Abstract
Previous studies have reported an association between circulating insulin and incident cardiovascular disease, but limited knowledge is available on the association across subgroups. We examined the associations of fasting insulin with incident coronary heart disease (CHD) and ischemic stroke in multiple subgroups of a biracial, middle-age cohort. A total of 12,323 subjects were included in the analysis. The incidence of CHD (n = 960) and ischemic stroke (n = 445) through 2005 was determined through annual interviews, repeat examinations, and community surveillance. Serum insulin was measured at baseline. Cox regression analysis was used to estimate the hazard ratios by quintile of fasting insulin at baseline and to determine the significance of effect modification. In the minimally adjusted models (age, gender, race, and field center), the baseline fasting insulin quintile was positively associated with both incident CHD (hazard ratio per quintile insulin = 1.12, p-trend <0.0001) and ischemic stroke (hazard ratio per quintile insulin = 1.11, p = 0.0018). The adjustment for high-density lipoprotein completely attenuated the association of insulin with CHD but not with stroke. The associations of insulin with CHD were stronger in nonsmokers (p-interaction = 0.018) and in those without hypertension (p-interaction = 0.0087). The associations of insulin with stroke were stronger in women (p-interaction = 0.037), whites (compared to blacks; p-interaction = 0.036), and those without hypertension (p-interaction = 0.0027).
It is well established that type 2 diabetes mellitus increases the risk of coronary heart disease (CHD) and stroke, but the mechanisms of these associations are incompletely understood. A recent meta-analysis reviewed prospective cohort studies examining the association between circulating insulin and incident CHD1 and concluded that circulating insulin is a modest risk factor for CHD but that larger studies are required, in particular so the association could be examined in subgroups and effect modification (interaction) evaluated. Fewer studies have examined the prospective association of circulating insulin with stroke as a discrete end point,2–8 and all, including an earlier Atherosclerosis Risk In Communities (ARIC) Study analysis8 had relatively few events (<150 stroke cases). The results from these studies have been inconclusive, and no previous studies have included extensive subgroup analysis. The ARIC study has follow-up through 2005 with large numbers of CHD and stroke events. Therefore, we examined the association of baseline circulating insulin levels with incident CHD and incident ischemic stroke in the ARIC study.
Methods
The ARIC study is a multicenter prospective investigation of cardiovascular disease.9 White and black men and women aged 45 to 64 years were recruited in 1987 to 1989 from 4 communities: Forsyth County, North Carolina; Jackson, Mississippi; suburban areas of Minneapolis, Minnesota; and Washington County, Maryland. A total of 15,792 subjects participated in the baseline examination. Three triennial follow-up examinations were performed. The institutional review board at each field center approved the study, and all participants gave informed consent.
Of the 15,792 ARIC participants, we excluded anyone self-reporting race other than white or black and nonwhite persons from Minneapolis and Washington County (n = 103). We also excluded anyone with prevalent cardiovascular disease (n = 1,394) or prevalent diabetes (n = 1,721) at baseline. Prevalent cardiovascular disease was defined as a self-reported history of physician-diagnosed myocardial infarction (MI) or stroke; previous MI detected by electrocardiogram; or previous cardiovascular surgery or coronary angioplasty. Prevalent diabetes was defined as the presence of any of the following: fasting serum glucose of ≥126 mg/dl (7.0 mmol/L); nonfasting serum glucose level of ≥200 mg/dl (11.1 mmol/L); self-reported physician diagnosis of type 2 diabetes; or pharmacologic treatment of diabetes in the past 2 weeks. Finally, we excluded anyone who had fasted <8 hours (n = 251). The total sample size for the analysis was 12,323.
