Summary
Background
Mannan-binding lectin (MBL) deficiency is determined by MBL gene polymorphisms and associated with an increased infection risk.
Methods
To clarify the role of MBL in allogeneic stem cell transplantation (SCT) 131 recipients/donors were analysed. MBL-genotypes were determined by PCR and heteroduplex analyses, MBL-serum levels by ELISA, MBL oligomers by Western blotting.
Results
MBL-levels <400ng/ml were associated with increased susceptibility to fungal pneumonia (7/12 vs. 35/111; p=0.04, adjusted p=0.002), HSV/VZV (7/12 vs. 26/111; p=0.03), CMV-reactivation and acute graft-versus-host-disease. Donor genotypes had no influence. Pre-SCT MBL-levels corresponded to recipients’ genotypes (p<0.001), changed significantly post-SCT, but were not influenced by donors’ genotypes. MBL oligomer profiles were similar pre-/post-SCT. Cultured CD34+ cells were not found to synthesize MBL.
Conclusions
Low MBL-levels pre-transplant predisposed patients to sepsis, fungal and viral infection. Donors’ MBL genotypes did not influence infection rates. Prospective studies should clarify the importance of MBL as prelude for MBL-replacement after SCT.
Keywords: Adolescent; Adult; Aged; Disease Susceptibility; Female; Genotype; Graft vs Host Disease; etiology; genetics; Humans; Male; Mannose-Binding Lectin; blood; genetics; Middle Aged; Mycoses; etiology; Phenotype; Prospective Studies; Retrospective Studies; Sepsis; etiology; Stem Cell Transplantation; adverse effects; mortality; Tissue Donors; Transplantation, Homologous
Keywords: Mannan-binding lectin (MBL) protein, MBL variants, sepsis, infection, allogeneic stem cell transplantation (SCT)
Introduction
Mannan-binding lectin (MBL) is a component of the innate immune system. The carbohydrate recognition domain of the MBL protein binds to polysaccharides of microorganisms and activates the complement system in an antibody independent fashion.1 Mutations in the structural and regulatory sequences of the MBL gene lead to great inter-individual variation.2 MBL variants due to single nucleotide polymorphisms in exon 1 - B (G54D), C (G57E), and D (R52C)1;3 - are found in approximately 30% of the general population.
Several studies have demonstrated increased infection rates in patients with low MBL levels4;5 and also in neutropenia following chemotherapy.6;7 Recently, MBL variants were associated with an increased risk of infections in myeloablative, TBI-conditioned transplantation.8 Conversely, patients with wildtype genotypes have a reduced sepsis risk during autologous stem cell transplantation (SCT).9 Limited studies show an influence of both recipients and donor genotypes10–12 on the infection risk in this setting. In solid organ recipients, MBL deficiency is associated with increased CMV reactivation.11 MBL may also influence the development of graft versus host disease (GvHD) and graft rejection in solid organ recipients.13
We analysed 131 stem cell recipients to evaluate the influence of MBL on transplant related mortality (TRM), infection, and acute GvHD (aGvHD) in the allo-SCT setting.
Patients and Methods
Patients
From January 2001 – October 2003 a total of 131 patients, who received allo-SCT from related/unrelated donors at the University of Hamburg, Germany, were enrolled in a combined pro-/retrospective study after ethical approval and patients’ informed consent.
MBL genotype and protein analyses
Blood for MBL pheno- and genotypes was collected pre-, on days 0–45 and days +46–75 post-transplant. MBL serum levels were determined by ELISA (Antibody Shop, Denmark). DNA was isolated from whole-blood samples (QIAamp DNA, blood mini kit, Qiagen, Hilden, Germany).
The MBL variants in Exon 1 B, C and D (codons 54, 57, and 52) were determined by PCR and heteroduplex analyses utilising polyacrylamide gel electrophoresis.14 Patients homozygous for wildtype alleles were denoted A/A, homozygous for variant alleles O/O, and heterozygous A/O. Promoter polymorphisms of MBL were determined similarly.15 The structural MBL-2 mutations B, C, and D are in linkage disequilibrium with the promoter region polymorphism X/Y, so only Y associates with variant alleles. Western blot analyses of MBL protein pre- and post-transplant were performed using SDS-Page gel (Hybond C extra, Amersham Biosciences, UK) and immunoblotting as described previously.16 To investigate whether MBL is synthesized by CD34+ cells, mononuclear cells were obtained from three bone marrow samples (donors with MBL wildtype) and CD34+ cells were separated and cultured as previous described.17 MBL concentrations in cultures were analysed on day 14 using the ELISA.
