (Figure 1 and Table 2).
3.4. Methamphetamine increases ROS in the heart
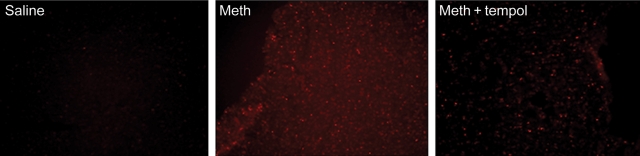
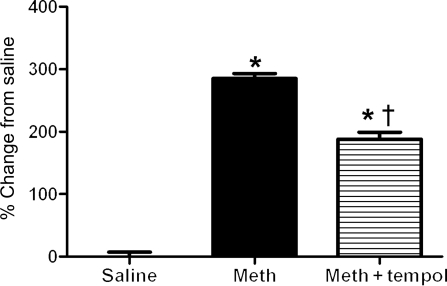
Separate groups of rats were subject to four binges of saline (n = 3), methamphetamine (n = 3), or methamphetamine plus tempol (n = 3). Echocardiography was used to confirm that four binges of methamphetamine had significantly increased EDD and decreased EF and that tempol had effectively blocked these effects. Figure 3 shows representative examples of DHE fluorescence in mid-left ventricular wall from a rat from each treatment group. Figure 4 compares the amount of DHE fluorescence (punctuate staining per pixel) between the groups. Treatment with methamphetamine significantly increased DHE fluorescence in the heart (Figures 3 and 4). Treatment with tempol reduced, but did not prevent the methamphetamine-mediated increase in ROS (Figures 3 and 4).
Figure 3.
Representative photomicrographs of dihydroethidium staining in frozen sections of the left ventricle from rats treated with four binges of saline, methamphetamine, or methamphetamine plus tempol. Magnification is ×200.
Figure 4.
Comparison of DHE staining in rats treated with four binges of saline, methamphetamine, or methamphetamine plus tempol. Fluorescence was quantified as described in Methods section. n = 3 rats per group with four sections being analysed from each heart. *P < 0.05 between saline and methamphetamine groups. †P < 0.05 significant difference between methamphetamine and methamphetamine plus tempol groups.
3.5. Nitration of cardiac proteins
To determine whether the increase in ROS detected by DHE staining resulted in the redox modification of cardiac proteins, we used 2D electrophoresis to detect proteins with nitrated tyrosine residues. Peroxynitrite leads to nitration of tyrosine residues on proteins. Peroxynitrite is formed by a diffusion-limited reaction of both superoxide and nitric oxide. After finishing the ventricular function studies, the hearts were removed and left ventricular tissue lysates were prepared from the hearts of three rats randomly selected from each of the three treatment groups. The pooled lysates from each group were immunoprecipitated using anti-nitrotyrosine antibody. N-Tyr immunoprecipitates were separated by two-dimensional PAGE; spots differentially nitrated between the saline and methamphetamine treatment groups were identified using MALDI-TOF-MS. Differentially nitrated proteins in the methamphetamine treatment group clustered into two classes: myofilament proteins (desmin, myosin light chain) and mitochondrial proteins (ATP synthase, NADH dehydrogenase, cytochrome c oxidase, and prohibitin) (Table 3). The same pattern of protein nitration in methamphetamine-treated hearts was observed in the hearts from rats treated with methamphetamine plus tempol; however, the staining of the spots in the tempol-treated samples was less intense. The level of nitrated protein in the 2D gels could not quantified.
Table 3.
MALDI-TOF identification of differentially nitrated proteins in the left ventricle of rats treated with four binges of methamphetamine
| Protein | Protein mol. wt. (kDa) | Protein score |
|---|---|---|
| NADPH dehydrogenase | 80.3 | 395 |
| Desmin | 53.4 | 842 |
| ATPase complex F1 | 44.3 | 157 |
| Prohibitin | 29.8 | 554 |
| Myosin light chain polypeptide | 22.3 | 350 |
| Cytochrome c oxidase | 12.5 | 202 |
| Actin | 39.5 | 149 |
4. Discussion
This is the first study to systematically show that repeated binge administration of methamphetamine produces left ventricular dilation as well as systolic and diastolic dysfunction in rats. Moreover, we demonstrate for the first time that the SOD-mimetic tempol can prevent the drug-induced structural and functional derangements, thereby indicating that oxidative stress is an important causative factor in the development of methamphetamine-induced cardiac dysfunction.
