Abstract
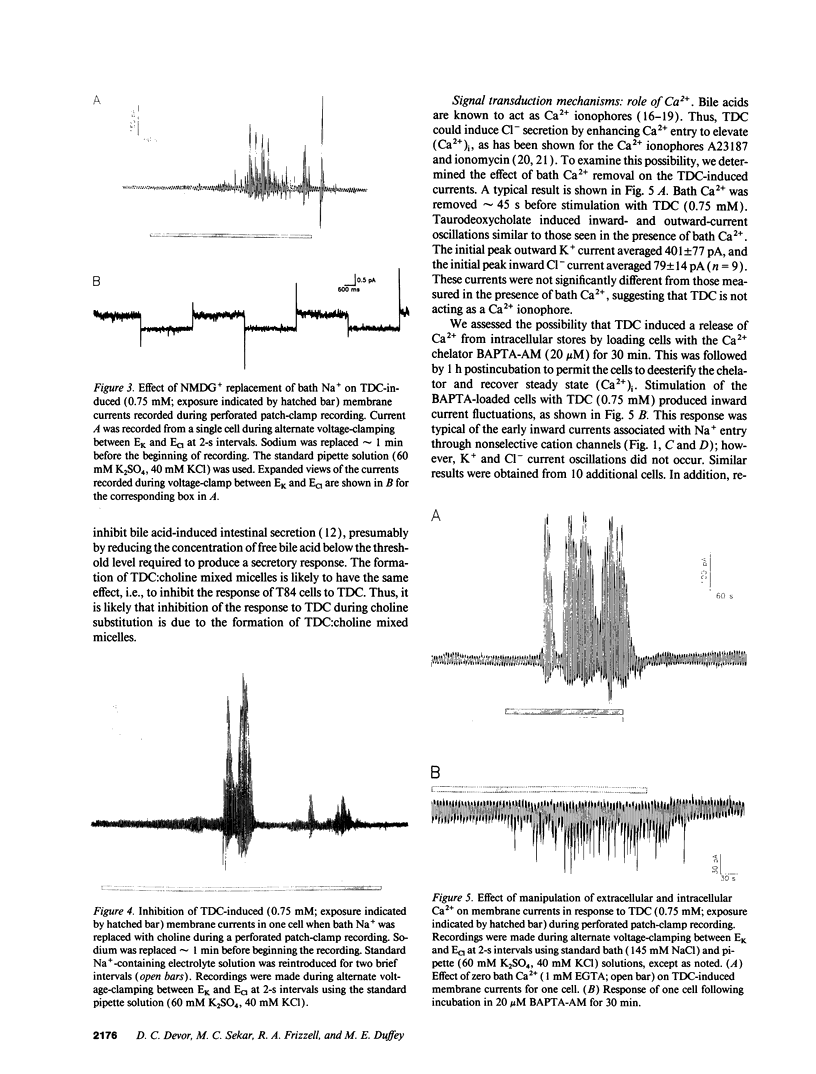
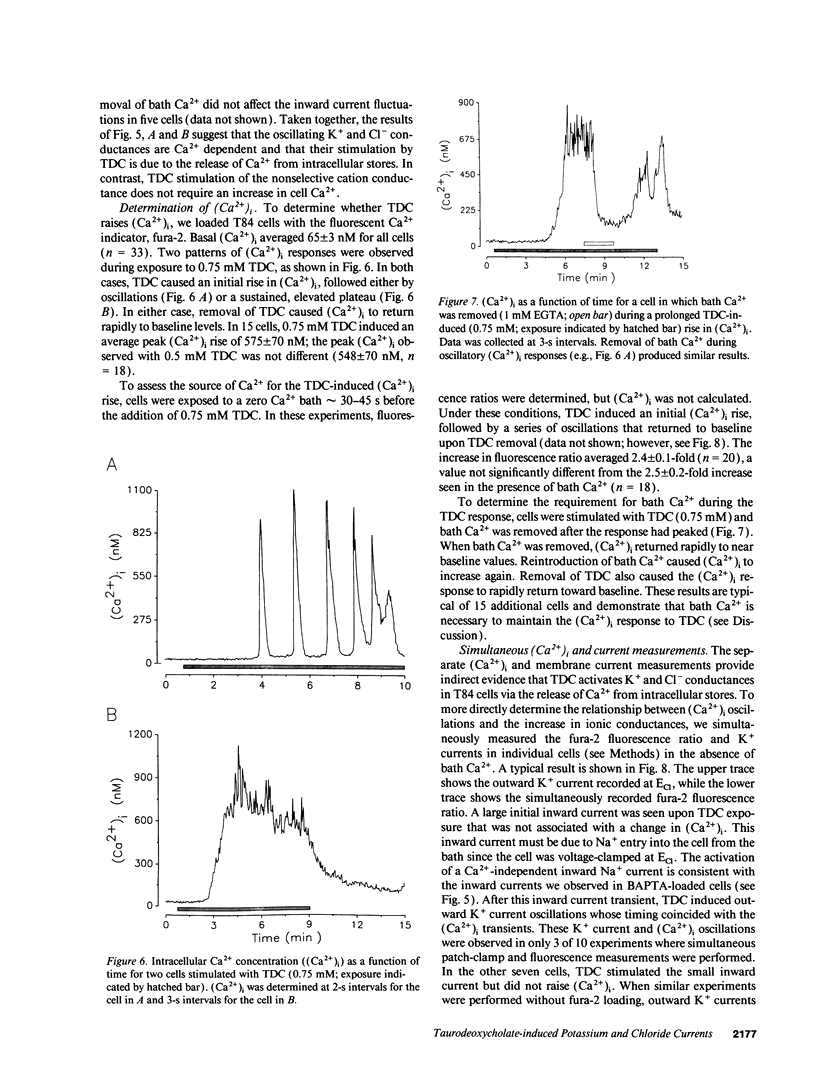
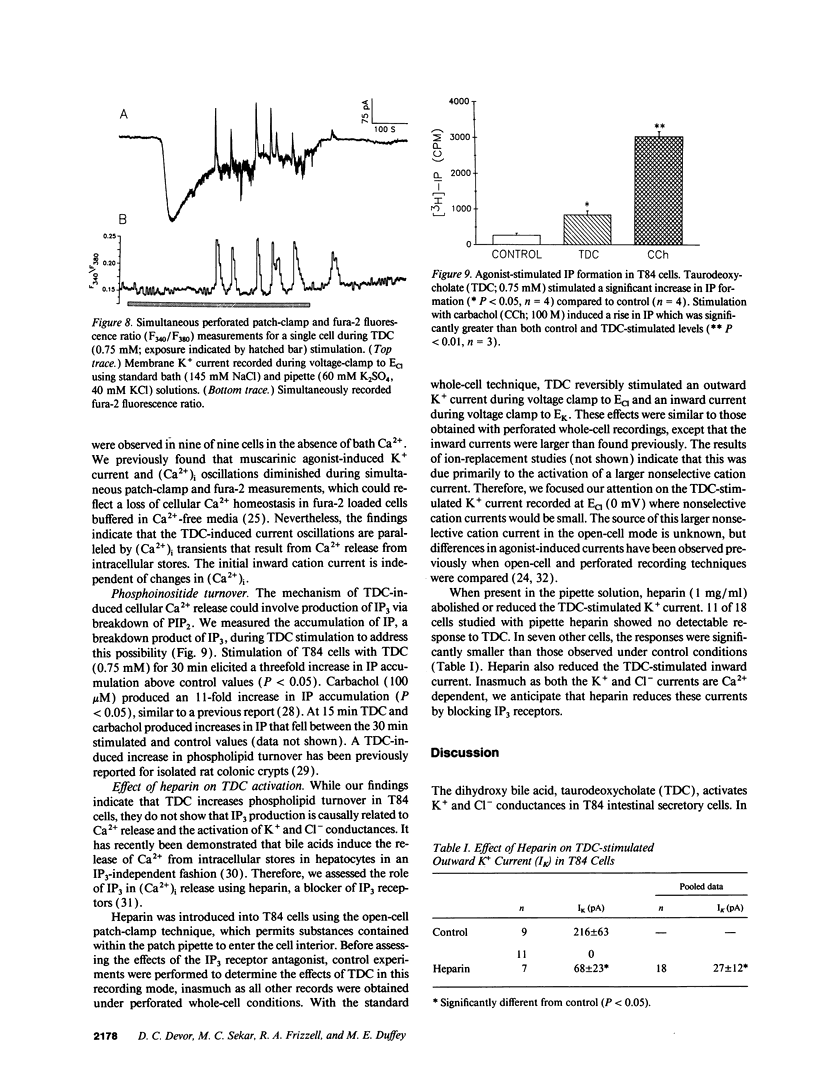
Whole-cell patch-clamp techniques and fluorescence measurements of intracellular Ca2+ concentration, (Ca2+)i, were used to investigate the mechanism of taurodeoxycholate (TDC) stimulation of Cl- secretion in the T84 colonic cell line. During perforated whole-cell recordings, the cell membrane voltage was alternately clamped to EK and ECl. Initially, TDC (0.75 mM) stimulated inward nonselective cation currents that were composed of discrete large conductance single-channel events. This initial response was followed by activation of K+ and Cl- currents with peak values of 385 +/- 41 pA and 98 +/- 28 pA, respectively (n = 12). The K+ and Cl- currents oscillated while TDC was present and returned to baseline levels upon its removal. The threshold for activation of the oscillatory currents was 0.1 mM TDC. Taurocholate, a bile acid that does not stimulate colonic Cl- secretion, induced no current response. The TDC-induced currents could be activated in Ca(2+)-free bathing solutions. Preincubation of cells with the Ca2+ chelator, bis-(o-aminophenoxy)-ethane-N,N,N',N'-tetraacetic acid, tetra(acetoxymethy)-ester (20 microM), (BAPTA-AM), eliminated the K+ and Cl- current responses, although the nonselective cation channel events were still present. Replacement of bath Na+ with NMDG+ inhibited the TDC-induced nonselective cation current but did not affect the K+ or Cl- currents. TDC induced a transient (Ca2+)i rise of 575 +/- 70 nM from a baseline of 71 +/- 5 nM (n = 15); thereafter, (Ca2+)i either plateaued or oscillated. TDC-induced (Ca2+)i oscillations were observed in the absence of bath Ca2+; however, removal of bath Ca2+ during the TDC response caused (Ca2+)i to return to near baseline values. Simultaneous K+ current and (Ca2+)i measurements confirmed that the initial nonselective cation current was independent of (Ca2+)i, while K+ current oscillations were in phase with the (Ca2+)i oscillations. TDC induced inositol monophosphate (IP) accumulation, reflecting production of inositol 1,4,5-trisphosphate (IP3) during