Abstract
To test the hypothesis that central changes in sympathoregulation might contribute to sympathoexcitation after cyclic intermittent hypoxia (CIH) we exposed male Sprague-Dawley rats to CIH or to room air sham (Sham) for 8 hours/d for 3 weeks. After completion of the exposure we assessed heart rate, mean arterial pressure and renal sympathetic nerve activity in conscious animals before and after intracerebroventricular (i.c.v.) administration of endothelin-1 (ET-1, 3 pmol). CIH-exposed animals had a significantly greater sympathetic response to ET-1 than did Sham-exposed animals (CIH 137.8 ± 15.6% of baseline; Sham 112.2 ± 10.0 % of baseline; CIH vs. Sham, P = 0.0373). This enhanced sympathetic response to i.c.v. ET-1 was associated with greater expression of endothelin receptor A (ETA) protein in the subfornical organs of CIH-exposed relative to Sham-exposed rats. We conclude that 3-week CIH exposure enhances central ET-1 receptor expression and the sympathetic response to i.c.v. ET-1 suggesting central endothelin may contribute to the sympathetic and hemodynamic response to cyclic intermittent hypoxia.
Keywords: Intermittent hypoxia, sympathetic nervous system, endothelin, subfornical organ, intracerebroventricular injection
2.0 INTRODUCTION
Patients who develop obstructive sleep apnea (OSA) are at risk to develop an increase in arterial pressure (Hla et al.1994; Peppard et al. 2000). The pathophysiological mechanisms that connect nocturnal upper airway obstructions, with associated sleep disruption and oxygen desaturation, to diurnal hypertension remain unclear, however. Two animal models have revealed aspects of the causal link between sleep-disordered breathing and elevated arterial pressure. The first model, which closely mimics the upper airway changes of obstructive sleep apnea, established that nocturnal cyclic intermittent hypoxia (CIH) rather than sleep fragmentation produced the increase in diurnal pressure. In this model, which uses a chronic dog preparation, a computer is used to activate a solenoid valve that occludes a tracheotomy tube whenever the animal sleeps (Brooks et al. 1997b). The occlusion is released when the animal awakens, mimicking human sleep apnea. Significantly, animals subjected to this protocol for 2 months developed sustained daytime elevations of arterial pressure but acoustic arousals, without desaturations, failed to change arterial pressure (Brooks et al. 1997a).
The second model represents an attempt to mimic the nocturnal fluctuations in oxygen saturation experienced by sleep apnea patients. In this model, rats are exposed to intermittent hypocapnic hypoxia for 8 hours each day induced by alterations in inspired oxygen concentration (Fletcher et al. 1992; Fletcher et al. 1995). Fletcher and colleagues, who developed this model, showed that CIH-induced hypertension in rats is mediated through sympathetic nervous system activation, as section of the renal nerves prior to the exposure to CIH prevented the rise in arterial pressure (Bao et al. 1997). In addition, these same investigators demonstrated that this sustained sympathoexcitation requires activation of the peripheral chemoreceptor, as carotid sinus nerve section also prevents the rise in arterial pressure associated with CIH-exposure (Bao et al. 1997).
Although this evidence suggests that CIH induces hypertension by activating the peripheral chemoreceptor, which in turn augments sympathetic outflow, there are reasons to believe that peripheral chemo-activation may not entirely account for the sustained sympathoexcitation that is a consequence of CIH-exposure. For example, our laboratory recently reported that CIH-exposure was associated with decreased expression of neuronal nitric oxide synthase (NOS) in the paraventricular nucleus of the hypothalamus, a site of central nervous system modulation of sympathetic activity (Huang et al. 2007). Substantial evidence indicates that nitric oxide (NO) is sympathoinhibitory so that a decrease in NO production would be expected to increase sympathetic activity (Zhang et al. 1997; Ma et al. 1999; Liu et al. 1999; Patel 2000). In congestive heart failure (CHF), another condition associated with sustained sympathoexcitation and enhanced peripheral chemosensitivity, substantial evidence indicates that neuromodulators in central sites of sympathoregulation also contribute to sympathetic activation (Zucker 2002; Zucker et al. 2004). As with CIH-exposure, CHF is associated with decreased NOS expression in the paraventricular nucleus (PVN) (Patel 2000; Wang et al. 2005). In addition, central angiotensin II acting through the angiotensin II, AT1 receptor in the subfornical organ is believed to enhance sympathetic activity in CHF (Liu et al. 1998; Liu et al. 1999; Wang et al. 2004). We reasoned that CIH-exposure might alter expression of other central sympathetic neuromodulators. In particular, endothelin-1 (ET-1), a 21-amino acid peptide that is found in endothelium, in Type 1 cells (glomus cells) in the carotid bodies, and in the central nervous system is known to play an important role in peripheral chemoreceptor activation after CIH exposure (Rey et al. 2006a; Rey et al. 2006b). We reasoned that endothelin might similarly contribute to CIH-induced sympathoexcitation by acting at central sites of sympathoregulation.
3.0 METHODS
3.1 Ethical approval
The Institutional Animal Care and Use Committee of Beth Israel Deaconess Medical Center and Harvard Medical School approved all surgical procedures and experimental protocols.
