The disaccharide trehalose accumulates as yeast cells enter quiescence. Glucose equivalents in the form of trehalose and glycogen lead to an increase in the apparent density of the cell. Upon exit from quiescence, trehalose stores are initially metabolized in preference over other energy sources to help drive cell cycle progression.
Abstract
When conditions are unfavorable, virtually all living cells have the capability of entering a resting state termed quiescence or G0. Many aspects of the quiescence program as well as the mechanisms governing the entry and exit from quiescence remain poorly understood. Previous studies using the budding yeast Saccharomyces cerevisiae have shown that upon entry into stationary phase, a quiescent cell population emerges that is heavier in density than nonquiescent cells. Here, we show that total intracellular trehalose and glycogen content exhibits substantial correlation with the density of individual cells both in stationary phase batch cultures and during continuous growth. During prolonged quiescence, trehalose stores are often maintained in favor over glycogen, perhaps to fulfill its numerous stress-protectant functions. Immediately upon exit from quiescence, cells preferentially metabolize trehalose over other fuel sources. Moreover, cells lacking trehalose initiate growth more slowly and frequently exhibit poor survivability. Together, our results support the view that trehalose, which is more stable than other carbohydrates, provides an enduring source of energy that helps drive cell cycle progression upon return to growth.
INTRODUCTION
For many microorganisms, the majority of their lives are spent in a resting or quiescent (G0) state. Only under favorable conditions will such a cell exit quiescence and begin a program of growth and proliferation. The budding yeast Saccharomyces cerevisiae represents an ideal model organism for studying quiescence (Gray et al., 2004). Yeast cells can survive long periods of nutrient starvation, cold temperatures, and even desiccation, conditions under which they must persist in a quiescent yet viable metabolic state. Thus, there has been considerable interest in using yeast to understand the changes in metabolism and physiology that function to maximize the survivability of a cell, or its chronological life span, under such unpredictable conditions in the wild (Powers et al., 2006; Fabrizio and Longo, 2007). Although several mutants have been identified that seem to enhance or compromise cell survivability, many aspects of the cellular quiescence program remain poorly understood.
In the laboratory, stationary phase batch cultures have traditionally been used to study quiescence in yeast (Gray et al., 2004). After rapid growth in rich medium and entry into stationary phase, yeast cells were discovered to form two distinct populations separable by density centrifugation (Allen et al., 2006). The heavier population of cells was found to be more viable and thermotolerant and displayed fewer markers of aging and oxidative stress compared with the lighter population. Thus, the properties of these heavy cells were likened to those of stem cells and were termed quiescent, whereas the light cells were termed nonquiescent (Allen et al., 2006). Morphological examination of the quiescent cell population revealed that they tended to be unbudded, lacked obvious mitochondria and endoplasmic reticulum, and contained substantial concentrations of glycogen. Numerous transcripts also were found to be enriched in either the quiescent or nonquiescent cell populations (Aragon et al., 2008). However, the determinants that contribute to the increased density of quiescent cells as well as the specific mechanisms underlying entry and exit from the quiescent metabolic state have remained unclear. Here, we describe how our studies of metabolic cycles in budding yeast led to the finding that the carbohydrate trehalose is a key determinant of the quiescent metabolic state and moreover functions as an energy reserve that helps fuel exit from quiescence.
MATERIALS AND METHODS
Yeast Strains and Media
The prototrophic CEN.PK strain background was used for all experiments (van Dijken et al., 2000). Δglc3, Δtps1, and Δglc3Δtps1 knockout strains were obtained by sporulation of a CEN.PK122 diploid strain in which one copy of the GLC3 gene was replaced by a kanR cassette and one copy of the TPS1 gene was replaced by a natR cassette via homologous recombination. Sporulated haploid knockout strains were isolated by growth on YPD plates containing 200 μg/ml G418-sulfate (kan) or 100 μg/ml nourseothricin (Nat). The NTH1-FLAG strain was constructed by appending a FLAG epitope cassette to the C terminus of the endogenous chromosomal copy of NTH1 by homologous recombination (Longtine et al., 1998). YPGal minus trehalose medium was made by treating YPGal medium (10 g/l yeast extract, 20 g/l peptone, and 2% galactose) with trehalase to deplete residual trehalose present in yeast extract and peptone. The treated medium was subsequently assayed for trehalose to ensure that it had been depleted of trehalose. SGal medium contained 6.7 g/l yeast nitrogen base without amino acids and 2% galactose. SGal plus trehalose medium contained 0.7 mg/ml trehalose (T9531; Sigma-Aldrich, St. Louis, MO) to mimic the concentration of trehalose present in YP medium.
Production of Metabolic Cycles during Continuous Culture Growth
Continuous culture conditions used to observe yeast metabolic cycles were described previously (Tu et al., 2005).
Batch Culture Growth
Wild-type, Δglc3, Δtps1, and Δglc3Δtps1 strains were grown in YPGal-T or SGal medium. Cells were first inoculated in 5 ml of the appropriate medium and grown at 30°C to saturation. Then, cultures were diluted into fresh medium and grown for several hours to OD600 = 0.7–2.0. These exponentially growing cultures were then diluted into 350 ml of YPGal-T or SGal medium to OD600 = 0.01 and grown at 30°C. Cell samples were then collected at indicated time points for density fractionation as well as trehalose and glycogen assays.
Density Fractionation
Fractionations of yeast cell cultures were performed as described previously, with minor modifications (Allen et al., 2006). Approximately 100 OD of yeast cells were collected for each sample and centrifuged at 3000 × g for 4 min at 4°C. After resuspension with 1 ml of ice-cold Tris buffer (50 mM Tris-HCl, pH 7.5), cell samples were kept on ice until they could be processed together. Such incubations on ice did not affect the density of cells within a span of 6 h. To make Percoll density gradients, Percoll PLUS (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom) was diluted 10:1 (vol/vol) with 1.5 M NaCl. Then, 10 ml of the mixed solution was put into 15-ml tubes and centrifuged at either 19,310 × g (batch cultures) or 14,350 × g (continuous cultures) for 15 min at 20°C. Cells resuspended in Tris buffer were overlaid carefully onto the preformed gradient and centrifuged at 400 × g for 30 min at 20°C. The fractionation of density standards was performed similarly using Density Marker Beads (GE Healthcare) (Supplemental Figure S1).