TDC stimulation. The response to TDC during standard whole-cell patch-clamp was similar to that observed with perforated whole-cell recordings, except the nonselective cation current was prolonged. When heparin (1 mg/ml) was added to the pipette under these conditions, the Ca(2+)-activated currents were inhibited, but the nonselective cation currents were unaffected. These data suggest that TDC induces a Ca(2+)-independent nonselective cation conductance, perhaps by directly permeabilizing the plasma membrane. TDC stimulates Cl- secretion by activating K+ and Cl- conductances via an IP3-mediated release of Ca2+ from intracellular stores.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson J. J., Shamoo A. E. Anionic detergents as divalent cation ionophores across black lipid membranes. J Membr Biol. 1979 Nov 30;50(3-4):241–255. doi: 10.1007/BF01868891. [DOI] [PubMed] [Google Scholar]
- Ammon H. V., Cho D. S., Loeffler R. L., Reetz K. L. Effects of taurodeoxycholate on in vivo water and solute transport in rat jejunum in absence and presence of calcium. Am J Physiol. 1986 Feb;250(2 Pt 1):G248–G251. doi: 10.1152/ajpgi.1986.250.2.G248. [DOI] [PubMed] [Google Scholar]
- Amédée T., Large W. A., Wang Q. Characteristics of chloride currents activated by noradrenaline in rabbit ear artery cells. J Physiol. 1990 Sep;428:501–516. doi: 10.1113/jphysiol.1990.sp018224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M. P., Welsh M. J. Calcium and cAMP activate different chloride channels in the apical membrane of normal and cystic fibrosis epithelia. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6003–6007. doi: 10.1073/pnas.88.14.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balistreri W. F., Heubi J. E., Suchy F. J. Bile acid metabolism: relationship of bile acid malabsorption and diarrhea. J Pediatr Gastroenterol Nutr. 1983;2(1):105–121. [PubMed] [Google Scholar]
- Binder H. J., Filburn C., Volpe B. T. Bile salt alteration of colonic electrolyte transport: role of cyclic adenosine monophosphate. Gastroenterology. 1975 Mar;68(3):503–508. [PubMed] [Google Scholar]