Blood samples were drawn from an antecubital vein into tubes containing ethylenediaminetetraacetic acid (lipids) or a serum separator gel (glucose and insulin). Serum insulin was measured with a radioimmunoassay (125Insulin Kit, Cambridge Medical Diagnostics, Baillerica, Massachusetts). The assay was not specific for insulin, and thus, some cross-reactivity occurred with pro-insulin. The details of the validity and reproducibility of this assay have been previously published.10 The total plasma cholesterol11 and triglyceride12 levels were determined using enzymatic methods. High-density lipoprotein (HDL) cholesterol was measured after dextran sulfate-magnesium precipitation of non-HDL lipoproteins.13 The low-density lipoprotein (LDL) cholesterol14 and non-HDL cholesterol levels were calculated. Trained technicians measured blood pressure 3 times. The mean of the last 2 measurements was used for analysis. Body mass index (BMI) was calculated from the participants' height and weight measured in scrub suits. A questionnaire was used to assess the participants' cigarette smoking history. Study participants were asked to bring all medication, vitamins, and supplements taken in the 2 weeks before the examination. Information on pharmacologic treatment of hypertension and diabetes was determined from the participants' self-reported use of any medication to treat high blood pressure or elevated glucose and the transcription and coding of all medication names. Hypertension was defined as systolic blood pressure of ≥140 mm Hg, diastolic blood pressure of ≥90 mm Hg, or use of antihypertensive medication. Left ventricular hypertrophy was determined using the Cornell voltage criteria for the resting electrocardiogram.15
Ascertainment of stroke and CHD events in the ARIC study has previously been described in detail.8,16 Events were identified using several methods. Interviewers contacted all participants annually by telephone to identify hospitalizations and death. Local death certificates and discharge lists, including International Classification of Diseases codes from local hospitals were also surveyed by the ARIC staff. For hospitalized patients, the records were abstracted for diagnostic information by trained staff. At the ARIC examinations, the technicians visually coded up to 3 12-lead electrocardiograms and evaluated the wave form evolution using side-by-side comparisons to identify silent MIs.16 For out-of-hospital coronary deaths, an interview with the next of kin, physician questionnaires, coroner reports, and autopsy reports were obtained when possible.
The CHD incidence was defined as definite, probable, or silent MI or definite CHD death. A committee of physicians reviewed and adjudicated all potential clinical CHD events using published criteria.17 For strokes, the relevant abstracted information was classified by computer algorithm and physician review. Differences were adjudicated by a second physician. Details on quality assurance for the ascertainment and classification of ischemic stroke events have been previously published.18 Incident ischemic stroke events were defined as validated definite or probable embolic or thrombotic brain infarctions.
All analyses were performed using Statistical Analysis Systems, version 9.1 (SAS Institute, Cary, North Carolina). For participants dying from CHD or with definite or probable CHD or stroke, the follow-up period was the interval from the baseline examination to the first event. For those with silent MI, the follow-up period ended at the midpoint between the ARIC examination that had revealed the electrocardiogram changes and the previous examination. For noncases, the follow-up period ended at the date of death, date of last contact, or December 31, 2005. Adjusted incidence rates were estimated using Poisson regression analysis.
The baseline fasting insulin levels were divided into 5 groups according to the quintile cut points of the entire distribution, and a variable coded 1 to 5 was created for the 5 categories. To test the association of fasting insulin with covariates, the covariates were regressed on this quintile variable to perform a test of linear trend. To test the association of the quintile variable with dichotomous outcomes, a chi-square test was used.
