Abstract
Although mechanical linkages between the proximal and distal limb are present in a range of species, their functional significance is unknown. We have investigated the mechanical function of the flexor cruris lateralis pars pelvica (FCLP), flexor cruris lateralis pars accessoria (FCLA) and gastrocnemius intermedia (GI), a system of linked muscles spanning proximal and distal limb segments in the guinea fowl (Numida meleagris) hind limb. The FCLP, which is in the anatomical position of a hamstring muscle, is the primary component of the linkage. It is connected to the distal femur via the FCLA, the tarsometatarsus via the tendon of insertion of the GI and the common Achilles tendon, and the tibiotarsus via a distal tendon of insertion. The FCLP may, therefore, potentially exert moments at the hip, knee and ankle joints depending on the joint angles and the relative states of activation in the three muscles. Evidence presented here suggests that the GI and FCLA act as actively controlled links that alter distal action of the FCLP. The FCLP and GI are coactive in the late swing and early stance phases of the stride, forming a triarticular complex, and likely act together to resist and control ankle flexion immediately after foot-down in addition to providing hip extension and knee flexion moments. The FCLP and FCLA are coactive from mid-through to late stance, acting together as a uniarticular hip extensor. Available evidence suggests that this role of the FCLP and FCLA is of increased importance in inclined running and accelerations. This linkage between a proximal muscle and alternate distal connections allows for functional flexibility, both in terms of the site at which the muscle exerts force and the nature of the muscle's mechanical function. The interactions generated between the proximal and distal limb by linkages of this type suggest that less emphasis should be placed on the distinct functional roles of specific anatomical classes of muscle within proximal and distal limb segments.
Keywords: avian, biomechanics, limb, locomotion, muscle
INTRODUCTION
The mechanical functions of muscle during legged locomotion are diverse and complex. Muscles may load elastic tissues to store mechanical energy, exert force to support body weight, do work to accelerate the body center of mass and body segments, or absorb work, acting as brakes. Studies of vertebrate limb muscle function have typically divided the proximal and distal muscles into two distinct anatomical and functional classes: the proximal musculature, with long fascicles and a limited series elastic component, and the distal musculature, typified by muscles with short fascicles and long, elastic tendons of insertion. During level running, some proximal muscle fascicles exhibit substantial length changes when active, doing work to accelerate the center of mass and limb segments and absorbing energy to stabilize joints (Gillis and Biewener, 2001; Roberts et al., 2007). As a functional group, the distal muscles have been considered to be primarily associated with isometric force production, tensioning elastic tendons to store mechanical energy (Kram and Taylor, 1990; Roberts et al., 1997; Biewener et al., 1998; Biewener and Roberts, 2000), although they may also contribute to work production during level and uphill running (Daley and Biewener, 2003; Gabaldón et al., 2004; Rubenson et al., 2006).
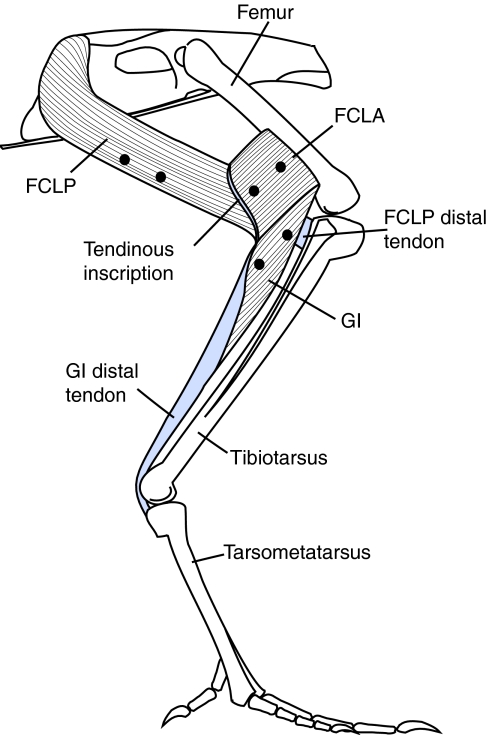
This functional division ignores the direct mechanical connections between proximal and distal limb muscles that are present in a number of birds and mammals. For example, the hamstrings of a number of quadrupedal mammals are connected to the ankle via long accessory tendons (Evans and Evans, 1993; Constantinescu, 2001). A similar, but functionally more complex, system exists in the avian hind limb (Ellerby et al., 2002). The proximally located flexor cruris lateralis pars pelvica (FCLP) may influence both proximal and distal limb function. The anatomical position of the FCLP resembles that of a mammalian ‘hamstring’ and is referred to as the ‘semitendinosus’ in some nomenclatures (George and Berger, 1966). It originates on the posterior part of the iliac crest and inserts distal to the knee via a tendon shared with the flexor cruris medialis (FCM) (Fig. 1). In some species, including the guinea fowl, this muscle is also connected to the distal femur by an accessory head (the flexor cruris lateralis pars accessoria, FCLA). The tendinous inscription that forms the boundary between the FCLP and FCLA is continuous with the tendon of insertion of the gastrocnemius intermedia (GI). The GI tendon forms a strong connective tissue band running between the lateral and intermediate heads of the gastrocnemius and joining the common Achilles tendon. Thus, in these birds, the FCLP can act at the hip, knee and ankle joints. The significance of linked muscle systems of this type and the nature and extent of the interactions they create between the proximal and distal limb are unknown, as previous studies of limb muscle function have typically been confined to single limb segments (e.g. Griffiths, 1991; Roberts et al., 1997; Daley and Biewener, 2003; Gabaldon et al., 2004; Nelson and Roberts, 2008). Characterizing these interactions is important as it may lead to a reevaluation of the extent to which proximal and distal limb muscles are viewed as separate functional entities.
Fig. 1.
Lateral view of the flexor cruris lateralis pars pelvica (FCLP), flexor cruris lateralis pars accessoria (FCLA) and gastrocnemius intermedia (GI) muscles. The overlying musculature has been removed to expose the muscles, their origins and insertions. The flexor cruris medialis (FCM) (not shown) runs parallel and medial to the FCLP. Note that the orientation of the longitudinal axis of the fascicles does not necessarily represent the orientation in vivo when the muscles are active.