- Bolton J. E., Field M. Ca ionophore-stimulated ion secretion in rabbit ileal mucosa: relation to actions of cyclic 3',5'-AMP and carbamylcholine. J Membr Biol. 1977 Jun 30;35(2):159–173. doi: 10.1007/BF01869947. [DOI] [PubMed] [Google Scholar]
- Böhme M., Diener M., Rummel W. Calcium- and cyclic-AMP-mediated secretory responses in isolated colonic crypts. Pflugers Arch. 1991 Sep;419(2):144–151. doi: 10.1007/BF00373000. [DOI] [PubMed] [Google Scholar]
- Capiod T., Combettes L., Noel J., Claret M. Evidence for bile acid-evoked oscillations of Ca2(+)-dependent K+ permeability unrelated to a D-myo-inositol 1,4,5-trisphosphate effect in isolated guinea pig liver cells. J Biol Chem. 1991 Jan 5;266(1):268–273. [PubMed] [Google Scholar]
- Cliff W. H., Frizzell R. A. Separate Cl- conductances activated by cAMP and Ca2+ in Cl(-)-secreting epithelial cells. Proc Natl Acad Sci U S A. 1990 Jul;87(13):4956–4960. doi: 10.1073/pnas.87.13.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combettes L., Berthon B., Doucet E., Erlinger S., Claret M. Characteristics of bile acid-mediated Ca2+ release from permeabilized liver cells and liver microsomes. J Biol Chem. 1989 Jan 5;264(1):157–167. [PubMed] [Google Scholar]
- Combettes L., Dumont M., Berthon B., Erlinger S., Claret M. Effect of the bile acid taurolithocholate on cell calcium in saponin-treated rat hepatocytes. FEBS Lett. 1988 Jan 25;227(2):161–166. doi: 10.1016/0014-5793(88)80889-5. [DOI] [PubMed] [Google Scholar]
- Combettes L., Dumont M., Berthon B., Erlinger S., Claret M. Release of calcium from the endoplasmic reticulum by bile acids in rat liver cells. J Biol Chem. 1988 Feb 15;263(5):2299–2303. [PubMed] [Google Scholar]
- Coquil J. F., Berthon B., Chomiki N., Combettes L., Jourdon P., Schteingart C., Erlinger S., Claret M. Effects of taurolithocholate, a Ca2(+)-mobilizing agent, on cell Ca2(+) in rat hepatocytes, human platelets and neuroblastoma NG108-15 cell line. Biochem J. 1991 Jan 1;273(Pt 1):153–160. doi: 10.1042/bj2730153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotting J., Lentze M. J., Reichen J. Effects of ursodeoxycholic acid treatment on nutrition and liver function in patients with cystic fibrosis and longstanding cholestasis. Gut. 1990 Aug;31(8):918–921. doi: 10.1136/gut.31.8.