Abstract
Nurses caring for traumatic brain injury (TBI) patients with intracranial hypertension (ICH) recognize that patients whose intracranial adaptive capacity is reduced are susceptible to periods of disproportionate increase in intracranial pressure (DIICP) in response to a variety of stimuli. It is possible DIICP signals potential secondary brain damage due to sustained or intermittent ICH. However, there are few clinically accessible intracranial pressure (ICP) measurement parameters that allow nurses and other critical care clinicians to identify patients at risk for DIICP. The purpose of this study was to investigate whether there are specific min-to-min trends in ICP variability during the first 48 hr of monitoring that might accurately predict DIICP in patients with severe TBI. Thirty-eight patients with severe TBI were sampled from the dataset of a randomized controlled trial testing bedside monitoring displays and cerebral perfusion pressure management in individuals with TBI or subarachnoid hemorrhage. The investigators retrospectively examined the rates of change (slope) in mean, standard deviation and variance of ICP on a 1-min basis for 30 consecutive min prior to a specified DIICP event. There was a significantly increasing linear and quadratic slope in mean ICP prior to the development of DIICP, compared with the comparison data set (p < .05). It is feasible to display moving averages in modern bedside monitoring. Such an arrangement may be useful to provide visual displays that provide immediate clinically relevant information regarding the patients with decreased adaptive capacity and therefore increased risk for DIICP.
Keywords: Traumatic brain injury, Intracranial adaptive capacity, Intracranial pressure, Intracranial hypertension, Disproportionate increase in intracranial pressure (DIICP), Signal variability
A number of studies have demonstrated that nursing care activities and other external stimuli can result in transient or sustained periods of increased intracranial pressure (ICP; (Kirkness, Mitchell, Burr, March, & Newell, 2000; March, Mitchell, Grady, & Winn, 1990; Mitchell, 1986a, 1986b; Mitchell & Mauss, 1978; Mitchell, Ozuna, & Lipe, 1981; Muwaswes, 1985; Rising, 1993; Tsementzis, Harris, & Loizou, 1982). A sustained period of increased ICP greater than 20–25 mmHg is called intracranial hypertension (ICH; Czosnyka & Pickard, 2004). ICH can superimpose secondary hypoxic/ischemic brain injury in patients already suffering primary injury from traumatic brain injury (TBI). It is likely that patients who manifest frequent and large ICP responses to internal and external stimuli cannot maintain intracranial pressure-volume equilibrium. The compensatory ability to maintain equilibrium has been called intracranial compliance (ΔV/ΔP) or intracranial elastance (ΔP/ΔV) by anesthesiologists and neurosurgeons and intracranial adaptive capacity by neuroscience nurses. The nursing diagnosis “decreased adaptive capacity” was coined over 2 decades ago to recognize this state of decompensation. It is manifested by “a disproportionate increase in intracranial pressure (DIICP) in response to a variety of noxious and nonnoxious stimuli” (Mitchell, 1986a).
Operationally, DIICP is defined as an increase in ICP, in response to internal or external stimuli, of more than 10 mmHg over baseline for 3 min or longer or an increase in ICP that triggers the protocol for medical intervention (Ackley & Ladwig, 2006). Frequent or sustained periods of increased ICP act through decreased cerebral perfusion to negatively influence mortality and morbidity for TBI patients (Chesnut et al., 1993; Jones et al., 1994; Lang & Chesnut, 1995; Marmarou et al., 1991; Resnick, Marion, & Carlier, 1997). Unfortunately, little progress has been made in identifying clinically available monitoring indices that will predict patients most at risk as opposed to leaving health care professionals to simply respond to the periods of elevated ICP or reduced cerebral perfusion pressure (CPP).
Over the past 40 years, a number of invasive methods have been developed and introduced in a limited way into clinical practice to measure the compensatory ability of the craniospinal axis space. These include the pressure-volume index (PVI; Maset et al., 1987), volume-pressure response (VPR; Miller & Garibi, 1972; Miller, Garibi, & Pickard, 1973), and the dependence of PVI on the CPP level (Gray & Rosner, 1987). Although these measures have been incorporated into some bedside monitoring devices (Yau et al., 2002), they are mainly helpful during the episode of increased ICP rather than during the period prior to the increase. Further, they are not routinely incorporated into clinical bedside monitoring systems to be used as a real-time, beat-by-beat continuous measurement tool (Kirkness et al., 2000). Therefore, a clinically accessible, real-time indicator of craniospinal compensatory ability in individuals with severe ICH is needed. If we can identify individuals who are at risk for rapid and perhaps prolonged increases in ICP, we can design individualized nursing interventions to either increase compensatory ability or decrease compensatory demand.