Past studies provide some information about this linked system in the guinea fowl (Numida meleagris) hind limb. Anatomical comparisons and regional blood flow indications of muscle energy expenditure suggest that the FCLP–FCLA–GI system is important in rapid terrestrial locomotion on the level and uphill. A robust FCLA connection between the FCLP and the femur is typical of species that locomote on the ground, whereas the FCLA, or both FCLA and FCLP, is absent in many aquatic and arboreal species with limited running abilities (George and Berger, 1966). Blood flow measurements support the association of the FCLP and FCLA with rapid locomotion on the ground. In guinea fowl, energy expenditure in the FCLP and FCLA during walking was not significantly greater than at rest but increased with the transition from walking to running (Ellerby et al., 2005). If the FCLP and FCLA were active simultaneously, these data would suggest a role for the muscle pair in generating increased hip extension at high speeds. Also, the increase in energy expenditure of these two muscles was disproportionately high compared with that in other hind-limb muscles when the birds switched from level to incline running (Rubenson et al., 2006), suggesting a role in net work production on an incline. The potential actions of the FCLP at the knee and ankle joints are less clear. The distal tendon may allow for the exertion of a knee flexor moment. If active in concert with the GI, the FCLP could also contribute to ankle extension.
The primary aim of the present study was to further establish how this linked muscle system functions during steady level and incline legged locomotion across a range of speeds, although we also offer some observations relevant to accelerations during walking. Muscle activity and length change in the FCLP, FCLA and GI muscles were measured during treadmill exercise in guinea fowl using sonomicrometry and electromyography. We hypothesized that as limb position and the relative states of activation of the muscular components of the linked system changed during the course of the stride, there would be a shift in FCLP function consistent with it acting in concert with the FCLA and GI as either a hip or ankle extensor. If the FCLP–FCLA linkage contributes to hip extension, then these muscles should be active together during stance. If the FCLP–GI linkage performs a role similar to other mono- or bi-articular ankle extensors, then both muscles should be active together during late swing or early stance phases to extend the ankle before foot-down or control ankle flexion during early stance. We further hypothesized that, during incline running, FCLP function would be modulated in a way consistent with increasing its net work production, primarily by increasing the extent to which it shortens while active with the FCLA to increase work done at the hip.
MATERIALS AND METHODS
Experimental animals
Guinea fowl (Numida meleagris L.) were obtained from The Guinea Farm (New Vienna, IA, USA) as hatchlings and were cage reared at the Northeastern University Division of Laboratory Medicine. The birds had access to food and water ad libitum and were maintained on a 12 h:12 h light:dark cycle. Their mean body mass was 1.35±0.10 kg (mean ± s.e.m., N=5). All procedures were approved by the Northeastern University Institutional Animal Care and Use committee.
For 2 months prior to experimentation, the birds were trained to run on a treadmill (Trimline 2600, Trimline, Vancouver, WA, USA; 120×44 cm tread area). The training regime consisted of running for 30 min per day, five days per week at speeds ranging from 1.5 to 2.5 m s–1. The guinea fowl ran inside a three-sided box, open at the back, with a mirror mounted on the side facing the running bird. The left side of the box was made of transparent Perspex to enable filming of the running birds. High-speed video was obtained at a frame rate of 500 Hz using a NAC HSAV-1000 video camera (NAC, Tokyo, Japan).
Sonomicrometry and electromyography
Changes in muscle fascicle length were measured directly in situ using sonomicrometry. Sonomicrometry uses the transit time of an ultrasonic pulse between pairs of piezoelectric transducers to determine length changes. Transducer pairs are implanted along the long axis of muscle fascicles. Changes in muscle length are detected as changes in the pulse transit time. Sonomicrometry transducers were constructed from 1 mm-diameter discs of piezoelectric material (Boston Piezo-optics, Boston, MA, USA) attached to twisted pairs of stainless steel wire (316SS3T; Medwire, Mount Vernon, NY, USA). The bared tips of the wires were attached to opposite faces of the piezoelectric disks using conductive epoxy resin (Chemtronics, Kennesaw, GA, USA). The disks were then encapsulated in standard, non-conductive epoxy resin (2 Ton Epoxy, Devcon, Danvers, MA, USA). A length of stainless steel wire was embedded in the epoxy capsule to form an anchorage point. This wire was sutured to the connective tissue fascia on the muscle surface after implantation.
Length-change data were collected using a Sonometrics TRX Series Sonomicrometer (Sonometrics, London, Ontario, Canada). Sound travels faster through the encapsulating layer of epoxy than through muscle. This difference in speed causes the sonomicrometer to calculate a shorter than actual distance between the transducer pairs. The offset was measured by attaching the transducers to a pair of vernier calipers and measuring the difference between the calculated and actual spacing over a range of inter-transducer distances. The offset for transducer pairs was typically 0.75 mm, and the calculated segment lengths were corrected accordingly. Sonomicrometry data were recorded at 1068 Hz.
Bipolar electromyography electrodes with an offset hook design (1.5 mm exposed tips, 3.0 mm tip offset) were constructed from twisted pairs of Teflon®-coated stainless steel wire (316SS3T; Medwire). Electrode and transducer wires were soldered to female, ultra-miniature, gold-plated strip connectors (Microtech, Boothwyn, PA, USA). After surgery, these were left exposed on the dorsal surface of the pelvis. Two-meter lightweight cables were used to link these connectors to the sonomicrometer and preamplifiers. Electromyogram (EMG), sonomicrometry and video data were synchronized using a square wave output from the Powerlab A/D converter. Onset of the synchronization wave coincided with the start of EMG recording. The wave was recorded as an additional channel with the length change data and could also be visualized on the video image via an input on the NAC camera.