3.2 Animals
We performed all experiments on male Sprague–Dawley rats (Charles River, Boston, MA) weighing 240–250 g at entry into the protocol. Rats were housed in standard rodent cages with a 12-h light: 12-h dark cycle and were given food and water ad libitum.
3.3 Cyclic hypoxic exposure
Rats were randomly assigned to exposure with either cyclic intermittent hypoxia or a similar sham exposure. During the hypoxic exposure we placed the animals each day in commercial hypoxic chambers (A84XOV, Biospherix, Redfield, NY 13437). These chambers were flushed with 100% nitrogen to FIO2 nadir of 8–10% for 1 min. The FIO2 progressively returned to 21% over the remainder of each cycle. The exposure cycle repeated every 4 min for 8 hours/day, 7 days/week for 3 weeks during the animal’s sleeping hours (light). We chose this duration exposure because preliminary studies suggested a longer exposure (5 weeks) produced significant elevations in arterial pressure that were not yet evident at 3 weeks. Sham control animals underwent identical handling and exposure but chambers were flushed with room air rather than nitrogen. After completing the exposure, animals were randomly assigned to either physiological investigations or molecular studies.
3.4 PHYSIOLOGICAL STUDIES
3.41 Physiological preparation
Renal sympathetic nerve activity
We recorded renal sympathetic nerve activity in conscious, freely moving animals after CIH or Sham exposure. We placed fine wire bipolar electrodes on the left renal nerve under direct visualization using a surgical microscope under pentobarbitol anesthesia. A left subcostal incision was made and the kidney was approached in the retroperitoneal space. A bundle of renal nerves were identified and gently freed from surrounding tissue. A pair of Teflon coated stainless steel electrodes attached to a transmitter (Telemetry Research) was placed around the nerve. To insulate the electrodes and the nerve from the surrounding tissue and to prevent dessication of the nerves, the electrodes and the nerve assembly were covered with a 2-component silicone elastomer (World Precision Instruments). A ground electrode and the telemetry unit were buried in the subcutaneous tissue over the abdomen. The incision was then closed in layers. Animals were allowed to recover for 48 hrs before conscious recordings. The signal was amplified and the raw signal was band-pass filtered between 100 Hz and 2 kHz. The raw signal was full wave rectified, visually inspected and recorded digitally using the PowerLab system. The integrated signal was corrected for noise by subtracting the integrated signal amplitude evident after a bolus of phenylephrine sufficient to abolish phasic sympathetic activity (approximately a MAP of 150 mmHg). Renal sympathetic nerve activity was expressed as a percentage of the basal signal in room air.
Intracerebroventricular Injection
At the same surgery as for placement of the renal electrodes we placed the rats in a cranial stereotaxic instrument with the skull leveled between the bregma and the lambda. The skull was exposed through a 0.8 – 1.0 cm incision on the midline of the scalp. Chronic intracerebroventricular cannulas (Plastics One, VA) were implanted into the left lateral ventricle using the coordinates 1.0 mm caudal, 1.5 mm lateral to the bregma and 3.7 mm below the skull surface. The cannulas were fixed to the skull with dental cement and stainless steel screws and were occluded with an obturator when not in use. In all cases, the cannulas were placed 48–72 hours prior to data collection. After recovery from anesthesia the animals returned to their cages to continue exposure (CIH or sham) until complete.
Hemodynamic Monitoring
At the same surgery, a catheter was placed in the femoral artery and tunneled to the nape of the neck, filled with heparinized saline and capped for later recording.
3.42 Protocol
ET-1 and BQ123, the ETA receptor antagonist, were obtained from Sigma ((St. Louis, MO) and were diluted in an artificial cerebrospinal fluid (NaCl 121; KCl 3.4; MgCl2 1.2; NaH2PO4 0.6; NaHCO3 29; Glucose 3.4; pH 7.4). On the day of study animals were placed in the recording chamber to which they were previously acclimated. Once exploring behavior had ceased and the animal was resting quietly either ET-1 (3 pmol in 5µl of artificial CSF), the ETA receptor antagonist BQ123 (15 nmol in 5µl of artificial CSF) or artificial CSF (5µl) was injected intra-cerebroventricularly over 1 minute. These doses were determined from preliminary investigations in which dose-response relationships were defined. Higher doses of ET-1 elicited extreme agitated behavior that prevented hemodynamic and sympathetic recordings. When BQ123 was injected we administered ET-1 10 min later using the same injection protocol. Injection of ET-1 without pre-administration of BQ123 invariably elicited stereotypical movements so data acquisition was deferred until these movements ceased, approximately 5 minutes.
3.43 Confirmation of the Injection Site
After completion of the experiment, 5 µl Alcian Blue dye was injected into the left lateral ventricle over 1 min. The cannula was left for 5 min. The animal was euthanized with an injection of pentobarbitol and the brain dissected out. Brain sections were mounted on slides and counterstained with 1% aqueous neutral red staining procedures for histological confirmation of injection sites. Presence of the blue dye along the wall of the ventricle was verified microscopically (Paxinos and Watson 1998).