Few studies have examined the trend or pattern of ICP variability prior to the development of ICH. Only one prior study, conducted by Contant et al. (1995), involved an attempt to clarify the pattern changes in ICP waveform prior to ICP change in both transient and refractory ICP. Changes in the level of ICP were examined at three different time periods in 109 individuals with severe head trauma: 10–60 min prior to the ICP increase, at the peak of the ICP increase, and after the ICP increase. There was a statistically significant (p < .005) increasing trend in ICP from 13.9 mmHg to 32 mmHg during the 10–60–min period prior to a sustained ICP increase. No information was provided regarding the frequency of transient ICP in each subject and how the baseline was defined.
Most ICP studies have focused on the relationship between the magnitude of ICP changes and patient outcome (Chambers, Treadwell, & Mendelow, 2001; Jones et al., 2003; Marmarou et al., 1991; McGraw, Howard, & O'Connor, 1983; Miller et al., 1981; Saul & Ducker, 1982; Stocchetti et al., 1999). A few who have studied ICP variability have focused on the relationship between the standard deviation (SD) or variance of the ICP and the clinical outcome. For example, Marmarou et al. (1991) used ICP variance (one value), which was calculated from the hourly ICP data, as an indirect measure of the volume/pressure stability. ICP variance as a single value was only poorly related to outcome. Jones et al. (2003) conducted a prospective observational study in a sample of 43 children to examine the pressure parameters of ICP, arterial blood pressure (ABP), and CPP and their relative variability (SD) over 1-min periods for 144 hr post head injury and the relationship of the SDs to patient outcomes 12 months after head injury. The variability of these three pressure parameters over the whole ICP monitoring time period was significantly higher in children who died than in the survivors at 12 months post head injury (Jones et al., 2003). For the survivors, the SD of ICP alone (from the first 48 hr post injury) was strongly related to the outcome (p = .008), with a larger SD in those who did not recover compared to those who recovered at 12 months post injury.
Elevation of the second peak, or P2, component of the ICP pulse waveform has also been suggested as a clinically accessible measure of decreased adaptive capacity (or decreased brain compliance; Cardoso, Rowan, & Galbraith, 1983; Germon, 1988). However, this morphologic feature of the ICP waveform is not uniquely predictive of DIICP episodes (Fan, Kirkness, Vicini, Burr, & Mitchell, 2008).
In individuals with TBI, there is a natural evolution of pathophysiologic states from the time of injury to recovery or death, and these may shift rapidly from compensated to uncompensated state, which may be manifested by DIICP before transitioning to sustained ICP. In other words, there is a “transition zone” between compensated and uncompensated states. In the study described here, the hypothesis was that, prior to the DIICP event, there are specific trends in the ICP dynamic that could serve as clinically oriented indicators to predict the real-time evolution of intracranial adaptive capacity on a time-dependent basis during the acute stage of hospitalization. The overall goal was early identification of individuals with severe TBI who were at risk of decreased intracranial adaptive capacity. The variability of ICP in terms of mean, SD, and variance in the 30 min prior to a DIICP event in individuals with severe TBI was analyzed on a min-by-min basis to determine whether there are any specific trends in ICP variability during this time period and to determine the predictive value of ICP variability for DIICP events. Data were drawn from an existing database in which monitoring occurred during the 48 hr immediately after the deployment of an ICP monitor.
Method
This secondary analysis used ICP data collected continuously as part of a previously reported prospective randomized controlled clinical trial examining a highly visible display of CPP and clinical outcome of patients with subarachnoid hemorrhage and TBI (Kirkness, Burr, Cain, Newell, & Mitchell, 2005, 2006; Mitchell, Burr, & Kirkness, 2002). This parent study will be referred to as the CPP study. Approval for secondary data analysis was obtained from the CPP study investigators and the Institutional Review Board of the University of Washington.
Data were sampled from the original database that comprised 157 patients (124 males and 33 females) with TBI who were enrolled in the parent CPP study. Each individual had invasive ICP (Camino fiberoptic transducer-tipped catheter) and ABP (intra-arterial catheter in the radial artery) monitoring devices in place. The specifications of these instruments have been described in previous publications (Kirkness et al., 2005, 2006; Mitchell et al., 2002). All signal data and information about the physiological parameters, injury severity, and clinical course were gathered during hospitalization in the intensive care unit. Data from the first 48 hr after insertion of the ICP monitor were used for this analysis. Both ICP and ABP data were stored in hourly segments comprised of 5-s averages. Each subject had a different number of hourly files, depending upon how long the ICP monitor was used.
Sample
Only data from individuals with TBI were included in the present study. Systematic sampling was used to select 38 TBI subjects from the parent CPP study, which had complete data for 48 hr, to be representative of the severity of injury and the range of ICP seen in the parent study. Those with craniectomy were excluded because of the dampening effect of craniectomy on the ICP pulse waveform. Details of the sampling frame have been previously reported (Fan et al., 2008).
