Abstract
Purpose of review
Tuberous sclerosis complex (TSC) is a multiorgan genetic disease caused by mutations in the TSC1 or TSC2 genes. TSC has been recognized for many years as an important cause of severe neurological disease with patients suffering from epilepsy, developmental delay, autism, and psychiatric problems. During the last year there has been enormous advances in basic and translational research pertaining to TSC.
Recent findings
In this review, I discuss the basic science findings that position the TSC1 and TSC2 genes as critical regulators of the mTOR kinase within mTORC1. In addition, I will discuss the development of new animal models, translational data, and recent clinical trials using mTORC1 inhibitors such as rapamycin.
Summary
The past few years have seen spectacular advances that have energized TSC related research and challenged existing symptomatic treatments. While it remains to be seen whether use of mTORC1 inhibitors will revolutionize the care of patients with TSC, the application of basic and translational research towards a specific clinical disorder emphasizes the potential and promise of molecular medicine.
Keywords: tuber, epilepsy, autism, cortical development, progenitor cell, mTOR, mTORC1
"O wonder!
How many goodly creatures are there here!
How beautious mankind is!
O brave new world,
That has such people in't! "
-Miranda, from “The Tempest” by William Shakespeare
Introduction
Tuberous Sclerosis Complex (TSC) is a relatively common multi-system disorder seen in approximately 1:6,000 people worldwide [1]. Children and adults with TSC are well known to neurologists as they have a very high prevalence of epilepsy, autism, developmental delay, mental retardation, and other neurological and psychiatric problems.
Genetics
TSC was initially described in the 19th century [2] but further progress on disease pathogenesis was quite slow. The eventual emergence of molecular biology and genetics allowed the cloning of the causative TSC1 and TSC2 genes that respectively encode hamartin and tuberin [3–5]. As these proteins physically bind and function within a multiprotein entity, loss of function of either TSC1 and TSC2 appears sufficient to cause TSC though patients with TSC2 mutations typically have more severe clinical findings than those with TSC1 [5]. TSC can be inherited as an autosomal dominant disorder though the majority of patients have apparent spontaneous mutations in TSC1 or TSC2 [6]. While some controversy remains, the prevailing model invokes a “two-hit hypothesis” with functional loss of both copies of either the TSC1 or TSC2 genes required to produce disease [7]. Evidence for such a model is readily found in kidney, skin, and lung lesions associated with TSC but has been quite difficult to demonstrate in brain malformations [8]. This lack of evidence has led some investigators to postulate that hamartin or tuberin may be inactivated through post-translational modifications such as phosphorylation by ERK [9].
Neuropathology
Hamartomas are the pathologic hallmark of TSC. These lesions contain cells that have undergone abnormal differentiation but generally behave as benign tumors. TSC associated hamartomas in the cerebral cortex (tubers) are severe malformations of cortical development with complete disruption of the normal laminar organization of the cerebral cortex. In addition, tubers contain abnormally large (“giant”) cells that variously express markers of both neuronal and glia differentiation. Epilepsy and autism in TSC are generally ascribed to the presence of these tubers though there is some evidence to suggest functional abnormalities in brain regions that appear to be tuber-free [10]. In addition to tubers, other brain hamartomas include subependymal nodules and subependymal giant cell astrocytomas (SEGAs). These latter lesions are clinically important as continued growth near the Foramen of Monro during the first 20 years of life can lead to CSF obstruction flow and may eventually cause hydrocephalus, visual loss, and death.