Infection, GVHD and TRM
Patients were monitored during the study period (days 0 to +75) for frequency of infections, GVHD and TRM. Blood cultures, C-reactive protein (CRP) and imaging were performed when infection was suspected. Definite sepsis was diagnosed in case of positive blood cultures and clinical and/or laboratory sepsis evidence. Clinical sepsis was diagnosed in absence of a positive blood culture. Diagnosis of sepsis was retrospectively made by two blinded clinicians. Viral reactivation, GVHD and TRM were also recorded. Fungal pneumonia was defined as presumed, possible or definitive.18
Statistical analysis
Patients were classified according to MBL exon-1 mutations and their corresponding promoter allotypes as follows: Patients with YA/YA, YA/XA, or XA/XA genotypes were wildtype (A/A), patients with YA/YD, YA/YB+YC, XA/YD or XA/YB+YC heterozygous (A/O), and patients with YO/YO MBL genotype homozygous (O/O).
Genotype/allele frequencies were analysed by Chi-square. Differences between groups (MBL serum levels) were compared by Mann-Whitney U Test. Changes in mean MBL values were performed by Wilcoxon’s signed rank test. The analyses of the risk of sepsis, viral reactivation, GVHD, and TRM according to MBL genotype and serum levels were performed by log-rank test and Kaplan-Meier. In addition, multivariable analysis (Cox proportional hazard model) was performed to estimate the independent effect of MBL level on fungal pneumonia, CMV-reactivation and acute graft-versus-host-disease adjusted for recipient and donor age, sex, diagnosis, type of transplant (BM vs. PBSC), type of donor (unrelated or related), HLA type (match or mismatch), conditioning (standard or reduced), type of GvHD prophylaxis (with or without antithymocyte globulin), MBL genotypes of recipient and donor. SPSS version 13.0 (SPSS, Inc, Chicago, IL, USA) and SAS v9.1 (SAS®, Cary, NC, USA) was used for analyses.
Results
Patients’ and donors’ characteristics
Characteristics of donor and recipients are summarised in Table 1.
Table 1.
Patient/Donor characteristics, complications and outcome (n=131)
| PATIENT | |
| Age at SCT in years (median) | 13–69 (46) |
| Sex of patients (m/f) | 83/48 |
| CMV positive | 75 (58%) |
| DONOR | |
| Sex of donors (m/f) | 90/41 |
| CMV positive | 73 (56%) |
| Matched/Mismatched | 107/24 |
| Related/Unrelated | 51/80 |
| DISEASE | |
| AML | 35 (27%) |
| ALL | 16 (12%) |
| Lymphoma | 11 (8%) |
| Multiple Myeloma | 20 (15%) |
| CML | 26 (20%) |
| Myelodysplastic Syndrome | 10 (8%) |
| Others | 13 (10%) |
| TYPE OF TRANSPLANT | |
| PBSC | 109 (83%) |
| BMT | 22 (17%) |
| CONDITIONING REGIMENS | |
| Bu/Cy+/−VP16 | 50 (38%) |
| TBI/Cy/VP16 | 19 (14%) |
| RIC | 62 (48%) |
| ENGRAFTMENT | |
| Leukocyte (≥ 1.0 ×109/L) | 6–26 days (mean 15) |
| Lymphocyte (×106/ml) | 20–3340 (mean 1008) |
| NK cells (×106/ml) | 8–858 (mean 239) |
| ACUTE GVHD | |
| Grade 0–I | 82 (63%) |
| Grade II–IV | 49 (37%) |
| Grade III–IV | 29 (22%) |
| INFECTIONS | |
| Definite sepsis | 50 (38%) |
| Clinical sepsis | 56 (43%) |
| Pneumonia | 76 (58%) |
| Fungal | 46 (35%) |
| Bacterial | 17 (13%) |
| Atypical | 8 (6%) |
| Viral | 5 (4%) |
| CMV infection/reactivation | |
| Yes | 46 (35%) |
| No | 85 (65%) |
| VZV/HSV infection/reactivation | |
| Yes | 33 (25%) |
| No | 98 (75%) |
| DEATH AND CAUSE | n=62 (47%) |
| Infection | 23 (17.5%) |
| TRM | 37 (28%) |
| Relapse | 20 |
| GvHD | 8 |
| GvHD with infection | 6 |
| Toxicity | 3 |
| Other | 2 (1.5%) |
PBCST, peripheral blood stem cell transplantation; BMT, bone marrow transplantation; NK cells, natural killer cells; Bu, Busulfan; Cy cyclophophamide; VP16, Etoposide; TBI, total body irradiation; RIC, reduced intensification conditioning
Analysis of MBL genotypes
MBL-2 genotypes were successfully determined in 130 patients and donors. Donors’ and recipients’ genotypes were similarly distributed and corresponded to previous data (Table 2).4;9;19
Table 2.