3.5 MOLECULAR STUDIES
3.51 Brain Punch
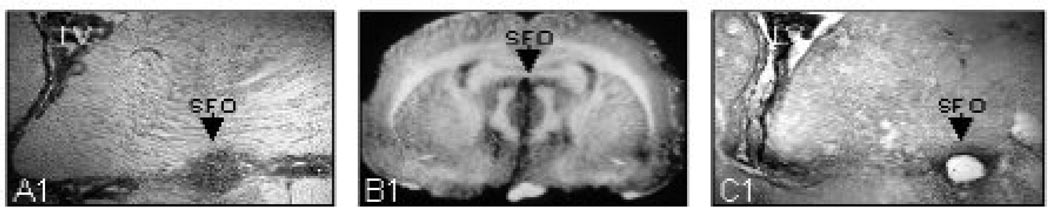
We performed Western Blot on micropunch samples of the SFO, and other brain regions of interest. The animals were anesthetized (7% Chloral Hydrate, 4ml/kg i.p.) and euthanized by decapitation. Their brains were quickly removed. We used a razor blade to resect a portion of brain stem and cerebellum to yield a flat base on which the brain could sit. The brain was then placed in a vertical position on the specimen holder, covered with Tissue-Tek® O.C.T. compound (Sakura Finetek U.S.A., Inc.) and snap-frozen in dry ice and finally stored in −80° C freezer. The morning of the day sectioning was performed the brain was transferred from the −80° C freezer to a Leica CM1800 cryostat (Leica Microsystems) where the temperature was set at −20° C for 3 hours. During that time, the block was adjusted so that the brain was bilaterally symmetrical and aligned properly in the dorsal-ventral plane. The temperature of the cryostat was changed from −20° C to −12° C for 2 hours. Using the cryostat at −8°C, serial sections (200 μm) of the brain was cut and mounted on microscope slides and immediately frozen on a block of dry ice. The SFO and other regions of interest were carefully dissected out on a bed surrounded by a mix of dry ice and ice under a dissecting microscope (Zeiss Opmi 1, Carl Zeiss Int.) with a sharpened luer stub adapter (23 gauge) (BD Biosciences) using Palkovits’ microdissection technique [2]. Each tissue slice was inspected after cryostat slicing and stained with 0.1 % cresyl violet (Sigma) and photographed after microdissection to verify accuracy and consistency of anatomic sampling. For each animal, all punches containing SFO were placed into a 1.5-ml centrifuge tube containing Trizol Reagent (Invitrogen) for RNA isolation or RIPA buff (50 mM Tris-HCL PH 7.4, 150 mM NaCl, 1% NP-40, 0.5% sodium decxycholate, 0.1% SDS) supplemented with 0.5 mM phenylmethylsulphonylfluoride, 1 mM Na3VO4 and protease inhibitor (Roche Diagnostics) for protein extraction. Figure 1 demonstrates our technique.
Figure 1.
Coronal section of rat brain showing the technique used to punch tissue of the subfornical organ (SFO) for Western blot analysis. Left panel (A) shows SFO in close-up, and middle panel (B) shows full coronal section with SFO identified. Right panel (C) shows section after punch is obtained with a blunt Luer adaptor.
3.52 Western Blot
Punched tissues were homogenized in the protein lysis buffer described above and homogenates were centrifuged at 14,000 × g for 10 min at 4°C. Supernatants were collected and stored in a −80°C freezer. Equivalent amounts of the proteins were electrophoresed in sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto Immobilon Transfer Membranes (Millipore). Gel concentration and blotting time was dependent on the molecular weight of the protein that will be tested. Nonspecific binding sites on the membrane were be blocked with 5% (w/v) non-fat dry milk or bovine serum albumin in PBS-T buffer (0.15M NaCl, 100mM sodium phosphate buffer, pH7.4 and 0.1% (v/v) Tween100) for 1 hour at room temperature. The membranes were then incubated overnight at 4°C with proper dilutions of the primary antibodies and washed in PBS-T buffer three times for 10 minutes at room temperature. Subsequently, the membranes were incubated for 1 hour at room temperature with horseradish peroxidase-conjugated secondary anti-rabbit (1:2000 dilutions in PBS-T) or anti-mouse (1:4000 dilutions in PBS-T) antibodies (Amersham Biosciences). After three washings in PBS-T buffer, the immunoreactive bands were visualized using the SuperSignal West Pico Chemiluminescent Substrate detection system (Pierce) according to the manufacturer’s instructions. The primary antibodies used are as follows: rabbit anti-endothelin A receptor polyclonal antibody (Abcam, 1:1000).
3.6 Data Analysis
We recorded arterial pressure, heart rate and integrated sympathetic activity during quiet waking within 15 min of ET-1 administration and analyzed the peak value of each variable. Peak sympathetic activity typically preceded peak arterial pressure and heart rate by 5 minutes. At least 60 sec of continuous nerve activity were analyzed. RSNA was expressed as a percent of baseline. We administered sodium nitroprusside (SNP) intravenously in an attempt to elicit a maximal sympathetic response so that RSNA could be expressed as percent of maximum. The response to this intervention was inconsistent, however, and the sympathetic response to the euthanizing dose of pentobarbitol was often greater. For this reason, we express RSNA as a percent of the baseline value obtained during quiet resting prior to ET-1 administration. The data were statistically analyzed using two-way ANOVA. P < 0.05 was considered to indicate statistical significance for Pre-Post comparisons. Comparisons between Sham and CIH were made using an unpaired t-Test on the differences assuming unequal variances (P < 0.05 considered significant).