We used Cox proportional hazards regression analysis to estimate the hazard ratios (HRs) for ischemic stroke and CHD by baseline level of fasting insulin. To test the association of fasting insulin with incident CHD and ischemic stroke, the quintile variable was included in the model to perform a test of linear trend. In separate models, the fasting insulin quintile was coded as a 4-degree of freedom class variable to determine the HR for CHD or stroke for each quintile of fasting insulin compared to the lowest quintile. Finally, regression models were run using log-transformed insulin as a continuous predictor variable. Minimally adjusted models were adjusted for age, race, gender, and ARIC field centers. In other models, the association was additionally adjusted for significant predictors of incident CHD or incident stroke from analyses of ARIC-specific CHD and ischemic stroke risk scores.19,20 These covariates included systolic blood pressure, HDL cholesterol, use of antihypertensive medication, current smoking for CHD and systolic blood pressure, current smoking, use of antihypertensive medication, and prevalent left ventricular hypertrophy for ischemic stroke. Additionally, we adjusted for BMI because of the strong relation between BMI and fasting insulin and for triglycerides and non-HDL cholesterol because of the hypothesized relations among insulin, triglycerides, and very-low-density lipoprotein. We evaluated the effect modification of the fasting insulin and CHD or stroke association by including interaction terms with each covariate of interest in the minimally adjusted linear trend model of fasting insulin and by performing regression analyses stratified by the potential covariates. We considered p <0.05 to be evidence of a significant effect modification to balance the typically low power for interaction analyses with the number of interaction tests performed.
Results
Of the 12,323 subjects included in the analysis, 56.8% were women and 24.5% were black. The mean age was 53.8 years at baseline. In the 16 to 18 years of follow-up, we identified 960 incident CHD events and 445 incident ischemic stroke events. Adjusted for age, gender, race, and ARIC center, the incidence rate for CHD was 4.59 per 1,000 person-years, and the incidence rate for ischemic stroke was 2.04 per 1,000 person-years. All proposed covariates were significantly associated with the quintiles of baseline fasting insulin (Table 1).
Table 1.
Baseline risk factors by baseline serum insulin quintile, the Atherosclerosis Risk In Communities (ARIC) Study
| Variable | Fasting Insulin (μU/ml) | p Value* | ||||
|---|---|---|---|---|---|---|
| <6 | 6 to <8 | 8 to <11 | 11 to <15 | ≥15 | ||
| Participants (n) | 2,680 | 2,243 | 2,736 | 2160 | 2,504 | |
| Age (years) | 53.5 | 53.7 | 54.0 | 54.1 | 54.0 | 0.0002 |
| Blacks | 16.0% | 18.5% | 22.2% | 28.2% | 38.2% | <0.0001 |
| Women | 60.8% | 59.2% | 54.2% | 53.1% | 56.2% | <0.0001 |
| Total cholesterol (mg/dl) | 207.2 | 213.7 | 214.9 | 215.8 | 217.1 | <0.0001 |
| High-density lipoprotein cholesterol (mg/dl) | 61.0 | 56.5 | 52.7 | 48.7 | 45.3 | <0.0001 |
| Non-high-density lipoprotein cholesterol (mg/dl) | 146.1 | 157.2 | 162.2 | 176.2 | 171.8 | <0.0001 |
| Low-density lipoprotein cholesterol (mg/dl) | 127.5 | 136.1 | 138.5 | 140.2 | 140.9 | <0.0001 |
| Triglycerides (mg/dl) | 93.0 | 106.3 | 119.5 | 137.0 | 162.1 | <0.0001 |
| Systolic blood pressure (mm Hg) | 115 | 117 | 120 | 123 | 126 | <0.0001 |
| Body mass index (m/kg2) | 23.9 | 25.3 | 26.7 | 28.7 | 31.8 | <0.0001 |
| Antihypertensive medication | 10.9% | 14.0% | 18.2% | 25.2% | 38.2% | <0.0001 |
| Current smoker | 31.9% | 28.0% | 24.5% | 23.4% | 21.1% | <0.0001 |
| Prevalent left ventricular hypertrophy (Cornell definition) | 1.2% | 1.1% | 1.7% | 2.1% | 2.9% | <0.0001 |
Data are presented as crude means or percentages.
Test for linear trend for continuous variables; chi-square test for overall difference for dichotomous variables.