Estimation of Average Cell Density
Pictures of the density-fractionated cells were taken with a black background by using a Sony digital camera at 5-megapixel resolution under uniform lighting. Each gradient tube in the picture was individually cropped, resized, sorted, labeled with black background, converted to grayscale, and inverted in Photoshop (Adobe Systems, Mountain View, CA). To estimate the average density of each sample, intensities of every 2 pixels of each tube from the meniscus (top) to the bottom were measured using ImageJ (National Institutes of Health, Bethesda, MD). With the top pixels set as zero intensity, the remaining pixel data were converted to intensity percentages relative to the sum of the intensity of pixels for the entire tube. These intensity percentages can be used to estimate cell number percentages at each position in the tube relative to the meniscus. The exact density at each distance relative to the meniscus was estimated from the sedimentation position of density marker beads using Prism (GraphPad Software, San Diego, CA) to fit a dose-response curve with variable slope (four parameters). The average density of each cell sample was calculated by summing the products of the intensity percentage multiplied by density at each distance to the meniscus.
Glycogen and Trehalose Assays
Glycogen and trehalose assays were performed as described previously, with minor modifications (Parrou and Francois, 1997). Cell samples were collected and pelleted in parallel with those used for density fractionations. Cell pellets were quickly washed with 1 ml of ice-cold H2O and then resuspended in 0.25 ml of 0.25 M Na2CO3 and stored at −80°C until processed. For batch cultures, 20 OD total cells were collected. After resuspension in H2O, 0.5 ml of cell suspensions was transferred to two capped Eppendorf tubes (one tube for glycogen assay and the other tube for trehalose assay). For continuous cultures, 10 OD total cells were collected twice directly into two capped Eppendorf tubes. When sample collections were complete, cell samples (in 0.25 M Na2CO3) were boiled at 95–98°C for 4 h, and then 0.15 ml of 1 M acetic acid and 0.6 ml of 0.2 M sodium acetate were added to each sample.
For controls, half of each sample was transferred to another Eppendorf tube, and the remaining half of the sample was incubated overnight with 1 U/ml amyloglucosidase (10115; Sigma-Aldrich) rotating at 57°C for the glycogen assay, or 0.025 U/ml trehalase (T8778; Sigma-Aldrich) at 37°C for the trehalose assay. Samples were then centrifuged at top speed for 3 min and assayed for glucose using a Glucose Assay kit (GAGO20; Sigma-Aldrich).
Glucose assays were done with modifications in a 96-well plate format. Samples were added into each well with or without dilution with H2O to fit into the linear concentration range of the assay (20–80 μg/ml). The total volume of sample (with or without dilution) in each well was 40 μl. The plate was preincubated at 37°C for 5 min, and then 80 μl of the assay reagent from the kit was added into each well to start the colorimetric reaction. After 30-min incubation at 37°C, 80 μl of 12 N H2SO4 was added to stop the reaction. Absorbance at 540 nm was determined to assess the quantity of glucose liberated from either glycogen or trehalose.
Triglyceride and Free Glycerol Assays
Triglyceride and free glycerol levels were measured using the Serum Triglyceride Determination kit (TR0100; Sigma-Aldrich), with minor modifications. Cells (10 ODs) were collected at each time point and rapidly pelleted. The pellets were stored at −80°C until further processing. Cell pellets from each sample were resuspended with 500 μl of ice-cold H2O and then broken open using bead-beating. After lysis, another 500 μl of H2O was added.
Triglyceride and free glycerol levels were then assayed largely as recommended: 4 parts of Free Glycerol Reagent was premixed with 1 part of water or 1 part of Triglyceride Reagent (contains lipoprotein lipase) to generate Triglyceride Reagent Blank and Triglyceride Working Reagent, respectively. Then, 100 μl of each generated reagent was added into two separate wells for each sample in a 96-well plate and prewarmed at 37°C for 5 min. The colorimetric reaction was started by adding 20 μl of each sample into two wells with different reagents. After 10-min incubation at 37°C, precipitate in each well was pelleted by centrifugation at top speed for 4 min, and then 80 μl of supernatant from each well was transferred to another plate and read at 540 nm.
Nth1p Immunoprecipitation and Activity Assay
Cells (50 ODs) were collected at each of 13 time points through one complete yeast metabolic cycle, or at various time points after regrowth in fresh medium. They were rapidly pelleted and stored at −80°C until further processing. Cell pellets were resuspended with 500 μl of lysis buffer (100 mM NaCl, 50 mM NaF, 100 mM Tris, pH 7.5, 1 mM EDTA, 1 mM EGTA, 0.1% Tween 20, 10% glycerol, 30 μM H-89, EMD Phosphatase Inhibitor Cocktail Set III [EMD Biosciences, San Diego, CA], Roche Protease Inhibitor Cocktail [Roche Diagnostics, Indianapolis, IN], 1 mM phenylmethylsulfonyl fluoride, and 14 mM β-mercaptoethanol), and then cells were broken open by bead-beating. After centrifugation, the supernatant was collected as cell lysate. The lysate was precleared with ∼15 μl of protein A/G agarose beads (Santa Cruz Biotechnology, Santa Cruz, CA). Another 25 μl of agarose beads were preconjugated with 2 μg of anti-FLAG antibody and then incubated with the precleared lysate for 3 h at 4°C with rotation. The agarose beads were then washed four times with wash buffer (50 mM HEPES, pH 7.5, and 100 mM NaCl).
After resuspension in 100 μl of wash buffer, 20 μl of agarose beads suspension from each sample was transferred into two tubes containing Antarctic phosphatase buffer to generate two sets of identical samples, one set for dephosphorylation of Nth1p and the other set as a control. Then, 1 μl of Antarctic phosphatase (New England Biolabs, Ipswich, MA) was added to one set of samples followed by 30-min incubation at 37°C. Nth1p activity was assayed by incubating with 180 μl of Trehalase Assay solution (50 mM imidazole, pH 7.0, and 50 mg/ml trehalose) at 30°C for 10 min. After the reaction was stopped by boiling for 5 min, the glucose generated in solution was assayed as described above.
RESULTS
During continuous growth under nutrient-poor conditions, budding yeast exhibit robust oscillations in oxygen consumption (von Meyenburg, 1969; Parulekar et al., 1986; Porro et al., 1988; Satroutdinov et al., 1992; Tu et al., 2005). Such cycles are characterized by phases of rapid oxygen consumption that alternate with phases where the rate of oxygen consumption is substantially lower. Numerous cellular and metabolic processes, including cell division, were found to be precisely orchestrated about these “yeast metabolic cycles” (YMCs) to defined temporal windows. Three phases of the YMC were defined by analysis of periodic gene expression patterns: an oxidative (OX) growth phase that coincides with a burst of mitochondrial respiration; a reductive, building phase (RB) where cells divide and up-regulate transcription of mitochondrial gene products; and a reductive, charging phase (RC) where many genes associated with starvation, stress-associated responses, and slow growth are induced (Tu et al., 2005; Tu and McKnight, 2006; Brauer et al., 2008). Yeast cell populations repeatedly undergo these three phases during continuous growth, and so the YMC can be interpreted to represent the life of a yeast cell under glucose-poor growth conditions (Tu and McKnight, 2006, 2007).