4.0 RESULTS
Figure 2 demonstrates Alcian blue confirmation of the ET-1 injection site in a representative animal. Figure 3 displays screen shots of representative recordings from a CIH-exposed and a Sham-exposed rat.
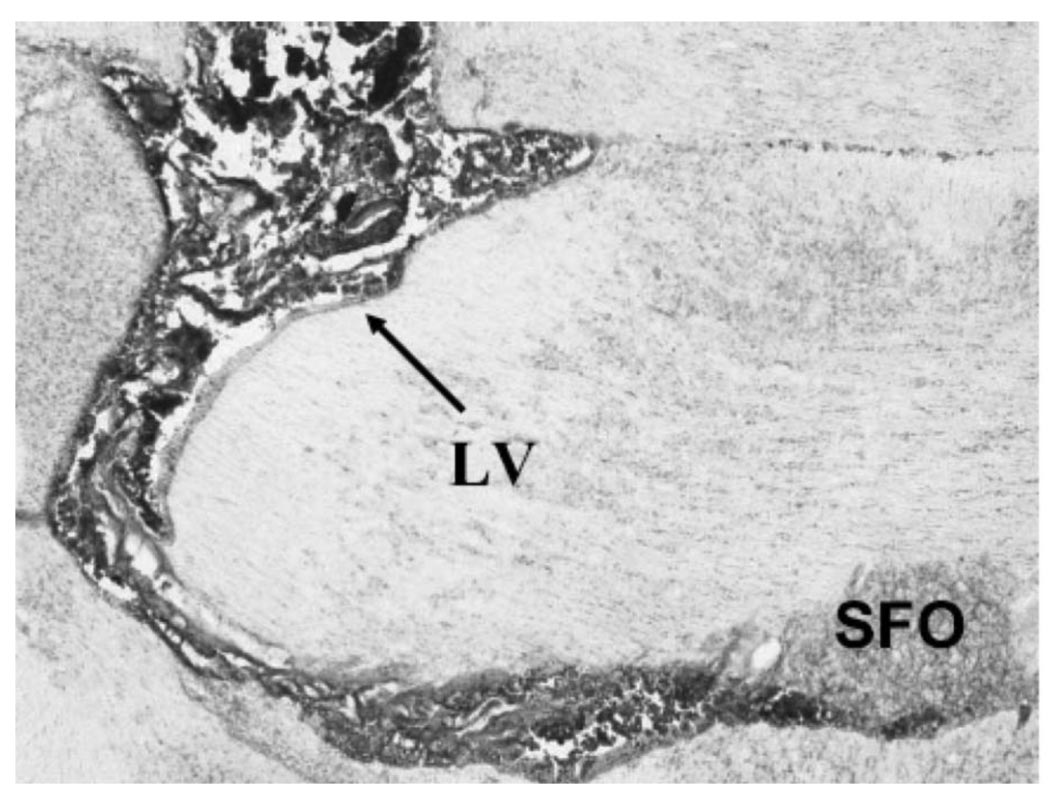
Figure 2.
Coronal section of rat brain showing confirmation of injection site. Alcian Blue dye was injected into the left lateral ventricle over 1 min. Brain sections were mounted on slides and counterstained with 1% aqueous neutral red staining procedures for histological confirmation of injection sites. In this section Blue dye outlines the lateral ventricle and the subfornical organ (SFO) is identified.
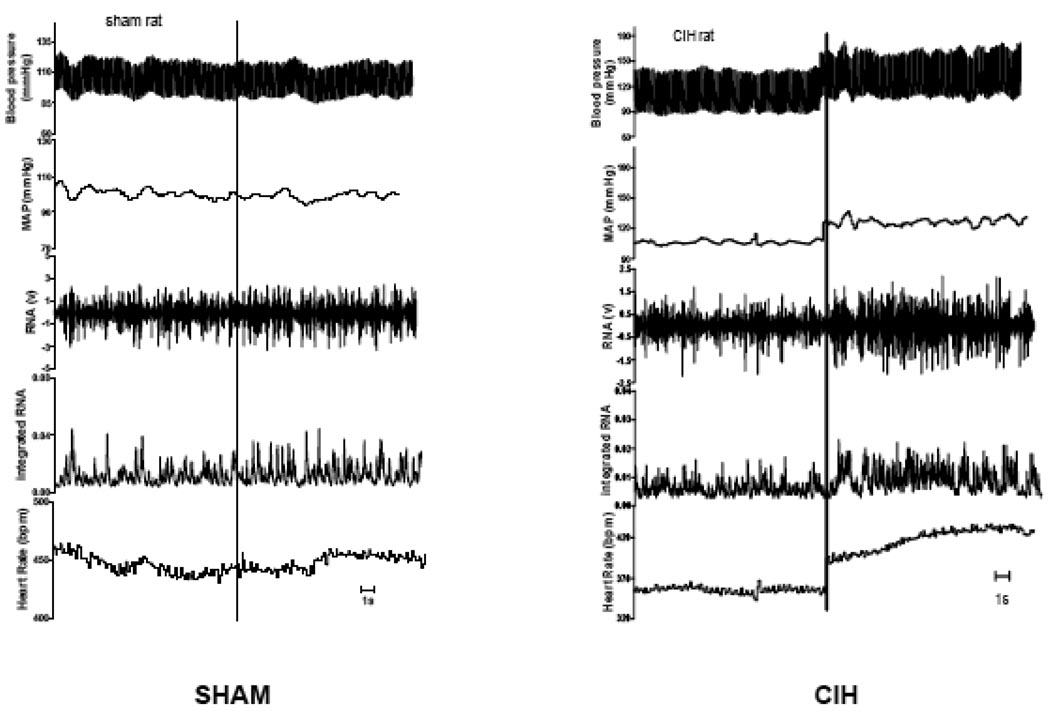
Figure 3.