The sample for this analysis comprised 31 males and 7 females, ranging in age from 16 to 89 years. The mean age was 40.0, with a range in age from 16 to 89 years. The median postresuscitation Glasgow Coma Scale (GCS) score was 7 with a range from 3 to 15. The characteristics of the individuals sampled for this analysis were similar to those of the complete sample in the parent CPP study, as shown in Table 1.
Table 1.
Characteristics of individuals with traumatic brain injury (TBI) for the current intracranial pressure (ICP) study and the parent cerebral perfusion pressure (CPP) study
|
M ± SD or N (%) |
||
|---|---|---|
| Characteristics | ICP Study (N = 38) | CPP Parent Study (N = 157) |
| Age (years) | 40.0 ± 22.0 | 37.1 ± 18.1 |
| Glasgow Coma Scale score postresuscitation | 7.9 ± 3.5 | 7.3 ± 3.1 |
| Male | 31 (81.6) | 124 (78.9) |
| Injury mode | ||
| Motor vehicle accident | 19 (50.0) | 71 (45.5) |
| Fall | 7 (18.4) | 32 (20.4) |
| Motorcycle | 4 (10.5) | 18 (11.5) |
| Bicycle | 3 (7.9) | 8 (5.1) |
| Assault | 2 (5.3) | 7 (4.5) |
| Pedestrian | 2 (5.3) | 14 (8.9) |
| Other | 1 (2.6) | 1 (0.6) |
| Gun shot wound | 3 (1.9) | |
| Hit by object | 2 (1.3) | |
| Injury Severity Score on admission | 28.2 ± 8.7 | 29.1 ± 9.6 |
| Abbreviated Injury Scale - head score | ||
| 0 (no visible pathology on head CT) | 1 (2.6) | 3 (1.9) |
| 1 (minor injury) | 0 (0) | 0 (0) |
| 2 (moderate injury) | 0 (0) | 1 (0.6) |
| 3 (severe but not life threatening) | 5 (13.2) | 16 (10.2) |
| 4 (life threatening but survival likely) | 17 (44.7) | 81 (51.6) |
| 5 (critical with uncertain survival) | 15 (39.5) | 56 (35.7) |
| Extended Glasgow Outcome Scale score at discharge | ||
| 1 (dead) | 6 (15.8) | 21 (13.4) |
| 2 (vegetative) | 2 (5.3) | 14 (8.9) |
| 3 (lower severe disability) | 28 (73.7) | 115 (73.2) |
| 4 (upper severe disability) | 2 (5.3) | 7 (4.5) |
Note. SD = standard deviation.
Data Preparation
The hourly files were first scanned for missing and out-of-range data. If 12 or fewer data points were missing and there was no systematic pattern, missing data were replaced based on the imputed mean of the five valid values before and after the missing data period. If there were more than 36 consecutive data points missing from an hourly file, that file was excluded from the analysis.
The consecutive hourly files for each person were then merged into a large data set. Then, every 12 data points (equal to 1 min) were aggregated into a “min value.” These min values were then used for statistical analysis of the min-to-min (within each min) trends in mean, SD and variance of the ICP prior to each DIICP event.
Operational Definition of DIICP
DIICP was operationally defined for this study as (1) an increase in ICP greater than 10mmHg over baseline for 5 min or longer if the baseline is less than 20 mmHg or (2) an increase in ICP to a value greater than or equal to 25 mmHg for 5 min or longer if the baseline is greater than 20 mmHg (the latter value being one that would trigger medical intervention.) For this study, the baseline reference was defined as a 10-min moving average of ICP. Any event of DIICP that met the above criteria was considered a discrete event. However, if a second DIICP episode occurred within 4 min or less following the end of the first, the second event was considered to be part of the first DIICP event. Once a DIICP event was discovered, the first data point of the prior 10-min moving average was used for this specific entire DIICP event. Only one DIICP event was used for analysis due to the highly variable number of DIICP events across subjects. In addition, 7 subjects without DIICP events and 1 who had only one DIICP event in the first 14 min of Hr 1 after the ICP monitor was deployed were excluded. A total of 30 TBI subjects were thus included in the final analysis model. SPSS-PC version 12 (SPSS Inc., Chicago) was used for data analysis and the significant level was set at p < .05.
Local Variability Approach
The initial step was to measure the within-min variability of the ICP in a time series of 30 min prior to the DIICP event and in a 30-min comparison period from the same individual in an hr file without DIICP. It was hypothesized that there would be an increase in the local variability on a min-by-min basis 30 min prior to the onset of the DIICP. Mean, SD, and variance were calculated on a min-by-min basis in the 30-min window. Repeated measures analysis of variance (Repeated measures ANOVA) was used to determine whether there was a significant trend for time in mean, SD, or variance from min to min in the 30-min period prior to the DIICP event, in the comparison period, or between pre-DIICP period and comparison period. The general linear model (GLM) repeated measures algorithms test within-subject and between-subjects factors and adjusts the degrees of freedom for the repeated measures.