Table 2 lists the HRs of CHD and ischemic stroke by quintile of fasting insulin. We tested the proportional hazards assumption for the association of fasting insulin with CHD and ischemic stroke in minimally adjusted models and found that this assumption was not violated in either case. In a minimally adjusted model (model 1), the baseline fasting insulin quintile was a significant predictor of incident CHD (p <0.0001). The HRs for CHD were increased across every quintile of fasting insulin. When additional covariates were included individually in the regression models, most attenuated the association of insulin and CHD somewhat, although the association remained significant. However, the inclusion of HDL in the regression model completely attenuated the association between insulin and CHD. A similar pattern was seen when log-transformed insulin was used as a continuous predictor variable. In the minimally adjusted model, log-insulin was a significant predictor (p <0.0001) of CHD; however, this association was completely attenuated after adjustment for HDL. To investigate this unexpected attenuation, we ran several additional models, adjusting for ethanol intake and exercise, removing all those receiving statin treatment or other cholesterol-lowering treatment, and stratifying by baseline HDL level (data not shown). Significant attenuation of the association after adjustment for HDL level was seen in all these models. We also ran additional Cox models excluding only patients with treated diabetes (data not shown). For that analysis, in the minimally adjusted model, the HRs were increased; however, nearly complete attenuation of the association in a model adjusted for HDL was still seen.
Table 2.
Hazard ratio (HR) and 95% confidence interval of incident coronary heart disease or ischemic stroke by fasting insulin quintile, the Atherosclerosis Risk In Communities (ARIC) Study, 1987 to 2005
| Variable | Fasting Insulin (μU/ml) | Per Quintile | p Value* | ||||
|---|---|---|---|---|---|---|---|
| <6 | 6 to <8 | 8 to <11 | 11 to <15 | ≥15 | |||
| Coronary heart disease | |||||||
| Events (n) | 164 | 147 | 208 | 173 | 268 | — | |
| Person-years | 42,075 | 35,496 | 43,157 | 33,718 | 38,623 | — | |
| Hazard ratio, model 1† (95% CI) | 1.00 (Referent) | 1.02 (0.82–1.28) | 1.13 (0.92–1.39) | 1.17 (0.94–1.44) | 1.59 (1.31–1.94) | 1.12 (1.07–1.17) | <0.0001 |
| Hazard ratio, model 2‡ (95% CI) | 1.00 (Referent) | 1.04 (0.83–1.30) | 1.09 (0.88–1.34) | 1.05 (0.83–1.34) | 1.28 (1.02–1.62) | 1.05 (1.00–1.11) | 0.058 |
| Hazard ratio, model 3§ (95% CI) | 1.00 (Referent) | 0.90 (0.72–1.13) | 0.92 (0.75–1.14) | 0.85 (0.67–1.07) | 1.01 (0.80–1.29) | 1.00 (0.95–1.06) | 0.99 |
| Ischemic stroke | |||||||
| Events (n) | 75 | 61 | 97 | 87 | 125 | — | |
| Person-years | 42,590 | 36,192 | 43,839 | 34,340 | 39,537 | — | |
| Hazard ratio, model 1† (95% CI) | 1.00 (Referent) | 0.90 (0.64–1.26) | 1.13 (0.84–1.53) | 1.21 (0.89–1.65) | 1.45 (1.09–1.94) | 1.11 (1.04–1.19) | 0.0018 |
| Hazard ratio, model 4¶ (95% CI) | 1.00 (Referent) | 0.89 (0.63–1.25) | 1.11 (0.82–1.51) | 1.10 (0.80–1.53) | 1.28 (0.92–1.78) | 1.07 (0.99–1.16) | 0.079 |
Test for linear trend across 5 categories of fasting insulin.
From proportional hazards regression model adjusted for age, gender, race, and ARIC study center.
Adjustments as for model 1, plus systolic blood pressure, current smoking, use of antihypertensive medication, and BMI.
Adjustments as for model 2, plus HDL and non-HDL cholesterol.
Adjustments as for model 1, plus systolic blood pressure, current smoking, use of antihypertensive medication, BMI, and prevalent left ventricular hypertrophy (Cornell definition).