Screen shots from representative Sham-exposed (Sham) and cyclic intermittent hypoxia-exposed (CIH) rats. Upper channel is arterial pressure. Other channels, in descending order, are: mean arterial pressure (MAP); raw renal sympathetic nerve (RNA); integrated sympathetic nerve; and heart rate. Recordings are discontinuous with intracerebroventricular (i.c.v.) injection of endothelin-1 (ET-1) indicated by the vertical line in each panel. Tracings after injection are from approximately 5 min after i.c.v. injection. One sec is indicated by the horizontal line in each panel.
By design, at three weeks, baseline arterial pressure and heart rate were not significantly different in CIH and Sham animals. Table 1 displays the peak changes in arterial pressure, RSNA, and heart rate after ET-1 for all animals. The increase in arterial pressure after ET-1 was significant for Sham (102.7 ± 6.9 to 109.1 ± 6.8 mmHg, P = 0.0076) and for CIH (98.3 ± 6.1 to 117.8 ± 11.3 mmHg, P = 0.0116) but the increase was greater in CIH than Sham animals (P = 0.0374). The increase in sympathetic activity also achieved statistical significance in CIH (137.8 ± 15.6%, P = 0.0057) but not Sham (Sham 112.2 ± 10.0%, P = 0.0523) and again, the increase was greater in CIH than Sham animals (P = 0.0373). Heart rate changes paralleled the other two variables.
Table 1.
Hemodynamic and sympathetic responses to intracerebroventricular injection of ET-1
| SHAM | MAP | Peak RSNA |
HR | ||
|---|---|---|---|---|---|
| Baseline | Max | Baseline | Max | ||
| 1 | 100.0 | 106.2 | 112 | 451 | 473 |
| 2 | 92.6 | 101.2 | 103 | 371 | 385 |
| 3 | 103.6 | 109.5 | 110 | 398 | 430 |
| 4 | 106.8 | 108.7 | 107 | 458 | 505 |
| 5 | 110.7 | 119.7 | 129 | 395 | 425 |
|
Mean ± SD |
102.7 + 6.9 |
109.1 + 6.8 |
112.2 + 10.0 |
414.6 + 38.0 |
443.6 + 46.4 |
|
Pre Vs. Post |
P = 0.0076 | P = 0.0523 | P = 0.0063 | ||
| CIH | |||||
| 1 | 96.2 | 126.5 | 134 | 444 | 527 |
| 2 | 102.2 | 111.4 | 116 | 452 | 495 |
| 3 | 95.7 | 123.9 | 154 | 421 | 459 |
| 4 | 91.0 | 101.0 | 133 | 437 | 503 |
| 5 | 106.6 | 126.2 | 152 | 357 | 417 |
|
Mean ± SD |
98.3 + 6.1 |
117.8 + 11.3 |
137.8 + 15.6 |
422.2 38.2 |
480.2 + 42.9 |
|
Pre vs. Post |
P = 0.0116 | P = 0.0057 | P = 0.002 | ||
|
SHAM vs. CIH |
P = 0.0374 | P = 0.0373 | P = 0.0211 | ||
MAP, mean arterial pressure at baseline and maximum after ET-1 injection; Peak RSNA, peak renal sympathetic activity as a percent of baseline before ET-1 injection; HR, heart rate at baseline and peak after ET-1 injection; Sham, animals exposed to cyclic intermittent air for 3 weeks; CIH, animals exposed to cyclic intermittent hypoxia for 3 weeks.