Similarly, individuals' slopes (rates of change) of mean, SD and variance were calculated by plotting each individual's mean ICP at each min prior to the DIICP event or each min of the comparison period. A paired sample t test was use to determine whether there was a difference in the change of slopes between DIICP and the comparison data set.
Results
The DIICP Events
In the 38 patients with TBI, 301 DIICP events occurred, with a range of 0–37 events per subject. Descriptive characteristics of the DIICP events are shown in Table 2.
Table 2.
Characteristics of the Disproportionate Increases in Intracranial Pressure (DIICPs) in 38 Individuals with Traumatic Brain Injury
| Characteristic | Value (No. (%) or M ± SD) |
|---|---|
| Total no. of events | 301 |
| Number with no events | 6 (15) |
| Number with 6 or fewer events | 16 (52 |
| Number of events less than 30 minutes | 81 (26.9) |
| Duration of events in minutes | 25.1 ± 41.7 |
Local Variability Approach
The local variability approach was used to retrospectively examine the changes in the mean, SD, and variance of ICP over the 30-min period prior to the DIICP event.
Mean
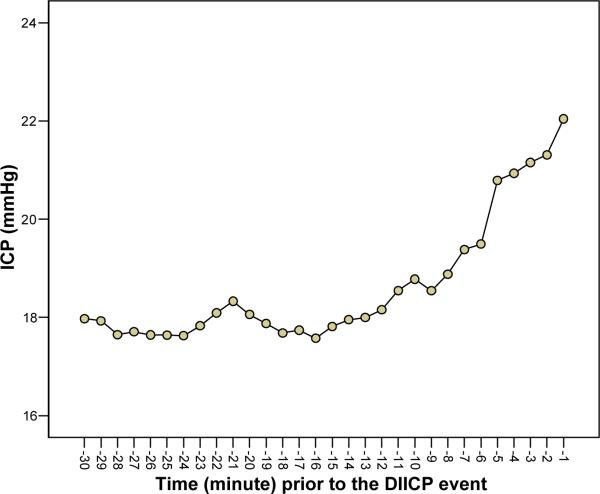
There was a statistically significant increasing trend for time on a min-basis in mean ICP prior to the DIICP event (F[3.71, 100.05] = 12.09, p < .001). For this analysis, the Greenhouse-Geisser epsilon was applied to correct for the degrees of freedom because the Mauchly's test of sphericity was significant (p < .001). Polynomial contrasts for the within-subjects factors showed significance for both a linear trend (p < .001) and a quadratic trend (p < .001). The mean for ICP at 1 min prior to the DIICP was larger than the mean for ICP at 2 min prior to the DIICP, which was larger than the mean for ICP at 3 min prior to the DIICP, etc. If there is a significant quadratic trend, a U- or inverted U-shaped pattern is shown in the graph. The significant trend is only of interest if the overall F value is significant, and it is a test of a trend, not a specific test of differences between mean ICP at specific time points. Further analysis, such as post hoc tests, is necessary. The increasing trend is shown in Figure 1.
Figure 1.
An increasing trend in intracranial pressure (ICP) 30 min prior to the disproportionate increase in intracranial pressure (DIICP) event. Data points are averages of the values for 30 subjects.
Standard Deviations (SDs)
There was not a specific trend in SD 30 min prior to the DIICP event (F[6.56, 177.10] =1.94, p = .07) after applying the Greenhouse-Geisser epsilon to correct for the degrees of freedom.
Variance
There was not a trend or pattern in variance 30 min prior to the DIICP event (F[4.24, 114.34] = 1.15, p = .336) after applying the Greenhouse-Geisser epsilon to correct for the degrees of freedom.
Verifying the Trend
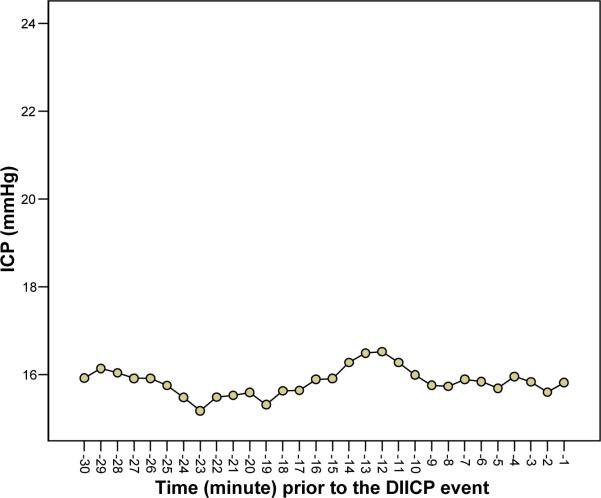
To examine whether the increasing trend was unique and only appeared prior to the DIICP event, 30 comparison sets were chosen based on the criteria described in the statistical analysis section. Figure 2 shows that there were no statistically significant trends or patterns in mean, SD, or variance on a min-by-min basis in the comparison group (F[4.24, 122.97] = 1.20, p = .312), (F[4.40, 127.70] = 1.56, p = .182), and (F[1.55, 44.93] = 1.36, p = .262), respectively, after applying the Greenhouse-Geisser epsilon to correct for the degrees of freedom.