In a minimally adjusted model (model 1), baseline fasting insulin was a significant predictor of incident ischemic stroke (p = 0.0018). Additional adjustment for systolic blood pressure, current smoking, use of antihypertensive medication, BMI, and prevalent left ventricular hypertrophy (model 4) attenuated the association (p = 0.079). However, the elevated pattern of HRs across the quintiles of fasting insulin remained. A similar pattern was seen when log-transformed insulin was used as a continuous variable. In the minimally adjusted model, log-insulin was a significant predictor (p <0.003) of stroke, but this association was attenuated (p = 0.09) after adjustment for additional covariates. The adjustment for HDL did not attenuate the association with ischemic stroke more than the other covariates. In analyses excluding only treated diabetes (data not shown), the magnitude of the HRs was increased with p-trend <0.0001 for the minimal model and p-trend = 0.015 for the fully adjusted model.
Figure 1 shows the HR of CHD per each quintile increase in baseline fasting insulin for gender, race, BMI, age, hypertension, HDL and non-HDL cholesterol, triglycerides, and smoking subgroups. In the minimally adjusted Cox regression models (age, gender, race, field center) for CHD, evidence was seen of significant (p <0.05) effect modification by current smoking (p interaction = 0.018) and hypertension (p interaction = 0.0087). The association of insulin and CHD was significantly stronger in those without hypertension and nonsmokers. Figure 1 shows the HR of ischemic stroke per quintile increase in fasting insulin in selected subgroups. In the minimally adjusted Cox regression models, evidence was seen of significant effect modification for ischemic stroke by hypertension (p interaction = 0.0027), race (p interaction = 0.036), and gender (p interaction = 0.037). The association of insulin and stroke was significantly stronger in those without hypertension, whites, and women. When the effect modification was tested in the fully adjusted models or was tested with a 4-degree of freedom class variable, instead of a 1 degree of freedom test of trend, only the interactions between insulin and hypertension remained significant (for both CHD and stroke). When the effect modification by hypertension was tested only in those not taking antihypertensive medication, the significance of the interactions was reduced because of a loss of power (p-interaction = 0.03 for CHD and p-interaction = 0.09 for stroke); however, the HR for CHD and stroke in the subgroup of nonmedicated patients with hypertension remained near 1.0 (HR 1.01 for CHD, HR 0.99 for stroke).
Figure 1.
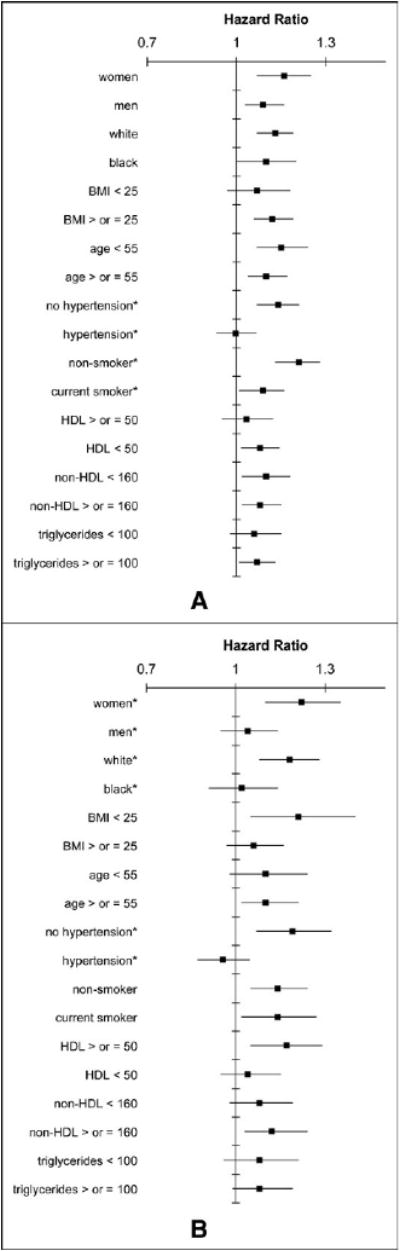
HR and 95% confidence interval of incident (A) CHD and (B) ischemic stroke per quintile of fasting insulin in selected subgroups from ARIC study, 1987 to 2005. *Significant effect modification (p <0.05) of association with insulin by subgroup. Cox regression models were adjusted for age, gender, race, and center.