In four animals (2 CIH and 2 Sham) we administered the ETA receptor blocker BQ123 prior to administering ET-1. BQ123 completely prevented the hemodynamic and sympathetic response to ET-1 in all animals for at least 15 min after injection. In 2 Sham animals BQ 123 had no effect on baseline arterial pressure or RSNA (change in MAP less than 1 mmHg for both animals; change in RSNA less than 1% from the baseline value for both animals) and there was no response to ET-1 after BQ123 in either parameter (again MAP within 1 mmHg and RSNA with 1% of baseline). In the CIH animals, one animal had no response to BQ123 (MAP within 1 mmHg and RSNA within 1% of baseline). In the other CIH animal, however, BQ123 induced small decreases in both variables (MAP declined 4 mmHg and RSNA decreased 5% from baseline). In these CIH animals the effect of ET-1 was also completely blocked by BQ123 (MAP within 1 mmHg and RSNA within 1% of baseline). Artificial CSF was injected i.c.v. in all animals prior to injection of ET-1 and had no effect on any parameter.
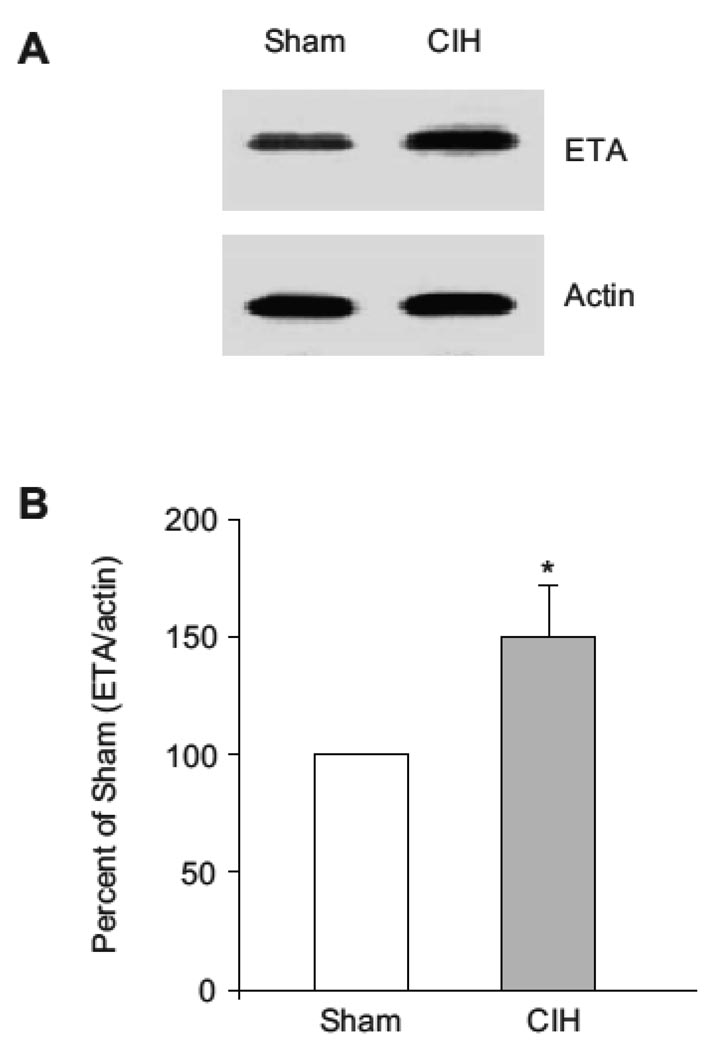
Results of Western Blotting from SFO punches obtained from CIH and Sham-exposed animals are displayed in Figure 4. Expression of ETA protein was significantly greater after a 3-week exposure to cyclic hypoxia than after a 3-week exposure to room air sham (P < 0.05).
Figure 4.
Western blot of showing enhanced expression of ETA receptor in the subfornical organ (SFO) of rats exposed to cyclic intermittent hypoxia-exposed (CIH) relative to room air sham (Sham) for 3 weeks. Homogenized tissue of SFO pooled from 10 rats in each group was used. A. Representative Western blot. B. Ratio of ETA to actin with Sham ratio considered 100%. In tissue from CIH animals, ratio of ETA to actin was significantly different from Sham (*P < 0.05). Data of Densitometric analysis were from three individual Western blots running in triplicates.
5.0 DISCUSSION
The major findings of this study are two: 1) intracerebroventricular injection of ET-1 elicits a significantly greater increase in renal sympathetic nerve activity and hemodynamics in rats exposed to cyclic intermittent hypoxia for 3 weeks than in rats exposed similarly to room air; and, 2) CIH exposure is associated with greater expression of the ETA receptor in the subfornical organ when compared to Sham-exposed animals. These findings suggest that central sympathetic processing may play a role in the sustained sympathoexcitation that results from exposure to CIH.