As demonstrated by serial echocardiography and the analysis of pressure–volume data, binge administration of methamphetamine significantly increased left ventricular volume and decreased ventricular wall thickness during systole and diastole. The ratio of 2 × PWd/EDD, a measure used to distinguish hypertrophic changes, was significantly smaller in methamphetamine than in saline-treated rats indicating the development of eccentric left ventricular dilation. These findings are consistent with clinical and autopsy reports which link chronic methamphetamine abuse with eccentric dilated cardiomyopathy.3,20–21 In contrast to the eccentric changes observed in our study, Yu et al.7 showed that the daily administration of escalating doses of methamphetamine (15–40 mg/kg, ip) in mice over a 12-week period produced concentric left ventricular hypertrophy and reduced ventricular performance. Whether the ability of methamphetamine to produce different hypertrophic responses in the hearts of rats and mice reflects differences in the dosing paradigms or is a species specific phenomenon remains to be determined.
The eccentric left ventricular dilation produced by the binge administration of methamphetamine was accompanied by impaired systolic performances indicated by decreases in EF, fractional shortening, and adjusted maximal power. Similar changes in EF and fractional shortening have also been reported in human case studies.20 Although methamphetamine decreased systolic function, ESPVR (a measure of contractility) was not significantly reduced by the administration of the drug. A possible explanation for this observation is the activation of compensatory neural/humoral and/or structural mechanisms during early systolic dysfunction preventing the expected decline in ESPVR.22–23 Evidence for cardiac compensation is provided by the fact that SV and CO in methamphetamine- and saline-treated rats were similar, in spite of the significant increase in left ventricular volume. We anticipate that continued administration of methamphetamine would ultimately lead to uncompensated heart failure and a significant decrease is ESPVR.
As evidenced by DHE staining, treatment with methamphetamine significantly increased ROS production resulting in increased nitration of contractile and mitochondrial proteins in the heart. More importantly, treatment with tempol blocked the left ventricular dilation and systolic dysfunction produced by the binge administration of methamphetamine. Tempol also significantly reduced, but did not eliminate methamphetamine-induced increases in ROS. Nevertheless, the reduction in ROS was sufficient to prevent methamphetamine-mediated decreases in systolic function and ventricular dilation.
The increased nitration of cardiac contractile and structural proteins provides further evidence that methamphetamine produces oxidative stress in the heart and may provide clues to the mechanisms underlying the systolic and diastolic dysfunction. Desmin, an intermediate filament, has structural, sensory, and signalling roles in normal and diseased cardiac myocytes.24 Increased nitration of desmin has been reported during cardiac hypertrophy and congestive heart failure.25 The specific effect nitration has on the function of this protein is unknown; however, we recently showed that desmin is nitrated after the binge administration of the amphetamine analogue 3,4-methylenedioxymethamphetamine which also induced structural changes and functional deficits in the rat heart.10
Methamphetamine also produced diastolic dysfunction. It is generally accepted that diastolic dysfunction may precede systolic dysfunction and contribute to the progression of heart failure through changes in compliance and relaxation.26–27 Treatment with methamphetamine significantly impaired relaxation as indicated by the increases in tau and −dP/dt; however, EDPVR was unchanged, suggesting that the diastolic impairment reflected changes in relaxation, rather than changes in compliance or the extracellular matrix. This possibility is further supported by the observed parallel increases in EDV and EDP (Table 2) and the nitration of mitochondrial and structural proteins known to contribute to ATP synthesis and relaxation.
Although we did not measure mitochondrial production of ATP, treatment with methamphetamine and the associated oxidative stress led to the nitration of complex I (NADH dehydrogenase), complex IV (cytochrome c oxidase), and complex V (ATP synthase) of the mitochondrial electron transport chain. The redox modification of components within the mitochondrial transport chain can decrease energy production and increase the production of ROS.25 For example, nitration of complexes I and V decreases the production of ATP.28–29 Redox-mediated changes in mitochondrial function are also thought to underlie many of the drug's neurotoxic effects.30 Although tempol significantly reduced ROS production and prevented methamphetamine-induced ventricular dilation and systolic dysfunction, it did not prevent the tyrosine nitration of the mitochondrial proteins or the diastolic dysfunction. These findings further support a link between mitochondrial energy production and diastolic function. Alternatively, methamphetamine may produce diastolic dysfunction independent of increased ROS.