Metabolic cycles occur under glucose-poor conditions that to a first approximation might parallel those encountered by batch cultures sometime upon entry into stationary phase. Moreover, because in every metabolic cycle approximately half of the cell population divides (Tu et al., 2005), we wondered whether cycling cells might transiently segregate into quiescent and nonquiescent populations that resemble those observed previously in rich medium stationary phase cultures (Allen et al., 2006).
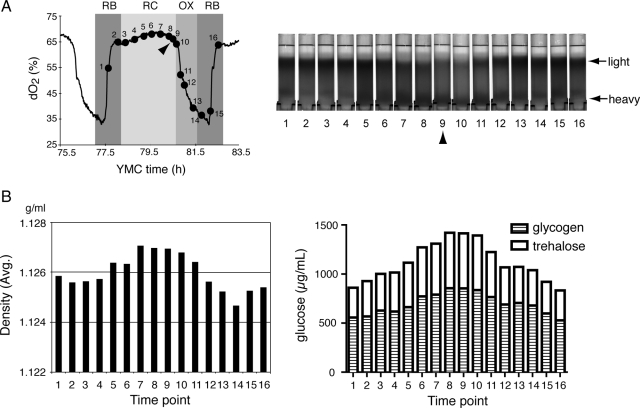
To test this possibility, we collected cells at 16 time points across one metabolic cycle and subjected them to centrifugation through density gradients to assess whether there were any differences in the density of individual cells as a function of the YMC (Figure 1A). Interestingly, we observed that during the late RC phase (time points 5–10), the majority of cells became noticeably heavier in density (Figure 1A). As they entered the OX growth phase (time points 11–14), a portion of the cell population became lighter in density (Figure 1A). We estimated the average density of the entire cell population by comparing the sedimentation patterns of cells with those of marker beads with known, predetermined density (Supplemental Figure S1). These calculations confirm that the average density of the cell population increases during the RC phase and subsequently decreases upon entry into the OX, growth phase. Thus, during late RC phase of the YMC, the majority of cells are reminiscent of stationary phase quiescent cells based on the criteria of individual cell density.
Figure 1.
Cyclic changes in individual cell density during the yeast metabolic cycle. (A) WT cells were collected at 16 time points spanning one complete metabolic cycle and fractionated according to density using Percoll gradients. “Light” refers to a population of cells at the top of the gradient that are lighter in density, whereas “heavy” refers to a population of cells at the bottom of the gradient that are heavier in density. Note that at time point 9 (arrow) in RC phase, the majority of the cells are heavy. (B) The average individual cell density of the entire population at each time point was estimated as described in text. Cell samples collected in parallel were assayed for trehalose and glycogen content. The bar graphs display the sum of glucose equivalents derived from intracellular trehalose (open) and glycogen (barred). Data are the means of three measurements from each time point. We confirmed that cells in the bottom fraction (heavy) indeed contained more trehalose and glycogen than cells in the top fraction (light) (see Supplemental Figure S6).
The precise determinants of the increased density of quiescent cells have been somewhat unclear (Allen et al., 2006). However, we noticed that the oscillating pattern of density changes that occurs as a function of the YMC correlates well with trehalose levels that were determined previously from metabolite profiling studies of the YMC (Tu et al., 2007). Trehalose, as well as glycogen, can constitute a large percentage of the mass of a yeast cell, up to 20% dry weight, and so their abundance could influence the apparent density of cells (Lillie and Pringle, 1980; Francois and Parrou, 2001). These carbohydrates, which are derived from glucose, are known to accumulate as growth rate decreases and as cells enter stationary phase (Lillie and Pringle, 1980; Sillje et al., 1997; Sillje et al., 1999; Boer et al., 2010).
We measured trehalose and glycogen content over the same 16 time points by using well established enzymatic assays, and we confirmed that the levels of these carbohydrates oscillate during the YMC, peaking during the end of RC phase (Figure 1B). Moreover, the pattern of trehalose and glycogen oscillation showed significant correlation to the observed density changes, with the time intervals where trehalose and glycogen were most abundant corresponding to the time intervals of highest cell density (Figure 1). These observations suggest that the total abundance of these storage carbohydrates might contribute to the overall apparent density of cells. The rapid decrease in trehalose and glycogen during the OX phase of the YMC suggests that stores of these carbohydrates are being metabolized to fuel a round of growth and proliferation. Thus, under such continuous culture conditions, yeast cells tend to accumulate trehalose and glycogen after the division process and then liquidate these carbohydrates during a subsequent round of growth (Futcher, 2006; Xu and Tsurugi, 2006; Tu et al., 2007).
We next wondered whether total intracellular trehalose and glycogen content also would correlate with changes in cell density observed previously in stationary phase batch cultures (Allen et al., 2006). To test this possibility, we determined individual cell densities and trehalose and glycogen content of batch-cultured cells at a variety of times after entry into stationary phase. We also asked whether cells that cannot synthesize either trehalose (Δtps1), glycogen (Δglc3), or both trehalose and glycogen (Δglc3Δtps1) could still become dense and form quiescent populations. Because mutants that cannot synthesize trehalose (Δtps1) are known to have defects in their ability to uptake and metabolize glucose (Thevelein and Hohmann, 1995), we grew cells in rich medium containing galactose (YPGal) instead of glucose as the carbon source to minimize experimental complications due to growth deficiencies of Δtps1 mutants in glucose.
During such experiments, we observed that Δtps1 cells unexpectedly contained trehalose when grown in YPGal medium (Supplemental Figure S2). We subsequently confirmed that trehalose present in the yeast extract and peptone of rich medium might explain the presence of intracellular trehalose detected in Δtps1 cells after growth in such medium. Thus, we performed the majority of our growth and density measurements in either YPGal medium that had been pretreated with the enzyme trehalase (YPGal-T) to remove trehalose from the medium or synthetic defined minimal medium (SGal) that does not contain trehalose (Figures 2 and 3).