Outflow from post-ganglionic renal sympathetic nerves is modulated by input from pre-ganglionic neurons in the spinal cord which, in turn, receive input from sympathetic pre-motor neurons in a number of locations in the central nervous system including the rostral ventral lateral medulla (RVLM), the medullary raphe, the A5 area of the pons, and the paraventricular nucleus of the hypothalamus (PVN). In addition, these premotor neurons receive input from a wide variety of CNS locations including neurons within the circumventricular organs (CVO) in the lamina terminalis (Dampney et al. 2003; Dampney et al. 2005). Evidence suggests that all these regions may be involved in states of pathological sympathoexcitation, with greatest attention recently on the role of the PVN, RVLM and subfornical organ (SFO) in several animal models of sustained hypertension - SHR and Dahl-sensitive rats (DiBona 2004) - and in the heightened sympathetic activity that is associated with congestive heart failure (CHF) (Felder et al. 2003). While modulation of sympathetic activity within the PVN is complex, a number of studies suggest that sustained sympathoexcitation can occur as a consequence of down regulation of neuronal nitric oxide synthase (nNOS) in neurons in the PVN because such neurons are sympathoinhibitory (Zhang et al. 1997; Campese et al. 2002; Campese et al. 2004). Also contributing to sympathoexcitation in these conditions is overexpression of the AT1 receptor in the RVLM and SFO. Angiotensin containing neurons are sympathoexcitatory in the PVN, RVLM, and CVO (Brooks et al. 2001; Zucker et al. 2004). We have previously reported that 5-week CIH exposure decreases expression of nNOS in the PVN (Huang et al. 2007).
The role of ET-1 in augmenting central sympathetic control after intermittent hypoxia has not previously been explored. Nor has there been substantial investigation of the role of central endothelin in sympathetic control in animal models of hypertension or CHF. Nevertheless, several lines of investigation suggest ET-1 plays a similar role to Ang II in the central nervous system. First, ETA receptors are widely distributed within the central nervous system, and are found in the subfornical organ, the PVN, and in the RVLM, with a particularly high concentration in the SFO (Kurokawa et al. 1997). In addition, injection of low doses of ET-1 intra-cerebroventricularly causes hypertension in rats, and this hypertensive response is prevented by lesioning the PVN (Kurokawa et al. 1997; Rossi et al. 2000; Rossi et al. 2001). This hypertensive response appears to be mediated through ETA receptors as high doses of ET-1 produce acute increases in arterial pressure followed by prolonged decreases; the decrease in pressure appears to be caused by NO release in the PVN produced by ETB receptor activation in the SFO (Rossi et al. 1997; Rossi et al. 2001). Finally, the hypertensive response to i.c.v. ET-1 is mediated through the sympathetic nervous system rather than through vasopressin release (Rossi et al. 2000). Our results indicate this pathway is augmented by CIH-exposure, suggesting central endothelin expression may contribute to sustained sympathoexcitation after cyclic intermittent hypoxia.
Although most studies of central sympathetic processing in states of sympathoexcitation have focused on the roles of angiotensin II and nitric oxide, there are reasons to believe endothelin may be an important mediator of sympathoexcitation after exposure to CIH. Kanagy and colleagues showed that rats exposed to CIH for 8 hours/d for 11 days developed elevated levels of circulating ET-1 (determined by radioimmunoassay) (Kanagy et al. 2001). Arterial pressure was significantly increased in CIH-exposed, but not sham-exposed animals and the non-selective endothelin receptor antagonist PD145065 prevented the increase in arterial pressure. Rey and co-workers showed that cats exposed to CIH for as few as 4 days demonstrated an increase in endothelin immunoreactivity in both carotid body and in plasma (Rey et al. 2006b). Interestingly, these same authors showed that this relatively brief hypoxic exposure increased carotid body expression of ETB but not ETA receptor (Rey et al. 2006a). Thus, endothelin seems to play a significant role in the homeostatic response to CIH.
While we believe these data suggest a role for endothelin in the sympathetic response to hypoxia after CIH exposure several aspects of our findings should be noted. First, although we assessed ET-1 receptor expression in the SFO, our method of endothelin administration does not permit us to precisely identify the site of action. Obviously, other structures border the lateral ventricle and we cannot exclude the possibility ET-1 is acting at one, or several, of these other sites. Nevertheless, the SFO is a major site of sympathetic regulation and Rossi has shown that direct injection of endothelin into the SFO of rats elicits hypertension mediated by projections from the SFO to the PVN. As in our studies, her data suggest the ETA receptor mediates the acute increase in arterial pressure to i.c.v. ET-1. A second aspect of our study worthy of mention is that although our data indicate this endothelin pathway is augmented after CIH exposure we do not yet know whether central endothelin plays a role in the development of sustained sympathoexcitation and hypertension caused by cyclic hypoxia. To establish a causal link we believe studies involving chronic blockade of central endothelin receptors would be most useful. Although we have shown that CIH-exposure increases expression of ETA protein in the SFO we do not know the precise pathways through which ET-1 is elevating RSNA. Further work needs to be done to understand the mechanisms by which ET-1 alters central sympathoregulation in these hypoxia-exposed animals. Third, in this series, CIH exposure did not alter baseline arterial pressure as hemodynamics in CIH-exposed and Sham-exposed animals were not significantly different prior to ET-1 administration. We deliberately chose the three week exposure because preliminary data suggested a 5-week CIH exposure produced a significant increase in arterial pressure. Our goal in this protocol was to assess the effect of ET-1 on sympathetic activity prior to a persistent change in arterial pressure. Future studies now are needed to determine if altered sympathoregulation by ET-1 plays a causal role in the change in arterial pressure. Finally, we must not assume that the altered central sympathoregulation we have demonstrated in these animals is specific to the hypoxic exposure employed in this protocol. We are specifically interested in this pattern of cyclic intermittent hypoxia but a similar duration exposure to continuous hypoxia may elicit the same response.
