SUMMARY
Penicillium aethiopicum produces two structurally interesting and biologically active polyketides: the tetracycline-like viridicatumtoxin 1 and the classic antifungal agent griseofulvin 2. Here, we report the concurrent discovery of the two corresponding biosynthetic gene clusters (vrt and gsf) by 454 shotgun sequencing. Gene deletions confirmed two nonreducing PKSs (NRPKS), vrtA and gsfA, are required for the biosynthesis of 1 and 2, respectively. Both PKSs share similar domain architectures and lack a C-terminal thioesterase domain. We identified gsfI as the chlorinase involved in the biosynthesis of 2, as deletion of gsfI resulted in the accumulation of decholorogriseofulvin 3. Comparative analysis with the P. chrysogenum genome revealed that both clusters are embedded within conserved syntenic regions of P. aethiopicum chromosomes. Discovery of the vrt and gsf clusters provided the basis for genetic and biochemical studies of the pathways.
INTRODUCTION
Fungal polyketides are an important class of secondary metabolites, which include blockbuster drugs, mycotoxins and pigments. There has been a growing interest in the engineering and heterologous expression of fungal polyketide synthase (PKS) genes and pathways to enable rational production of novel compounds (Schümann and Hertweck, 2006). Understanding the molecular genetic basis of the biosynthesis of these structurally diverse compounds is the underlying key to achieve this goal. Over the past decade, there has been a steady growth of knowledge on the mechanisms of fungal polyketide biosynthetic pathways (Cox, 2007). Numerous fungal polyketides have been linked to their PKS genes and gene clusters (Hoffmeister and Keller, 2007), including some from the cryptic metabolic pathways uncovered via genome mining (Bergmann et al., 2007; Bok et al., 2006; Chiang et al., 2008). Nevertheless, the biosynthetic pathways of many previously known fungal metabolites, some of which have important pharmaceutical applications, still remain to be elucidated.
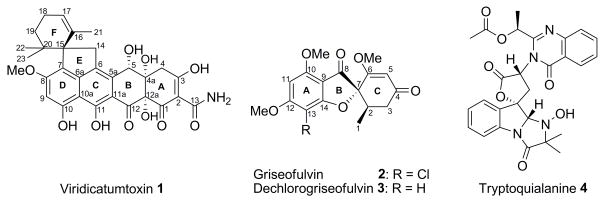
The filamentous fungus Penicillium aethiopicum Frisvad is known to produce a number of interesting secondary metabolites, including viridicatumtoxin (1), griseofulvin (2), and tryptoquialanine (4) (Figure 1), which are used as chemotaxonomic markers for the species (Frisvad and Samson, 2004). Tryptoquialanine is a tetrapeptide structurally similar to tryptoquivaline, which is a known tremorgen (Ariza et al., 2002; Clardy et al., 1975). Viridicatumtoxin is a hybrid polyketide-isoprenoid compound and is a rare example of tetracycline-like compounds produced by fungi. Compound 1 shares a common tetracyclic carboxamide core with the well-known tetracycline intermediate anhydrotetracycline. 1 has been reported to cause nephrotoxicity (Hutchison et al., 1973) as well as exhibiting modest antitumor activity (Raju et al., 2004). An epoxide derivative of the compound, viridicatumtoxin B, was isolated from Penicillium sp. FR11 along with 1; both were shown to inhibit the growth of methicilin- and quinolone-resistant Staphylococcus aureus at 8–64 times higher activity than tetracycline (Zheng et al., 2008). Another known fungal compound with a tetracyclic carboxamide core is anthrotainin (TAN-1652) (Ishimaru et al., 1993; Wong et al., 1993), which is an inhibitor of neuropeptide substance P binding and is a potential non-steroidal anti-inflammatory agent. The biosynthetic convergence of tetracycline-like compounds across the bacterial and fungal kingdoms, and their various bioactivities, further suggest that compounds of this structural type contain an “evolutionarily privileged scaffold”, which is capable of interacting with a variety of biological targets. The co-occurrence of tetracyclic carboxamide core in both the tetracyclines from bacteria and 1 from fungi provides an excellent example to study the convergence of the polyketide pathway. Previous isotope feeding study (de Jesus et al., 1982) showed that 1 is produced by a mechanism significantly different from the bacterial tetracyclines (Thomas, 2001). The formation and attachment of the spirobicyclic ring of isoprenoid origin on a tetracycline scaffold also warrants detailed biosynthetic investigation.
Figure 1.
Structures of the major secondary metabolites produced by P. aethiopicum. The numbering schemes for the carbon atoms for 1–3 are based on previous studies (de Jesus et al., 1982; Simpson and Holker, 1977).
Griseofulvin, which affects the function of mitotic spindle microtubules in mitosis, is an antifungal drug and has been in use for many years in medical and veterinary applications (Finkelstein et al., 1996). Although the use of 2 is mostly superseded by newer and more effective synthetic antifungal agents, it remains useful for the treatment of some dermatophytes such as Tinea capitis (ringworm of the scalp) and Tinea pedis (athlete’s foot). Recently, there is a renewed interest in 2 owing to its specific antiproliferative and antimitotic activities towards cancer cells (Panda et al., 2005; Rebacz et al., 2007). The recent studies suggest that 2 acts by inhibiting centrosomal clustering in tumor cells with supernumerary centrosomes, causing multipolar mitoses, and subsequently, apoptosis (Rebacz et al., 2007). Interestingly, 2 has also been shown to suppress hepatitis C virus replication in vitro (Jin et al., 2008). The discovery of the new potentials of 2 has led to new synthetic efforts to search for superior analogs (Rønnest et al., 2009). Structurally, 2 has an interesting cyclization pattern, where the polyketide backbone is folded to allow formation of orcinol and phloroglucinol ring structures through a Claisen and an aldol reactions, respectively. A stereospecific oxidative coupling reaction was proposed for the formation of the grisan structure of 2 (Barton and Cohen, 1957). The biosynthetic pathway of 2 has been extensively studied using isotopic incorporation (Harris et al., 1976; Lane et al., 1982; Rhodes et al., 1963; Simpson and Holker, 1977), but the genes and enzymes involved in the biosynthesis remain unknown.
The significant biological properties and unusual structural features of 1 and 2 have motivated us to perform a genome scanning of P. aethiopicum with DNA pyrosequencing technology and search for the two corresponding gene clusters. A bioinformatic search for PKS genes and comparative analysis with Penicillium chrysogenum genome revealed the putative biosynthetic gene clusters for both compounds, which were confirmed by targeted gene deletions and RNA-silencing. The two gene clusters provide an important step towards understanding the enzymatic basis of biosynthesis of 1 and 2.
RESULTS AND DISCUSSION
Genome-wide analysis of P. aethiopicum PKS genes revealed two putative gene clusters for viridicatumtoxin and griseofulvin biosynthesis
The 454 shotgun sequencing of P. aethiopicum IBT 5753 with the GS FLX Titanium series generated a total of ~572 million bases with an average sequencing read length of 367.5 bases. Assembly of the unpaired shotgun sequence reads resulted in 1522 contigs, which consist of 28,925,551 non-redundant bases, with an N50 of 149.2 kilobases. Based on the phylogenetic analysis of β-tubulin sequences, P. aethiopicum is closely related to P. chrysogenum, and both species are grouped under Penicillium subgenus Penicillium, section Chrysogena (Samson et al., 2004). Assuming that the size of P. aethiopicum genome is close to the sequenced P. chrysogenum genome (32.19 Mb), the total non-redundant bases were estimated to cover about 90% of the fungal genome.
Using a local BLASTP program queried against a database consists of all the contigs with an arbitrary KS domain sequences, a total of 30 putative, complete PKS genes were found in the P. aethiopicum genome. Out of them, there are a total of 7 non-reducing PKSs (NRPKSs), 17 highly-reducing PKSs (HRPKS), 3 partially-reducing PKSs (PRPKSs) and 3 HRPKS-nonribosomal peptide synthetases (NRPSs) hybrids. For simplicity, the PKSs are designated as PaPKS[contig number] (Table S1).
Compared to P. chrysogenum, which has a total of 21 putative PKS genes, the P. aethiopicum genome contains more putative PKS genes. Based on a chemotaxonomic study, the two closely related species are known to produce distinct sets of secondary metabolites (Frisvad and Samson, 2004). Since P. chrysogenum is not known to produce 1 and 2, we reasoned that the orthologous PKSs are not involved in the biosynthesis of the two compounds. Sequence alignments and phylogenetic analysis implied that out of the 30 putative PKSs genes in P. aethiopicum, 9 are orthologous to P. chrysogenum (Table S1 and Figure S1).
We reasoned that NRPKSs are likely involved in the biosynthesis of the carbon skeletons of 1 and 2, since these compounds are derived from aromatic polyketide precursors. Among the P. aethiopicum NRPKSs that are non-orthologous to those found in P. chrysogenum, PaPKS0274 and PaPKS0880 fall into the same clade as the recently identified Aspergillus nidulans asperthecin synthase (AptA) (Szewczyk et al., 2008) and Aspergillus terreus atrochrysone carboxylic acid synthase (ACAS) (Awakawa et al., 2009), both of which produce fused-ring aromatic compounds (Figure S1). Both PaPKS0274 and PaPKS0880 consist of starter unit:ACP transacylase (SAT) (Crawford et al., 2006), ketosynthase (KS), malonyl-CoA:ACP transacylase (MAT), product template (PT) (Crawford et al., 2009) and acyl carrier protein (ACP) domains, but lack a thioesterase/Claisen cyclase (TE/CLC) domain (Fujii et al., 2001) at the C-terminal for chain release. Since the ACAS product, atrochrysone carboxylic acid, has a similar structure to the BCD rings of 1, we predicted that one of these two PKSs may be involved in the biosynthesis of 1.