Discussion
Our results for the association of insulin and CHD largely support the findings from previously published studies. In a meta-analysis of studies (all smaller than the present study) examining the association between circulating insulin and incident CHD, the HR for CHD was 1.12 per tertile of fasting insulin,1 slightly less than the HR of 1.12 per quintile in our minimal model. The meta-analysis did not find a discernable difference in the magnitude of the association with greater adjustment, in contrast to our finding about the importance of adjusting for HDL cholesterol.
Previous evidence of a role for HDL in the association of circulating insulin and CHD is limited. The 3 largest published studies to date examining the prospective association of circulating insulin and incident CHD in nondiabetic populations include the Malmö study, which did not include an adjustment for HDL21; an earlier analysis of the ARIC study, which showed less attenuation resulting from HDL than the present analysis; and the Prospective Epidemiological Study of Myocardial Infarction (PRIME) study, which showed considerable attenuation of the CHD HR with adjustment for HDL (and several other covariates) in part of the cohort (Belfast) but not in another section of the cohort (Paris).22 The association between HDL and both circulating insulin and CHD is strong, and our results have indicated that HDL might be an important confounder of the relation between fasting insulin and CHD. However, it might be that HDL resides in the causal pathway between increased circulating insulin and CHD incidence, making an adjustment for such a variable inappropriate. Finally, given that the assay the ARIC study used to measure insulin has some cross-reactivity with pro-insulin,16 it might be that an association between HDL and pro-insulin was confounding the insulin and CHD relation in our analysis. HDL was not a major confounder of the relation between fasting insulin and ischemic stroke, suggesting that the mechanisms of the associations of circulating insulin with the 2 ischemic processes (ie, stroke and CHD) are not identical. Additional research should be pursued to better understand the biologic inter-relations among CHD, insulin, and HDL.
Although some previous studies examining the association of incident stroke with circulating insulin have found nonsignificant associations,2,3 these studies included few cases of stroke. Other studies have found modest positive associations between circulating insulin and incident strokes. In a prospective cohort study of British women, the HR of stroke per SD increase in fasting insulin was 1.14.7
We identified several effect modifiers of both the association of fasting insulin with ischemic stroke and the association of fasting insulin with CHD. Hypertension was the most robust effect modifier of the association of insulin for both CHD and stroke. The effect of hypertension on the association between insulin and CHD or stroke appeared to be independent of the use of antihypertensive medication, because the association was null for both outcomes in both medicated and unmedicated patients with hypertension. An earlier ARIC analysis of the baseline fasting insulin level and incident ischemic stoke (when the median follow-up period for participants in the study was 7.2 years8) suggested that the association of insulin and stroke differed by race, although the interaction term did not reach statistical significance. The findings from our updated analysis support this earlier finding, with the association demonstrated to be significantly stronger in whites than in blacks. The differing associations might be driven by different risk factor distributions or different genetic risk factors underlying the traits in the 2 populations.
In all cases of significant effect modification for both outcomes, the magnitude of the association was smaller in the strata of higher risk—those with hypertension, blacks, current smokers, and men. This pattern suggests that the modest association of circulating insulin with cardiovascular disorders is overwhelmed and not detectable in the presence of stronger cardiovascular risk factors. In the future, should the associations between insulin and CHD be proven causal, and should focused treatment of hyperinsulinemia be proposed for the prevention of heart disease, such treatment might prove more beneficial in otherwise low-risk populations.