Methamphetamine treatment also resulted in the nitration of prohibitin. Prohibitin is a highly conserved protein that is predominantly located in the inner mitochondrial membrane. This protein is thought to regulate mitosis, apoptosis, and assembly of mitochondrial respiratory chain enzymes.31 Although its role in the heart is unclear, there is evidence that prohibitin is involved in the maintenance and function of complex I. Whether the nitration of prohibitin decreases ATP production by altering the assembly of mitochondrial electron transport chain complexes remains to be tested.
4.1. Limitations
As methamphetamine can increase arterial pressure, we cannot completely discount the possibility that haemodynamic factors, such as methamphetamine-induced increases in afterload could have produced eccentric left ventricular dilation and oxidative stress. However, we previously reported that binge doses of methamphetamine (3 mg/kg, iv, bid), elicits a two-part pressor response in conscious, telemetered rats consisting of a rapid increase in arterial pressure (∼28 mmHg) lasting <30 s, followed by a smaller, more prolonged increase in pressure (∼10 mmHg) lasting 15–20 min.8 Although sensitization developed to initial pressor response elicited by the drug between binges, this sensitization was only evident for the first four doses within in the binge. Given the relatively short duration of the pressor responses and the fact that drug treatment did not increase baseline arterial pressure, it is unlikely that increased afterload was responsible for the observed eccentric left ventricular dilation. Moreover, increased afterload would have been expected to produce concentric, rather than eccentric ventricular dilation. Our data describe a very early stage of methamphetamine-induced heart failure; whether haemodynamic factors would be involved if the heart progressed to overt or decompensated failure with continued drug administration is not known. Other mechanisms that could potentially be involved in the evolution to decompensated failure include neurohumoral activation, changes in beta-adrenergic receptor number or signalling, or coronary vascular dysfunction (e.g. changes in eNOS, endothelin, or others). Supporting these possible mechanisms, we previously showed that the binge administration of methamphetamine significantly impaired the vasodilator response to acetylcholine and nitroprusside, possibly due to increased oxidative stress and/or impairment of the nitric oxide system.8 In contrast, the arterial pressure and heart rate responses to phenylephrine and isoproterenol were not altered after the binge administration of the methamphetamine.8
4.2. Summary and conclusions
To our knowledge, this study provides the first conclusive evidence that oxidative stress plays an important role in mediating the left ventricular dilation and dysfunction produced in response to the binge administration of methamphetamine. The mechanism(s) whereby this drug increases oxidative stress in the heart remains largely unknown. Clinical and experimental reports show that exposure to high levels of circulating catecholamine produces a pattern of cardiac toxicity which in many ways resembles that produced by methamphetamine.32 Given that methamphetamine increases synaptic and circulating levels of catecholamines, many authors have speculated that excessive catecholaminergic stimulation is involved in the drug's cardiotoxic actions. High levels of catecholamine can produce oxidative stress by both direct and indirect actions. In high concentrations, catecholamines can undergo auto-oxidation or degradation by monoamine oxidase, resulting in the formation of quinones and semiquinones which can undergo redox cycling thus producing large amounts of superoxide.33,34 High catecholamine levels during binge dosing may also produce episodes of catecholamine-mediated coronary vasoconstriction followed by reperfusion leading to the activation of xanthine oxidase, leucocyte activation, and/or mitochondrial dysfunction, all of which would increase the production of ROS and/or reactive nitrogen species. Identification of the mechanisms responsible for generating methamphetamine-induced oxidative stress will be important for devising treatments to protect individuals from the cardiotoxic actions of this drug.
Funding
This work was supported by the American Heart Association [615646 to S.K.S., 655796 to K.J.V.] and National Institutes of Health [HL63318 to P.A.L., P20 RR18766].
Acknowledgements
We would like to thank Dr Jason Gardner for his editorial comments.
Conflict of interest: none declared.