Given that genes involved in fungal secondary metabolic pathways are often clustered together (Hoffmeister and Keller, 2007), we searched the genes surrounding the two putative PKS genes for clues about the final polyketide products. Preliminary bioinformatic analysis of contig 00274 identified a pair of genes related to isoprenoid pathway downstream of PaPKS0274 gene. The presence of both genes may be associated with the isoprenoid portion of the spirobicyclic ring in 1. Other genes encoding putative oxygenases, aminotransferase and O-methyltransferase were also found in the vicinity of the PaPKS0274 gene, which respectively match the structural features present in 1. On contig 00880, the presence of three putative O-methyltransferase genes and a halogenase gene flanking the PaPKS0880 gene immediately points to a potential correlation to the structure of 2.
VrtA and GsfA are two PKSs essential for the biosynthesis of viridicatumtoxin and griseofulvin respectively
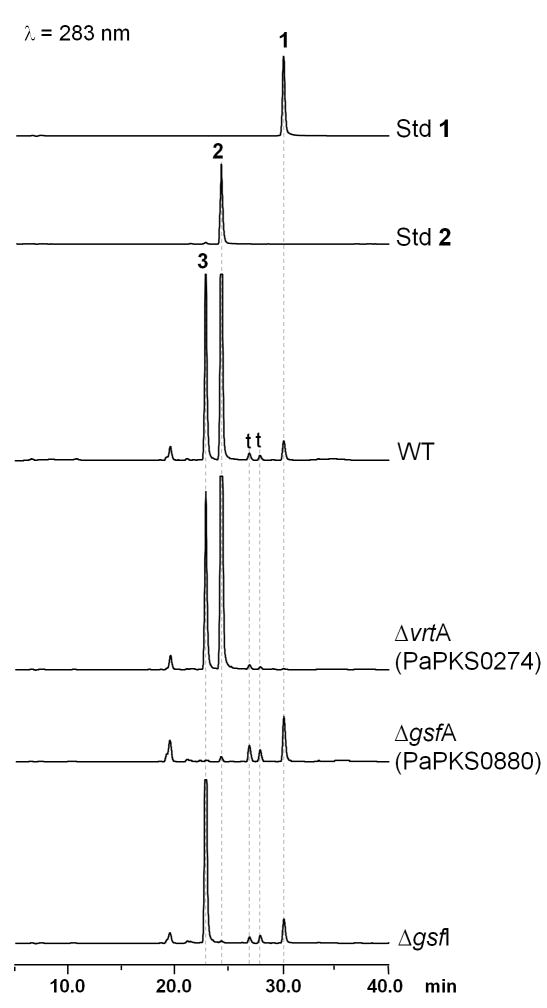
Liquid chromatography-mass spectrometry (LC-MS) analysis of the ethyl acetate extract from P. aethiopicum wild type (WT) stationary culture grown in yeast malt extract glucose (YMEG) medium showed six distinct peaks at 283 nm. Two of the peaks have the retention time, UV spectra and m/z value matching the authentic standards of 1 (RT = 30 min, m/z 548 [M+H-H2O]+) and 2 (RT = 24 min, m/z 353 [M+H]+) (Figure 2). Another peak at RT = 22 min showed a UV spectrum that is similar to 2 and m/z 319 [M+H]+, which matches with the molecular weight of dechlorogriseofulvin 3 (Figure 1). The compound was purified from a large culture of P. aethiopicum and the structure was confirmed to be 3 by comparing the 1H NMR and 13C NMR spectra to the previously published spectra for 2 (Simpson and Holker, 1977) and epidechlorogriseofulvin (Jarvis et al., 1996) (Table S3). Two additional peaks (RT = 27 and 28 min) with UV spectra similar to previously reported for tryptoquivalines and tryptoquialanines were also detected (Ariza et al., 2002; Clardy et al., 1975). Both peaks have a mass of [M+H]+ m/z = 519, which matches with the molecular weight of 4. The additional peak could be an isomer of 4, analogous to tryptoquivaline and isotryptoquivaline (Yamazaki et al., 1976). The identity of the remaining peak at RT = 19.5 min is undetermined.
Figure 2.
HPLC analysis (283 nm) of metabolites produced by wild type and mutant P. aethiopicum strains. The mutant ΔvrtA, ΔgsfA or ΔgsfI no longer produced 1, 2 or 3, respectively. Standards of compounds 1 and 2 are shown. The identity of 3 was established by NMR characterization. Peaks labeled with t have UV spectra and m/z values matching to 4 and can be isomers.
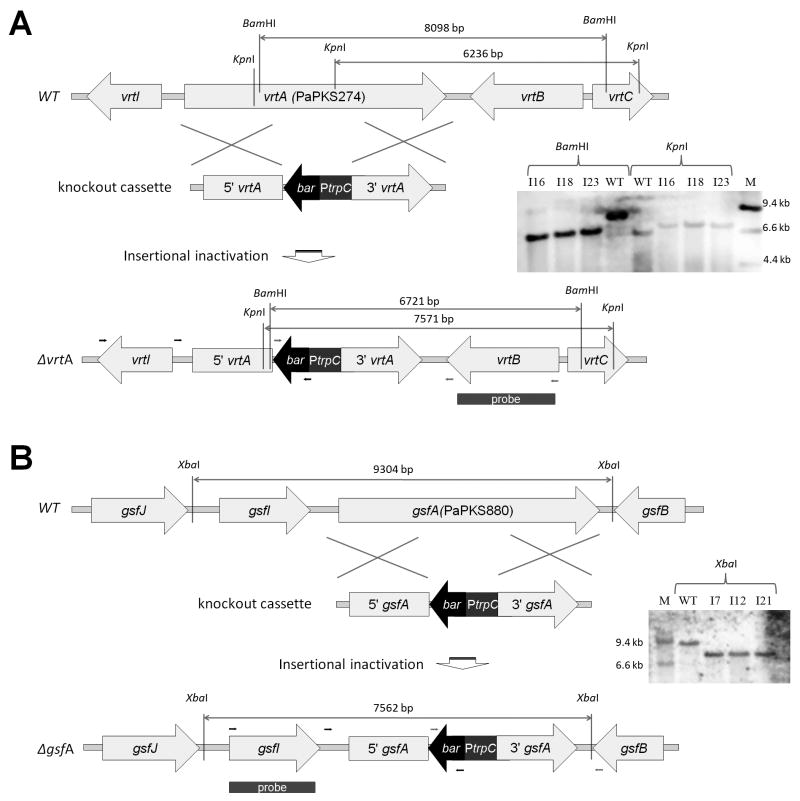
To verify the proposed associations between the putative gene clusters and the metabolites 1 and 2, we developed a transformation system for P. aethiopicum based on existing methods for A. nidulans using bar resistant marker (Chooi et al., 2008; Nayak et al., 2006). Using double-homologous deletion cassettes with a bar resistant marker, the PaPKS0274 and PaPKS0880 genes were deleted by double crossover recombination (Figure 3). Approximately 100 glufosinate-resistant transformants were picked and screened with PCR using a bar gene primer and primers outside of the deletion cassette (Table S2). A total of 6 and 5 potential recombinants were identified for PaPKS0274 and PaPKS880 respectively. Three PCR positive clones from each knockout experiments were chosen and confirmed by Southern hybridization (Figure 3). All the PCR positive clones (ΔPKS0274-I16, ΔPKS0274-I18, ΔPKS0274-I23, ΔPKS880-I7, ΔPKS880-I12 and ΔPKS880-I9) showed the expected band size for correct insertion of deletion cassette by double crossover recombination into the corresponding genomic copy of the two PKS genes. Analysis of the PaPKS0274 disruptants showed that the production of 1 was totally abolished (Figure 2), while the other metabolites were unaffected. For the PaPKS0880 disruptants, only the production of 2 and 3 were abolished (Figure 2). These results confirmed that PaPKS0274 is essential for the biosynthesis of 1, while PaPKS0880 is required for the biosynthesis of 2 and 3.
Figure 3.
Homologous recombination schemes for deletion of vrtA and gsfA genes in P. aethiopicum. The knockout cassettes for (A) vrtA (PaPKS0274) (B) gsfA (PaPKS880) are shown with marked restriction sites. The corresponding Southern blot results for the wild type and the mutants are displayed on the right. Small arrows indicate approximate binding sites of the primers used in PCR screening.
The two PKS gene clusters for 1 and 2 were designated as vrt and gsf respectively, and the two NRPKS genes were designated as vrtA and gsfA, respectively (Table 2 and 3). Interestingly, both PKSs have the same domain architecture and lack a fused TE/CLC domain. The functional assignment of VrtA and GsfA, which produce polyketides with different chain lengths and backbone folding patterns in 1 and 2, suggested that this family of “TE-less” PKSs (assigned as clade IV NR-PKSs here, Figure S1) may account for a greater structural diversity among fungal polyketides.