Epilepsy
There is an incredibly high prevalence of seizure disorders in TSC, affecting at least 90% of all patients [11]. Many different types of partial and generalized seizures are seen in TSC and are often very difficult to treat with conventional therapies. Of note, infantile spasms (IS) are seen in up to 50% of children with TSC. While typically a devastating form of seizures in young children, IS in TSC patients respond exceptionally well to vigabatrin [12]. As vigabatrin inhibits the catabolism of GABA to increase brain levels of this inhibitory neurotransmitter, the pathophysiology of infantile spasms in TSC may relate to abnormal GABAergic inhibition within the brain. Despite the rapid cessation of IS in most patients with TSC treated with vigabatrin and the near normalization of their EEG, the long term prognosis remains poor for many patients though importantly, a subset have normal intelligence or mild cognitive impairment [13•]. While the response of IS to vigabatrin is an important clue to the early pathophysiology of TSC, much more clearly needs to be learned about the development and progression of epilepsy and other neurological and psychiatric abnormalities in these patients.
Signaling Pathways
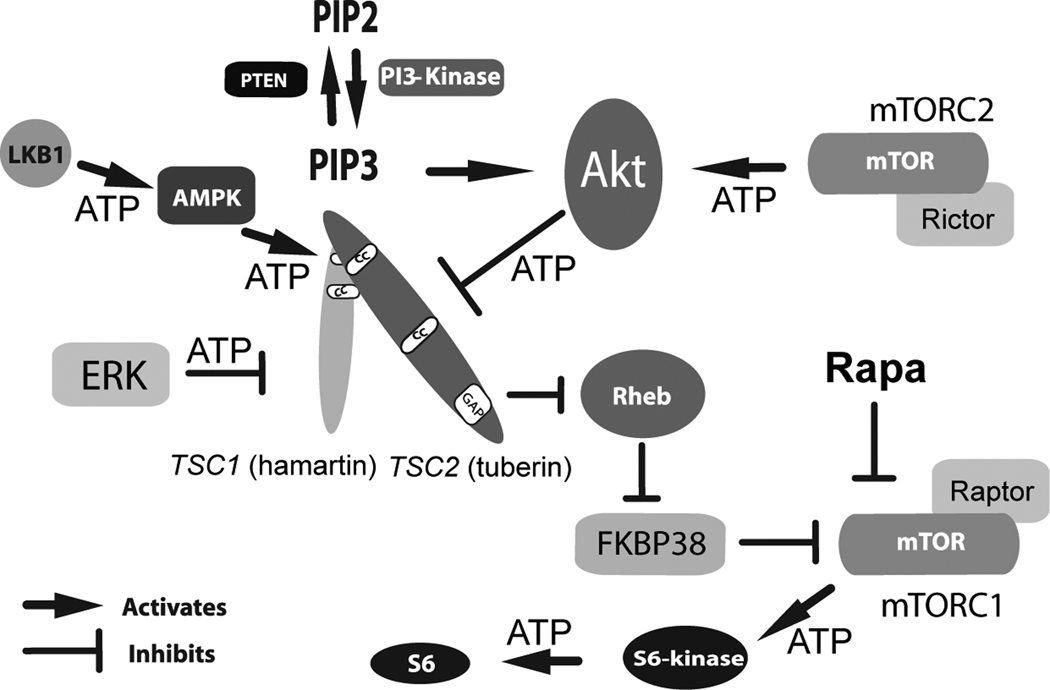
The main catalyst for the accelerated pace of research discoveries in this field was the identification of TSC1/TSC2 as upstream regulators of Rheb and the mTOR kinase (Figure 1, reviewed in [14]). These findings almost immediately suggested that inhibitors of mTOR might be a rational and effective treatment for patients with TSC. Furthermore, the placement of mTOR downstream of TSC1/TSC2 was quite plausible as mTOR was known to participate in multiple processes relevant to TSC including control of cell size and proliferation [15]. Other studies later determined that mTOR could be found within two molecularly and functionally distinct complexes, mTORC1 and mTORC2. mTORC1 includes mTOR, mLST8, and raptor and is rapamycin sensitive while mTORC2 includes mTOR, mLST8, mSIN1, and rictor and is relatively rapamycin insensitive [16]. Within mTORC1, mTOR phosphorylates ribosomal S6 kinase that in turn phosphorylates ribosomal S6 to impact mRNA translation. In contrast, mTORC2 phosphorylates and activates AKT, a central kinase in the regulation of cell division and proliferation. Loss of TSC1 or TSC2 then allows continued phosphorylation and activity of S6-Kinase and S6. While increased mRNA translation is postulated to underlie the abnormal cellular function in TSC, very little is known about what subsets of mRNAs may be responsible. In addition to the regulation of mTORC1, hamartin/tuberin appear to physically interact with mTORC2 and are required for AKT phosphorylation and activation [17••]. This last pathway may explain why TSC-associated lesions are generally benign if AKT activation depends on functional alleles of TSC1 and TSC2.