References
- 1.Network DAW. National Estimates of drug-related emergency department visits. DAWN Series D-30. 2006 DHHS Publication (SMA) [Google Scholar]
- 2.Westover AN, Nakonezny PA, Haley RW. Acute myocardial infarction in young adults who abuse amphetamines. Drug Alcohol Depend. 2008;96:49–56. doi: 10.1016/j.drugalcdep.2008.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karch SB, Stephens BG, Ho CH. Methamphetamine-related deaths in San Francisco: demographic, pathologic, and toxicologic profiles. J Forensic Sci. 1999;44:359–368. [PubMed] [Google Scholar]
- 4.Wijetunga M, Seto T, Lindsay J, Schatz I. Crystal methamphetamine-associated cardiomyopathy: tip of the iceberg? J Toxicol Clin Toxicol. 2003;41:981–986. doi: 10.1081/clt-120026521. [DOI] [PubMed] [Google Scholar]
- 5.He SY, Matoba R, Fujitani N, Sodesaki K, Onishi S. Cardiac muscle lesions associated with chronic administration of methamphetamine in rats. Am J Forensic Med Pathol. 1996;17:155–162. doi: 10.1097/00000433-199606000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Islam MN, Jesmine K, Kong Sn Molh A, Hasnan J. Histopathological studies of cardiac lesions after long term administration of methamphetamine in high dosage—Part II. Legal Med. 2009;11:S147–S150. doi: 10.1016/j.legalmed.2009.02.035. [DOI] [PubMed] [Google Scholar]
- 7.Yu Q, Montes S, Larson DF, Watson RR. Effects of chronic methamphetamine exposure on heart function in uninfected and retrovirus-infected mice. Life Sci. 2002;71:953–965. doi: 10.1016/s0024-3205(02)01769-1. [DOI] [PubMed] [Google Scholar]
- 8.Varner KJ, Ogden BA, Delcarpio J, Meleg-Smith S. Cardiovascular responses elicited by the ‘binge’ administration of methamphetamine. J Pharmacol Exp Ther. 2002;301:152–159. doi: 10.1124/jpet.301.1.152. [DOI] [PubMed] [Google Scholar]
- 9.Jiang JP, Downing SE. Catecholamine cardiomyopathy: review and analysis of pathogenetic mechanisms. Yale J Biol Med. 1990;63:581–591. [PMC free article] [PubMed] [Google Scholar]
- 10.Shenouda SK, Lord KC, McIlwain E, Lucchesi PA, Varner KJ. Ecstasy produces left ventricular dysfunction and oxidative stress in rats. Cardiovasc Res. 2008;79:662–670. doi: 10.1093/cvr/cvn129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Acikgoz O, Gonenc S, Kayatekin BM, Uysal N, Pekcetin C, Semin I, et al. Methamphetamine causes lipid peroxidation and an increase in superoxide dismutase activity in the rat striatum. Brain Res. 1998;813:200–202. doi: 10.1016/s0006-8993(98)01020-8. [DOI] [PubMed] [Google Scholar]
- 12.Melega WP, Lacan G, Harvey DC, Way BM. Methamphetamine increases basal ganglia iron to levels observed in aging. Neuroreport. 2007;18:1741–1745. doi: 10.1097/WNR.0b013e3282f0d4f4. [DOI] [PubMed] [Google Scholar]
- 13.Davidson C, Gow AJ, Lee TH, Ellinwood EH. Methamphetamine neurotoxicity: necrotic and apoptotic mechanisms and relevance to human abuse and treatment. Brain Res Rev. 2001;36:1–22. doi: 10.1016/s0165-0173(01)00054-6. [DOI] [PubMed] [Google Scholar]
- 14.Imam SZ, Newport GD, Itzhak Y, Cadet JL, Islam F, Slikker W, Jr, et al. Peroxynitrite plays a role in methamphetamine-induced dopaminergic neurotoxicity: evidence from mice lacking neuronal nitric oxide synthase gene or overexpressing copper–zinc superoxide dismutase. J Neurochem. 2001;76:745–749. doi: 10.1046/j.1471-4159.2001.00029.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Banerjee A, Banks WA, Ercal N. N-acetylcysteine amide protects against methamphetamine-induced oxidative stress and neurotoxicity in immortalized human brain endothelial cells. Brain Res. 2009;1275:87–95. doi: 10.1016/j.brainres.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kadota T, Kadota K. Neurotoxic morphological changes induced in the medial prefrontal cortex of rats behaviorally sensitized to methamphetamine. Arch Histol Cytol. 2004;67:241–251. doi: 10.1679/aohc.67.241. [DOI] [PubMed] [Google Scholar]
- 17.Hisaki R, Fujita H, Saito F, Kushiro T. Tempol attenuates the development of hypertensive renal injury in Dahl salt-sensitive rats. Am J Hypertens. 2005;18:707–713. doi: 10.1016/j.amjhyper.2004.11.045. [DOI] [PubMed] [Google Scholar]