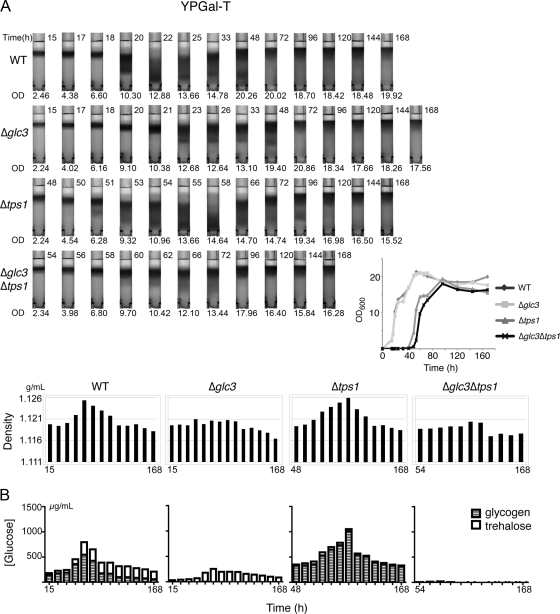
Figure 2.
Changes in cell density during growth in rich medium exhibit significant correlation with glucose equivalents in the form of glycogen and trehalose. (A) Density gradients of WT, Δglc3, Δtps1, and Δglc3Δtps1 cultures as they enter stationary phase in YPGal minus trehalose medium. Cells were collected at various times according to the growth curves (inset) and were subsequently density fractionated. The average density of the population at each time point was estimated for comparison. For density fractionation, time (hours) of collection is indicated at the top right corner of each tube and optical density of the culture (OD600) at the bottom. All the bar graphs only contain time labels for the first and last time points, the time points in between correspond to the time labels in the top panel. Time 0 of the growth curve refers to the time when cells were diluted into fresh medium. (B) Cell samples collected in parallel were assayed for trehalose and glycogen content. Results of trehalose and glycogen content are the means of three measurements from each time point, represented by glucose equivalents.
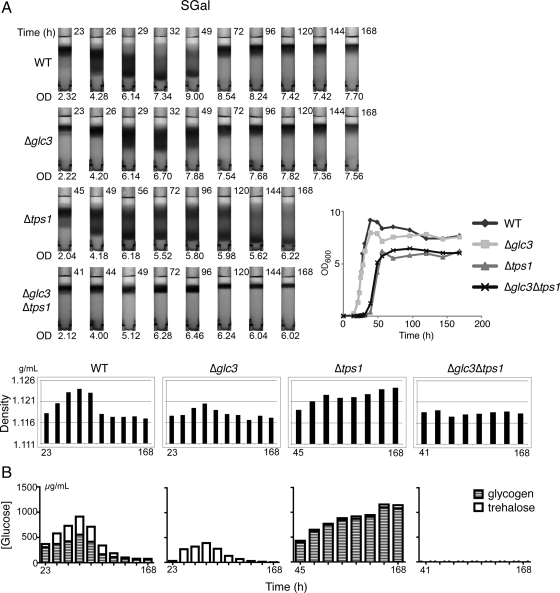
Figure 3.
Changes in cell density during growth in minimal medium exhibit significant correlation with glucose equivalents in the form of glycogen and trehalose. (A) Density gradients of WT, Δglc3, Δtps1, and Δtps1Δglc3 cells as they enter stationary phase in SGal medium. Cell samples were collected according to the growth curves (inset) and were subsequently density fractionated. Average density of the population at each time point was estimated for comparison. (B) Cell samples collected in parallel were assayed for trehalose and glycogen content as described in text (see Figure 2).
These four strains were cultured in YPGal-T medium to log phase, diluted into fresh YPGal-T medium, and then grown for 7 d, conditions under which cells have well entered stationary phase. Cells that cannot synthesize glycogen (Δglc3) grew comparably to wild-type (WT) cells (Figure 2A). However, cells that cannot synthesize trehalose (Δtps1), and cells that cannot synthesize both trehalose and glycogen (Δglc3Δtps1) exhibited significant defects in growth (Figure 2A). All strains eventually saturated at a similar high cell density (OD600 > 15) after 72 h.
On exit from exponential phase in YPGal-T (OD600 = 2–5), cells from all four strains tested were light in density. Beginning at ∼20 h, we noticed that a fraction of the WT cell population became heavier in density, similar to quiescent populations reported previously (Allen et al., 2006; Figure 2A). Both heavy and light populations of cells were observable until ∼72 h after inoculation. Beyond this point, the majority of cells were light up until 168 h. We observed that cells that cannot synthesize trehalose (Δtps1) also could become dense upon entry into stationary phase, whereas cells that cannot synthesize either glycogen (Δglc3) or both trehalose and glycogen (Δglc3Δtps1) also became dense but not nearly to the same extent as WT cells (Figure 2A).
We then measured the abundance of both trehalose and glycogen during the same time points that cell densities were determined for all four strains. The sum of trehalose and glycogen in terms of glucose equivalents showed significant correlation with average cell density (Figure 2B) for all strains tested. In WT cells, although both trehalose and glycogen levels initially increased during early stationary phase as expected, later on in stationary phase glycogen levels actually decreased whereas trehalose levels were maintained. After 7 days of growth, >3 times as much trehalose as glycogen was typically present within cells (Figure 2B and Supplemental Figure S2), indicating that trehalose is preferentially retained upon entry into long-term stationary phase, or quiescence, after growth in YP rich medium.
In Δtps1 cells, which can become dense to the same extent as WT cells upon entry into stationary phase, the concentration of glycogen was much greater than in WT cells, suggesting that glycogen synthesis is up-regulated to compensate for the inability of cells to synthesize trehalose (Figure 2B). In the absence of Tps1p, UDP-glucose equivalents that are a precursor for both trehalose and glycogen synthesis may be directed solely toward glycogen. In Δglc3 cells, as expected, no glycogen was detected. Similar to WT cells, trehalose levels increased upon entry into stationary phase and then decreased but were maintained (Figure 2B). However, due to the lack of glycogen, these cells contained fewer total glucose equivalents than WT cells, which may explain why they do not become as dense (Figure 2). In Δglc3Δtps1 cells, which barely increased in density upon entry into stationary phase, there was almost no detectable glycogen or trehalose.
We then repeated the same experiments by growing the set of four strains in synthetic defined minimal medium with galactose as the carbon source (SGal). Again, for all strains tested, we observed a substantial correlation between total glucose equivalents in the form of trehalose and glycogen and the overall density of cells (Figure 3). Together, these results strongly suggest that changes in individual cell density can largely be explained by the changes in glucose equivalents in the form of glycogen and trehalose within cells. However, under such synthetic minimal medium conditions, trehalose stores were not preferentially maintained compared with glycogen (Figure 3).