The primary strength of the present analysis was the comprehensive examination of possible effect modification. The limitations of the present analysis included the cross-reactivity of the ARIC insulin assay with pro-insulin and the inclusion of only whites and blacks. The replication of these results in large cohorts with varied ethnic representation, in particular to examine the role of HDL in the association of circulating insulin and CHD, and the effect modification of the relation between circulating insulin and cardiovascular disorders by hypertension, might help to clarify the nature of the association between circulating insulin levels and atherosclerotic processes.
Acknowledgments
We thank the staff and participants of the ARIC study for their important contributions.
The Atherosclerosis Risk In Communities Study was performed as a collaborative study supported by contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, and N01-HC-55022 from the National Heart, Lung, and Blood Institute, Bethesda, Maryland. Dr. Rasmussen-Torvik was supported by training grant HL07779.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sarwar N, Sattar N, Gudnason V, Danesh J. Circulating concentrations of insulin markers and coronary heart disease: a quantitative review of 19 Western prospective studies. Eur Heart J. 2007;28:2491–2497. doi: 10.1093/eurheartj/ehm115. [DOI] [PubMed] [Google Scholar]
- 2.Adachi H, Hirai Y, Tsuruta M, Fujiura Y, Imaizumi T. Is insulin resistance or diabetes mellitus associated with stroke? An 18-year follow-up study. Diabetes Rev Clin Pract. 2001;51:215–223. doi: 10.1016/s0168-8227(00)00236-9. [DOI] [PubMed] [Google Scholar]
- 3.Lakka HM, Lakka TA, Tuomilehto J, Sivenius J, Salonen JT. Hyperinsulinemia and the risk of cardiovascular death and acute coronary and cerebrovascular events in men: the Kuopio ischaemic heart disease risk factor study. Arch Intern Med. 2000;160:1160–1168. doi: 10.1001/archinte.160.8.1160. [DOI] [PubMed] [Google Scholar]
- 4.Kuusisto J, Mykkanen L, Pyorala K, Laakso M. Non-insulin-dependent diabetes and its metabolic control are important predictors of stroke in elderly subjects. Stroke. 1994;25:1157–1164. doi: 10.1161/01.str.25.6.1157. [DOI] [PubMed] [Google Scholar]
- 5.Pyorala M, Miettinen H, Laakso M, Pyorala K. Hyperinsulinemia and the risk of stroke in healthy middle-aged men: the 22-year follow-up results of the Helsinki policemen study. Stroke. 1998;29:1860–1866. doi: 10.1161/01.str.29.9.1860. [DOI] [PubMed] [Google Scholar]
- 6.Burchfiel CM, Sharp DS, Curb JD, Rodriguez BL, Abbott RD, Arakaki R, Yano K. Hyperinsulinemia and cardiovascular disease in elderly men; the Honolulu Heart Program. Arterioscler Thromb Vasc Biol. 1998;18:450–457. doi: 10.1161/01.atv.18.3.450. [DOI] [PubMed] [Google Scholar]
- 7.Lawlor DA, Fraser A, Ebrahim S, Smith GD. Independent associations of fasting insulin, glucose, and glycated haemoglobin with stroke and coronary heart disease in older women. PLoS Med. 2007;4:e263. doi: 10.1371/journal.pmed.0040263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Folsom AR, Rasmussen ML, Chambless LE, Howard G, Cooper LS, Schmidt MI, Heiss G. The Atherosclerosis Risk In Communities (ARIC) Study Investigators. Prospective associations of fasting insulin, body fat distribution, and diabetes with risk of ischemic stroke. Diabetes Care. 1999;22:1077–1083. doi: 10.2337/diacare.22.7.1077. [DOI] [PubMed] [Google Scholar]