Frequency of MBL Exon-1 and X/Y promoter polymorphisms MBL Genotype Recipient
| MBL Genotype | Recipient n (%) | Donor n (%) |
|---|---|---|
| YA/YA | 48 (36.6) | 46 (35.1) |
| YA/XA | 30 (22.9) | 32 (24.4) |
| XA/XA | 9 (6.9) | 6 (4.6) |
| Wildtype (A/A) | 87 (66.4) | 84 (64.1) |
| YA/YD | 12 (9.2) | 17 (13.0) |
| YA/YB+YC | 20 (15.2) | 15 (11.5) |
| XA/YD | 3 (2.3) | 2 (1.5) |
| XA/YB+YC | 3 (2.3) | 2 (1.5) |
| Heterozygous (A/O) | 38 (29.0) | 36 (27.5) |
| Homozygous (O/O) | 5 (3.8) | 10 (7.6) |
| Missing | 1 (0.8) | 1 (0.8) |
| Total | 131 (100) | 131 (100) |
Patients with either YA/YA, YA/XA, XA/XA or genotype were summarized as A/A; YA/YD, YA/YB+YC, XA/YD or XA/YB+YC patients were defined as A/O and patients with YO/YO MBL genotype as O/O.
Infection
Fifty-six patients suffered from one clinical septic episode or more. Definite (blood culture positive, most frequently with gram-positive organisms) sepsis was observed in 50 patients (n=72). There were 18 gram negative septic episodes and seven patients developed fungal sepsis. The first episode occurred between days 0 and +252 post-transplant (median 52). Over 50% of patients suffered from pneumonia, a third from CMV reactivation, and a quarter from VZV/HSV reactivation (Table 1).
MBL genotype and infection
There was no significant association between MBL genotypes and sepsis. 37/87 MBL wildtype patients developed bacterial sepsis compared to 13/43 patients with variant alleles (p=0.32). No association was observed between recipients’ MBL genotypes and pneumonia (p=0.26) or CMV reactivation (p=0.96). VZV/HSV reactivation was higher in patients with variant alleles (15/43 compared to 18/87 patients with MBL wildtype, p=0.08). Donor MBL genotype was not associated with any above parameter.
MBL genotypes of recipients and donors did not influence the severity of aGVHD (p=0.76 and 0.28) or TRM (p=0.44 and 0.26).
MBL phenotype analysis
Pre-SCT MBL levels (available from 123 patients) were significantly related to MBL-2 genotypes (p < 0.001) (Figure 1). In 29/87 A/A genotype patients, where MBL levels were available pre-SCT, on days 0–45 and +46–75 (Figure 2a), MBL levels increased significantly from a mean of 3580 ng/ml pre-transplant to 5767 ng/ml by day +45 (p<0.001). Levels declined over the next 30 days to a mean of 4136 ng/ml (p=0.15). In a robustness analysis excluding two patients of this group because of extreme values, results were similar (3000 ng/ml to 4343 ng/ml by day +45; p=0.02 and 3758 ng/ml by day 75; p=0.20).
Figure 1.
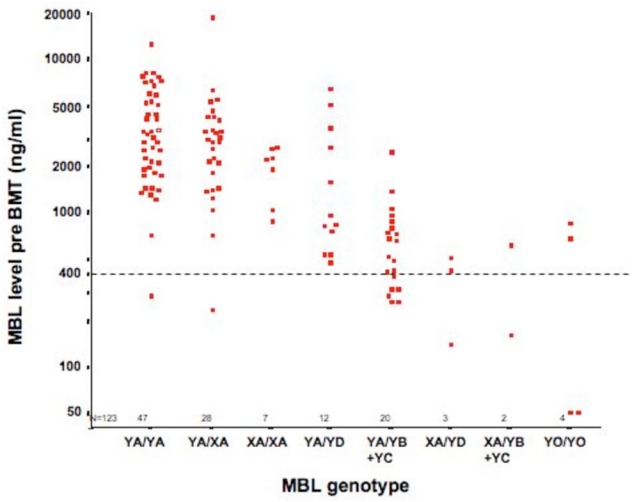
Correlation of MBL genotype and phenotype pre SCT.