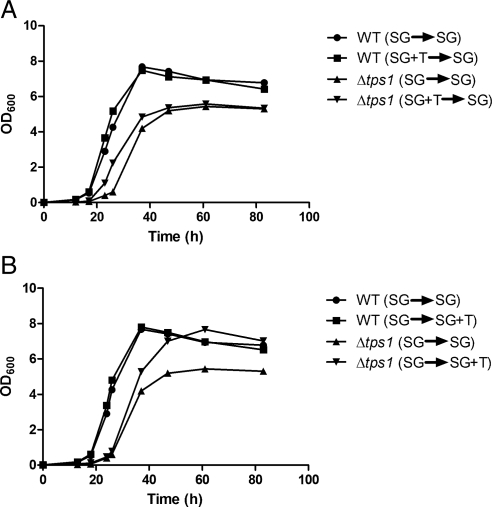
Because we observed a delay in the growth of cells lacking trehalose but not glycogen (Figures 2A and 3A), we next asked whether addition of trehalose to Δtps1 strains could rescue growth of these cells upon dilution into fresh medium. Significantly, we observed that prior growth of Δtps1 cells in SGal medium that had been supplemented with trehalose partially improved growth upon dilution into SGal medium (Figure 4A). In contrast, for WT cells, there was no difference in growth when trehalose was supplemented to the medium. We also tested whether Δtps1 cells grown in SGal medium lacking trehalose altogether would benefit from the presence of trehalose in the new growth medium. In this scenario, although there was not a detectable difference in the initial rates of growth, eventually these cells grew at a faster rate compared with Δtps1 cells that were diluted into regular SGal medium (Figure 4B). In contrast, there was no difference in the growth rates of WT cells if trehalose was supplemented to the new growth medium. Thus, the presence of trehalose can partially rescue the overall growth rates of Δtps1 cells lacking trehalose. Moreover, we observed an increased number of heavy, quiescent cells after 7 d of growth in medium containing trehalose, compared with medium lacking trehalose (Supplemental Figure S3).
Figure 4.
Trehalose partially rescues growth of Δtps1 cells. (A) To test whether prior growth in medium containing trehalose can partially rescue growth of Δtps1 cells, WT and Δtps1 cells were grown in SGal or SGal plus trehalose medium to log phase and then diluted into fresh SGal medium. OD600 was measured at the indicated time points. (B) To test whether subsequent growth in medium containing trehalose can improve growth of Δtps1 cells, WT and Δtps1 cells were grown in SGal medium to log phase and then inoculated into either fresh SGal or SGal plus trehalose medium. OD600 was measured at the indicated time points.
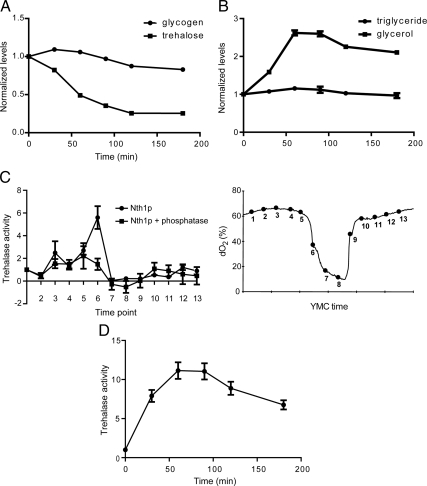
The growth defect exhibited by Δtps1 strains and the retention of trehalose during long-term starvation or quiescence (Figure 2B) suggest there might be a specialized role for trehalose in fueling metabolic demands of a cell upon exit from quiescence. We grew WT cells for 7 d in rich medium, conditions under which they enter a quiescent state, and then diluted them into fresh rich medium to induce growth. We then measured glycogen, trehalose, and triglyceride content of cells at several time points immediately after dilution into fresh medium. We observed that trehalose stores were depleted rapidly, whereas glycogen stores initially increased during the first 30 min of regrowth (Figure 5A). Triglyceride levels remained largely constant, whereas free glycerol levels increased significantly (Figure 5B). We observed previously an increase in intracellular glycerol during the OX, growth phase of the YMC, which might be required for up-regulation of lipid synthesis needed to build a new cell (Tu et al., 2007). As growth progressed, total trehalose and glycogen stores decreased to levels expected of cells in exponential growth. Cells lacking glycogen (Δglc3), which do not exhibit growth defects, also rapidly metabolized trehalose stores upon exit from quiescence (Supplemental Figure S5). However, cells lacking trehalose (Δtps1) did not rapidly metabolize their glycogen stores. The inability of these cells to metabolize trehalose stores could explain why they exit quiescence more slowly (Figures 2, 3, and 4, and Supplemental Figures S2 and S5). These data support the idea that intracellular trehalose stores play an important role in fueling cell cycle progression upon exit from quiescence.
Figure 5.
Intracellular trehalose stores are rapidly metabolized as yeast cells exit quiescence. (A) Trehalose stores are metabolized before glycogen upon regrowth. WT cells were grown in YPGal minus trehalose medium for 168 h and diluted 10 times in volume with fresh prewarmed medium. Cell samples were collected quickly for 180 min thereafter. Samples were assayed for trehalose and glycogen content. Data are shown as mean ± SEM from three measurements in terms of glucose equivalents, normalized to levels before initiation of regrowth. (B) Total intracellular triglyceride levels do not decrease upon exit from quiescence. WT yeast cells were grown, diluted into fresh medium, and samples were collected as described above. Samples were assayed for triglyceride and free glycerol levels. Results are shown as mean ± SEM from three measurements, normalized to levels present before regrowth. (C) Trehalase activity is up-regulated substantially during the switch to the OX growth phase. Cells expressing Nth1p-FLAG were harvested at 13 times points across one metabolic cycle. Nth1p was immunoprecipitated using FLAG epitope-conjugated beads and immediately assayed for trehalase activity with or without prior treatment with general phosphatases. Activity was normalized against the activity observed for time point 1. The x-axis tick marks denote 1-h time intervals. (D) Trehalase is rapidly activated upon exit from batch culture-induced quiescence. Nth1p-FLAG was immunoprecipitated from cells at the same time points as in A after regrowth and assayed for trehalase activity. Activity was normalized against the activity observed just before initiation of regrowth.