Figure 1. Schematic of Upstream and Downstream Signaling Pathways in TSC.
A complex series of phosphorylation events and other protein interactions regulate hamartin/tuberin and activity of the mTOR kinase. Initial growth factor binding to transmembrane receptors (not shown) activates PI-3 Kinase resulting in increased production of PIP3 with AKT activation that directly phosphorylates and inhibits tuberin. ERK can also phosphorylate and inactivate tuberin. In contrast, AMPK phosphorylation at distinct amino acid residues serves to activate tuberin. Loss of the TSC1 or TSC2 genes leads to constitutive activation of mTOR within mTORC1 with greatly increased levels of phosphorylated ribosomal S6-kinase and phosphorylated ribosomal S6. Rapamycin inhibits mTOR activity within mTORC1 to restore inhibition of this kinase and downstream components within this signaling pathway. ATP indicates phosphorylation events.
AKT (proto-oncogene also known as PKB); AMPK, AMP-activated protein kinase; ERK, Extracellular Signal-Regulated Kinases; FKBP38, FK506-binding protein 38; LKB1, Peutz-Jeghers Syndrome Kinase; mTOR, mammalian target of rapamycin; mTORC1, mammalian target of rapamycin complex 1; mTORC2, mammalian target of rapamycin two; PIP2, phosphatidylinositol bisphosphate; PIP3, phosphatidylinositol (3,4,5)-trisphosphate; PTEN, phosphatase and tensin homolog; Rapa, rapamycin; Raptor, regulatory associated protein of mTOR, Rictor, rapamycin-insensitive companion of mTOR; Rheb, Ras homolog enriched in brain; TSC1, (hamartin); TUBEROUS SCLEROSIS COMPLEX GENE ONE; TSC2, (tuberin) TUBEROUS SCLEROSIS COMPLEX GENE TWO.
Animal Models
Determining the utility of mTORC1 inhibitors necessitated the concomitant development of preclinical animal models of TSC. Such models are essential to validate treatment advances as well as investigate toxicity. Along these lines, both conventional and conditional knockouts of the mouse Tsc1 and Tsc2 genes have been reported. Overall, conventional gene knockout models have not been illuminating as complete loss of either gene led to embryonic lethality prior to any substantial brain development [18,19]. Heterozygous (+/−) mice have minimal brain pathology with the exception of some astrocytosis [20]. However, Tsc2+/− mice exhibit moderate increased mTORC1 activity and learning deficits that are reversible by rapamycin (see below). Conditional knockout models using the Cre-LoxP system have thus been extremely advantageous to elucidate abnormalities in specific neural populations including astrocytes [21] and post-mitotic neurons [22]. Both of these conditional knockout mouse models have been used to determine if their specific neuropathology is mTORC1-dependent and thus reversible through postnatal treatment with rapamycin or RAD001, a related compound (see below). Homozygous conditional knockout (CKO) mice for the Tsc1 gene in astrocytes (Tsc1GFAP CKO) were reported several years ago. These animals have well documented seizures that increase in frequency over time culminating in death around 3 months of age. Increased numbers of astrocytes are found in the cerebral neocortex as well as the hippocampus. Astrocyte mediated abnormalities of both glutamate transport [23] and potassium uptake [24] were reported in brains from Tsc1GFAP CKO mice. Either of these alterations in astrocyte homeostasis are expected to lower the seizure threshold either via glutamate mediated excitotoxicity or abnormal neuronal depolarization. These findings wonderfully illustrate how animal models can expand the scope of a field of research and also focus attention back to human tissues for validation [25•].