Table 2.
Putative genes within and flanking the gsf cluster.
| Gene | Size (bp/aa) | BLASTP homolog | Identity/Similarity (%) | Conserved Domain | E value |
|---|---|---|---|---|---|
| gsfA | 5672/1790 | B. fuckeliana, PKS14 | 57/71 | SAT-KS-MAT-PT-ACP | |
| A. terreus, ATEG_08451 (ACAS) | 42/60 | ||||
| gsfB | 1442/419 | P. marneffei, PMAA_079120 | 41/61 | pfam00891, O-methyltransferase | 7e-33 |
| G. fujikuroi, Bik3 bikaverin O-methyltransferase3 | 29/45 | COG2226, UbiE, Methylase involved in ubiquinone/menaquinone biosynthesis | 9e-07 | ||
| gsfC | 1191/396 | N. haematococca, NECHADRAFT_81295 | 32/51 | pfam00891, O-methyltransferase | 2e-20 |
| A. flavus, omtB aflatoxin O-methyltransferase4 | 24/44 | ||||
| gsfD | 378/1301 | P. chrysogenum, Pc12g06140 | 26/41 | pfam00891, O-methyltransferase | 1e-21 |
| A. flavus, omtB aflatoxin O-methyltransferase4 | 27/45 | ||||
| gsfE | 1134/377 | A. nidulans, AN9028.2 | 61/74 | COG0451, WcaG, Nucleoside-diphosphate-sugar epimerases/dehydratases | 1e-09 |
| gsfF | 1603/462 | N. haematococca, NECHADRAFT_3047 | 37/53 | pfam00067, p450, Cytochrome P450 | 2e-37 |
| gsfG | 1033/299 | C. globosum, CHGG_02201 | 24/37 | cd00204, ANK, ankyrin repeats; ankyrin repeats mediate protein-protein interactions | 7e-37 |
| gsfH | 889/237 | A. oryzae, AO090001000095 | 81/89 | cd01012, YcaC_related, YcaC related amidohydrolases | 1e-31 |
| gsfI | 1976/533 | C. chiversii, RadH flavin-dependent halogenase | 61/75 | pfam04820, tryptophan halogenase | 3e-15 |
| COG0644, FixC, dehydrogenases (flavoproteins) | 3e-17 | ||||
| gsfJ | 2031/554 | A. nidulans, AN8459.2 | 47/61 | TIGR00711, efflux_EmrB, drug resistance transporter, EmrB/QacA subfamily | 1e-24 |
| gsfK | 1028/251 | P. chrysogenum, Pc12g16460 | 40/59 | PRK06953, short chain dehydrogenase | 4e-24 |
| gsfR1 | 2118/688 | A. nidulans, AN8460.2 | 52/68 | pfam04082, fungal specific transcription factor domain | 2e-05 |
| gsfR2 | 1248/415 | Sclerotinia sclerotiorum, SS1G_05579 | 53/67 | smart00066, GAL4-like Zn(II)2Cys6 binuclear cluster DNA-binding domain | 8e-09 |
| orf1′ | 1246/351 | P. chrysogenum, Pc06g01050 | 86/90 | No conserved domain detected | |
| orf2′ | 1310/366 | P. chrysogenum, Pc06g01040 | 96/99 | cd03445, Thioesterase II repeat2 | 7e-27 |
| cd03444, Thioesterase II repeat1 | 2e-20 | ||||
| orf3′ | 765/211 | P. chrysogenum, Pc06g01030 | 80/86‡ | No conserved domain detected | |
| orf4′ | 2187/653 | P. chrysogenum, Pc06g01020 | 92/96 | PRK08310, amidase | 4e-30 |
| orf5′ | 2352/783 | P. chrysogenum, Pc06g01000 | 94/96 | pfam04082, fungal specific transcription factor domain | 2e-16 |
| orf6′ | 807/157 | P. chrysogenum, Pc06g00990 | 90/94 | No conserved domain detected | |
| orf7′ | 1346/411 | P. chrysogenum, Pc06g00980 | 94/96 | pfam07942, N2227-like protein | 1e-85 |
| orf8′ | 2268/702 | P. chrysogenum, Pc06g00970 | 98/98 | COG0443, DnaK, Molecular chaperone | 2e-17 |
Genus abbreviation: A. -Aspergillus, B. -Botryiotinia, C. - Chaetomium, G. - Giberrella, N. - Nectria, P. - Penicillium.
based on Pc06g01030 conceptual translation as predicted by FGENESH, different from the version in GenBank. Sequence of the gsf gene cluster was submitted to GenBank under the accession GU574478.
We also tested whether the production of 1 and 2 in P. aethiopicum can be attenuated or abolished by using RNA silencing. This strategy has been previously employed in the identification of the chaetoglobosin gene cluster from P. expansum (Schumann and Hertweck, 2007), and in the study of tailoring oxidations during tenellin biosynthesis in Beauveria bassiana (Halo et al., 2008). Gene fragments of PaPKS0274 (3.8 kB) and PaPKS0880 (2.5kB) were separately cloned into the pBARGPE1 plasmid (Pall and Brunelli, 1993) in the antisense orientation under the control of a gpdA promoter. The resultant plasmids, pBAR274si and pBAR880si, were randomly integrated into the P. aethiopicum genome. We recovered 9 clones for PaPKS0274 and 6 clones for PaPKS0880 that contain the full length gpdA promoter with contiguous PKS gene fragments. For the vrtA silencing, 3 out of the 9 PCR positive clones screened did not produce 1, while the other 6 clones showed various degree of reduced viridicatumtoxin production compared to wild type (Figure S2). In the case for gsfA, all 6 clones continued to produce 2 and 3, but at various lower titers compared to wild type (Figure S3). The level of silencing of the two PKS genes is likely linked to the copy number of the silencing constructs integrated in the transformants, the expression level of the antisense gene driven by the gpdA promoter and the endogenous transcript levels of the two corresponding PKS genes. The inability to silence gsfA completely may due to the high level of endogenous gene expression as reflected in the relatively large amount of 2 produced in the culture.
Comparative genomics analysis revealed the vrt and gsf cluster embedded within conserved syntenic regions of P. aethiopicum genome
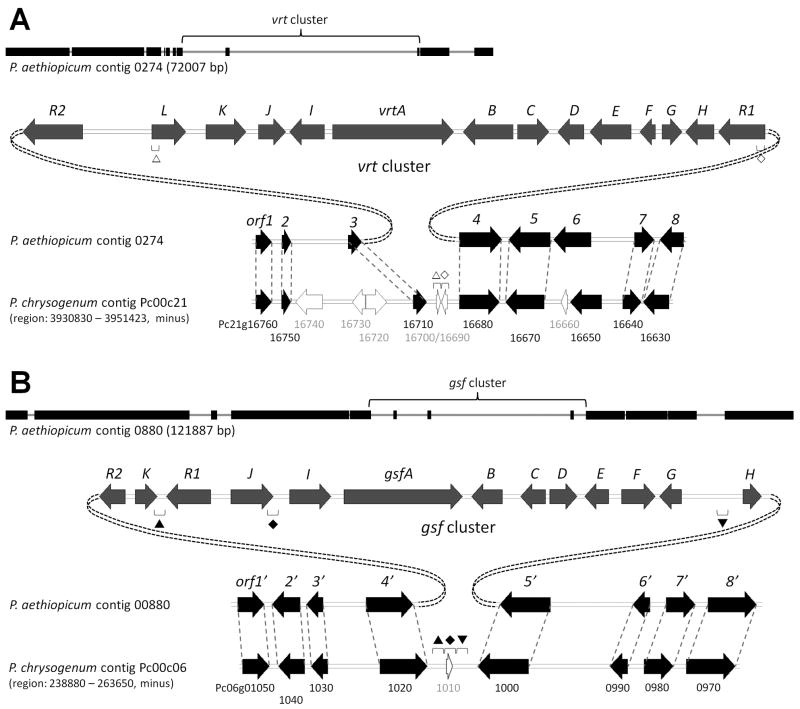
A more detailed bioinformatic analysis of the two biosynthetic loci revealed adjacent genes that are highly similar and syntenic to those found in P. chrysogenum genome (80–99% identity). Except the regions surrounding vrtA and gsfA, the conserved syntenic blocks span the whole contig 0274 and 0880, and they correspond to a specific locus in P. chrysogenum contig Pc00c21 and Pc00c06, respectively (Figure 4). Under the assumption that secondary metabolic genes could be unique to individual species while housekeeping and primary metabolic genes are usually conserved among closely related organisms, we used those syntenic sets of orthologous genes to tentatively assign the boundaries of the two putative clusters (Figure 4).
Figure 4.
Putative vrt and gsf clusters embedded within conserved syntenic regions of P. aethiopicum genome. (A) vrt cluster on contig 0274 and the corresponding locus on P. chrysogenum contig Pc00c21. (B) gsf cluster on contig 0880 and the corresponding locus on P. chrysogenum contig Pc00c06. Blocks on the thin lines indicate conserved syntenic blocks on the contigs. The symbols (△ ◇ ▲ ◆ ▼) indicate short conserved regions within the vrt and gsf clusters that are remained at the corresponding loci in P. chrysogenum. Block arrows: black, genes predicted not to be involved in the biosynthesis of 1 and 2; white, putative pseudogenes.