- 9.ARIC Investigators. The Atherosclerosis Risk In Communities (ARIC) study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 10.Eckfeldt JH, Chambless LE, Shen YL. Short-term, within-person variability in clinical chemistry test results: experience from the atherosclerosis risk in communities study. Arch Pathol Lab Med. 1994;118:496–500. [PubMed] [Google Scholar]
- 11.Siedel J, Hagele EO, Ziegenhorn J, Wahlefeld AW. Reagent for the enzymatic determination of serum total cholesterol with improved lipolytic efficiency. Clin Chem. 1983;29:1075–1080. [PubMed] [Google Scholar]
- 12.Nagele U, Hagele EO, Sauer G, Wiedemann E, Lehmann P, Wahlefeld AW, Gruber W. Reagent for the enzymatic determination of serum total triglycerides with improved lipolytic efficiency. J Clin Chem Clin Biochem. 1984;22:165–174. doi: 10.1515/cclm.1984.22.2.165. [DOI] [PubMed] [Google Scholar]
- 13.Warnick GR, Benderson J, Albers JJ. Dextran sulfate-Mg2+ precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin Chem. 1982;28:1379–1388. [PubMed] [Google Scholar]
- 14.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 15.Crow RS, Prineas RJ, Rautaharju P, Hannan P, Liebson PR. Relation between electrocardiography and echocardiography for left ventricular mass in mild systemic hypertension (results from Treatment of Mild Hypertension Study) Am J Cardiol. 1995;75:1233–1238. [PubMed] [Google Scholar]
- 16.Folsom AR, Szklo M, Stevens J, Liao F, Smith R, Eckfeldt JH. A prospective study of coronary heart disease in relation to fasting insulin, glucose, and diabetes: the Atherosclerosis Risk In Communities (ARIC) study. Diabetes Care. 1997;20:935–942. doi: 10.2337/diacare.20.6.935. [DOI] [PubMed] [Google Scholar]
- 17.White AD, Folsom AR, Chambless LE, Sharret AR, Yang K, Conwill D, Higgins M, Williams OD, Tyroler HA. Community surveillance of coronary heart disease in the Atherosclerosis Risk In Communities (ARIC) study: methods and initial two years' experience. J Clin Epidemiol. 1996;49:223–233. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 18.Rosamond WD, Folsom AR, Chambless LE, Wang CH, McGovern PG, Howard G, Copper LS, Shahar E. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk In Communities (ARIC) cohort. Stroke. 1999;30:736–743. doi: 10.1161/01.str.30.4.736. [DOI] [PubMed] [Google Scholar]
- 19.Chambless LE, Heiss G, Shahar E, Earp MJ, Toole J. Prediction of ischemic stroke risk in the Atherosclerosis Risk In Communities study. Am J Epidemiol. 2004;160:259–269. doi: 10.1093/aje/kwh189. [DOI] [PubMed] [Google Scholar]
- 20.Chambless LE, Folsom AR, Sharrett AR, Sorlie P, Couper D, Szklo M, Nieto FJ. Coronary heart disease risk prediction in the Atherosclerosis Risk In Communities (ARIC) study. J Clin Epidemiol. 2003;56:880–890. doi: 10.1016/s0895-4356(03)00055-6. [DOI] [PubMed] [Google Scholar]
- 21.Nilsson P, Nilsson JA, Hedblad B, Eriksson KF, Berglund G. Hyperinsulinaemia as long-term predictor of death and ischaemic heart disease in nondiabetic men: the Malmö Preventive Project. J Intern Med. 2003;253:136–145. doi: 10.1046/j.1365-2796.2003.01064.x. [DOI] [PubMed] [Google Scholar]
- 22.Bataille V, Perret B, Troughton J, Amouyel P, Arveiler D, Woodside J, Dallongeville J, Haas B, Bingham A, Ducimetiere P, Ferrieres J. Fasting insulin concentrations and coronary heart disease incidence in France and Northern Ireland: the PRIME study. Int J Cardiol. 2006;108:189–196. doi: 10.1016/j.ijcard.2005.04.024. [DOI] [PubMed] [Google Scholar]