Two additional CKO models of TSC were created using either the synapsin (Tsc1Synapsin CKO mice) or CamKII promoters (Tsc1CamKII CKO mice) to mediate Tsc1 gene inactivation in post-mitotic neurons [26,27]. Tsc1Synapsin CKO mice have been more extensively described by two independent groups and will be further reviewed though Tsc1CamKII CKO mice have similar abnormalities. Tsc1Synapsin CKO mice have increased mTORC1 activity in glutamatergic and GABAergic neurons as well as increased neuronal size [22]. Some discrepancies are apparent with reports of either mild [28] or moderate cortical disorganization [22]. Clearly no overt tuber-like malformations were seen though a hypomyelinated cortex was present suggesting a secondary alteration in oligodendrocyte function. Tsc1Synapsin CKO mice also suffer from poor growth, spontaneous seizures, and premature death by 65 days of life. While these studies point to the requirement of the Tsc1 gene for postmitotic neuronal development and function, the formation of tubers in patients with TSC likely requires loss of the Tsc1 gene at earlier stages of brain development. Given the multilineage abnormalities seen in brains of patients with TSC, loss of the TSC1 or TSC2 genes within neural progenitor cells are most likely responsible for tuber formation. Testing this hypothesis will require the development of mouse models of TSC targeting neural progenitor cells at early stages of brain development.
Treatment of mouse models with mTORC1 inhibitors
A remarkable trio of publications during the last year have provided important animal data to support the use mTORC1 inhibitors as therapeutic agents in TSC. The laboratory of Michael Wong [29••] found that seizures and premature death of Tsc1GFAP CKO mice were preventable by treatment with rapamycin. More importantly, earlier treatment with rapamycin before seizures first appeared was found to prevent later epilepsy altogether. These results suggest that rapamycin may function as a bona fide anti-epileptic drug able to prevent seizure initiation or progression in TSC. The authors go on to show that the likely mechanism of rapamycin in these mice involves the restoration of glutamate homeostasis within the synaptic cleft of excitatory neurons. Further support for such a mechanism was seen as stopping rapamycin treatment again allowed seizure initiation and disease progression. This report was particularity compelling as the authors used prolonged EEG monitoring of these mice to precisely determine seizure frequency and their response to early versus late initiation of rapamycin.
Another important publication from the laboratory of David Kwiatkowski [26••] reported on the response of Tsc1Synapsin CKO mice to treatment with rapamycin as well as RAD001, a related mTORC1 inhibitor. Treatment of Tsc1Synapsin CKO mice with either agent markedly increased survival and prevented mTORC1 hyperactivity within the brain. Furthermore, the rapamycin treated mice had a near resolution of cell size defects and impaired myelination that was previously noted [22]. Finally, the authors report that rapamycin treatment led to restoration of AKT activation in the brains of the neuronal knockout mice. This finding is of interest as TSC1 and TSC2 deficient cells have impaired AKT activation, likely due to loss of mTORC2 activity [17•]. Given the powerful oncogenic properties of activated AKT in many human cancers, these findings may help explain why the tumors seen in TSC are generally not malignant. The usage of mTORC1 inhibitors should be viewed with caution for patents with TSC as this finding suggests that mTORC1 inhibition may promote tumor growth through AKT activation. However it should be stressed that there is no data to support this concern either in mouse models of TSC or in TSC patients treated with rapamycin (see below).