In the case of the vrt gene cluster, 14 putative genes (designated as vrtA-L, vrtR1 and vrtR2) were found between orf3 and orf4, which share 93% and 92% identity to Pc21g16710 and Pc21g16780, respectively (Figure 4A and Table 1). Most of the genes upstream of orf3 and downstream of orf4 also share syntenies and high similarities with genes in the corresponding locus on the P. chrysogenum genome, except that several putative P. chrysogenum pseudogenes in the locus appear to have been replaced with the vrt cluster. The gsf cluster consists of 13 putative genes (designated as gsfA-K, gsfR1 and gsfR2) and was found inserted between orf4′ and orf5′, which are highly similar to Pc06g01020 and Pc06g01000 (92% and 94% identity respectively). The gsf cluster replaces Pc06g01010 in the P. chrysogenum genome (Figure 4B and Table 2). The genes immediately flanking the gsf cluster also remained syntenic with the corresponding locus in the P. chrysogenum genome. These observations of vrt and gsf clusters embedded within conserved syntenic regions led to speculation that P. aethiopicum may have acquired the two gene clusters via horizontal gene transfer (Walton, 2000). However, careful examinations of the Pc00c21 and Pc00c06 contigs revealed the presence of small conserved regions (some annotated as pseudogenes), which appeared to be fragments of vrt and gsf gene clusters, in the corresponding loci in P. chrysogenum (Figure 4). This suggests that P. chrysogenum may have possessed the vrt and gsf gene clusters in the past but have lost them during evolution.
Table 1.
Putative genes within and flanking the vrt cluster.
| Gene | Size (bp/aa) | BLASTP homolog | Identity/Similarity (%) | Conserved Domain | E value |
|---|---|---|---|---|---|
| vrtA | 5578/1824 | A. nidulans, AN6000.3 (AptA) | 60/75 | SAT-KS-MAT-PT-ACP | |
| vrtB | 2312/723 | Pyrenophora tritici-repentis, PTRG_06131 | 51/64 | PRK03584, acetoacetyl-CoA synthetase | < 1.0e-180 |
| Rattus norvegicus, AACS_RAT | 40/56 | ||||
| vrtC | 1452/483 | Microsporum canis, MCYG_03599 | 45/59 | TIGR03429, aromatic prenyltransferase | 2e-73 |
| A. fumigatus, FgaPT2 [3I4X] | 20/36 | ||||
| vrtD | 1206/349 | Neurospora crassa, FACPS_NEUCR [Q92250] | 54/68 | cd00685, trans-isoprenyl diphosphate synthases | 7e-46 |
| vrtE | 1898/520 | A. terreus, ATEG_04107 | 33/52 | pfam00067, cytochrome P450 | 5e-24 |
| vrtF | 717/238 | A. niger, An11g07340 | 55/65 | pfam08242, methyltransferase domain | 1e-04 |
| Polyangium cellulosum, JerF | 35/49 | type 12 | |||
| vrtG | 924/307 | A. nidulans, AN6001.3 (AptB) | 60/74 | smart00849, metallo-β-lactamase superfamily | 2e-24 |
| vrtH | 1305/413 | A. nidulans, AN6002.3 (AptC) | 50/69 | COG0654, UbiH and related FAD-dependent oxidoreductases | 4e-11 |
| vrtI | 1597/413 | A. clavatus, ACLA_098360 | 39/50 | COG3491, PcbC, IPNS and related | 2e27 |
| Nicotiana tabacum, Ntc12 | 18/35 | dioxygenases | |||
| gibberillin 20-oxidase | pfam03171, 2OG-Fe(II) oxygenase | 2e-11 | |||
| vrtJ | 1249/388 | Sclerotinia sclerotiorum, SS1G_01438 | 47/61 | cd06502, low-specificity threonine aldolase (TA), PLP-dependent aspartate aminotransferase superfamily (fold I) | 1e-77 |
| Candida albicans, Gly1 [GLY1_CANAL]6 | 37/53 | pfam01212, Beta-eliminating lyase | 4e-70 | ||
| vrtK | 1847/534 | A. flavus, verB desaturase | 37/54 | pfam00067, p450, Cytochrome P450 | 3e-41 |
| A. nidulans, stcL desaturase | 31/47 | ||||
| vrtL | 1570/503 | A. niger, An13g00720 | 80/89 | cd06174, MFS, The Major Facilitator Superfamily transporters | 1e-31 |
| vrtR1 | 2148/715 | Neosartorya fischeri, NFIA_025860 | 70/81 | pfam04082, fungal specific transcription factor domain | 2e-09 |
| vrtR2 | 2757/836 | A. niger, An11g07350 | 34/48 | smart00066, GAL4-like Zn(II)2Cys6 binuclear cluster DNA-binding domain | 2e-05 |
| orf1 | 789/262 | P. chrysogenum, Pc21g16760 | 91/95 | COG0400, Predicted esterase | 8e-12 |
| orf2 | 444/147 | P. chrysogenum, Pc21g16750 | 99/100 | No conserved domain detected | |
| orf3 | 675/224 | P. chrysogenum, Pc21g16710 | 93/95 | COG4297, uncharacterized protein with double-stranded beta helix domain | 3e-14 |
| orf4 | 1974/657 | P. chrysogenum, Pc21g16680 | 92/94 | No putative conserved domains detected | |
| orf5 | 1499/462 | P. chrysogenum, Pc21g16670 | 85/92 | cd05120, aminoglycoside 3′-phosphotransferase (APH) and choline kinase (ChoK) family. | 1e-04 |
| orf6 | 1722/573 | Uncinocarpus reesii, UREG_05338 | 26/40 | cd00204, ANK, ankyrin repeats; mediate protein-protein interactions | 0.004 |
| orf7 | 888/263 | P. chrysogenum, Pc21g16640 | 97/99 | pfam09177, Syntaxin 6, N-terminal | 2e-21 |
| pfam05739, SNARE domain | 2e-08 | ||||
| orf8 | 1112/319 | P. chrysogenum, Pc21g16630 | 95/98 | pfam00956, NAP, Nucleosome assembly protein | 1e-09 |
Genus abbreviation: A. -Aspergillus, P. - Penicillium.
Sequence of the vrt gene cluster was submitted to GenBank under the accession GU574477.
Both clusters have two transcription factors (vrtR1 and vrtR2, gsfR1 and gsfR2) that contain Zn(II)2Cys6 DNA-binding domains, which are similar to pathway specific regulators such as aflR and ctnA for aflatoxin and citrinin biosynthesis respectively (Ehrlich et al., 1999; Shimizu et al., 2007). It is unclear if both or only one of the transcription factors is involved in the regulation of the corresponding cluster.
The putative biosynthetic pathways for the biosynthesis of viridicatumtoxin
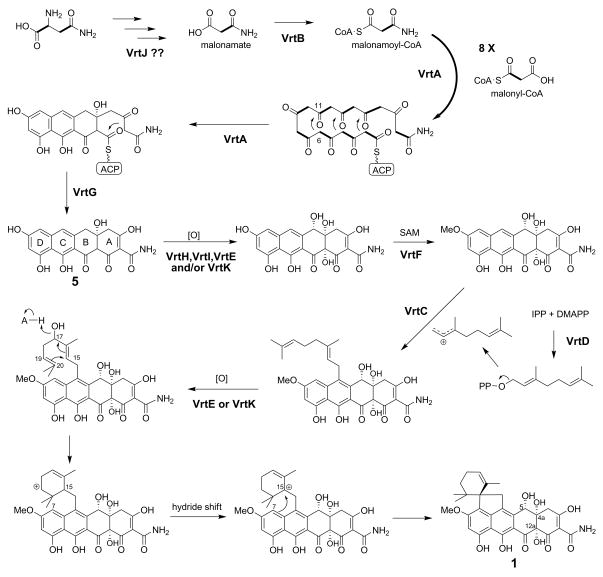
Previous isotope incorporation study showed that the biosynthesis of the tetracyclic carboxamide core of 1 is significantly different from tetracyclines in bacteria (de Jesus et al., 1982), including: 1) differences in the cyclization regioselectivity of the carbon backbone (Thomas, 2001); 2) the non-acetate origin of C3; and 3) the retention of the oxygen at C4a of 1 from [1-13C18O]-acetate. These differences exclude the participation of a fully aromatic tetracene intermediate, such as the pretetramid intermediate in oxytetracycline biosynthesis (Thomas and Williams, 1983; Zhang et al., 2007). Based on the acetate labeling pattern and the new gene cluster data, a putative pathway for biosynthesis of 1 can be envisioned (Figure 5). The PT domain of a NRPKS has recently been shown to mediate aromatic polyketide cyclization (Crawford et al., 2009). Since the PT domain of VrtA shares high similarity to those from AptA and ACAS (61% and 50% identity), which were shown to produce polyketide products with C6–C11 first-ring cyclization regioselectivity, we reasoned that the cyclization of the polyketide backbone of 1 by VrtA may also proceed via a similar route. We hypothesized that VrtA may utilize a malonamoyl-CoA starter unit, which is generated by VrtB, followed by sequential condensation of eight malonyl-CoA units to form the polyketide backbone. The cyclizations of the BCD rings were assumed to follow the pattern observed in atrochrysone biosynthesis (Awakawa et al., 2009), in which the oxygen at C4a is retained from an acetate unit (Figure 5). Cyclization of the last ring (ring A) along with offloading of the intermediate 5 could be mediated by VrtG, which has protein sequence similarity to the recently discovered β-lactamase-type TE (AptB and ACTE, 60% and 49% protein identity) (Awakawa et al., 2009; Szewczyk et al., 2008).