EMG signals were amplified using DAM 50 preamplifiers (WPI, Sarasota, FL, USA) and downloaded to a G4 PowerMac via a Powerlab 16SP A-to-D converter (ADinstruments, Colorado Springs, CO, USA). Data were recorded at 2500 Hz. The amplifiers were operated in differential AC mode with gain set to 1000. 3 kHz low-pass and 10 Hz high-pass filters were applied to the EMG signal. EMG data were transferred to Igor Pro (Version 3.16; Wavemetrics, Lake Oswego, OR, USA) for processing. Where necessary, 700 Hz low-pass and 50 Hz high-pass digital filters were applied to the data to remove both high-frequency noise and low-frequency movement artifacts. EMG signals were rectified and then integrated using a trapezoidal integration. Mean EMG amplitude was calculated as integrated burst magnitude/burst duration. Mean EMG amplitude for a given electrode was expressed as a proportion of the mean value measured with that electrode at a level running speed of 2.5 m s–1. EMG bursts in the FCLA and GI typically showed a division into low-amplitude and high-amplitude segments. Within-burst amplitude transitions were initially identified by visually inspecting the EMG traces. The precise timing of transitions was determined from the relationship between the rectified burst integral and time. Sharp transitions in EMG amplitude produced marked changes in the slope of the integrated EMG–time relationship, which indicated the division between low- and high-amplitude phases. Further confirmation of the division was obtained by comparing the mean EMG amplitude of the segments. A segment was confirmed as being of low amplitude if the mean EMG amplitude of the segment was 20% or less than that of the adjacent higher amplitude segment.
An electromechanical delay (EMD) between EMG activity and force production was assumed when estimating the timing of muscle force production. The EMD has not been measured in the FCLP, FCLA or GI, but activation and relaxation EMDs of approximately 25 and 60 ms, respectively, have been measured for other guinea fowl and turkey leg muscles during treadmill exercise (Daley and Biewener, 2003; Roberts and Gabaldon, 2008) and are the basis for our estimates. A precise relationship between EMG amplitude and muscle force production is difficult to establish but, at least within a given phase of the stride, force production by avian leg muscle is approximately proportional to EMG amplitude (Roberts and Gabaldon, 2008). On this basis, the high-amplitude segment of the EMG burst has been taken to indicate the primary period of force production in the FCLA and GI muscles.
Surgical procedures
We anesthetized the birds with isoflurane (Isothesia, Vetus, St Paul, MN, USA) via a mask at a concentration of 3% to induce and 1–1.5% to maintain anesthesia. A heating pad placed under a sterile drape beneath the bird kept the bird warm (approximately 40°C). Feathers were removed from the lateral and posterior thigh of the left leg and the left side of the pelvic region. The skin surface was sterilized by swabbing with Betadine solution, and the area around the surgical field was covered with sterile drapes. A 15 mm skin incision was made on the dorsal surface of the pelvis, parallel to, and immediately to the left of, the vertebral column. A 30 mm skin incision was also made on the posterior margin of the thigh parallel to the femur. Curved blunt-blunt scissors were used to separate the skin from the underlying connective tissue between the two incisions. A length of 5 mm-diameter plastic tubing was inserted under the skin between the incisions and used to route the sonomicrometer and electromyography wires from the back to the thigh. The tubing was withdrawn, leaving the connectors exposed at the back of the bird and the sonomicrometry transducers and EMG electrodes adjacent to the target muscles.
The tip of a No. 11 scalpel blade was used to create a 1 mm incision in the superficial fascia of the muscle, parallel to the fascicles, at the insertion sites for the sonomicrometry transducers. A blunt probe was used to separate the fascicle prior to insertion of a transducer. Sonomicrometry transducers were inserted with plastic inserters made from 1.0 mm-diameter polyurethane tubing. The wire anchors attached to the transducers were sutured to the fascia with 6-0 silk suture (Look 752, Ethicon, Somerville, NJ, USA).
We inserted electromyography electrodes into the muscles using 25 G hypodermic needles with the internal cutting edge blunted to avoid damaging the wires. The electrode tips were inserted into the opening of the needle and bent back to form a hook before insertion. Two electrodes were inserted parallel to and on either side of the fascicle segment between each transducer pair. Wires were secured to the superficial fascia with 6-0 silk suture. Skin incisions were closed with Ethilon 4-0 nylon monofilament suture (Ethicon). The birds were allowed to recover overnight before data collection.
Kinematic analyses
The approximate points of rotation of the hip, knee and ankle joints were marked with white paint. In all sequences, the timing of foot-up and foot-down was recorded to determine the transition between swing and stance phases of the stride. The limb kinematics of four birds were analyzed to determine hind-limb joint angles. We considered a stride suitable for joint angle analysis if the foot position relative to the treadmill and the joint angles were approximately the same at the beginning and end of the stride. Joint angles during the course of a stride were determined using NIH image software (NIH version 1.62). As small changes in pelvic pitch were not readily visible during running, the hip angle was expressed relative to the horizontal plane. An estimate of pelvic pitch angle over the course of the stride was added to the measured hip angle.
Statistical analyses
General linear models (GLMs) were used to test for changes in the timing of EMG onset and offset, FCLP shortening, and mean EMG amplitude with respect to changing speed and incline. Speed and incline were included in the GLMs as independent variables. A GLM was also used to test for changes in FCLP shortening velocity when estimated to be exerting force concurrently with the GI versus when estimated to be exerting force concurrently with the FCLA. In this GLM, relative timing within the stride was included as an independent variable in addition to speed and incline. An individual identifier for each bird was also included as a random factor in all GLMs. If a GLM detected significant changes in a variable in relation to speed or incline, Scheffé's post-hoc test was used to make pair-wise comparisons between mean values. All data expressed as proportions or percentages were arcsine transformed to preserve a normal distribution. The transformed value was equal to the arcsine of the square root of the proportional value (Sokal and Rohlf, 1981). Untransformed data are presented in the text and figures. All statistical analyses were carried out using SPSS (version 14.0; SPSS Inc., Chicago, IL, USA).