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven P. A., Pfanstiel J., DeRubertis F. R. Role of activation of protein kinase C in the stimulation of colonic epithelial proliferation and reactive oxygen formation by bile acids. J Clin Invest. 1987 Feb;79(2):532–541. doi: 10.1172/JCI112844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor D. C., Ahmed Z., Duffey M. E. Cholinergic stimulation produces oscillations of cytosolic Ca2+ in a secretory epithelial cell line, T84. Am J Physiol. 1991 Mar;260(3 Pt 1):C598–C608. doi: 10.1152/ajpcell.1991.260.3.C598. [DOI] [PubMed] [Google Scholar]
- Devor D. C., Duffey M. E. Carbachol induces K+, Cl-, and nonselective cation conductances in T84 cells: a perforated patch-clamp study. Am J Physiol. 1992 Oct;263(4 Pt 1):C780–C787. doi: 10.1152/ajpcell.1992.263.4.C780. [DOI] [PubMed] [Google Scholar]
- Devor D. C., Simasko S. M., Duffey M. E. Carbachol induces oscillations of membrane potassium conductance in a colonic cell line, T84. Am J Physiol. 1990 Feb;258(2 Pt 1):C318–C326. doi: 10.1152/ajpcell.1990.258.2.C318. [DOI] [PubMed] [Google Scholar]
- Dharmsathaphorn K., Huott P. A., Vongkovit P., Beuerlein G., Pandol S. J., Ammon H. V. Cl- secretion induced by bile salts. A study of the mechanism of action based on a cultured colonic epithelial cell line. J Clin Invest. 1989 Sep;84(3):945–953. doi: 10.1172/JCI114257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson K. E., Frizzell R. A., Sekar M. C. Activation of T84 cell chloride channels by carbachol involves a phosphoinositide-coupled muscarinic M3 receptor. Eur J Pharmacol. 1992 Apr 10;225(4):291–298. doi: 10.1016/0922-4106(92)90102-2. [DOI] [PubMed] [Google Scholar]
- Fasano A., Budillon G., Guandalini S., Cuomo R., Parrilli G., Cangiotti A. M., Morroni M., Rubino A. Bile acids reversible effects on small intestinal permeability. An in vitro study in the rabbit. Dig Dis Sci. 1990 Jul;35(7):801–808. doi: 10.1007/BF01536791. [DOI] [PubMed] [Google Scholar]
- Freel R. W. Dihydroxy bile salt-induced secretion of rubidium ion across the rabbit distal colon. Am J Physiol. 1987 Apr;252(4 Pt 1):G554–G561. doi: 10.1152/ajpgi.1987.252.4.G554. [DOI] [PubMed] [Google Scholar]