The horizontal dashed line represents MBL levels of 400ng/ml.
Figure 2.
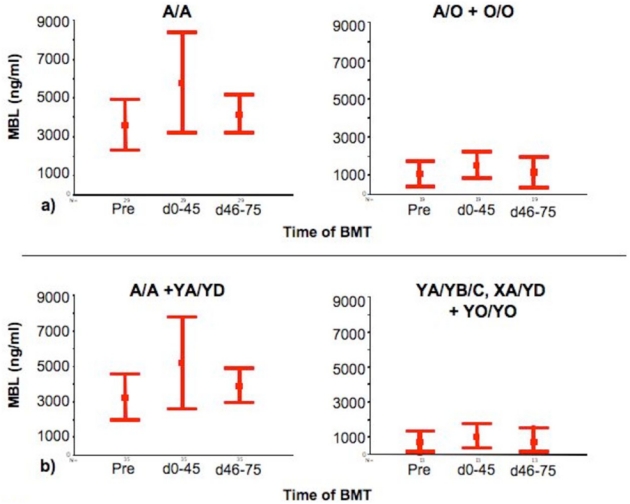
Serial measurements of MBL serum levels pre and post SCT in patients with a) wild type MBL genotype (A/A) and heterozygous (A/O) or homozygous (O/O) MBL genotype and b) with A/A + YA/YD and YA/YB/C, XA/YD + YO/YO MBL genotype. Levels are expressed as Mean +/−2SE.
In 19/43 A/O and O/O patients, samples were available for all three time points. MBL levels increased from a mean of 1021 ng/ml pre-transplant to 1476 ng/ml by day +45 (p=0.32) and fell to a mean of 594 ng/ml by day 46–75 (p=0.21, Figure 2a). If YA/YD individuals (whose levels are frequently in the same range as in A/A) were included in the wildtype group, the pattern remained similar (Figure 2b).
To investigate whether MBL levels were influenced by donor derived cells, levels were measured in 14 variant patients (A/O or O/O) with wildtype donors. Levels did not change significantly pre- to post-transplant (p=0.26) and did not alter in wildtype recipients and variant donors (n=11, p=0.92).
To explore MBL expression following SCT, Western blot analysis was performed from a YA/YD recipient who received SCT from a XA/XA donor. MBL concentrations pre-transplant were 843 ng/ml and 993 ng/ml post-transplant. MBL protein profiles pre- and post-SCT were similar, and did not increase in high order oligomers as seen in WT individuals (Figure 3). Finally, MBL was not detectable from CD34+ cells cultured from three donor marrows with wildtype MBL genotype.
Figure 3.
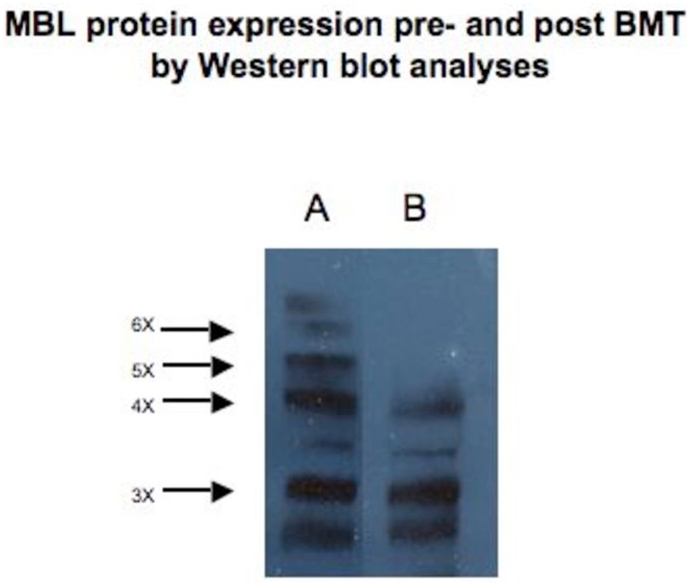
MBL protein expression pre- and post SCT by Western blot analyses.
MBL tetramers and pentamers are expressed as x4 and x5 respectively. MBL protein pre SCT is seen in lane A and post BMT in lane B. Correspondent MBL concentration pre SCT was 843 ng/ml and 993 ng/ml post SCT. MBL genotype of recipient was YA/YD (A/O) and of the donor were XA/XA (A/A).