Figure 5.
Proposed pathway for the biosynthesis of viridicatumtoxin 1. DMAPP – dimethylallyl pyrophosphate; IPP – isopentenyl pyrophosphate.
The hypothesis of a malonamoyl-CoA starter unit produced by VrtB is supported by the high sequence similarity of VrtB to acetoacetyl-CoA synthetases (AACSs) (44% protein identity with the rat homolog, AACS_RAT), and the structural similarity of the AACS substrate, acetoacetate, to malonamate. AACS activates acetoacetate as an acyl-AMP intermediate followed by ligation to the free thiol of CoA to form acetoacetyl-CoA (Fukui et al., 1982). Similarly, the formation of malonamoyl-CoA from malonamate could be catalyzed by VrtB. Furthermore, P. aethiopicum has an additional copy of putative AACS gene (PaAACS1086) on contig1086, which is 96% identical to the only AACS homolog (Pc13g10810) in P. chrysogenum, while VrtB shares only 50% protein identity with the putative PcAACS (Pc13g10810). This implies that VrtB is most likely dedicated to the vrt pathway, while PaAACS1086 is involved in the primary metabolism.
VrtJ has a pyridoxal 5′-phosphate (PLP) binding site and a conserved domain similar to those found in threonine aldolase (TA), aspartate aminotransferase and β-eliminating lyase (Table 1). The closest characterized homolog of VrtJ is Gly1 from Candida albicans (39% protein identity), which is a TA that catalyzes degradation of L-threonine to glycine and acetaldehyde (McNeil et al., 2000). The presence of an additional copy of TA-like gene (PaTA0310) in P. aethiopicum, which shares higher identity (92%) to the only homolog in P. chrysogenum (Pc12g01020) than VrtJ (54%), again implies that PaTA0310 is most likely to be the primary TA involved in glycine metabolism. VrtJ is therefore likely to be dedicated to the vrt pathway and may be involved in the synthesis of the malonamate substrate for VrtB. A malonamoyl-CoA starter unit is also likely involved in oxytetracyline biosynthesis, however, the malonate-derived carboxamide of oxytetracyline is in contrast to the acetate-derived carboxamide of 1 (Thomas and Williams, 1983). One possibility could be a malonamoyl-CoA starter derived from asparagine, presumably synthesized by VrtJ and VrtB collaboratively (Figure 5). Such a pathway, however, does not match the previous acetate labeling study as incorporation of an asparagine-derived malonamoyl-CoA would result in the C3 of 1 being labeled by the C2 of [1,2-13C]-acetate. This is due to asparagine is originating from oxaloacetate, and the formation of oxaloacetate from [1,2-13C2]-acetate proceeds via a symmetrical succinate intermediate in the tricarboxylic acid cycle (Ogasawara and Liu, 2009). Further gene targeting along with biochemical characterization of the four gene products, VrtA/B/G/J, are likely to shed light to the enzymatic reactions involved in the biosynthesis of the tetracyclic carboxamide core.
The proposed post-PKS tailoring steps following the formation of intermediate 5 are two hydroxylations and an O-methylation (Figure 5). The hydroxylations at C5 and C12a are predicted to be catalyzed by one or two of the oxygenases (VrtH, VrtI, VrtE, and VrtK), while the methoxy group is likely to be formed by the O-methyltransferase (VrtF). The pathway that leads to formation of the spirobicyclic ring of 1 is proposed to involve at least three gene products in the cluster (Figure 5). VrtD, which is similar to trans-isoprenyl diphosphate synthases (Table 1) such as the Neurospora crassa farnesyl pyrophosphate synthase (Homann et al., 1996), is predicted to catalyze the formation of geranylpyrophosphate. Based on the shared homology of VrtC with aromatic prenyltransferases, such as A. fumigatus dimethylallyltryptophan synthase FgaPT2 (Metzger et al., 2009) (Table 1), the protein is predicted to catalyze the attachment of the geranyl moiety to the C ring of 1 to yield 6 (Figure 5). Most aromatic prenyltransferases identified in fungi transfer the prenyl group to a tryptophan or indole moiety (Heide, 2009). Intriguingly, VrtC is proposed to catalyze the transfer of a geranyl group to the aromatic C ring of the tetracyclic polyketide intermediate of 1. Prenylation at the same C6 position is also observed in a similar fungal tetracyclic compound, hypomycetin, which has an acetyl group in place of the carboxamide in 1 (Breinholt et al., 1997). Thus, VrtC and the corresponding prenyltransferase in hypomycetin pathway can be considered as potential enzymes that can modify tetracycline compounds at C6.
We propose that the cyclization of the geranyl moiety of 6 can be initiated by a cytochrome P450 enzyme-catalyzed hydroxylation (VrtE or VrtK) at the allylic C17, which upon protonation of the hydroxyl can lead to a C17 carbocation (Figure 5). Attack of C20 on the bridging C15 forms the C15–C20 bond and transfers the positive charge to C19. A hydride shift from C15 to C19 is followed by C7 attack on C15 to form the spirobicyclic ring (Figure 5). The 1,3-hydride shift was previously proposed to rationalize the incorporation pattern of 2H-labeled mevalonate (Horak et al., 1988). The cyclization of the geranyl side chain to the spirobicyclic ring in 1 is most likely enzyme-mediated; however, no terpene cyclase gene is found in the vicinity of the vrt cluster. Thus, formation of the spirobicyclic ring in 1 could be mediated by the prenyltransferase (VrtC) as similarly proposed in paspaline biosynthesis (Saikia et al., 2006) or facilitated by the P450 enzyme.
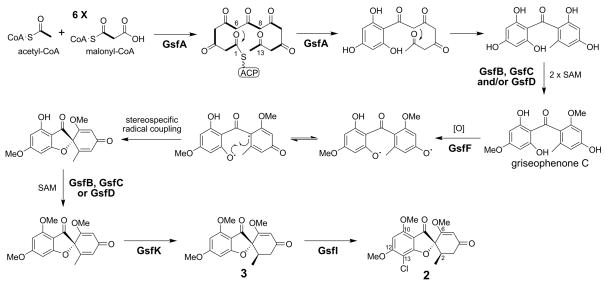
The putative biosynthetic pathway for the biosynthesis of griseofulvin
The biosynthesis of 2, as implied by the previously established pathway (Harris et al., 1976), required at least seven biosynthetic enzymes (Figure 6). Formation of the heptaketide backbone by GsfA is initiated by priming with acetyl-CoA, followed by sequential condensations of six malonyl-CoA units (Simpson and Holker, 1977). Since neither a fused TE/CLC nor a stand-alone TE is present, we proposed that the C1–C6 Claisen cyclization of the polyketide backbone could be mediated by the PT domain of GsfA, while the C8–C13 aldol cyclization could occur spontaneously to afford the benzophenone intermediate of 2 (Figure 6). Alternatively, the PT domain may mediate the cyclization of both aromatic rings. As PT domains of fungal PKSs are so far known to only catalyze aldol cyclizations (Crawford et al., 2009), the mechanism of folding and cyclization of the polyketide backbone by GsfA requires further investigation.
Figure 6.
Proposed biosynthesis of griseofulvin 2. The function of the halogenase GsfI has been confirmed using knockout as shown in Figure 2.
Isotope feeding experiment by Harris et al. (1976) established that two of the O-methylation steps at C6 and C12 hydroxyl groups occur immediately after the formation of the benzophenone intermediate to yield griseophenone C, while the methylation of the hydroxyl group at C10 occurs at a later stage (Figure 6). Three O-methyltransferases (GsfB/C/D) are encoded in the gene cluster and can therefore catalyze the required methylation steps.
An interesting feature in the biosynthesis of 2 is the formation of the heterocyclic ring by a stereospecific phenol oxidative coupling, which leads to the unique spiro structure of 2. Geodin is another grisan compound similar to 2, which also undergoes a similar stereospecific oxidative coupling. It has been established that the A. terreus dihydrogeodin oxidase (DHGO), which converts the benzophenone dihydrogeodin to (+)-geodin, is a multicopper blue protein similar to laccases (Fujii et al., 1987; Huang et al., 1995). The structural similarity of 2 to geodin suggests that a similar enzyme may be responsible for the oxidative coupling reaction, but no such enzyme is encoded in the gsf cluster. A P450 oxygenase (GsfF) was found in the gsf cluster, although no oxidation is required en route to 2. We proposed that GsfF may catalyze the stereospecific oxidative coupling reaction of griseophenone C to form the grisan core (Figure 6). Oxidative coupling reactions catalyzed by cytochrome P450 enzymes are not unprecedented. A well-known example is OxyB involved in the phenol coupling reaction during the biosynthesis of vancomycin (Zerbe et al., 2004). GsfK, a putative NAD(P)-dependent oxidoreductase, is likely to catalyze the stereospecific reduction step at the C-ring resulting in the R conformation at the C2, forming the second chiral center in 2 and 3 (Figure 6).