Figure 2.
In the 30 comparison data sets of intracranial pressure over 30-min periods that did not precede disproportionate increase in intracranial pressure (DIICP) events, there was no trend or pattern in mean intracranial pressure (ICP). Data points are averages of the values for 30 subjects.
The Change of Raw Slopes Prior to DIICP Events
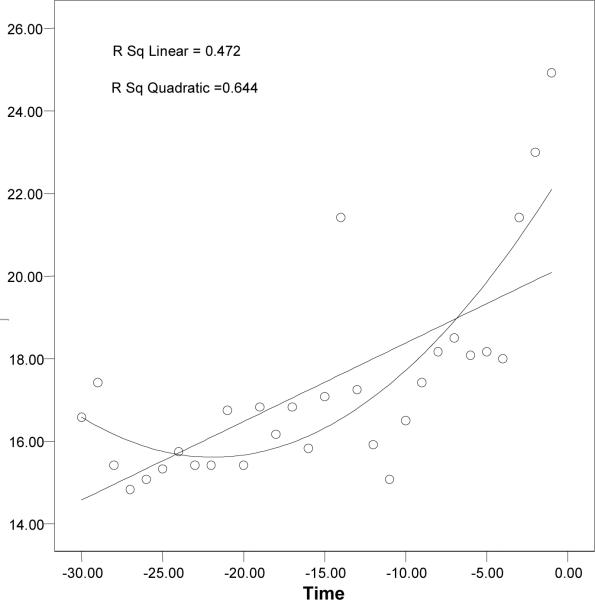
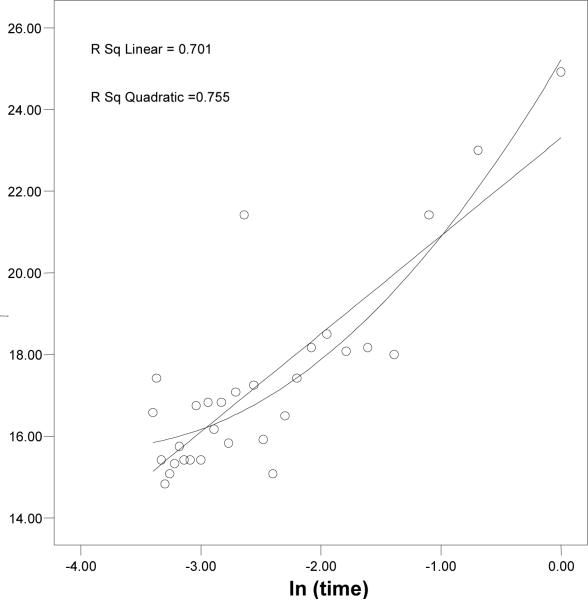
According to the result from the local variability analysis, there was a statistically significant increasing trend (linear and quadratic) in mean ICP on a 1-min basis 30 min prior to the DIICP event. Further analysis was done to determine whether there was a difference in the change of slopes for mean ICP between DIICP and the comparison data set. Before studying the change of the slopes, the investigators examined whether the linear or quadratic approach was appropriate. The investigators plotted each subject's ICP on the Y axis and the time parameter on the X axis. Figure 3 shows an example where a quadratic trend fit better than a linear trend when the X axis was labeled as real time. After the time was transformed to a natural log (ln) format (ln[time]), a fairly linear relationship between ICP and ln(time) became apparent (Figure 4). This suggests some sort of power, or exponential, relationship between ICP and time before DIICP.
Figure 3.
Quadratic trend fits better than the linear trend when the X axis represents real time.
Figure 4.
Both linear and quadratic trends fit equally well when the X axis represents ln (time).
Based on the foregoing data, the X axis variable was transformed to ln(time) from real time for ease of individual slope analysis. The slope of ICP vs. ln(time) was significantly increased prior to the DIICP event in the DIICP data set compared to the mean slope in the comparison data set (t[29] = 3.057, p = .005).