MBL phenotype and infection
Sepsis rates were not significantly associated with MBL levels <400ng/ml (p=0.57), but MBL levels in the first or last quartile (low and high MBL levels, 790 ≥ MBL > 3418 ng/ml) showed an increased risk of culture proven sepsis (p=0.05) and a trend towards clinical sepsis (p=0.07) and fungal pneumonia (p=0.06) when compared with the 2nd/3rd quartiles (normal MBL levels, 790 < MBL ≤ 3418 ng/ml). Twelve patients had levels of <400ng/ml (lowest 10%) and suffered significantly more from fungal pneumonia (7/12 vs. 35/111; p=0.04), HSV/VZV infection/reactivation (7/12 vs. 26/111; p=0.03), and also CMV infection/reactivation was higher (6/12; 50% vs. 36/111; 32%; n.s., Figure 4a–c). When multivariable analysis was performed, the risk of fungal pneumonia was significantly higher in male patients (HR= 2.9, p=0.008) and in patients with serum MBL levels ≤400 ng/ml (HR= 4.1, p=0.002). MBL was the only one factor associated with risk of sepsis, HSV/VZV and CMV infection/reactivation.
Figure 4.
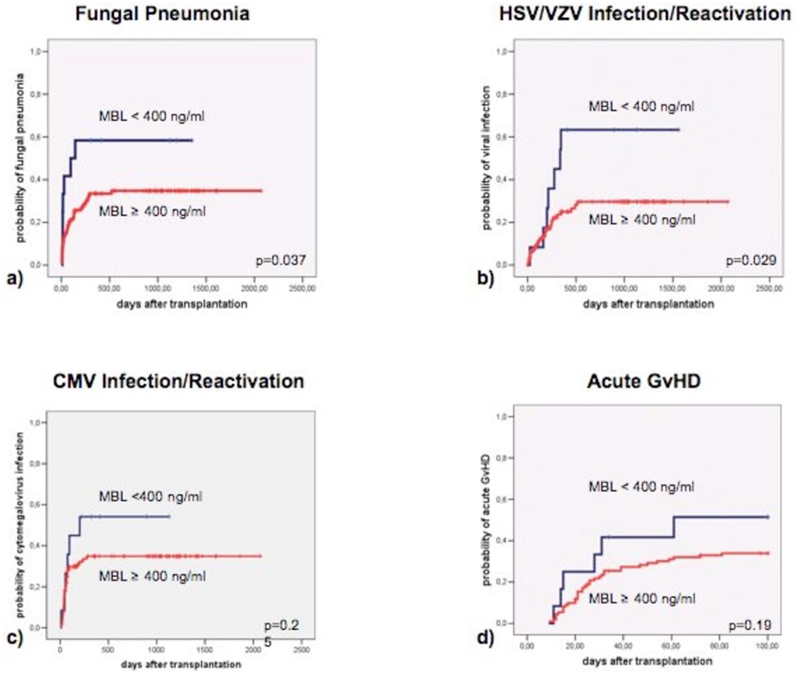
Association of very low MBL serum levels (<400ng/ml) with infection and acute GvHD.
MBL phenotype and risks of GVHD and TRM
There was no significant association between MBL levels and TRM or acute GVHD. However 7/12 patients with MBL levels <400 ng/ml suffered from aGVHD compared to 37/111 with MBL levels ≥ 400 ng/ml (58% versus 33%, Figure 4d), half of the patients with MBL levels <400 ng/ml suffered from TRM compared to 31 out of 111 (50% versus 28%).
Discussion
Infections still contribute to TRM in recipients of allo-SCT. Previous studies suggested an influence of MBL status on infection in neutropenic patients.6;7 We analysed 131 stem cell recipients to clarify the role of donors’ and recipients’ MBL genotypes and MBL serum levels on infectious and transplant related complications.