A final publication by the laboratory of Alcino Silva [27••] used conventional Tsc2 heterozygous knockout mice to assess learning abnormalities and response to rapamycin. These mice do not have cortical or hippocampal structural abnormalities though an increase in mTORC1 levels was reported in the hippocampus. The authors further show that Tsc2+/− mice have impaired long term potentiation (LTP) and abnormalities in hippocampal-dependent learning tasks such as spatial memory in the Morris water maze. Rapamycin was able to completely suppress mTORC1 activity and was associated with normalization of LTP and spatial learning. These results suggest that even partial dysregulation of mTORC1 may underlie some of the functional deficits seen in patients with TSC and that “tuber-less” regions of the brain may contribute to their morbidity. Similar results were previously reported in Tsc1+/− mice though the effect of mTORC1 inhibitors on impaired learning was not studied [30].
Clinical Trials
Given the lack of effective treatments for TSC other than symptomatic treatment of seizure disorders, recognition of the above signaling pathways quickly lead to the suggestion that mTORC1 inhibitors such as rapamycin maybe be rational therapies to treat and possibly prevent clinical aspects of TSC. As a practical matter, rapamycin is a readily available FDA approved drug that has been used for many years as an immunosuppressant to prevent rejection of transplanted solid organs and has a well-known side effect profile. Thus far, there have been two reports of rapamycin use in patients with neurological disease from TSC [31••, 32]. Both publications reported the treatment of SEGAs in patients who were considered to be poor surgical candidates. SEGA regression was seen in all patients and likely obviated the need for any surgical intervention. Patients appeared to tolerate rapamycin therapy well without any major side effects. Treatment duration was from 2.5 months to greater than 9 months. One patient was reported to have recurrence of SEGA growth after rapamycin was stopped though resumption of therapy was again associated with tumor regression.
In addition to the CNS, rapamycin has been employed for the serious kidney and lung manifestations seen in TSC or lymphangioleiomyomatosis, a genetically related disorder [33••, 34••]. Use of rapamycin in these adult patients was notable for shrinkage of kidney lesions and a potential slowing in the progression of the pulmonary disease. Side effects were again manageable consisting mainly of aphthous ulcers, diarrhea, and mild respiratory infections.
These initial clinical results are very encouraging and appear to validate the accruing biochemical, cell culture, and animal model based research. However, as these studies reported treatment of relatively few subjects, rare but serious side effects may not have been detected in patients with TSC. From these results it seems likely that mTORC1 inhibitors will require continuous use to be efficacious for patients with TSC. Negative effects from chronic exposure to mTORC1 inhibitors especially in young children are essentially unknown. These may include immunosuppression, alterations in growth, and abnormalities of lipid metabolism. Finally, it is unknown whether human patients with TSC will respond to mTORC1 inhibitors to ameliorate the devastating neurological impact of epilepsy and autism.
Conclusion
Aldous Huxley in Brave New World (1932) used Miranda’s words to caution against a possible future dystopia where an overreliance on drugs is the cure for all societal problems. Similar caution may now be applied to the stimulating research findings before us and their logical but unclear application to patients with TSC. While the research in this field is tremendously exciting and promising, rigorous clinical trials are required to determine the proper usage of mTORC1 inhibitors in the treatment of TSC and related disorders.
Acknowledgements
Dr. Ess is supported by the NINDS, NIH and the Tuberous Sclerosis Alliance. Support was also provided by the Vanderbilt Kennedy Center for Research on Human Development.