- Freel R. W., Hatch M., Earnest D. L., Goldner A. M. Dihydroxy bile salt-induced alterations in NaCl transport across the rabbit colon. Am J Physiol. 1983 Dec;245(6):G808–G815. doi: 10.1152/ajpgi.1983.245.6.G808. [DOI] [PubMed] [Google Scholar]
- Frizzell R. A. Active chloride secretion by rabbit colon: calcium-dependent stimulation by ionophore A23187. J Membr Biol. 1977 Jun 30;35(2):175–187. doi: 10.1007/BF01869948. [DOI] [PubMed] [Google Scholar]
- Halm D. R., Rechkemmer G. R., Schoumacher R. A., Frizzell R. A. Apical membrane chloride channels in a colonic cell line activated by secretory agonists. Am J Physiol. 1988 Apr;254(4 Pt 1):C505–C511. doi: 10.1152/ajpcell.1988.254.4.C505. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Horn R., Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988 Aug;92(2):145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan M. M. Medical approaches to primary sclerosing cholangitis. Semin Liver Dis. 1991 Feb;11(1):56–63. doi: 10.1055/s-2008-1040423. [DOI] [PubMed] [Google Scholar]
- Kaplan M. M. Medical treatment of primary biliary cirrhosis. Semin Liver Dis. 1989 May;9(2):138–143. doi: 10.1055/s-2008-1040505. [DOI] [PubMed] [Google Scholar]
- Maenz D. D., Forsyth G. W. Calcium ionophore activity of intestinal secretory compounds. An in vitro porcine model for the effects of bile acids, hydroxy-fatty acids and dioctyl sulfosuccinate. Digestion. 1984;30(3):138–150. doi: 10.1159/000199098. [DOI] [PubMed] [Google Scholar]
- Maenz D. D., Forsyth G. W. Ricinoleate and deoxycholate are calcium ionophores in jejunal brush border vesicles. J Membr Biol. 1982;70(2):125–133. doi: 10.1007/BF01870222. [DOI] [PubMed] [Google Scholar]
- Mekjian H. S., Phillips S. F., Hofmann A. F. Colonic secretion of water and electrolytes induced by bile acids: perfusion studies in man. J Clin Invest. 1971 Aug;50(8):1569–1577. doi: 10.1172/JCI106644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris A. P., Kirk K. L., Frizzell R. A. Simultaneous analysis of cell Ca2+ and Ca2(+)-stimulated chloride conductance in colonic epithelial cells (HT-29). Cell Regul. 