Localization of the Change of the Slopes
Because the ICP vs. ln(time) slope was significantly increased within the 30 min prior to the DIICP event, the next step was to determine which time window was the most crucial. In the first scenario, the individuals' slopes were computed from the data up to the DIICP event at different time points (such as 5, 10 or 15 min) and at different time windows (20–30 min, 10–20 min, 5-20 min) prior to the DIICP event. The final results showed that there were differences in the change of slopes among these three time windows (F[1.53, 51.34] = 6.532, p = .006). Pairwise comparison with Bonferroni adjustment was used to distinguish the mean difference in slope among these three time windows. The post-hoc comparison showed that there were statistically significant decreases in slopes in the 10–20–min window contrasted with the 20–30–min window (mean difference [MD] = .752, p = .007), as well as from the 5–20–min window to the 20–30–min window (MD = .769, p = .049). There was no mean difference in slope between the 10–20–min and the 5–20–min time windows (MD = .017, p = 1.000). This analysis would not be useful in a clinical setting because it would require foreknowledge of the timing of the DIICP event. However, it does show that the rate of change in slope was a precursor for predicting a DIICP event.
In the second scenario, the individuals' slopes were computed based on the existing data, and care was taken not to overlap the time windows at different time intervals. There was a statistically significant change of slopes between ln(time) window 15–25 and ln(time) 5–15 window at the 10-min interval (F[1.75, 50.80] = 3.612, p = .04). The mean difference in slope was 1.7 (between ln(time) window 15–25 and ln(time) window 5–15).
Discussion
This study demonstrated the value of viewing the trend or pattern change in the level of ICP as a predictor of an episode of DIICP. The nonlinear and rapidly rising increase in mean ICP in the 30 min prior to DIICP as shown in Figure 1 should be visible on the graphic mode of a clinical monitor, particularly if the moving average is incorporated into the monitoring system. Our analyses using calculations of changes in slope further confirm the change in pattern that immediately precedes the DIICP event. The comparison data set for each individual subject further indicates the specificity of this change in mean and slope as a valid predictor of DIICP.
These findings are consistent with those of Contant et al. (1995), who found that there was an increase in ICP level prior to transient ICH in individuals with severe TBI. It also confirms the findings of Rauch, Mitchell, and Tyler (1990) that the increased level of ICP is one of the clinical risk factors to identify an individual with subsequent episodes of DIICP. Our study extends the prior findings in that we were able to quantify the rate as well as level of increase. The increasing rate in ICP was statistically significant in the 5–15 min prior to developing DIICP. As a result, it may be important for critical care providers to recognize this increasing trend, to be aware of the status of intracranial adaptive capacity at this time, and to be able to deliver interventions minimizing or preventing the secondary insults. Other prior work that used variability measures, moving averages, and min-to-min variations have related these changes to outcomes rather than to predicting adverse periods of ICH within the monitoring period (Jones et al., 2003; Marmarou et al., 1991).
Advantages and Limitations
There are two advantages to the analytic approach used in this study. First, using moving average as a baseline not only takes into account the nonstationary state of the craniospinal system, but also reflects the conditions of intracranial dynamics. Second, using min-basis ICP values provides trend information and counters the criticism that an averaged ICP smooths too much of the data time course and loses too much information. However, these scientific advantages pose a limitation in terms of the usual bedside monitoring equipment, which is not usually programmed to provide data in this format.
The primary limitation to generalizing these findings is that only a quarter of the individuals with TBI from the parent study were selected. It is not clear that the same findings would hold for the rest of the individuals with TBI in the CPP project, in particular, those who underwent craniectomy surgery (where one might expect dampened ICP levels). Because only one DIICP event per subject was included in the statistical analysis, it is unknown if the predictors held true for all events in any given individual. Second, the time period of 48 hr after the deployment of ICP monitoring might not be sufficient to capture the natural evolution of the pathophysiologic states in TBI in relation to treatment events.
Clinical Implications
It is vital to provide simple and reliable clinical indicators in real time for critical care staff to assess or evaluate the craniospinal volume-pressure relationship and cerebrovascular pathophysiology. Our findings suggest that 1) there appears to be a visually recognizable transient increase in ICP that occurs shortly before a DIICP event, 2) this short-term increase may reflect the poor status of craniospinal adaptive capacity during this period, and 3) intervention during this window of increase may reduce potential risk for secondary insult. It should be relatively easy to display the ICP trend in a clinical bedside monitoring system and test the value of this increasing trend in everyday practice.
Acknowledgments
We would like to thank Dr. Martha J. Lentz for her guidance and assistance during with data analysis. The study from which the data were obtained was funded by grant R01 NR04901 from the National Institute of Nursing Research to Pamela H. Mitchell and Catherine Kirkness, principal investigators.