RESULTS
Joint angles during running
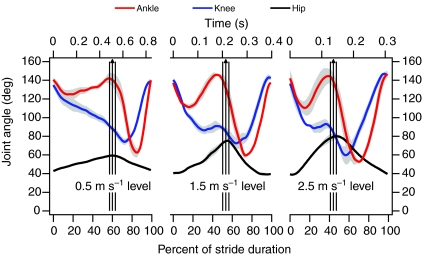
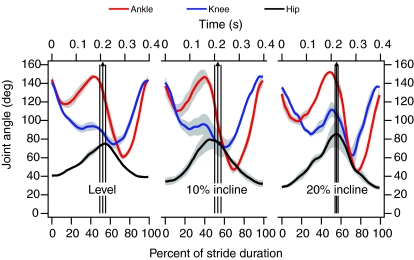
Joint angles at a range of speeds and inclines are shown in Figs 2 and 3. The same general patterns were apparent regardless of speed or incline. The hip was extended throughout stance and flexed throughout the swing phase, reaching maximum extension at the end of stance. The knee was maximally extended just prior to foot-down and was flexed overall through most of stance and in early swing phase before being re-extended in late swing. At speeds greater than 0.5 m s–1, the knee extended in late stance, and this extension increased with speed and incline. The ankle was maximally extended at foot-down, and again in late stance, undergoing cycles of flexion and re-extension between each of these points. Increasing speed was associated with a number of kinematic changes (Fig. 2): increased hip extension; increased late-stance knee extension; increased swing-phase knee flexion; and greater amplitude of ankle flexion and re-extension during both swing and stance. Similar trends were apparent in hip, knee and ankle kinematics with increasing incline (Fig. 3), the primary difference being a more marked flexion and re-extension of the knee in late stance at the steepest incline.
Fig. 2.
Ankle, knee and hip angles of guinea fowl during level treadmill running. Data are plotted in relation to normalized and absolute stride durations, with 0 representing foot-down. Shaded areas around each line indicate ± 1 s.e.m. Vertical arrows indicate the mean timing of toe-off, bounded by vertical lines indicating ± 1 s.e.m. Data were obtained from four birds.
Fig. 3.
Ankle, knee and hip angles of guinea fowl during treadmill running at 1.5 m s–1. Data are plotted in relation to normalized and absolute stride durations, with 0 representing foot-down. Shaded areas around each line indicate ± 1 s.e.m. Vertical arrows indicate the mean timing of toe-off, bounded by vertical lines indicating ± 1 s.e.m. Level running data were obtained from four birds, and incline data from three birds.
Muscle recruitment and length change
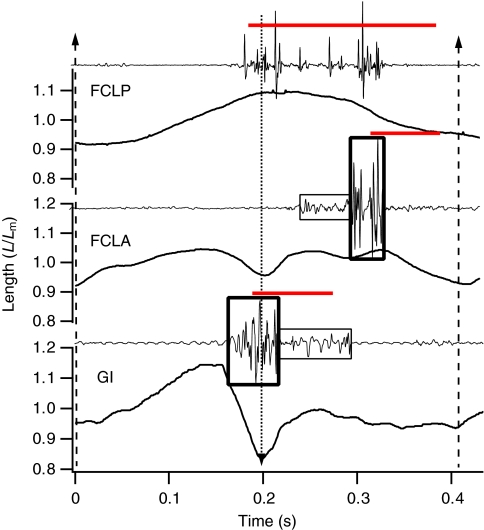
Fig. 4 shows typical patterns of activity and length change during level running at 1.5 m s–1 in the FCLP, FCLA and GI muscles. FCLP activity started in late swing and persisted through most of the stance phase (Figs 4 and 5). The FCLA was active from foot-down until late stance (Figs 4 and 5). The initial part of the EMG burst was of low amplitude, with high-amplitude FCLA activity occurring from mid- until late stance (Figs 4 and 5). The FCLP shortened throughout stance. Its shortening velocity was significantly greater while estimated to be exerting force in combination with the FCLA than when exerting force in combination with the GI (GLM, F1,1168=264, P<0.001). Relative FCLP shortening during the estimated period of concurrent FCLP and FCLA force production changed significantly with both gradient (GLM, F2,8=18.8, P<0.001) and speed (GLM, F4,16=29.2, P<0.001) (Fig. 6). FCLA fascicle length remained relatively constant through early stance, with some shortening in late stance (Fig. 4). GI activity started during late swing with a high-amplitude EMG burst that ended in early stance (Figs 4 and 5), which was followed by low-amplitude EMG activity that persisted until mid-stance. The GI was lengthened while active in early stance. GI length was relatively constant through the latter part of stance, with a large passive stretch and subsequent shortening during the swing phase.
Fig. 4.
Representative muscle segment length and electromyogram (EMG) traces from the guinea fowl flexor cruris lateralis pars pelvica (FCLP), flexor cruris lateralis pars accessoria (FCLA) and gastrocnemius intermedia (GI) during treadmill running at 1.5 m s–1. The timings of foot-down and toe-off are indicated by vertical dotted and dashed arrows, respectively. Segment length, L, is expressed relative to mean segment length, Lm. High- and low-amplitude FCLP and GI EMG segments are bounded by thick and thin bordered boxes, respectively. Red horizontal lines indicate the estimated timing of muscle force production associated with high-amplitude EMG activity. The period of force production was calculated assuming activation and relaxation electromechanical delays of 25 and 60 ms, respectively.
Fig. 5.