Dr. Ess receives research funding from the NINDS, NIH and the Tuberous Sclerosis Alliance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med. 2006;355:1345–1356. doi: 10.1056/NEJMra055323. [DOI] [PubMed] [Google Scholar]
- 2.Bourneville DM. Tuberous Sclerosis with Cortical Abnormalities: Mental Retardation and Hemiplegic Epilepsy. Archives de neurologie. 1880;1:81–91. [Google Scholar]
- 3.van Slegtenhorst M, de Hoogt R, Hermans C, Nellist M, Janssen B, Verhoef S, Lindhout D, van den Ouweland A, Halley D, Young J, et al. Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science. 1997;277:805–808. doi: 10.1126/science.277.5327.805. [DOI] [PubMed] [Google Scholar]
- 4.Consortium ECTS. Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell. 1993;75:1305–1315. doi: 10.1016/0092-8674(93)90618-z. [DOI] [PubMed] [Google Scholar]
- 5.Sancak O, Nellist M, Goedbloed M, Elfferich P, Wouters C, Maat-Kievit A, Zonnenberg B, Verhoef S, Halley D, van den Ouweland A. Mutational analysis of the TSC1 and TSC2 genes in a diagnostic setting: genotype--phenotype correlations and comparison of diagnostic DNA techniques in Tuberous Sclerosis Complex. Eur J Hum Genet. 2005;13:731–741. doi: 10.1038/sj.ejhg.5201402. [DOI] [PubMed] [Google Scholar]
- 6.Dabora SL, Jozwiak S, Franz DN, Roberts PS, Nieto A, Chung J, Choy YS, Reeve MP, Thiele E, Egelhoff JC, et al. Mutational analysis in a cohort of 224 tuberous sclerosis patients indicates increased severity of TSC2, compared with TSC1, disease in multiple organs. American Journal of Human Genetics. 2001;68:64–80. doi: 10.1086/316951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parry L, Maynard JH, Patel A, Clifford SC, Morrissey C, Maher ER, Cheadle JP, Sampson JR. Analysis of the TSC1 and TSC2 genes in sporadic renal cell carcinomas. British Journal of Cancer. 2001 October 19;85(8):1226–1230. doi: 10.1054/bjoc.2001.2072. 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henske EP, Scheithauer BW, Short MP, Wollmann R, Nahmias J, Hornigold N, van Slegtenhorst M, Welsh CT, Kwiatkowski DJ. Allelic loss is frequent in tuberous sclerosis kidney lesions but rare in brain lesions. American Journal of Human Genetics. 1996;59:400–406. [PMC free article] [PubMed] [Google Scholar]
- 9.Jozwiak J, Jozwiak S, Wlodarski P. Possible mechanisms of disease development in tuberous sclerosis. Lancet Oncol. 2008;9:73–79. doi: 10.1016/S1470-2045(07)70411-4. [DOI] [PubMed] [Google Scholar]
- 10.Wong M. The utility of tuberless models of tuberous sclerosis. Epilepsia. 2007;48:1629–1630. doi: 10.1111/j.1528-1167.2007.01178_1.x. author reply 1632-1624. [DOI] [PubMed] [Google Scholar]
- 11.Thiele EA. Managing epilepsy in tuberous sclerosis complex. J Child Neurol. 2004;19:680–686. doi: 10.1177/08830738040190090801. [DOI] [PubMed] [Google Scholar]
- 12.Elterman RD, Shields WD, Mansfield KA, Nakagawa J. Randomized trial of vigabatrin in patients with infantile spasms. Neurology. 2001 October 23;57(8):1416–1421. doi: 10.1212/wnl.57.8.1416. 2001. [DOI] [PubMed] [Google Scholar]
- 13. Muzykewicz DA, Costello DJ, Halpern EF, Thiele EA. Infantile spasms in tuberous sclerosis complex: Prognostic utility of EEG. Epilepsia. 2008 doi: 10.1111/j.1528-1167.2008.01788.x. The authors analyzed a large and well characterized TSC clinic population for EEG findings predictors of clinical outcomes for patients with infantile spasms and suggest early detection and treatment may allow for best cognitive outcomes.