References
- Ackley BJ, Ladwig GB, editors. Nursing diagnosis handbook. Mosby, Inc; St. Louis: 2006. [Google Scholar]
- Cardoso ER, Rowan JO, Galbraith S. Analysis of the cerebrospinal fluid pulse wave in intracranial pressure. Journal of Neurosurgery. 1983;59:817–821. doi: 10.3171/jns.1983.59.5.0817. [DOI] [PubMed] [Google Scholar]
- Chambers IR, Treadwell L, Mendelow AD. Determination of threshold levels of cerebral perfusion pressure and intracranial pressure in severe head injury by using receiver-operating characteristic curves: an observational study in 291 patients. Journal of Neurosurgery. 2001;94:412–416. doi: 10.3171/jns.2001.94.3.0412. [DOI] [PubMed] [Google Scholar]
- Chesnut RM, Marshall LF, Klauber MR, Blunt BA, Baldwin N, Eisenberg HM, et al. The role of secondary brain injury in determining outcome from severe head injury. Journal of Trauma. 1993;34:216–222. doi: 10.1097/00005373-199302000-00006. [DOI] [PubMed] [Google Scholar]
- Contant CF, Jr., Robertson CS, Crouch J, Gopinath SP, Narayan RK, Grossman RG. Intracranial pressure waveform indices in transient and refractory intracranial hypertension. Journal of Neuroscience Methods. 1995;57(1):15–25. doi: 10.1016/0165-0270(94)00106-q. [DOI] [PubMed] [Google Scholar]
- Czosnyka M, Pickard JD. Monitoring and interpretation of intracranial pressure. Journal of Neurology, Neurosurgery, and Psychiatry. 2004;75:813–821. doi: 10.1136/jnnp.2003.033126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan JY, Kirkness C, Vicini P, Burr R, Mitchell P. Intracranial pressure waveform morphology and intracranial adaptive capacity. American Journal of Critical Care. 2008;17:545–554. [PubMed] [Google Scholar]
- Germon K. Interpretation of ICP pulse waves to determine intracerebral compliance. Journal of Neuroscience Nursing. 1988;20:344–351. doi: 10.1097/01376517-198812000-00004. [DOI] [PubMed] [Google Scholar]
- Gray WJ, Rosner MJ. Pressure-volume index as a function of cerebral perfusion pressure. Part 1: The effects of cerebral perfusion pressure changes and anesthesia. Journal of Neurosurgery. 1987;67:369–376. doi: 10.3171/jns.1987.67.3.0369. [DOI] [PubMed] [Google Scholar]
- Jones PA, Andrews PJ, Midgley S, Anderson SI, Piper IR, Tocher JL, et al. Measuring the burden of secondary insults in head-injured patients during intensive care. Journal of Neurosurgical Anesthesiology. 1994;6(1):4–14. [PubMed] [Google Scholar]
- Jones PA, Minns RA, Lo TY, Andrews PJ, Taylor GS, Ali S. Graphical display of variability and inter-relationships of pressure signals in children with traumatic brain injury. Physiological Measurement. 2003;24:201–211. doi: 10.1088/0967-3334/24/1/315. [DOI] [PubMed] [Google Scholar]
- Kirkness CJ, Burr RL, Cain KC, Newell DW, Mitchell PH. Relationship of cerebral perfusion pressure levels to outcome in traumatic brain injury. Acta Neurochirurgica Supplement. 2005;95:13–16. doi: 10.1007/3-211-32318-x_3. [DOI] [PubMed] [Google Scholar]
- Kirkness CJ, Burr RL, Cain KC, Newell DW, Mitchell PH. Effect of continuous display of cerebral perfusion pressure on outcomes in patients with traumatic brain injury. American Journal of Critical Care. 2006;15:600–610. [PubMed] [Google Scholar]
- Kirkness CJ, Mitchell PH, Burr RL, March KS, Newell DW. Intracranial pressure waveform analysis: clinical and research implications. Journal of Neuroscience Nursing. 2000;32:271–277. doi: 10.1097/01376517-200010000-00007. [DOI] [PubMed] [Google Scholar]
- Lang EW, Chesnut RM. Intracranial pressure and cerebral perfusion pressure in severe head injury. New Horizones. 1995;3:400–409. [PubMed] [Google Scholar]
- March K, Mitchell P, Grady S, Winn R. Effect of backrest position on intracranial and cerebral perfusion pressures. Journal of Neuroscience Nursing. 1990;22:375–381. doi: 10.1097/01376517-199012000-00008. [DOI] [PubMed] [Google Scholar]
- Marmarou A, Anderson RL, Ward JD, Choi SC, Young HF, Eisenberg HM, et al. Impact of ICP instability and hypotension on outcome in patients with severe head trauma. Journal of Neurosurgery. 1991;75(5S):S59–S66. [Google Scholar]