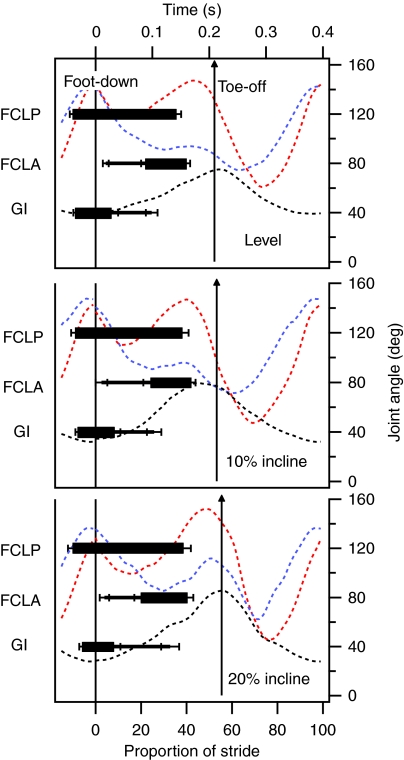
Timing of electromyogram (EMG) activity in the guinea fowl flexor cruris lateralis pars pelvica (FCLP), flexor cruris lateralis pars accessoria (FCLA) and gastrocnemius intermedia (GI) muscles during treadmill running at 1.5 m s–1. Horizontal bars show the timing of EMG activity relative to normalized stride duration, with 0 indicating foot-down. Thick bars represent the primary EMG burst for each muscle. Thinner bars indicate low mean EMG amplitude (mean amplitude <20% of mean amplitude in the primary burst) in the FCLA and GI. FCLP data were obtained from five individuals; FCLA and GI data from four individuals. Data are show as means ± s.e.m. Red, blue and black broken lines show mean joint angles of the ankle, knee and hip joints, respectively.
Fig. 6.
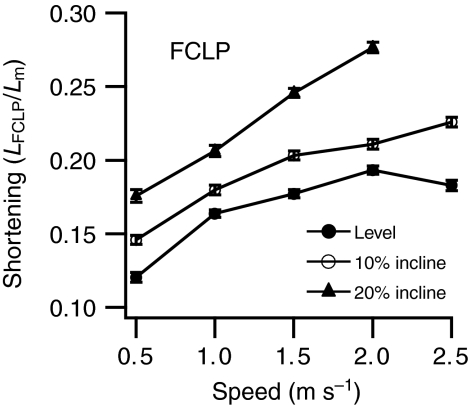
Extent of shortening in the flexor cruris lateralis pars pelvica (FCLP) during estimated concurrent force production by the FCLP and flexor cruris lateralis pars accessoria (FCLA). Shortening LFCLP is expressed relative to mean segment length Lm. Data are shown as means ± s.e.m. (N=5).
Overall, we detected significant differences between muscles in the timing of the start of high-amplitude EMG activity (GLM, F2,1378=113, P<0.01). Pair-wise comparisons showed that there was no significant difference in EMG onset timing between the FCLP and GI (Scheffé, P>0.05). There was a significant difference overall in the timing of high-amplitude EMG offset in the three muscles (GLM, F2,1378=87.0, P<0.01). Pair-wise comparisons showed that there was no significant difference in EMG offset timing between the FCLP and FCLA (Scheffé, P>0.05).
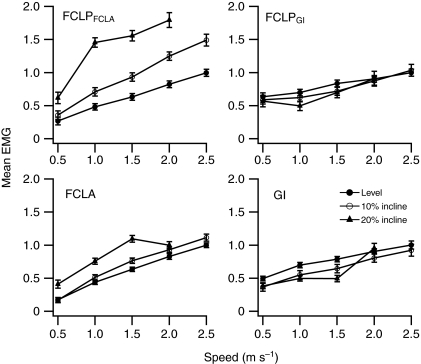
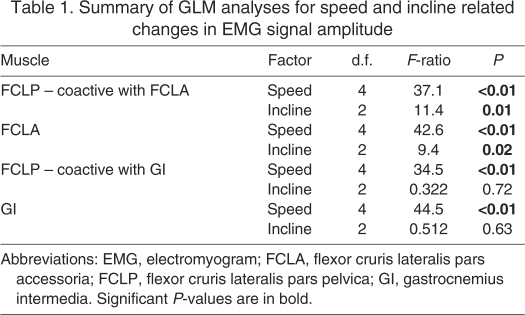
Mean EMG amplitudes at a range of speeds and inclines are shown in Fig. 7. Mean EMG amplitude in the FCLA, and the FCLP while coactive with the FCLA, changed significantly with both speed and incline (Table 1). Mean EMG amplitude in the GI, and in the FCLP while coactive with the GI, changed significantly with speed but not with incline (Table 1).
Fig. 7.
Mean electromyogram (EMG) amplitude in the guinea fowl flexor cruris lateralis pars pelvica (FCLP), flexor cruris lateralis pars accessoria (FCLA) and gastrocnemius intermedia (GI) during treadmill running. Values are expressed relative to the mean EMG amplitude measured during level running at 2.5 m s–1. Mean EMG amplitude was calculated as the rectified EMG burst integral divided by the burst duration. FCLP data were obtained from five individuals; FCLA and GI data from four individuals. The FCLP EMG burst was divided into the sections of coactivity with the GI (FCLPGI) and FCLA (FCLPFCLA) for calculation of mean EMG amplitude. Data are shown as means ± s.e.m.
Table 1.
Summary of GLM analyses for speed and incline related changes in EMG signal amplitude
Muscle and tendon dimensions
The dimensions of the FCM, FCLP and their distal connective tissue linkages to the tiobiotarsus and GI are shown in Table 2.
Table 2.
Dimensions of the FCLP, FCM and their distal tendons
DISCUSSION
Although direct mechanical connections between the proximal and distal limb are present in a number of species (Evans and Evans, 1993; Constantinescu, 2001; Ellerby et al., 2002), their functional significance is largely unexplored. The nature and extent of the limb segment interactions generated by these connections are dependent on three main factors: (1) the arrangement of the muscular and connective tissue linkages in relation to the joint centers of rotation, (2) the changing orientation of these linked elements during the stride and (3) the relative timing of activity in the muscular components.