GsfI is a halogenase that shares high similarity (60% identity) to the recently discovered flavin-dependent chlorinase (RadH) in the biosynthesis of radicicol (Wang et al., 2008). In this current study, 3 is a major product in the P. aethiopicum culture when grown on stationary YMEG liquid culture, albeit often produced at a slightly lower ratio to 2. To confirm the function of GsfI, the gene was disrupted in the same manner as the two PKSs. Two positive gsfI disruptants, ΔgsfI-IV5 and ΔgsfI-IV11, were found after screening approximately 100 glufosinate-resistant transformants. LC-MS analysis showed that disruption of gsfI completely abolished the production of 2, while production of 3 remained (Figure 2). This confirmed the role of GsfI as a halogenase responsible for the regiospecific chlorination of 3 at the C13 position to form 2. The result also suggests that chlorination does not affect the formation of the grisan ring and could therefore be one of the last steps in the 2 biosynthetic pathway in P. aethiopicum (Figure 6).
In conclusion, we have successfully associated two polyketide products to their corresponding gene clusters by bioinformatics and comparative analysis of the P. aethiopicum genome, and subsequently confirmed the functions of three of the biosynthetic genes by targeted gene replacement. As opposed to the previously reported low gene targeting efficiency in other Penicillium spp. (Casqueiro et al., 1999; Schumann and Hertweck, 2007), our results showed that gene targeting by double homologous recombination in P. aethiopicum is relatively feasible. The P. aethiopicum transformation system developed in this study will facilitate future mechanistic studies of the two pathways, as well as the numerous other PKSs found in the genome of this fungus.
SIGNIFICANCE
The present study unveiled the biosynthetic gene clusters of two interesting polyketide spiro compounds, 1 and 2, 1 is a rare example of fungal compound that is structurally similar to the tetracycline antibiotics from bacteria, while 2 is an important antifungal drug that has been in use for a long time for treating dermatophyte infections. Localization of the two gene clusters within conserved syntenic regions of the P. aethiopicum genome raises interesting questions regarding the evolution of clustering of secondary metabolite genes in fungi and may provide insights to the underlying genetic basis of Penicillium chemotaxanomy.
The vrt cluster is an interesting example of a biosynthetic pathway that encodes for a polyketide-isoprenoid hybrid compound in fungi. Understanding the exact mechanism of which the anhydrotetracycline-like core of 1 is formed by further targeted gene deletion and biochemical characterization of the gene products may provide new chemical insights. The unusual folding and cyclization of the isoprenoid moiety to form the spirobicylic ring also deserves further investigation. More importantly, unveiling the clustered genes for biosynthesis of 1 has opened up the possibilities to generate novel tetracycline analogs using combinatorial biosynthetic strategies by integrating bacterial and fungal genes that can act on the tetracycline scaffolds.
There has been a renewed interest in 2 owing to its newly discovered anti-cancer and anti-viral properties. It is significant that this is the first report of the genetic basis of biosynthesis of 2. Further functional characterization of the gene cluster will advance our understanding of biosynthesis of 2 and could lead to isolation of intermediates that may have important implications in producing useful structural analogs either by enzymatic or chemical modifications.
EXPERIMENTAL PROCEDURES
Strains and culture conditions
P. aethiopicum, IBT 5753, was obtained from the IBT culture collection (Kgs. Lyngby, Denmark) and maintained on YMEG agar (4 g/l yeast extract, 10g/l malt extract, 16 g/l agar) or glucose minimal medium (GMM) (Cove, 1966) at 28°C.
454 sequencing and bioinformatic analysis
The genomic DNA used for sequencing was prepared as described (Gauch et al., 1998) from mycelium grown in stationary liquid culture. The shotgun sequencing was performed at the GenoSeq (UCLA Genotyping and Sequencing Core) with the GS FLX Titanium system (Roche).
The 454 sequencing reads were assembled into contigs with the GS De Novo Assembler software (Roche). The contigs were converted into BLAST database format for local BLAST search using stand-alone BLAST software (ver. 2.2.18) downloaded from the NCBI website. Gene predictions were carried out using the FGENESH program (Softberry) and manually checked by comparing with homologous gene/proteins in the GenBank database. Functional domains in the translated protein sequences were predicted using Conserved Domain Search (NCBI) or InterproScan (EBI). Phylogenetic analysis was performed with MEGA4 software (Tamura et al., 2007).
Fungal transformation and gene disruption in P. aethiopicum
Polyethylene glycol-mediated transformation of P. aethiopicum was done essentially as described previously for A. nidulans (Andrianopoulos and Hynes, 1988; Chooi et al., 2008), except that the protoplasts were prepared with 3 mg/ml lysing enzymes (Sigma-Aldrich) and 2 mg/ml Yatalase (Takara Bio). Construction of fusion PCR knockout cassettes containing the bar gene were carried out as described (Szewczyk et al., 2007), except that the homologous regions flanking the resistant marker were increased to 2 kb. Fusion PCR products were gel purified and sequenced before using for transformation. The bar gene with the trpC promoter was amplified from the plasmid pBARKS1 (Pall and Brunelli, 1993), which was obtained from the Fungal Genetics Stock Center (FGSC). RNA-silencing plasmids were constructed from pBARGPE1 (Pall and Brunelli, 1993) obtained from FGSC. Glufosinate used for the selection of bar transformants was prepared by extracting twice with equal volume of 1-butanol from commercial herbicide Finale (Bayer), which contains 11.33 % (w/v) glufosinate-ammonium (Hays and Selker, 2000). The resulting aqueous phase was filter-sterilized and used directly at 40 μl per ml of GMM with 1.2M sorbitol and 10 mM ammonium tartrate as the sole nitrogen source. Miniprep genomic DNA from P. aethiopicum transformants was used for PCR screening of gene deletants and was prepared as described previously for A. nidulans (Chooi et al., 2008). Primers used for amplification of fusion PCR products and screening of transformants, were listed in Table S2. Southern hybridizations were performed with DIG-High Prime DNA labeling and detection starter kit II (Roche Applied Science) following manufacturer’s protocol.
Chemical analysis and compound isolation
For small scale analysis, the P. aethiopicum wild type and transformants were grown in YMEG liquid medium (20mL) for 7 days at 28°C without shaking. The cultures were extracted with equal volume of ethyl acetate and evaporated to dryness. The dried extracts were dissolved in methanol for LC-MS analysis. LC-MS was conducted with a Shimadzu 2010 EV Liquid Chromatography Mass Spectrometer by using both positive and negative electrospray ionization and a Phenomenex Luna 5μ 2.0 × 100 mm C18 reverse-phase column. Samples were separated at a flow rate of 0.1 ml/min on a linear gradient of 5 to 95% solvent B in 30 min followed by isocratic 95% solvent B for another 15 mins (solvent A: 0.1% (v/v) formic acid, solvent B: CH3CN with 0.1% (v/v) formic acid). The identity of 1 and 2 were confirmed by comparing the UV spectra, retention time and m/z value to the authentic standards: 1 (a gift from Dr. Wongon Kim, Korea Research Institute of Bioscience & Biotechnology) and 2 (Sigma-Aldrich).
Dechlorogriseofulvin was isolated using a solvent-solvent partitioning scheme as described previously (Hutchison et al., 1973) followed by crystallization in MeOH. Briefly, the ethyl acetate extract from a two liters stationary liquid culture of a ΔgsfI mutant was evaporated to dryness and partition between CHCl3/H2O. The CHCl3 layer was further partitioned with hexane/MeOH (90%). The 90% MeOH layer was dried completely and redissolved in a small volume of MeOH (1 ml). Formation of white crystals, which was later confirmed to be 3, was observed when the MeOH extract was left undisturbed at room temperature (further crystallized at 4°C). The crystals (28 mg) were collected by filtration, washed in cold methanol and dried. Purity of the compound was checked by LC-MS and the structure was confirmed by NMR with reference to published spectra (Table S3).
HIGHLIGHTS
454 sequencing unveiled the gene clusters for viridicatumtoxin 1 and griseofulvin 2.
vrt and gsf clusters lie within conserved syntenic regions of P. aethiopicum genome.
VrtA and GsfA are nonreducing PKSs required for biosynthesis of 1 and 2.
gsfI encodes chlorinase involved in the biosynthesis of 2.