The observation that trehalose stores are metabolized preferentially upon exit from quiescence predicts that the trehalase enzymes will be specifically activated during the switch to growth. We used the YMC system to immunoprecipitate the major trehalase enzyme Nth1p from cells to assay its activity during either the quiescent-like (RC) or growth and division (OX and RB) phases of the YMC. We observed a significant increase in trehalase activity at a single time point in the midst of the OX growth phase, just after the exit from the RC phase that can be likened to quiescence (Figure 5C). Similarly, we found a significant up-regulation of trehalase activity upon regrowth after 7 d in stationary phase using a normal batch culture model of quiescence (Figure 5D). Moreover, because Nth1p has been reported to be a substrate of the cAMP-dependent protein kinase A (van der Plaat, 1974; Uno et al., 1983), we tested whether the activity of Nth1p would be dependent on phosphorylation. Prior treatment of immunoprecipitated Nth1p with general phosphatases significantly decreased its activity especially in the OX phase (Figure 5C), indicating that phosphorylation events play an important role in regulating Nth1p activity. Although we observed that the NTH1 gene is up-regulated significantly in RC phase (Supplemental Figure S4), the Nth1p protein levels do not change significantly between the quiescent and growth phases of the YMC (Supplemental Figure S4). Thus, it can be predicted that newly synthesized Nth1p protein accumulates during quiescence so that it may be rapidly activated by posttranslational mechanisms (e.g., phosphorylation) in response to appropriate metabolic or nutritional cues to help fuel regrowth of cells.
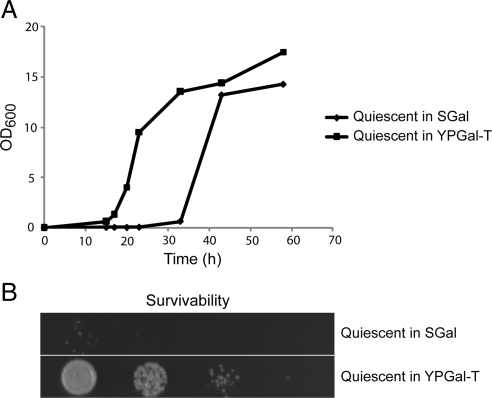
Lastly, our data predict that quiescent cells that contain less trehalose might recover more slowly and exhibit decreased survivability. To test this hypothesis, we determined the survivability of 7-d-old quiescent cells that were grown previously in either SGal or YPGal-T medium. We know that cells grown in SGal contain virtually no trehalose and small quantities of glycogen after 7 d, whereas cells grown in YPGal-T contain significant amounts of both trehalose and glycogen (Figures 2 and 3). We then tested the regrowth rates and survivability of these two populations of quiescent cells. Strikingly, the quiescent cells grown in SGal exhibited poor survivability and recovered much more slowly than quiescent cells grown in YPGal-T (Figure 6). Thus, these observations suggest that intracellular trehalose stores could affect cell survivability and chronological life span.
Figure 6.
Intracellular trehalose stores correlate with quiescent cell survivability. WT cells were inoculated into either SGal or YPGal minus trehalose medium and grown for 7 d. (A) Quiescent cells previously grown in SGal exhibit slower rates of regrowth. Cells from the two 7-d-old quiescent cultures were diluted into YPGal-T medium, and OD600 was monitored as a function of time. (B) Quiescent cells previously grown in SGal that contains virtually no trehalose exhibit poor survivability. As a measure of cell survivability, from left to right, 10,000, 1000, 100, and 10 cells from the quiescent cultures were spotted onto a YPGal agar plate. The plate was incubated at 30°C for 2 d to assess cell viability. Similar results were obtained in replicate experiments.
DISCUSSION
Here, our data strongly suggest that the primary reason quiescent cells observed during stationary phase become more dense is because these cells have accumulated more trehalose and glycogen stores. In turn, these cells can use these stored carbohydrates to help fuel cell cycle progression upon regrowth. This provides an explanation as to why these heavy, quiescent cells can begin division more readily than light, nonquiescent cells (Allen et al., 2006). However, we do not understand why only a fraction of cells in standard batch cultures become quiescent upon entry into stationary phase. Under such non–steady-state growth conditions, we speculate that certain nutrients or secreted metabolites might induce some cells to metabolize their trehalose and glycogen stores in an attempt to enter the cell cycle.
During continuous, steady-state growth conditions, cells in the RC phase of the YMC accumulate trehalose and glycogen in a manner similar to batch-cultured cells that enter stationary phase (Figures 1 and 2). Interestingly, the expression of many RC phase genes was found to be negatively correlated with increasing growth rate (Brauer et al., 2008). Thus, based on the criteria of gene expression and cell density, cells in the RC phase of the YMC are reminiscent of slow-growing cells that are about to enter quiescence. On entry into the OX growth phase, cells metabolize these carbohydrate stores and up-regulate numerous growth genes to fuel a round of growth and proliferation (Tu et al., 2005; Tu et al., 2007). Thus, there are continuous alternations between quiescent-like (RC) and growth (OX) phases during the YMC.
As cells enter prolonged stationary phase after growth in YP medium, trehalose levels tend to be maintained, whereas glycogen stores decrease (Figure 2 and Supplemental S2). Some of these glycogen and trehalose stores may be used to support basal metabolic activities as cells persist in stationary phase; however, it is clear that trehalose is preferentially maintained over glycogen as the quiescent state progresses. On exit from quiescence, trehalose stores are metabolized before glycogen and reduced to levels typical of exponential phase growth (Figure 5A).
Because mutants that cannot synthesize trehalose exhibit severe defects in the reinitiation of growth, and mutants that cannot synthesize glycogen do not, together our results suggest an important role for trehalose as a fuel reserve that enables yeast cells to survive starvation conditions and then rapidly proliferate upon return to favorable growth conditions. The rapid activation of the trehalase enzyme Nth1p during the switch to growth supports the idea that trehalose stores are quickly metabolized upon exit from quiescence to help fuel growth. Thus, the ability of a cell to accumulate sufficient trehalose stores is a key determinant of whether it can enter and exit quiescence successfully.
Trehalose has been widely reported to function as a stress protectant by acting as a chemical chaperone or osmolyte (Crowe et al., 1992; Singer and Lindquist, 1998b). Trehalose levels accrue under a variety of stress conditions, and increased levels of trehalose correlate with increased survivability after stresses such as heat shock, cold shock, and desiccation (Singer and Lindquist, 1998a; Kandror et al., 2004). We offer an additional interpretation to these previous observations in that trehalose stores in parallel might also function as an energy source that yeast cells initially utilize upon return to favorable growth conditions. Without sufficient trehalose stores, yeast cells would exhibit defects not only in their ability to persist in a quiescent state but also in their ability to exit the quiescent state induced by various stresses. Thus, although trehalose may directly function as a chaperone to help minimize protein aggregation or as an osmolyte to minimize water loss (Crowe et al., 1992; Singer and Lindquist, 1998b), it may simultaneously serve as a critical energy reserve.