We have determined the patterns of muscle activity and length change in the FCLP–FCLA–GI linked muscle system of the guinea fowl hind limb. The FCLP originates on the iliac crest and has three distal insertions: first, via an accessory head (FCLA) to the distal femur; second, via a tendon shared with the FCM to the proximal tibiotarsus; and third, via the GI muscle to the common Achilles tendon. A linked system of this type may allow for functional flexibility. A typical muscle–tendon unit has tensile elements made from connective tissue with fixed mechanical properties. Incorporating muscle tissue into an in-series linkage allows for active modulation of the connection's properties. Further flexibility can be achieved if a muscle has alternate insertions. Changes in the relative timing of activity in the primary muscle and its in-series links during the course of a stride could allow the linked system to alter its mechanical function in two ways: by changing the location at which the primary muscle exerts force and by switching between alternate connections with distinct mechanical characteristics. A distal connection with limited connective tissue would allow a muscle that shortened while active to function effectively in work production whereas a connection incorporating a long tendon of insertion would be better suited to near-isometric force production and loading of the in-series elastic element (Biewener and Roberts, 2000). In short, a single primary muscle acting as part of a linked system could fulfill roles typically assigned to separate functional groupings of limb muscle.
Coactivation of the FCLP and GI
The anatomy of the muscles precludes direct measurement of force production in the linked system. The relative timing of force production by the muscles has been inferred from EMG data, factoring in an electromechanical delay. Although we do not know the volume of active muscle, the relative force production of the active fibers is defined by the rate of shortening as measured from sonomicrometry. Thus, clear shifts in the relative timing of activation and shortening velocity of the muscular components of the linkage allow the muscle interactions to be identified and placed in context within the stride cycle. High-amplitude coactivation of the FCLP and GI occurred from late swing until early stance (Figs 4 and 5). During this period of coactivity, the ankle was extended prior to foot-down, then flexed during early stance (Figs 2, 3 and 5). Onset of EMG activity did not change with speed or incline, occurring 51±4 and 45±4 ms before foot-down in the FCLP and GI, respectively (grand mean ± s.d.). An electromechanical activation delay of 25 ms would mean that the muscles were not exerting force until the ankle was already fully extended and therefore had a limited role in swing-phase ankle extension (Figs 2 and 3). This conclusion is supported by regional blood flow measurements, showing that distal limb loading does not change FCLP and GI energy expenditure (Ellerby and Marsh, 2006), and swing-phase inverse dynamics, showing that the net muscle moment in late swing is in flexion not extension (Rubenson and Marsh, 2009).
Inverse dynamics measurements indicate that, immediately after foot-down, the hip and the ankle require net extensor muscle moments, and the net muscle moment at the knee is in flexion (Roberts and Scales, 2004; Daley et al., 2007). The combined activity of the GI and FCLP could contribute to the net moment at all these joints. During early stance, the ankle and knee flex. The hip extends in early stance, but at a slower rate than that occurring in late stance (Fig. 4). Hip extension and knee flexion would tend to shorten the FCLP whereas ankle flexion would tend to lengthen it. Correspondingly, the FCLP fibers shorten slowly during early stance. By contrast, the GI fibers are lengthened, presumably due to the dominant effect of ankle flexion. The primary function of these muscles while coactive in early stance could be in resisting and controlling ankle flexion at foot-down and in early stance, a role similar to that of the guinea fowl digital flexors and lateral gastrocnemius (Daley and Biewener, 2003). Coactivity of the FCM with the FCLP (Gatesy, 1999) suggests that it may share in this role but, given that its cross-sectional area is one-tenth that of the FCLP, its contribution to force production is likely much less than the FCLP (Table 2). Mean EMG amplitude increased with speed in both muscles, likely indicating an increase in the volume of active fibers. This measure of increased recruitment is consistent with these muscles resisting a speed-related increase in the peak ankle flexor moment created by the ground reaction force. Interestingly, unlike the major ankle extensors in the lower leg, any stored energy in the GI tendon could not be recovered in late stance because the high-amplitude activity of the GI ceases before ankle extension. One potential benefit of the linkage between the ankle and the hip is that this system could provide a mechanical communication system that links variation in ankle moment in early stance to the hip extensor moment provided by the FCLP. This linkage may have a role in controlling the response of the limb to variations in ankle extension at foot-down due to changes in substrate height.
Given the restriction of coactivity in the FCLP–GI linkage to early-stance ankle flexion, these muscles are unlikely to contribute to the increased net work requirement of uphill running because net work output occurs in late stance. Accordingly, there was no change in mean EMG amplitude with incline in the GI or the simultaneously active FCLP (Fig. 7). The absence of a role in uphill locomotion is supported by regional blood flow measurements, which indicate that energy expenditure by the GI during incline running is the same as that during level running at the same organismal metabolic rate (Rubenson et al., 2006). The fibularis longus and gastrocnemius lateralis, which increase both their work production (Daley and Biewener, 2003; Gabaldón et al., 2004) and energy expenditure (Rubenson et al., 2006) during incline running, are more likely contributors to increased work at the ankle.
Coactivation of the FCLP and FCLA
FCLP activity persisted after the offset of high-amplitude activity in the GI. This later period of activity coincided with a period of high-amplitude activity in the FCLA from just before mid-stance until offset of activity in both muscles in late stance (Figs 4 and 5). The speed- and incline-related patterns of change in the mean EMG amplitude of both muscles were similar during coactivity (Fig. 7) but differed from those in the FCLP–GI linkage. These patterns suggest they share mechanical function different from that of the FCLP–GI linkage. The increased mean EMG amplitude in both muscles with increasing speed is consistent with the FCLP exerting a hip extensor moment via the FCLA to contribute to the generation of increased extension work at the hip with speed (Fig. 2). Distally, the force of the FCLP could be shared between the FCLA and the FCLP tendon inserting on the tibiotarsus (Fig. 1). If this is the case, the late-stance knee extension could transfer work to the hip, as has been suggested for the biarticular hamstrings in humans (Roberts and Belliveau, 2005). Unlike the coactive FCLP and GI, the coactive FCLP and FCLA showed an increase in mean EMG amplitude with increasing incline (Fig. 7). This increase, coupled with the disproportionately high incline-related increase in energy expenditure by the FCLP and FCLA compared with other stance-phase muscles (Rubenson et al., 2006), suggests they contribute to the net work production required for incline running. These results led us to hypothesize that FCLP function would be modulated in a way consistent with increased work production on switching from level to incline running. In support of this hypothesis, the degree of FCLP shortening while estimated to be exerting force in combination with the FCLA increased markedly with incline (Fig. 6), likely allowing the linkage to contribute to the net work done at the hip during incline running.