Supplementary Material
Acknowledgments
We are grateful to W.G. Kim (Korea Research Institute of Bioscience & Biotechnology) for providing a standard of 1. We thank Dr. W. Zhang for initial identification of 1 from P. aethiopicum.; X. Xie and W. Xu for assistance with NMR of 3; and Prof. John Vederas and Prof. Neil Garg for helpful discussions. We also like to acknowledge the suggestions provided by a reviewer on the biosynthetic pathway of 1. This project was supported by a David and Lucile Packard Fellowship and NIH (1R01GM085128).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrianopoulos A, Hynes MJ. Cloning and analysis of the positively acting regulatory gene amdR from Aspergillus nidulans. Mol Cell Biol. 1988;8:3532–3541. doi: 10.1128/mcb.8.8.3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariza MR, Larsen TO, Duus JO, Barrero AF. Penicillium digitatum metabolites on synthetic media and citrus fruits. J Agric Food Chem. 2002;50:6361–6365. doi: 10.1021/jf020398d. [DOI] [PubMed] [Google Scholar]
- Awakawa T, Yokota K, Funa N, Doi F, Mori N, Watanabe H, Horinouchi S. Physically discrete β-lactamase-type thioesterase catalyzes product release in atrochrysone synthesis by iterative type I polyketide synthase. Chem Biol. 2009;16:613–623. doi: 10.1016/j.chembiol.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Barton D, Cohen T. Festschrift Prof Dr Arthur Stoll. Birkhäuser Verlag; Basel: 1957. Some biogenetic aspects of phenol oxidation; p. 117. [Google Scholar]
- Bergmann S, Schumann J, Scherlach K, Lange C, Brakhage AA, Hertweck C. Genomics-driven discovery of PKS-NRPS hybrid metabolites from Aspergillus nidulans. Nat Chem Biol. 2007;3:213–217. doi: 10.1038/nchembio869. [DOI] [PubMed] [Google Scholar]
- Bok JW, Hoffmeister D, Maggio-Hall LA, Murillo R, Glasner JD, Keller NP. Genomic mining for Aspergillus natural products. Chem Biol. 2006;13:31–37. doi: 10.1016/j.chembiol.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Breinholt J, Jensen GW, Kjæ r A, Olsen CE, Rosendahl CN. Hypomycetin - an antifungal, tetracyclic metabolite from Hypomyces aurantius: production, structure and biosynthesis. Acta Chem Scand. 1997;51:855–860. [Google Scholar]
- Caruthers JM, Kang I, Rynkiewicz MJ, Cane DE, Christianson DW. Crystal structure determination of aristolochene synthase from the blue cheese mold, Penicillium roqueforti. J Biol Chem. 2000;275:25533–25539. doi: 10.1074/jbc.M000433200. [DOI] [PubMed] [Google Scholar]
- Casqueiro J, Gutierrez S, Banuelos O, Hijarrubia MJ, Martin JF. Gene targeting in Penicillium chrysogenum: disruption of the lys2 gene leads to penicillin overproduction. J Bacteriol. 1999;181:1181–1188. doi: 10.1128/jb.181.4.1181-1188.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang YM, Szewczyk E, Nayak T, Davidson AD, Sanchez JF, Lo HC, Ho WY, Simityan H, Kuo E, Praseuth A, et al. Molecular genetic mining of the Aspergillus secondary metabolome: discovery of the emericellamide biosynthetic pathway. Chem Biol. 2008;15:527–532. doi: 10.1016/j.chembiol.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chooi YH, Stalker DM, Davis MA, Fujii I, Elix JA, Louwhoff SHJJ, Lawrie AC. Cloning and sequence characterization of a non-reducing polyketide synthase gene from the lichen Xanthoparmelia semiviridis. Mycol Res. 2008;112:147–161. doi: 10.1016/j.mycres.2007.08.022. [DOI] [PubMed] [Google Scholar]
- Clardy J, Springer JP, Buechi G, Matsuo K, Wightman R. Tryptoquivaline and tryptoquivalone, two tremorgenic metabolites of Aspergillus clavatus. J Am Chem Soc. 1975;97:663–665. doi: 10.1021/ja00836a045. [DOI] [PubMed] [Google Scholar]
- Cove DJ. The induction and repression of nitrate reductase in the fungus Aspergillus nidulans. Biochim Biophys Acta. 1966;113:51–56. doi: 10.1016/s0926-6593(66)80120-0. [DOI] [PubMed] [Google Scholar]
- Cox R. Polyketides, proteins and genes in fungi: programmed nano-machines begin to reveal their secrets. Org Biomol Chem. 2007;5:2010–2026. doi: 10.1039/b704420h. [DOI] [PubMed] [Google Scholar]
- Crawford JM, Dancy BCR, Hill EA, Udwary DW, Townsend CA. Identification of a starter unit acyl-carrier protein transacylase domain in an iterative type I polyketide synthase. Proc Natl Acad Sci USA. 2006;103:16728–16733. doi: 10.1073/pnas.0604112103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford JM, Korman TP, Labonte JW, Vagstad AL, Hill EA, Kamari-Bidkorpeh O, Tsai SC, Townsend CA. Structural basis for biosynthetic programming of fungal aromatic polyketide cyclization. Nature. 2009;461:1139–1143. doi: 10.1038/nature08475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jesus A, Hull W, Steyn P, van Heerden F, Vleggaar R. Biosynthesis of viridicatumtoxin, a mycotoxin from Penicillium expansum. J Chem Soc, Chem Commun. 1982:902–904. [Google Scholar]
- Ehrlich KC, Montalbano BG, Cary JW. Binding of the C6-zinc cluster protein, AFLR, to the promoters of aflatoxin pathway biosynthesis genes in Aspergillus parasiticus. Gene. 1999;230:249–257. doi: 10.1016/s0378-1119(99)00075-x. [DOI] [PubMed] [Google Scholar]
- Finkelstein E, Amichai B, Grunwald MH. Griseofulvin and its uses. Int J Antimicrob Agents. 1996;6:189–194. doi: 10.1016/0924-8579(95)00037-2. [DOI] [PubMed] [Google Scholar]
- Frisvad JC, Samson RA. Polyphasic taxonomy of Penicillium subgenus Penicillium. A guide to identification of food and air-borne terverticillate Penicillia and their mycotoxins. In: Samson RA, Frisvad JC, editors. Studies in Mycolgy 49: Penicillium subgenus Penicillium: new taxonomic schemes, mycotoxins and other extrolites. Utrecht, The Netherlands: Centraalbureau voor Schimmelcultures; 2004. pp. 1–174. [Google Scholar]
- Fujii I, Iijima H, Tsukita S, Ebizuka Y, Sankawa U. Purification and properties of dihydrogeodin oxidase from Aspergillus terreus. J Biochem. 1987;101:11–18. doi: 10.1093/oxfordjournals.jbchem.a121881. [DOI] [PubMed] [Google Scholar]
- Fujii I, Watanabe A, Sankawa U, Ebizuka Y. Identification of Claisen cyclase domain in fungal polyketide synthase WA, a naphthopyrone synthase of Aspergillus nidulans. Chem Biol. 2001;8:189–197. doi: 10.1016/s1074-5521(00)90068-1. [DOI] [PubMed] [Google Scholar]
- Fukui T, Ito M, Tomita K. Purification and characterization of acetoacetyl-CoA synthetase from Zoogloea ramigera I-16-M. Eur. J Biochem. 1982;127:423–428. doi: 10.1111/j.1432-1033.1982.tb06889.x. [DOI] [PubMed] [Google Scholar]
- Gauch S, Hermann R, Feuser P, Oelmuller U, Bastian H. Isolation of nucleic acids using anion-exchange chromatography: Qiagen-tip based methods. In: Karp A, Ingram DS, Isaac PG, editors. Molecular Tools for Screening Biodiversity. London: Chapman and Hall; 1998. pp. 54–59. [Google Scholar]
- Halo LM, Heneghan MN, Yakasai AA, Song Z, Williams K, Bailey AM, Cox RJ, Lazarus CM, Simpson TJ. Late stage oxidations during the biosynthesis of the 2-pyridone tenellin in the entomopathogenic fungus Beauveria bassiana. J Am Chem Soc. 2008;130:17988–17996. doi: 10.1021/ja807052c. [DOI] [PubMed] [Google Scholar]
- Harris C, Roberson J, Harris T. Biosynthesis of griseofulvin. J Am Chem Soc. 1976;98:5380–5386. doi: 10.1021/ja00433a053. [DOI] [PubMed] [Google Scholar]
- Hays S, Selker E. Making the selectable marker bar tighter and more economical. Fungal Genet Newsl. 2000;47:107. [Google Scholar]
- Heide L. Prenyl transfer to aromatic substrates: genetics and enzymology. Curr Opin Chem Biol. 2009;13:171–179. doi: 10.1016/j.cbpa.2009.02.020. [DOI] [PubMed] [Google Scholar]
- Hoffmeister D, Keller N. Natural products of filamentous fungi: enzymes, genes, and their regulation. Nat Prod Rep. 2007;24:393–416. doi: 10.1039/b603084j. [DOI] [PubMed] [Google Scholar]
- Homann V, Mende K, Arntz C, Ilardi V, Macino G, Morelli G, Böse G, Tudzynski B. The isoprenoid pathway: cloning and characterization of fungal FPPS genes. Curr Genet. 1996;30:232–239. doi: 10.1007/s002940050126. [DOI] [PubMed] [Google Scholar]
- Horak R, Maharaj V, Marais S, Heerden F, Vleggaar R. Stereochemical studies on the biosynthesis of viridicatumtoxin: evidence for a 1,3-hydride shift in the formation of the spirobicyclic ring system. J Chem Soc, Chem Commun. 1988;1988:1562–1564. [Google Scholar]