- 14.Kwiatkowski DJ. Rhebbing up mTOR: new insights on TSC1 and TSC2, and the pathogenesis of tuberous sclerosis. Cancer Biol Ther. 2003;2:471–476. doi: 10.4161/cbt.2.5.446. [DOI] [PubMed] [Google Scholar]
- 15.Mak BC, Yeung RS. The tuberous sclerosis complex genes in tumor development. Cancer Invest. 2004;22:588–603. doi: 10.1081/cnv-200027144. [DOI] [PubMed] [Google Scholar]
- 16.Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 17. Huang J, Dibble CC, Matsuzaki M, Manning BD. The TSC1-TSC2 complex is required for proper activation of mTOR complex 2. Mol Cell Biol. 2008;28:4104–4115. doi: 10.1128/MCB.00289-08. This study elegantly describes the complicated signaling pathways in TSC and underscores how basic science are rapidly translatable to neurological practice.
- 18.Kobayashi T, Minowa O, Sugitani Y, Takai S, Mitani H, Kobayashi E, Noda T, Hino O. A germ-line Tsc1 mutation causes tumor development and embryonic lethality that are similar, but not identical to, those caused by Tsc2 mutation in mice. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:8762–8767. doi: 10.1073/pnas.151033798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Onda H, Lueck A, Marks PW, Warren HB, Kwiatkowski DJ. Tsc2(+/−) mice develop tumors in multiple sites that express gelsolin and are influenced by genetic background. Journal of Clinical Investigation. 1999;104:687–695. doi: 10.1172/JCI7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uhlmann EJ, Apicelli AJ, Baldwin RL, Burke SP, Bajenaru ML, Onda H, Kwiatkowski D, Gutmann DH. Heterozygosity for the tuberous sclerosis complex (TSC) gene products results in increased astrocyte numbers and decreased p27-Kip1 expression in TSC2+/− cells. Oncogene. 2002;21:4050–4059. doi: 10.1038/sj.onc.1205435. [DOI] [PubMed] [Google Scholar]
- 21.Uhlmann EJ, Wong M, Baldwin RL, Bajenaru ML, Onda H, Kwiatkowski DJ, Yamada K, Gutmann DH. Astrocyte-specific TSC1 conditional knockout mice exhibit abnormal neuronal organization and seizures. Annals of Neurology. 2002;52:285–296. doi: 10.1002/ana.10283. [DOI] [PubMed] [Google Scholar]
- 22.Meikle L, Talos DM, Onda H, Pollizzi K, Rotenberg A, Sahin M, Jensen FE, Kwiatkowski DJ. A mouse model of tuberous sclerosis: neuronal loss of Tsc1 causes dysplastic and ectopic neurons, reduced myelination, seizure activity, and limited survival. J Neurosci. 2007;27:5546–5558. doi: 10.1523/JNEUROSCI.5540-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong M, Ess KC, Uhlmann EJ, Jansen LA, Li W, Crino PB, Mennerick S, Yamada KA, Gutmann DH. Impaired glial glutamate transport in a mouse tuberous sclerosis epilepsy model. Ann Neurol. 2003;54:251–256. doi: 10.1002/ana.10648. [DOI] [PubMed] [Google Scholar]
- 24.Jansen LA, Uhlmann EJ, Crino PB, Gutmann DH, Wong M. Epileptogenesis and reduced inward rectifier potassium current in tuberous sclerosis complex-1-deficient astrocytes. Epilepsia. 2005;46:1871–1880. doi: 10.1111/j.1528-1167.2005.00289.x. [DOI] [PubMed] [Google Scholar]
- 25. Sosunov AA, Wu X, Weiner HL, Mikell CB, Goodman RR, Crino PD, McKhann GM., 2nd Tuberous sclerosis: a primary pathology of astrocytes? Epilepsia. 2008;49 Suppl 2:53–62. doi: 10.1111/j.1528-1167.2008.01493.x. This study looked at human brain sections resected from epilepsy patients and showed upregulation of mTORC1 activity and prominent astrocyte abnormalities.