In addition to acting at the ankle and hip, the distal tibiotarsal tendon potentially allows the FCLP and FCM to exert a knee flexor moment. During stance, the orientation of the ground reaction force shifts from knee extension early in stance to knee flexion in mid-through late stance (Clark and Alexander, 1975; Roberts et al., 1998) (R.L.M. and J. Rubenson, unpublished observations). Knee flexor muscles likely resist extension in early stance and, later in stance, stabilize the knee through co-contraction with knee extensors such as the femorotibialis (Gatesy, 1999). The contribution of the FCLP and FCM to knee stabilization is likely to be small. The mid-point of their shared tendon of insertion on the tibiotarsus is only 5 mm from the center of rotation of the knee, allowing for a limited flexor moment. Also, the tibiotarsal tendon has a small cross-sectional area relative to that of the connection to the GI and the physiological cross sections of the muscles (Table 2). The ratio of the total physiological cross section of the FCLP and FCM muscles to that of the tendon is 222:1. This ratio is beyond the measured range of muscle: tendon cross-sectional ratios previously reported in avian muscle, the mean ratio being approximately 60 (Van Snik et al., 1994), suggesting that the tibiotarsal tendon is not a major route for force transmission to flex the knee.
Simultaneous activation of all three muscles
In addition to periods of coactivity with a large mean EMG amplitude, the GI and FCLA both show extended periods of activity with a low mean EMG amplitude, typically less than 20% of that in the primary EMG burst (Figs 4 and 5). Low-level activity in the FCLA coincides with high-amplitude activity in the GI and vice versa (Figs 4 and 5). This low-level activity may provide some tension in the linkage that is not the main route of force transmission at that given moment, maintaining alignment of the linked elements.
Role of linked muscles in unsteady movements
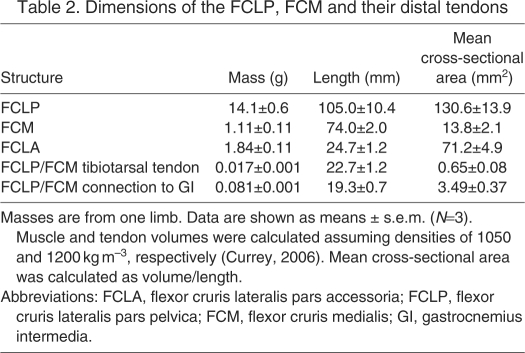
The FCLP–FCLA–GI linkage may also have a role in unsteady movements. The positive mechanical power required for acceleration in running birds is primarily generated through increased angular extension of the hip and ankle joints (Roberts and Scales, 2002; Roberts and Scales, 2004; Gabaldón et al., 2004). Given its likely role in supplying net work at the hip during incline running, the FCLP–FCLA linkage is a likely contributor to the increased work done at the hip during accelerations. As our experiments involved treadmill running at constant average speeds, we could not examine the role of the linkage in maximal accelerations. Unsteady movements on the treadmill do, however, suggest a role for the linkage in this context. At a treadmill speed of 0.5 m s–1, activity in the FCLP and FCLA was intermittent. There was no activity during steady walking, or if the bird drifted backwards by walking at a lower speed than the tread. Activity only occurred during strides where the birds accelerated forwards on the tread, suggesting a role for the linkage in creating the increased hip extension associated with the acceleration (Fig. 8).
Fig. 8.
Forward center of mass (COM) velocity, hip angle and electromyogram activity of the flexor cruris lateralis pars pelvica (FCLP) and flexor cruris lateralis pars accessoria (FCLA) muscles during walking at a treadmill velocity of 0.5 m s–1. The plot shows data from two consecutive strides. During the first stride, the bird decelerated, then accelerated forward during the second stride to maintain its position on the treadmill. Up and down arrows show the timing of toe-off and foot-down, respectively.
Conclusions
The changing coactivation patterns of the FCLP–FCLA–GI linkage are summarized in Fig. 9. The FCLP, by virtue of its connections to other muscles, shows considerable flexibility of function. In steady-speed level running, coactivity with the GI occurs in late swing and early stance when the FCLP is nearly isometric and likely allows the FCLP–GI linkage to assist in resisting ankle flexion in early stance (Fig. 9), a role typically associated with distal limb muscles. In mid-stance, activation of the GI decreases and activation of the FCLA increases, switching the function of the FCLP to only hip extension. Coactivity with the FCLA continues through late stance (Fig. 9) and, during this period, the FCLP shortens substantially, indicating work production. Thus, when coactive with the FCLA, the FCLP contributes to the net work output required of the hip joint during extension, which is a more typical role for a proximal muscle. The role of FCLP in hip work when coactive with the FCLA is reinforced by data showing increases in EMG amplitude and shortening during mid to late stance in uphill running and accelerations, activities requiring increases in net work during the stride. The presence of linked systems of this type therefore calls into question the typical functional division of limbs into proximal and distal segments with distinct mechanical functions.
Fig. 9.
Schematic showing the timing of electromyogram (EMG) activity in the flexor cruris lateralis pars pelvica (FCLP), flexor cruris lateralis pars accessoria (FCLA) and gastrocnemius intermedia (GI) muscles relative to the stride cycle. Red shading denotes high-amplitude muscle EMG activity.
Supported by NIH grant AR47337 to R.L.M. We are grateful to Julia Vasic, Havalee Henry, Jennifer Carr, Cindy Buchanan and Karen Bioski for assistance in data collection and analysis. Deposited in PMC for release after 12 months.