There are several significant reasons why trehalose might be a preferred energy source for survival of a variety of adverse conditions. Trehalose is a nonreducing disaccharide formed from two molecules of glucose. Because the glycosidic linkage exists between the 1-carbons of two glucose molecules, trehalose is more resistant than most carbohydrates to heat and acid-induced decomposition (Higashiyama, 2002). Thus, trehalose represents a source of energy that itself would endure a variety of harsh conditions that yeast might encounter in the wild. Moreover, cleavage of one glycosidic bond in trehalose would release two glucose equivalents that can be used for energy. In contrast, a glycogen polymer would liberate only one glucose equivalent per glycosidic bond cleaved. Trehalose is also an abundant component of plant seeds and fungal spores (Higashiyama, 2002; Elbein et al., 2003). In addition to protecting against desiccation, these trehalose stores also may be used as a source of energy to drive germination.
For these reasons, it becomes sensible as to why cells might favor the storage of trehalose over glycogen as time spent in the quiescent state increases. In many previous studies, a distinction between trehalose and glycogen in their roles as energy reserves has not been clear (Lillie and Pringle, 1980; Sillje et al., 1997, 1999; Guillou et al., 2004). In the YMC system, both glycogen and trehalose accumulate to a similar extent during RC phase and both become degraded upon entry into OX growth phase (Figure 1). However, the quiescent-like state in these situations persists on the order of hours, rather than days. As time spent in the quiescent state increases, cells may begin redirecting glucose equivalents from glycogen to trehalose to enhance survivability. Moreover, our data predict that trehalose synthesis may induce other important aspects of the quiescence program and that trehalose metabolism will be coupled to cell cycle progression upon exit from quiescence. The precise mechanisms by which trehalose metabolism may be linked to the cell cycle, especially under nutrient-poor conditions, will be of great interest in future studies.
Lastly, our findings regarding trehalose and quiescence offer a slightly different perspective on chronological aging and life span in yeast. We speculate that the perturbation of pathways that negatively impact trehalose accumulation or metabolism might compromise the ability of a cell to rapidly exit the quiescent state, and thus its survivability (Figure 6). Conversely, those that enhance trehalose storage or reduce trehalose metabolism while persisting in the quiescent state might improve survivability. In nature, yeast cells are more likely to undergo periodic bursts of rapid growth that alternate with long periods of quiescence, rather than continuous rapid growth that typically occurs under laboratory growth conditions (Sillje et al., 1999; Lu et al., 2009). Such conditions necessitate the accumulation of trehalose and other reserves (e.g., glycogen) so that cells can readily enter and exit quiescence when appropriate. The cycles of trehalose storage and breakdown that occur in yeast cell populations as a function of metabolic cycles during glucose-limited growth in essence depict such a strategy (Futcher, 2006; Xu and Tsurugi, 2006; Tu et al., 2007). We conclude that in addition to its numerous stress-protectant functions, a critical role for trehalose as an enduring energy reserve that in turn helps fuel rapid exit from quiescence cannot be discounted.
Supplementary Material
ACKNOWLEDGMENTS
We thank M. Werner-Washburne and members of the Tu laboratory for helpful discussions. This work was supported by a Burroughs Wellcome Career Award in Biomedical Sciences; the Welch Foundation; and the University of Texas Southwestern Medical Center Endowed Scholars Program. B.P.T. is a W. W. Caruth, Jr., Scholar in Biomedical Research.
Abbreviations used:
- YMC
yeast metabolic cycle.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E10-01-0056) on April 28, 2010.
REFERENCES
- Allen C., et al. Isolation of quiescent and nonquiescent cells from yeast stationary-phase cultures. J. Cell Biol. 2006;174:89–100. doi: 10.1083/jcb.200604072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragon A. D., Rodriguez A. L., Meirelles O., Roy S., Davidson G. S., Tapia P. H., Allen C., Joe R., Benn D., Werner-Washburne M. Characterization of differentiated quiescent and nonquiescent cells in yeast stationary-phase cultures. Mol. Biol. Cell. 2008;19:1271–1280. doi: 10.1091/mbc.E07-07-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boer V. M., Crutchfield C. A., Bradley P. H., Botstein D., Rabinowitz J. D. Growth-limiting intracellular metabolites in yeast growing under diverse nutrient limitations. Mol. Biol. Cell. 2010;21:198–211. doi: 10.1091/mbc.E09-07-0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer M. J., Huttenhower C., Airoldi E. M., Rosenstein R., Matese J. C., Gresham D., Boer V. M., Troyanskaya O. G., Botstein D. Coordination of growth rate, cell cycle, stress response, and metabolic activity in yeast. Mol. Biol. Cell. 2008;19:352–367. doi: 10.1091/mbc.E07-08-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe J. H., Hoekstra F. A., Crowe L. M. Anhydrobiosis. Annu. Rev. Physiol. 1992;54:579–599. doi: 10.1146/annurev.ph.54.030192.003051. [DOI] [PubMed] [Google Scholar]
- Elbein A. D., Pan Y. T., Pastuszak I., Carroll D. New insights on trehalose: a multifunctional molecule. Glycobiology. 2003;13:17R–27R. doi: 10.1093/glycob/cwg047. [DOI] [PubMed] [Google Scholar]
- Fabrizio P., Longo V. D. The chronological life span of Saccharomyces cerevisiae. Methods Mol. Biol. 2007;371:89–95. doi: 10.1007/978-1-59745-361-5_8. [DOI] [PubMed] [Google Scholar]
- Francois J., Parrou J. L. Reserve carbohydrates metabolism in the yeast Saccharomyces cerevisiae. FEMS Microbiol. Rev. 2001;25:125–145. doi: 10.1111/j.1574-6976.2001.tb00574.x. [DOI] [PubMed] [Google Scholar]