MBL-genotype did not significantly influence infections, GvHD, or TRM in this study, whereas previous studies found an association between MBL genotype and transplant-related complications.8,10 However, we found an association between low MBL levels and fungal pneumonia, HSV/VZV infection and observed a relationship between sepsis and low and high MBL levels. Thus, MBL levels may be more important than genotypes in this setting. MBL serum levels increased significantly following SCT before returning to pre-transplant levels by day +75. Overall the mean MBL levels pre-transplant were elevated to twice those in healthy populations which may reflect the pro-inflammatory state of patients undergoing SCT.1
We investigated the hypothesis that donor genotypes influence MBL levels.10,15 Levels did not significantly change in variant patients (A/O or O/O) with WT donors. Also, MBL levels did not alter in WT recipients who received SCT from variant donors, and donor genotypes did not influence the risk of transplant-related complications. We also failed to detect MBL in cultured CD34+ stem cells or a change to the oligomeric MBL profile in a patient with a heterozygous MBL genotype who received a wildtype donor SCT. Our results therefore support the study of Kilpatrick et al. where there was no effect of donor genotype on circulating MBL levels in two patients receiving SCT.20 In contrast, after liver transplantation, the genotype of the donor confers the subsequent MBL phenotype. Donations of livers (the main MBL source) from variant donors to wildtype recipients resulted in rapidly decreasing MBL serum levels.21 This keeps with previous studies22 where donors’ genotypes did not influence transplant-related complications. Therefore, it is unclear how to explain the findings of Mullighan et al. and Granell et al. that transplantation outcome depended on MBL genotypes of both recipients and donors.10,12
The observations from this study that the only clinically significant associations were related to MBL levels indicate that phenotype may be more important than genotype in predicting susceptibility to infection. 58% of patients with low MBL levels developed presumed fungal pneumonia and HSV/VZV reactivation, as compared to 31% and 23% in patients with pre-SCT levels >400ng/ml. Two patients with wildtype genotype and MBL levels <400ng/ml developed sepsis and fungal pneumonia. The low levels might be related to pre-transplant clinical status. Surprisingly, two homozygous O/O patients had MBL serum levels >400ng/ml although there is normally a strong correlation between MBL genotype and phenotype. One hypothesis might be repeated erythrocyte transfusions from donors with higher MBL but this alone is unlikely to fully explain these findings. The MBL levels of these two patients decreased post-transplant. It should be noticed that the only other independent predictor of susceptibility of infection was male sex. This result is not explained by current biological knowledge, and therefore remains to be confirmed in other studies. Also, a selection bias that could have generated this spurious association might be discussed.
MBL deficiency has emerged as a risk factor for mycosis probably due to the presence of MBL ligands such as mannan on various fungi.23 We also observed a relationship to HSV/VZV reactivation.
There is considerable debate about the definition of low MBL levels. Previous studies indicated that susceptibility to infection may vary at different MBL levels depending upon clinical circumstances and patient age. The use of different assays might also contribute to MBL level variability.24 Previously we found that MBL levels <400ng/ml did not activate complement following incubation with bacteria and failed to enhance MBL mediated opsonophagocytosis.25 Levels ≤400ng/ml correlated highly with YO/YO, XA/YB, or XA/YC genotypes in 500 healthy children (own unpublished observations).
Our data correlate with previous reports demonstrating an association of low MBL levels and increased infection rates in haematological patients with neutropenia6;7 and of a protective role of WT MBL genotype during autologous SCT.9 Additionally, we observed an increased rate of sepsis, not only in patients with low MBL levels but also in patients with very high levels. Schlapbach et al. saw similar results in a study of 94 paediatric cancer patients with treatment-associated neutropenia.7
Why high MBL levels should predispose patients to sepsis remains unclear. MBL might influence the host inflammatory response to invading microorganisms. In ex vivo experiments, MBL levels had a dose-dependent effect on the inflammatory response to Neisseria meningitides,26 and MBL influenced anti-inflammatory cytokines.27 The balance between pro- and anti-inflammatory responses has been shown to influence the susceptibility to sepsis,28 so high and low MBL levels might increase sepsis rates. High MBL levels may also merely reflect the inflammatory response to infection.
In conclusion, low MBL levels predispose patients undergoing SCT to sepsis, fungal pneumonia, and HSV/VZV infection. Larger prospective studies should fully evaluate the effect of MBL status after SCT and the potential role of MBL replacement therapy to prevent infection in this clinical setting.
Acknowledgments
We would like to thank the staff of the Infectious Diseases and Microbiology Unit and for assistance with the MBL assays. RT was supported by a grant from the Agence nationale de recherches sur le sida et les hépatites virales (ANRS).