- EMG
- electromyogram
- EMD
- electromechanical delay
- FCLA
- flexor cruris lateralis pars accessoria
- FCLP
- flexor cruris lateralis pars pelvica
- FCM
- flexor cruris medialis
- GI
- gastrocnemius intermedia
- GLM
- general linear models
REFERENCES
- Biewener A. A., Roberts T. J. (2000). Muscle and tendon contributions to force, work and elastic energy savings: a comparative perspective. Exerc. Sci. Sport Sci. Rev. 28, 99-107 [PubMed] [Google Scholar]
- Biewener A. A., Konieczynski D. D., Baudinette R. V. (1998). In vivo muscle force-length behavior during steady speed hopping in tammar wallabies. J. Exp. Biol. 201, 1681-1694 [DOI] [PubMed] [Google Scholar]
- Clark J., Alexander R. McN. (1975). Mechanics of running by quail (Coturnix). J. Zool. Lond. 176, 87-113 [Google Scholar]
- Constantinescu G. M. (2001). Guide To Regional Ruminant Anatomy Based on the Dissection of the Goat Oxford: Blackwell; [Google Scholar]
- Currey J. D. (2006). Bones: Structure and Mechanics Princeton: Princeton University Press; [Google Scholar]
- Daley M. A., Biewener A. A. (2003). Muscle force–length dynamics during level versus incline locomotion: a comparison of in vivo performance of two guinea fowl ankle extensors. J. Exp. Biol. 206, 2941-2958 [DOI] [PubMed] [Google Scholar]
- Daley M. A., Felix G., Biewener A. A. (2007). Running stability is enhanced by a proximo-distal gradient in joint neuromechanical control. J. Exp. Biol. 210, 383-394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellerby D. J., Marsh R. L. (2006). The energetic costs of trunk and distal-limb loading during walking and running in guinea fowl Numida meleagris. J. Exp. Biol. 209, 2064-2075 [DOI] [PubMed] [Google Scholar]
- Ellerby D. J., Marsh R. L., Buchanan C. I., Carr J. A. (2002). Mechanical function of a ‘hamstring’ muscle in running guinea fowl. Physiologist 45, 311 [Google Scholar]
- Ellerby D. J., Henry H. T., Carr J. A., Buchanan C. I., Marsh R. L. (2005). Blood flow in guinea fowl Numida meleagris as an indicator of energy expenditure by individual muscles during walking and running. J. Physiol. 564, 631-648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans H. E., Evans S. A. (1993). Miller’s Anatomy of the Dog, 3rd edition Amsterdam: Elsevier; [Google Scholar]
- Gabaldón A. M., Nelson F. E., Roberts T. J. (2004). Mechanical function of two ankle extensors in wild turkeys: shifts from energy production to energy absorption during incline versus decline running. J. Exp. Biol. 207, 2277-2288 [DOI] [PubMed] [Google Scholar]
- Gatesy S. M. (1999). Guineafowl hind limb function. II: Electromyographic analysis and motor pattern evolution. J. Morphol. 240, 127-142 [DOI] [PubMed] [Google Scholar]
- George J. C., Berger A. J. (1966). Avian Myology New York and London: Academic Press; [Google Scholar]
- Gillis G. B., Biewener A. A. (2001). Hindlimb muscle function in relation to speed and gait: in vivo patterns of strain and activation in a hip and knee extensor of the rat (Rattus norvegicus). J. Exp. Biol. 204, 2717-2731 [DOI] [PubMed] [Google Scholar]
- Griffiths R. I. (1991). Shortening of muscle fibers during stretch of the active cat medial gastrocnemius muscle the role of tendon compliance. J. Physiol. Lond. 436, 219-236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kram R., Taylor C. R. (1990). Energetics of running: a new perspective. Nature 346, 265-267 [DOI] [PubMed] [Google Scholar]
- Nelson F. E., Roberts T. J. (2008). Task-dependent force sharing between muscle synergists during locomotion in turkeys. J. Exp. Biol. 211, 1211-1220 [DOI] [PubMed] [Google Scholar]
- Roberts T. J., Belliveau R. A. (2005). Sources of mechanical power for uphill running in humans. J. Exp. Biol. 208, 1963-1970 [DOI] [PubMed] [Google Scholar]
- Roberts T. J., Gabaldon A. M. (2008). Interpreting muscle function from EMG: lessons learned from direct measurements of muscle force. Int. Comp. Biol. 48, 312-320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts T. J., Scales J. A. (2002). Mechanical power output during running accelerations in wild turkeys. J. Exp. Biol. 205, 1485-1494 [DOI] [PubMed] [Google Scholar]
- Roberts T. J., Scales J. A. (2004). Adjusting muscle function to demand: joint work during acceleration in wild turkeys. J. Exp. Biol. 207, 4165-4174 [DOI] [PubMed] [Google Scholar]
- Roberts T. J., Marsh R. L., Weyand P. G., Taylor C. R. (1997). Muscular force in running turkeys: the economy of minimizing work. Science, 275, 1113-1115 [DOI] [PubMed] [Google Scholar]
- Roberts T. J., Kram R., Weyand P. G., Taylor C. R. (1998). Energetics of bipedal running I. Metabolic cost of generating force. J. Exp. Biol. 201, 2745-2751 [DOI] [PubMed] [Google Scholar]
- Roberts T. J., Higginson B. K., Nelson F. E., Gabaldón A. M. (2007). Muscle strain is modulated more with running slope than speed in wild turkey knee and hip extensors. J. Exp. Biol. 210, 2510-2517 [DOI] [PubMed] [Google Scholar]
- Rubenson J., Marsh R. L. (2009). Mechanical efficiency of limb swing during walking and running in guinea fowl (Numida meleagris). J. Appl. Physiol. 106, 1618-1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenson J., Henry H. T., Dimoulas P. M., Marsh R. L. (2006). The cost of running uphill: linking organismal and muscle energy use in guinea fowl (Numida meleagris). J. Exp. Biol. 209, 2395-2408 [DOI] [PubMed] [Google Scholar]
- Sokal R. R., Rohlf F. J. (1981). Biometry: The Principles and Practice of Statistics in Biological Research, 2nd edn.New York: W. H. Freeman and Company; [Google Scholar]
- Van Snik G., Olmos M., Casinos A., Planell J. A. (1994). Stresses of leg tendons in birds. Neth. J. Zool. 44, 1-14 [Google Scholar]