- 18.Linares E, Giorgio S, Augusto O. Inhibition of in vivo leishmanicidal mechanisms by tempol: nitric oxide down-regulation and oxidant scavenging. Free Radic Biol Med. 2008;44:1668–1676. doi: 10.1016/j.freeradbiomed.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 19.Xiang L, Dearman J, Abram SR, Carter C, Hester RL. Insulin resistance and impaired functional vasodilation in obese Zucker rats. Am J Physiol Heart Circ Physiol. 2008;294:H1658–H1666. doi: 10.1152/ajpheart.01206.2007. [DOI] [PubMed] [Google Scholar]
- 20.Hong R, Matsuyama E, Nur K. Cardiomyopathy associated with the smoking of crystal methamphetamine. J Am Med Assoc. 1991;265:1152–1154. [PubMed] [Google Scholar]
- 21.Yeo KK, Wijetunga M, Ito H, Efird JT, Tay K, Seto TB, et al. The association of methamphetamine use and cardiomyopathy in young patients. Am J Med. 2007;120:165–171. doi: 10.1016/j.amjmed.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 22.Paulus WJ, Brutsaert DL. Relaxation abnormalities in cardiac hypertrophy. Eur Heart J. 1982;3(Suppl. A):133–137. doi: 10.1093/eurheartj/3.suppl_a.133. [DOI] [PubMed] [Google Scholar]
- 23.Brutsaert DL. Role of endocardium in cardiac overloading and failure. Eur Heart J. 1990;11(Suppl. G):8–16. doi: 10.1093/eurheartj/11.suppl_g.8. [DOI] [PubMed] [Google Scholar]
- 24.Heling A, Zimmermann R, Kostin S, Maeno Y, Hein S, Devaux B, et al. Increased expression of cytoskeletal, linkage, and extracellular proteins in failing human myocardium. Circ Res. 2000;86:846–853. doi: 10.1161/01.res.86.8.846. [DOI] [PubMed] [Google Scholar]
- 25.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madeira H. Diastolic heart failure: fact or myth? Rev Port Cardiol. 2006;25:883–886. [PubMed] [Google Scholar]
- 27.Melenovsky V, Borlaug BA, Rosen B, Hay I, Ferruci L, Morell CH, et al. Cardiovascular features of heart failure with preserved ejection fraction versus nonfailing hypertensive left ventricular hypertrophy in the urban Baltimore community: the role of atrial remodeling/dysfunction. J Am Coll Cardiol. 2007;49:198–207. doi: 10.1016/j.jacc.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 28.Choksi KB, Boylston WH, Rabek JP, Widger WR, Papaconstantinou J. Oxidatively damaged proteins of heart mitochondrial electron transport complexes. Biochim Biophys Acta. 2004;1688:95–101. doi: 10.1016/j.bbadis.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 29.Han Z, Chen YR, Jones CI, 3rd, Meenakshisundaram G, Zweier JL, Alevriadou BR. Shear-induced reactive nitrogen species inhibit mitochondrial respiratory complex activities in cultured vascular endothelial cells. Am J Physiol Cell Physiol. 2007;292:C1103–C1112. doi: 10.1152/ajpcell.00389.2006. [DOI] [PubMed] [Google Scholar]
- 30.Berman SB, Hastings TG. Dopamine oxidation alters mitochondrial respiration and induces permeability transition in brain mitochondria: implications for Parkinson's disease. J. Neurochem. 1999;73:1127–1137. doi: 10.1046/j.1471-4159.1999.0731127.x. [DOI] [PubMed] [Google Scholar]
- 31.Da Cruz S, Parone PA, Gonzalo P, Bienvenut WV, Tondera D, Jourdain A, et al. SLP-2 interacts with prohibitins in the mitochondrial inner membrane and contributes to their stability. Biochim Biophys Acta. 2008;1783:904–911. doi: 10.1016/j.bbamcr.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 32.Osadchii OE, Norton GR, McKechnie R, Deftereos D, Woodiwiss AJ. Cardiac dilatation and pump dysfunction without intrinsic myocardial systolic failure following chronic beta-adrenoreceptor activation. Am J Physiol Heart Circ Physiol. 2007;292:H1898–H1905. doi: 10.1152/ajpheart.00740.2006. [DOI] [PubMed] [Google Scholar]
- 33.Fornstedt B. Role of catechol autooxidation in the degeneration of dopamine neurons. Acta Neurol Scand Suppl. 1990;129:12–14. doi: 10.1111/j.1600-0404.1990.tb02595.x. [DOI] [PubMed] [Google Scholar]
- 34.Alagarsamy S, Phillips M, Pappas T, Johnson KM. Dopamine neurotoxicity in cortical neurons. Drug Alcohol Depend. 1997;48:105–111. doi: 10.1016/s0376-8716(97)00118-x. [DOI] [PubMed] [Google Scholar]