Reference List
- 1.Turner MW. Mannose-binding lectin: the pluripotent molecule of the innate immune system. Immunol Today. 1996;17:532–540. doi: 10.1016/0167-5699(96)10062-1. [DOI] [PubMed] [Google Scholar]
- 2.Minchinton RM, Dean MM, Clark TR, Heatley S, Mullighan CG. Analysis of the relationship between mannose-binding lectin (MBL) genotype, MBL levels and function in an Australian blood donor population. Scand J Immunol. 2002;56:630–641. doi: 10.1046/j.1365-3083.2002.01167.x. [DOI] [PubMed] [Google Scholar]
- 3.Sastry K, Herman GA, Day L, Deignan E, Bruns G, Morton CC, et al. The human mannose-binding protein gene. Exon structure reveals its evolutionary relationship to a human pulmonary surfactant gene and localization to chromosome 10. J Exp Med. 1989;170:1175–1189. doi: 10.1084/jem.170.4.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smithson A, Munoz A, Suarez B, Soto SM, Perello R, Soriano A, et al. Association between mannose-binding lectin deficiency and septic shock following acute pyelonephritis due to Escherichia coli. Clin Vaccine Immunol. 2007;14:256–261. doi: 10.1128/CVI.00400-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garred P, Strom J, Quist L, Taaning E, Madsen HO. Association of mannose-binding lectin polymorphisms with sepsis and fatal outcome, in patients with systemic inflammatory response syndrome. J Infect Dis. 2003;188:1394–1403. doi: 10.1086/379044. [DOI] [PubMed] [Google Scholar]
- 6.Vekemans M, Robinson J, Georgala A, Heymans C, Muanza F, Paesmans M, et al. Low mannose-binding lectin concentration is associated with severe infection in patients with hematological cancer who are undergoing chemotherapy. Clin Infect Dis. 2007;44:1593–1601. doi: 10.1086/518171. [DOI] [PubMed] [Google Scholar]
- 7.Schlapbach LJ, Aebi C, Otth M, Luethy AR, Leibundgut K, Hirt A, et al. Serum levels of mannose-binding lectin and the risk of fever in neutropenia pediatric cancer patients. Pediatr Blood Cancer. 2007;49:11–16. doi: 10.1002/pbc.21097. [DOI] [PubMed] [Google Scholar]
- 8.Mullighan CG, Heatley SL, Danner S, Dean MM, Doherty K, Hahn U, et al. Mannose-binding lectin status is associated with risk of major infection following myeloablative sibling allogeneic hematopoietic stem cell transplantation. Blood. 2008;112:2120–2128. doi: 10.1182/blood-2007-07-100222. [DOI] [PubMed] [Google Scholar]
- 9.Molle I, Peterslund NA, Thiel S, Steffensen R. MBL2 polymorphism and risk of severe infections in multiple myeloma patients receiving high-dose melphalan and autologous stem cell transplantation. Bone Marrow Transplant. 2006;38:555–560. doi: 10.1038/sj.bmt.1705466. [DOI] [PubMed] [Google Scholar]
- 10.Mullighan CG, Bardy PG. Mannose-binding lectin and infection following allogeneic hemopoietic stem cell transplantation. Leuk Lymphoma. 2004;45:247–256. doi: 10.1080/1042819031000146983. [DOI] [PubMed] [Google Scholar]
- 11.Manuel O, Pascual M, Trendelenburg M, Meylan PR. Association between mannose-binding lectin deficiency and cytomegalovirus infection after kidney transplantation. Transplantation. 2007;83:359–362. doi: 10.1097/01.tp.0000251721.90688.c2. [DOI] [PubMed] [Google Scholar]
- 12.Granell M, Urbano-Ispizua A, Suarez B, Rovira M, Fernandez-Aviles F, Martinez C, et al. Mannan-binding lectin pathway deficiencies and invasive fungal infections following allogeneic stem cell transplantation. Exp Hematol. 2006;34:1435–1441. doi: 10.1016/j.exphem.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Berger SP, Roos A, Mallat MJ, Fujita T, de Fijter JW, Daha MR. Association between mannose-binding lectin levels and graft survival in kidney transplantation. Am J Transplant. 2005;5:1361–1366. doi: 10.1111/j.1600-6143.2005.00841.x. [DOI] [PubMed] [Google Scholar]