- Maset AL, Marmarou A, Ward JD, Choi S, Lutz HA, Brooks D, et al. Pressure-volume index in head injury. Journal of Neurosurgery. 1987;67:832–840. doi: 10.3171/jns.1987.67.6.0832. [DOI] [PubMed] [Google Scholar]
- McGraw CP, Howard G, O'Connor C. Outcome associated with management based on ICP monitoring. In: Ishii S, Nagai H, Brock M, editors. Intracranial Pressure V. Springer-Verlag; Berlin: 1983. pp. 58–561. [Google Scholar]
- Miller JD, Butterworth JF, Gudeman SK, Faulkner JE, Choi SC, Selhorst JB, et al. Further experience in the management of severe head injury. Journal of Neurosurgery. 1981;54:289–299. doi: 10.3171/jns.1981.54.3.0289. [DOI] [PubMed] [Google Scholar]
- Miller JD, Garibi J. Intracranial volume/pressure relationshipsduring continous monitoring of ventricular fluid pressure. In: Brock M, Dietz H, editors. Intracranial Pressure. Springer-Verlag; Berlin: 1972. pp. 270–274. [Google Scholar]
- Miller JD, Garibi J, Pickard JD. Induced changes of cerebrospinal fluid volume. Effects during continuous monitoring of ventricular fluid pressure. Archives of Neurology. 1973;28:265–269. doi: 10.1001/archneur.1973.00490220073011. [DOI] [PubMed] [Google Scholar]
- Mitchell PH. Decreased adaptive capacity, intracranial: a proposal for a nursing diagnosis. Journal Neuroscience Nursing. 1986a;18:170–175. doi: 10.1097/01376517-198608000-00002. [DOI] [PubMed] [Google Scholar]
- Mitchell PH. Intracranial hypertension: influence of nursing care activities. Critical Care Nursing Clinical North America. 1986b;21:563–576. [PubMed] [Google Scholar]
- Mitchell PH, Burr RL, Kirkness CJ. Information technology and CPP management in neuro intensive care. Acta Neurochirurgica Supplement (Wien) 2002;81:163–165. doi: 10.1007/978-3-7091-6738-0_42. [DOI] [PubMed] [Google Scholar]
- Mitchell PH, Mauss NK. Relationship of patient-nurse activity to intracranial pressure variations: a pilot study. Nursing Research. 1978;27:4–10. [PubMed] [Google Scholar]
- Mitchell PH, Ozuna J, Lipe HP. Moving the patient in bed: effects on intracranial pressure. Nursing Research. 1981;30:212–218. [PubMed] [Google Scholar]
- Muwaswes M. Increased intracranial pressure and its systemic effects. Journal of Neurosurgical Nursing. 1985;17:238–243. doi: 10.1097/01376517-198508000-00006. [DOI] [PubMed] [Google Scholar]
- Rauch ME, Mitchell PH, Tyler ML. Validation of risk factors for the nursing diagnosis decreased intracranial adaptive capacity. Journal of Neuroscience Nursing. 1990;22:173–178. doi: 10.1097/01376517-199006000-00008. [DOI] [PubMed] [Google Scholar]
- Resnick DK, Marion DW, Carlier P. Outcome analysis of patients with severe head injuries and prolonged intracranial hypertension. Journal of Trauma. 1997;42:1108–1111. doi: 10.1097/00005373-199706000-00021. [DOI] [PubMed] [Google Scholar]
- Rising CJ. The relationship of selected nursing activities to ICP. Journal of Neuroscience Nursing. 1993;25:302–308. doi: 10.1097/01376517-199310000-00006. [DOI] [PubMed] [Google Scholar]
- Saul TG, Ducker TB. Effect of intracranial pressure monitoring and aggressive treatment on mortality in severe head injury. Journal of Neurosurgery. 1982;56:498–503. doi: 10.3171/jns.1982.56.4.0498. [DOI] [PubMed] [Google Scholar]
- Stocchetti N, Rossi S, Buzzi F, Mattioli C, Paparella A, Colombo A. Intracranial hypertension in head injury: management and results. Intensive Care Medicine. 1999;25:371–376. doi: 10.1007/s001340050860. [DOI] [PubMed] [Google Scholar]
- Tsementzis SA, Harris P, Loizou LA. The effect of Routine nursing care procedures on the ICP in severe head injuries. Acta Neurochirurgica Supplement (Wien) 1982;65:153–166. doi: 10.1007/BF01405841. [DOI] [PubMed] [Google Scholar]
- Yau Y, Piper I, Contant C, Citerio G, Kiening K, Enblad P, et al. Multi-centre assessment of the Spiegelberg compliance monitor: interim results. Acta Neurochirurgica Supplement (Wien) 2002;81:167–170. doi: 10.1007/978-3-7091-6738-0_43. [DOI] [PubMed] [Google Scholar]