- 26.Meikle L, Pollizzi K, Egnor A, Kramvis I, Lane H, Sahin M, Kwiatkowski DJ. Response of a neuronal model of tuberous sclerosis to mammalian target of rapamycin (mTOR) inhibitors: effects on mTORC1 and Akt signaling lead to improved survival and function. J Neurosci. 2008;28:5422–5432. doi: 10.1523/JNEUROSCI.0955-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ehninger D, Han S, Shilyansky C, Zhou Y, Li W, Kwiatkowski DJ, Ramesh V, Silva AJ. Reversal of learning deficits in a Tsc2+/− mouse model of tuberous sclerosis. Nat Med. 2008;14:843–848. doi: 10.1038/nm1788. The authors report learning abnormalities in mice heterozygous for the Tsc2 gene and demonstrate reversal of these deficits with rapamycin. This finding is of considerable interest as these mice do not exhibit neuronal disorganization or frank tubers.
- 28. Wang Y, Greenwood JS, Calcagnotto ME, Kirsch HE, Barbaro NM, Baraban SC. Neocortical hyperexcitability in a human case of tuberous sclerosis complex and mice lacking neuronal expression of TSC1. Ann Neurol. 2007;61:139–152. doi: 10.1002/ana.21058. This study analyzed and compared Tsc1Synapsin CKO mice and human cortex resected from a patient with TSC. They found abnormal increased synaptic excitation in both mouse and human tissue despite the absence of tuber-like lesions.
- 29. Zeng LH, Xu L, Gutmann DH, Wong M. Rapamycin prevents epilepsy in a mouse model of tuberous sclerosis complex. Ann Neurol. 2008;63:444–453. doi: 10.1002/ana.21331. The authors demonstrated for the first time that seizures and astrocyte abnormalities in a mouse model of TSC can be treated by rapamycin. More importantly, earlier rapamycin treatment was able to completely suppress seizure development.
- 30.Goorden SM, van Woerden GM, van der Weerd L, Cheadle JP, Elgersma Y. Cognitive deficits in Tsc1+/− mice in the absence of cerebral lesions and seizures. Ann Neurol. 2007;62:648–655. doi: 10.1002/ana.21317. [DOI] [PubMed] [Google Scholar]
- 31.Franz DN, Leonard J, Tudor C, Chuck G, Care M, Sethuraman G, Dinopoulos A, Thomas G, Crone KR. Rapamycin causes regression of astrocytomas in tuberous sclerosis complex. Ann Neurol. 2006;59:490–498. doi: 10.1002/ana.20784. [DOI] [PubMed] [Google Scholar]
- 32.Koenig MK, Butler IJ, Northrup H. Regression of subependymal giant cell astrocytoma with rapamycin in tuberous sclerosis complex. J Child Neurol. 2008;23:1238–1239. doi: 10.1177/0883073808321764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bissler JJ, McCormack FX, Young LR, Elwing JM, Chuck G, Leonard JM, Schmithorst VJ, Laor T, Brody AS, Bean J, et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med. 2008;358:140–151. doi: 10.1056/NEJMoa063564. This study demonstrated for the first time that rapamycin (sirolimus) appears to cause regression of kidney lesions in patients with TSC. Some improvement in lung manifestations was also reported. Both manifestations may require prolonged or continuous therapy.
- 34. Davies DM, Johnson SR, Tattersfield AE, Kingswood JC, Cox JA, McCartney DL, Doyle T, Elmslie F, Saggar A, de Vries PJ, et al. Sirolimus therapy in tuberous sclerosis or sporadic lymphangioleiomyomatosis. N Engl J Med. 2008;358:200–203. doi: 10.1056/NEJMc072500. This study like Bissler et al [33] also reported use of sirolimus for kidney and lung manifestation of TSC and lymphangioleiomyomatosis. They found modest reductions in the size of the kidney angiomyolipomas. In contrast, lung function did not improve. While only a few patients were treated, these results coupled with Bissler et al. are encouraging.