- 14.Jack D, Bidwell J, Turner M, Wood N. Simultaneous genotyping for all three known structural mutations in the human mannose-binding lectin gene. Hum Mutat. 1997;9:41–46. doi: 10.1002/(SICI)1098-1004(1997)9:1<41::AID-HUMU7>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 15.Madsen HO, Garred P, Thiel S, Kurtzhals JA, Lamm LU, Ryder LP, et al. Interplay between promoter and structural gene variants control basal serum level of mannan-binding protein. J Immunol. 1995;155:3013–3020. [PubMed] [Google Scholar]
- 16.Lipscombe RJ, Sumiya M, Summerfield JA, Turner MW. Distinct physicochemical characteristics of human mannose binding protein expressed by individuals of differing genotype. Immunology. 1995;85:660–667. [PMC free article] [PubMed] [Google Scholar]
- 17.Fiegel HC, Lioznov MV, Cortes-Dericks L, Lange C, Kluth D, Fehse B, et al. Liver-specific gene expression in cultured human hematopoietic stem cells. Stem Cells. 2003;21:98–104. doi: 10.1634/stemcells.21-1-98. [DOI] [PubMed] [Google Scholar]
- 18.Kibbler C. Defining invasive fungal infections in neutropenic or stem cell transplant patients. J Antimicrob Chemother. 2005;56 (Suppl 1):i12–i16. doi: 10.1093/jac/dki219. [DOI] [PubMed] [Google Scholar]
- 19.Mullighan CG, Heatley S, Doherty K, Szabo F, Grigg A, Hughes TP, et al. Mannose-binding lectin gene polymorphisms are associated with major infection following allogeneic hemopoietic stem cell transplantation. Blood. 2002;99:3524–3529. doi: 10.1182/blood.v99.10.3524. [DOI] [PubMed] [Google Scholar]
- 20.Kilpatrick DC, Stewart K, Allan EK, McLintock LA, Holyoake TL, Turner ML. Successful haemopoietic stem cell transplantation does not correct mannan-binding lectin deficiency. Bone Marrow Transplant. 2005;35:179–181. doi: 10.1038/sj.bmt.1704746. [DOI] [PubMed] [Google Scholar]
- 21.Bouwman LH, Roos A, Terpstra OT, de KP, van HB, Verspaget HW, et al. Mannose binding lectin gene polymorphisms confer a major risk for severe infections after liver transplantation. Gastroenterology. 2005;129:408–414. doi: 10.1016/j.gastro.2005.06.049. [DOI] [PubMed] [Google Scholar]
- 22.Rocha V, Franco RF, Porcher R, Bittencourt H, Silva WA, Jr, Latouche A, et al. Host defense and inflammatory gene polymorphisms are associated with outcomes after HLA-identical sibling bone marrow transplantation. Blood. 2002;100:3908–3918. doi: 10.1182/blood-2002-04-1033. [DOI] [PubMed] [Google Scholar]
- 23.Ip WK, Lau YL. Role of mannose-binding lectin in the innate defense against Candida albicans: enhancement of complement activation, but lack of opsonic function, in phagocytosis by human dendritic cells. J Infect Dis. 2004;190:632–640. doi: 10.1086/422397. [DOI] [PubMed] [Google Scholar]
- 24.Turner MW, Johnson M, Booth C, Klein N, Rolland J, Davies J. Assays for human mannose-binding lectin. J Immunol Methods. 2003;276:147–149. doi: 10.1016/s0022-1759(03)00107-8. [DOI] [PubMed] [Google Scholar]
- 25.Neth O, Jack DL, Johnson M, Klein NJ, Turner MW. Enhancement of complement activation and opsonophagocytosis by complexes of mannose-binding lectin with mannose-binding lectin-associated serine protease after binding to Staphylococcus aureus. J Immunol. 2002;169:4430–4436. doi: 10.4049/jimmunol.169.8.4430. [DOI] [PubMed] [Google Scholar]
- 26.Jack DL, Read RC, Tenner AJ, Frosch M, Turner MW, Klein NJ. Mannose-binding lectin regulates the inflammatory response of human professional phagocytes to Neisseria meningitidis serogroup B. J Infect Dis. 2001;184:1152–1162. doi: 10.1086/323803. [DOI] [PubMed] [Google Scholar]
- 27.Moller-Kristensen M, Ip WK, Shi L, Gowda LD, Hamblin MR, Thiel S, et al. Deficiency of mannose-binding lectin greatly increases susceptibility to postburn infection with Pseudomonas aeruginosa. J Immunol. 2006;176:1769–1775. doi: 10.4049/jimmunol.176.3.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allen ML, Peters MJ, Goldman A, Elliott M, James I, Callard R, et al. Early postoperative monocyte deactivation predicts systemic inflammation and prolonged stay in pediatric cardiac intensive care. Crit Care Med. 2002;30:1140–1145. doi: 10.1097/00003246-200205000-00031. [DOI] [PubMed] [Google Scholar]