1990 Nov;1(12):951–963. doi: 10.1091/mbc.1.12.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelberg D. G., Wang L. B., Sackman J. W., Adcock E. W., Lester R., Dubinsky W. P. Bile salt-induced calcium fluxes in artificial phospholipid vesicles. Biochim Biophys Acta. 1988 Jan 22;937(2):289–299. doi: 10.1016/0005-2736(88)90251-9. [DOI] [PubMed] [Google Scholar]
- Plantavid M., Rossignol L., Chap H., Douste-Blazy L. Studies of endogenous polyphosphoinositide hydrolysis in human platelet membranes. Evidence that polyphosphoinositides remain inaccessible to phosphodiesterase in the native membrane. Biochim Biophys Acta. 1986 Feb 12;875(2):147–156. doi: 10.1016/0005-2760(86)90163-3. [DOI] [PubMed] [Google Scholar]
- Potter G. D., Sellin J. H., Burlingame S. M. Bile acid stimulation of cyclic AMP and ion transport in developing rabbit colon. J Pediatr Gastroenterol Nutr. 1991 Nov;13(4):335–341. doi: 10.1097/00005176-199111000-00001. [DOI] [PubMed] [Google Scholar]
- Simon B., Cyzgan P., Stiehl A., Kather H. Human colonic adenylate cyclase: effects of bile acids. Eur J Clin Invest. 1978 Oct;8(5):321–323. doi: 10.1111/j.1365-2362.1978.tb00849.x. [DOI] [PubMed] [Google Scholar]
- Talamini M. A., Gadacz T. R. Gallstone dissolution. Surg Clin North Am. 1990 Dec;70(6):1217–1230. doi: 10.1016/s0039-6109(16)45280-1. [DOI] [PubMed] [Google Scholar]
- Walters R. J., Sepúlveda F. V. A basolateral K+ conductance modulated by carbachol dominates the membrane potential of small intestinal crypts. Pflugers Arch. 1991 Nov;419(5):537–539. doi: 10.1007/BF00370802. [DOI] [PubMed] [Google Scholar]
- Wingate D. L., Phillips S. F., Hofmann A. F. Effect of glycine-conjugated bile acids with and without lecithin on water and glucose absorption in perfused human jejunum. J Clin Invest. 1973 May;52(5):1230–1236. doi: 10.1172/JCI107290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worley P. F., Baraban J. M., Supattapone S., Wilson V. S., Snyder S. H. Characterization of inositol trisphosphate receptor binding in brain. Regulation by pH and calcium. J Biol Chem. 1987 Sep 5;262(25):12132–12136. [PubMed] [Google Scholar]