- Huang K-x, Fujii I, Ebizuka Y, Gomi K, Sankawa U. Molecular cloning and heterologous expression of the gene encoding dihydrogeodin oxidase, a multicopper blue enzyme from Aspergillus terreus. J Biol Chem. 1995;270:21495–21502. doi: 10.1074/jbc.270.37.21495. [DOI] [PubMed] [Google Scholar]
- Hutchison RD, Steyn PS, van Rensburg SJ. Viridicatumtoxin, a new mycotoxin from Penicillium viridicatum Westling. Toxicol Appl Pharmacol. 1973;24:507–509. doi: 10.1016/0041-008x(73)90057-4. [DOI] [PubMed] [Google Scholar]
- Ishimaru T, Tsuboya S, Saijo T. Tetracyclic compounds, its production and use thereof. 06–040995 JP. 1993
- Jarvis B, Zhou Y, Jiang J, Wang S, Sorenson W, Hintikka E, Nikulin M, Parikka P, Etzel R, Dearborn D. Toxigenic molds in water-damaged buildings: dechlorogriseofulvins from Memnoniella echinata. J Nat Prod. 1996;59:553–554. doi: 10.1021/np960395t. [DOI] [PubMed] [Google Scholar]
- Jin H, Yamashita A, Maekawa S, Yang P, He L, Takayanagi S, Wakita T, Sakamoto N, Enomoto N, Ito M. Griseofulvin, an oral antifungal agent, suppresses hepatitis C virus replication in vitro. Hepatol Res. 2008;38:909–918. doi: 10.1111/j.1872-034X.2008.00352.x. [DOI] [PubMed] [Google Scholar]
- Lane M, Nakashima T, Vederas J. Biosynthetic source of oxygens in griseofulvin. Spin-echo resolution of oxygen-18 isotope shifts in carbon-13 NMR spectroscopy. J Am Chem Soc. 1982;104:913–915. [Google Scholar]
- McNeil J, Flynn J, Tsao N, Monschau N, Stahmann K, Haynes R, McIntosh E, Pearlman R. Glycine metabolism in Candida albicans: characterization of the serine hydroxymethyltransferase (SHM1, SHM2) and threonine aldolase (GLY1) genes. Yeast. 2000;16:167. doi: 10.1002/(SICI)1097-0061(20000130)16:2<167::AID-YEA519>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Metzger U, Schall C, Zocher G, Unsöld I, Stec E, Li S, Heide L, Stehle T. The structure of dimethylallyl tryptophan synthase reveals a common architecture of aromatic prenyltransferases in fungi and bacteria. Proc Natl Acad Sci USA. 2009;106:14309. doi: 10.1073/pnas.0904897106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak T, Szewczyk E, Oakley CE, Osmani A, Ukil L, Murray SL, Hynes MJ, Osmani SA, Oakley BR. A versatile and efficient gene targeting system for Aspergillus nidulans. Genetics. 2006;172:1557–1566. doi: 10.1534/genetics.105.052563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogasawara Y, Liu HW. Biosynthetic studies of aziridine formation in azicemicins. J Am Chem Soc. 2009;131:18066–18068. doi: 10.1021/ja907307h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pall M, Brunelli J. A series of six compact fungal transformation vectors containing polylinkers with multiple unique restriction sites. Fungal Genet Newsl. 1993;40:59. [Google Scholar]
- Panda D, Rathinasamy K, Santra M, Wilson L. Kinetic suppression of microtubule dynamic instability by griseofulvin: implications for its possible use in the treatment of cancer. Proc Natl Acad Sci USA. 2005;102:9878–9883. doi: 10.1073/pnas.0501821102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju MS, Wu GS, Gard A, Rosazza JP. Microbial transformations of natural antitumor agents. 20 Glucosylation of viridicatumtoxin. J Nat Prod. 2004;45:321–327. [Google Scholar]
- Rebacz B, Larsen T, Clausen M, Ronnest M, Loffler H, Ho A, Kramer A. Identification of griseofulvin as an inhibitor of centrosomal clustering in a phenotype-based screen. Cancer Res. 2007;67:6342. doi: 10.1158/0008-5472.CAN-07-0663. [DOI] [PubMed] [Google Scholar]
- Rhodes A, Somerfield GA, Mcgonagle MP. Biosynthesis of griseofulvin. Observations on the incorporation of (14C)griseophenone C and (36C)griseophenones B and A. Biochem J. 1963;88:349–357. doi: 10.1042/bj0880349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rønnest M, Rebacz B, Markworth L, Terp A, Larsen T, Kra mer A, Clausen M. Synthesis and structure-activity relationship of griseofulvin analogues as inhibitors of centrosomal clustering in cancer cells. J Med Chem. 2009;52:3342–3347. doi: 10.1021/jm801517j. [DOI] [PubMed] [Google Scholar]
- Saikia S, Parker E, Koulman A, Scott B. Four gene products are required for the fungal synthesis of the indole-diterpene, paspaline. FEBS letters. 2006;580:1625–1630. doi: 10.1016/j.febslet.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Samson RA, Seifert KA, Frisvad JC. Phylogenetic analysis of Penicillium subgenus Penicillium using partial -tubulin sequences. In: Samson RA, Frisvad JC, editors. Studies in Mycolgy 49: Penicillium subgenus Penicillium: new taxonomic schemes, mycotoxins and other extrolites. Utrecht, The Netherlands: Centraalbureau voor Schimmelcultures; 2004. pp. 175–200. [Google Scholar]
- Schumann J, Hertweck C. Molecular basis of cytochalasan biosynthesis in fungi: gene cluster analysis and evidence for the involvement of a PKS-NRPS hybrid synthase by RNA silencing. J Am Chem Soc. 2007;129:9564–9565. doi: 10.1021/ja072884t. [DOI] [PubMed] [Google Scholar]
- Schümann J, Hertweck C. Advances in cloning, functional analysis and heterologous expression of fungal polyketide synthase genes. J Biotechnol. 2006;124:690–703. doi: 10.1016/j.jbiotec.2006.03.046. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Kinoshita H, Nihira T. Identification and in vivo functional analysis by gene disruption of ctnA, an activator gene involved in citrinin biosynthesis in Monascus purpureus. Appl Environ Microbiol. 2007;73:5097–5103. doi: 10.1128/AEM.01979-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson TJ, Holker JSE. 13C-NMR studies on griseofulvin biosynthesis and acetate metabolism in Penicillium patulum. Phytochemistry. 1977;16:229–233. [Google Scholar]
- Szewczyk E, Chiang Y, Oakley C, Davidson A, Wang C, Oakley B. Identification and characterization of the asperthecin gene cluster of Aspergillus nidulans. Appl Environ Microbiol. 2008;74:7607. doi: 10.1128/AEM.01743-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szewczyk E, Nayak T, Oakley CE, Edgerton H, Xiong Y, Taheri-Talesh N, Osmani SA, Oakley BR. Fusion PCR and gene targeting in Aspergillus nidulans. Nat Protocols. 2007;1:3111–3120. doi: 10.1038/nprot.2006.405. [DOI] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Thomas R. A biosynthetic classification of fungal and streptomycete fused-ring aromatic polyketides. ChemBioChem. 2001;2:612–627. doi: 10.1002/1439-7633(20010903)2:9<612::AID-CBIC612>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Thomas R, Williams D. Oxytetracycline biosynthesis: mode of incorporation of [1-13C]-and [1, 2-13C2]-acetate. J Chem Soc, Chem Commun. 1983;1983:128–130. [Google Scholar]
- Walton JD. Horizontal gene transfer and the evolution of secondary metabolite gene clusters in fungi: an hypothesis. Fung Genet Biol. 2000;30:167–171. doi: 10.1006/fgbi.2000.1224. [DOI] [PubMed] [Google Scholar]
- Wang S, Xu Y, Maine E, Wijeratne E, Espinosa-Artiles P, Gunatilaka A, Molnár I. Functional characterization of the biosynthesis of radicicol, an Hsp90 inhibitor resorcylic acid lactone from Chaetomium chiversii. Chem Biol. 2008;15:1328–1338. doi: 10.1016/j.chembiol.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Wong S, Kullnig R, Dedinas J, Appell K, Kydd G, Gillum A, Cooper R, Moore R. Anthrotainin, an inhibitor of substance P binding produced by Gliocladium catenulatum. J Antibiot. 1993;46:214–221. doi: 10.7164/antibiotics.46.214. [DOI] [PubMed] [Google Scholar]
- Yamazaki M, Fujimoto H, Okuyama E. Structure determination of six tryptoquivaline-related metabolites from Aspergillus fumigatus. Tetrahedron Lett. 1976;17:2861–2864. [Google Scholar]
- Zerbe K, Woithe K, Li DB, Vitali F, Bigler L, Robinson JA. An oxidative phenol coupling reaction catalyzed by OxyB, a cytochrome P450 from the vancomycin-producing microorganism. Angew Chem Int Ed. 2004;43:6709–6713. doi: 10.1002/anie.200461278. [DOI] [PubMed] [Google Scholar]
- Zhang W, Watanabe K, Wang CCC, Tang Y. Investigation of early tailoring reactions in the oxytetracycline biosynthetic pathway. J Biol Chem. 2007;282:25717–25725. doi: 10.1074/jbc.M703437200. [DOI] [PubMed] [Google Scholar]
- Zheng CJ, Yu HE, Kim EH, Kim WG. Viridicatumtoxin B, a new anti-MRSA agent from Penicillium sp FR11. J Antibiot. 2008;61:633–637. doi: 10.1038/ja.2008.84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.