- Futcher B. Metabolic cycle, cell cycle, and the finishing kick to start. Genome Biol. 2006;7:107. doi: 10.1186/gb-2006-7-4-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J. V., Petsko G. A., Johnston G. C., Ringe D., Singer R. A., Werner-Washburne M. “Sleeping beauty”: quiescence in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2004;68:187–206. doi: 10.1128/MMBR.68.2.187-206.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillou V., Plourde-Owobi L., Parrou J. L., Goma G., Francois J. Role of reserve carbohydrates in the growth dynamics of Saccharomyces cerevisiae. FEMS Yeast Res. 2004;4:773–787. doi: 10.1016/j.femsyr.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Higashiyama T. Novel functions and applications of trehalose. Pure Appl. Chem. 2002;74:1263–1269. [Google Scholar]
- Kandror O., Bretschneider N., Kreydin E., Cavalieri D., Goldberg A. L. Yeast adapt to near-freezing temperatures by STRE/Msn2,4-dependent induction of trehalose synthesis and certain molecular chaperones. Mol Cell. 2004;13:771–781. doi: 10.1016/s1097-2765(04)00148-0. [DOI] [PubMed] [Google Scholar]
- Lillie S. H., Pringle J. R. Reserve carbohydrate metabolism in Saccharomyces cerevisiae: responses to nutrient limitation. J. Bacteriol. 1980;143:1384–1394. doi: 10.1128/jb.143.3.1384-1394.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine M. S., McKenzie A., 3rd, Demarini D. J., Shah N. G., Wach A., Brachat A., Philippsen P., Pringle J. R. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998;14:953–961. doi: 10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Lu C., Brauer M. J., Botstein D. Slow growth induces heat-shock resistance in normal and respiratory-deficient yeast. Mol. Biol. Cell. 2009;20:891–903. doi: 10.1091/mbc.E08-08-0852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrou J. L., Francois J. A simplified procedure for a rapid and reliable assay of both glycogen and trehalose in whole yeast cells. Anal. Biochem. 1997;248:186–188. doi: 10.1006/abio.1997.2138. [DOI] [PubMed] [Google Scholar]
- Parulekar S. J., Semones G. B., Rolf M. J., Lievense J. C., Lim H. C. Induction and elimination of oscillations in continuous cultures of Saccharomyces cerevisiae. Biotechnol. Bioeng. 1986;28:700–710. doi: 10.1002/bit.260280509. [DOI] [PubMed] [Google Scholar]
- Porro D., Martegani E., Ranzi B. M., Alberghina L. Oscillations in continuous cultures of budding yeast: a segregated parameter analysis. Biotechnol. Bioeng. 1988;32:411–417. doi: 10.1002/bit.260320402. [DOI] [PubMed] [Google Scholar]
- Powers R. W., 3rd, Kaeberlein M., Caldwell S. D., Kennedy B. K., Fields S. Extension of chronological life span in yeast by decreased TOR pathway signaling. Genes Dev. 2006;20:174–184. doi: 10.1101/gad.1381406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satroutdinov A. D., Kuriyama H., Kobayashi H. Oscillatory metabolism of Saccharomyces cerevisiae in continuous culture. FEMS Microbiol. Lett. 1992;77:261–267. doi: 10.1016/0378-1097(92)90167-m. [DOI] [PubMed] [Google Scholar]
- Sillje H. H., Paalman J. W., ter Schure E. G., Olsthoorn S. Q., Verkleij A. J., Boonstra J., Verrips C. T. Function of trehalose and glycogen in cell cycle progression and cell viability in Saccharomyces cerevisiae. J. Bacteriol. 1999;181:396–400. doi: 10.1128/jb.181.2.396-400.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillje H. H., ter Schure E. G., Rommens A. J., Huls P. G., Woldringh C. L., Verkleij A. J., Boonstra J., Verrips C. T. Effects of different carbon fluxes on G1 phase duration, cyclin expression, and reserve carbohydrate metabolism in Saccharomyces cerevisiae. J. Bacteriol. 1997;179:6560–6565. doi: 10.1128/jb.179.21.6560-6565.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M. A., Lindquist S. Multiple effects of trehalose on protein folding in vitro and in vivo. Mol. Cell. 1998a;1:639–648. doi: 10.1016/s1097-2765(00)80064-7. [DOI] [PubMed] [Google Scholar]
- Singer M. A., Lindquist S. Thermotolerance in Saccharomyces cerevisiae: the yin and yang of trehalose. Trends Biotechnol. 1998b;16:460–468. doi: 10.1016/s0167-7799(98)01251-7. [DOI] [PubMed] [Google Scholar]
- Thevelein J. M., Hohmann S. Trehalose synthase: guard to the gate of glycolysis in yeast? Trends Biochem. Sci. 1995;20:3–10. doi: 10.1016/s0968-0004(00)88938-0. [DOI] [PubMed] [Google Scholar]
- Tu B. P., Kudlicki A., Rowicka M., McKnight S. L. Logic of the yeast metabolic cycle: temporal compartmentalization of cellular processes. Science. 2005;310:1152–1158. doi: 10.1126/science.1120499. [DOI] [PubMed] [Google Scholar]
- Tu B. P., McKnight S. L. Metabolic cycles as an underlying basis of biological oscillations. Nat. Rev. Mol. Cell Biol. 2006;7:696–701. doi: 10.1038/nrm1980. [DOI] [PubMed] [Google Scholar]
- Tu B. P., McKnight S. L. The yeast metabolic cycle: insights into the life of a eukaryotic cell. Cold Spring Harb. Symp. Quant. Biol. 2007;72:339–343. doi: 10.1101/sqb.2007.72.019. [DOI] [PubMed] [Google Scholar]
- Tu B. P., Mohler R. E., Liu J. C., Dombek K. M., Young E. T., Synovec R. E., McKnight S. L. Cyclic changes in metabolic state during the life of a yeast cell. Proc. Natl. Acad. Sci. USA. 2007;104:16886–16891. doi: 10.1073/pnas.0708365104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno I., Matsumoto K., Adachi K., Ishikawa T. Genetic and biochemical evidence that trehalase is a substrate of cAMP-dependent protein kinase in yeast. J. Biol. Chem. 1983;258:10867–10872. [PubMed] [Google Scholar]
- van der Plaat J. B. Cyclic 3′,5′-adenosine monophosphate stimulates trehalose degradation in baker's yeast. Biochem. Biophys. Res. Commun. 1974;56:580–587. doi: 10.1016/0006-291x(74)90643-3. [DOI] [PubMed] [Google Scholar]
- van Dijken J. P., et al. An interlaboratory comparison of physiological and genetic properties of four Saccharomyces cerevisiae strains. Enzyme Microb. Technol. 2000;26:706–714. doi: 10.1016/s0141-0229(00)00162-9. [DOI] [PubMed] [Google Scholar]
- von Meyenburg H. K. Energetics of the budding cycle of Saccharomyces cerevisiae during glucose limited aerobic growth. Arch. Microbiol. 1969;66:289–303. doi: 10.1007/BF00414585. [DOI] [PubMed] [Google Scholar]
- Xu Z., Tsurugi K. A potential mechanism of energy-metabolism oscillation in an aerobic chemostat culture of the yeast Saccharomyces cerevisiae. FEBS J. 2006;273:1696–1709. doi: 10.1111/j.1742-4658.2006.05201.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.