In summary, we provide evidence that exposure of rats to cyclic intermittent hypoxia for 3 weeks results in increased expression of the ETA receptor in the subfornical organ. Further, these receptors are functional since stimulation with infused ET-1 results in augmented renal sympathetic nerve activity in CIH-exposed, relative to sham-exposed animals. These findings are consistent with a possible role for central endothelin in the sustained sympathoexcitation that occurs as a result of exposure to cyclic intermittent hypoxia and warrant further investigation.
ACKNOWLEDGEMENTS
These studies were performed with support from the National Institutes of Health (HL075184).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE LIST
- 1.Bao G, Metrevelli N, Li R, Taylor A, Fletcher EC. Blood pressure response to chronic episodic hypoxia: role of the sympathetic nervous system. J Appl Physiol. 1997;83:95–101. doi: 10.1152/jappl.1997.83.1.95. [DOI] [PubMed] [Google Scholar]
- 2.Brooks D, Horner RL, Kimoff RJ, Kozar LF, Render-Texeira CL, Phillipson EA. Effect of obstructive sleep apnea versus sleep fragmentation on responses to airway occlusion. Am. J. Respir. Crit. Care Med. 1997a;155:1609–1617. doi: 10.1164/ajrccm.155.5.9154865. [DOI] [PubMed] [Google Scholar]
- 3.Brooks D, Horner RL, Kozar LF, Render-Texeira CL, Phillipson EA. Obstructive sleep apnea as a cause of systemic hypertension. Evidence from a canine model. Jour Clin Invest. 1997b;99:109. doi: 10.1172/JCI119120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brooks VL, Scrogin KE, McKeogh DF. The interaction of angiotensin II and osmolality in the generation of sympathetic tone during changes in dietary salt intake: an hypothesis. Ann NY Acad Sci. 2001;940:380–394. doi: 10.1111/j.1749-6632.2001.tb03692.x. [DOI] [PubMed] [Google Scholar]
- 5.Campese VM, Ye S, Zhong H. Downregulation of neuronal nitric oxide synthase and interleukin-1b mediates angiotensin II-dependent stimulation of sympathetic nerve activity. Hypertension. 2002;39:519–524. doi: 10.1161/hy0202.102815. [DOI] [PubMed] [Google Scholar]
- 6.Campese VM, Ye S, Zhong H, Yanamadala V, Ye Z, Chiu J. Reactive oxygen species stimulate central and peripheral sympathetic nervous system activity. Am J of Phys Heart Circ Physiol. 2004;287:H695–H703. doi: 10.1152/ajpheart.00619.2003. [DOI] [PubMed] [Google Scholar]
- 7.Dampney RAL, Horiuchi J, Killinger S, Sherrif MJ, Tan PSP, McDowall LM. Long-term regulation of arterial pressure by hypothalamic nuclei: some critical questions. Clin Exp Pharmacol Physiol. 2005;32:419–425. doi: 10.1111/j.1440-1681.2005.04205.x. [DOI] [PubMed] [Google Scholar]
- 8.Dampney RAL, Horiuchi J, Tagawa T, Fontes MAP, Potts PD, Polson JW. Medullary and supramedullary mechanisms regulating sympathetic vasomotor tone. Acta Physiologica Scandin. 2003;177:209–218. doi: 10.1046/j.1365-201X.2003.01070.x. [DOI] [PubMed] [Google Scholar]
- 9.DiBona GF. The sympathetic nervous system and hypertension: recent developments. Hypertension. 2004;43:147–150. doi: 10.1161/01.HYP.0000113047.47711.fa. [DOI] [PubMed] [Google Scholar]
- 10.Felder RB, Francis J, Zhang Z-H, Wei S-G, Weiss RM, Johnson AK. Heart failure and the brain: new perspectives. Am J of Physiol Regul Integr Comp Physiol. 2003;284:R259–R276. doi: 10.1152/ajpregu.00317.2002. [DOI] [PubMed] [Google Scholar]
- 11.Fletcher EC, Bao G, Miller CCI. Effect of recurrent episodic hypocapnic, eucapnic, and hypercapnic hypoxia on systemic blood pressure. J Appl Physiol. 1995;78:1516–1521. doi: 10.1152/jappl.1995.78.4.1516. [DOI] [PubMed] [Google Scholar]
- 12.Fletcher EC, Lesske J, Qian W, Miller CCI, Unger T. Repetitive, episodic hypoxia causes diurnal elevation of blood pressure in rats. Hypertension. 1992;19:555–561. doi: 10.1161/01.hyp.19.6.555. [DOI] [PubMed] [Google Scholar]
- 13.Hla KM, Young TB, Bidwell T, Palta M, Skatrud JB, Dempsey J. Sleep Apnea and Hypertension: A Population-based Study. Ann Intern Med. 1994;120:382–388. doi: 10.7326/0003-4819-120-5-199403010-00005. [DOI] [PubMed] [Google Scholar]
- 14.Huang J, Tamisier R, Ji E, Tong J, Weiss JW. Chronic intermittent hypoxia modulates nNOS mRNA and protein expression in the rat hypothalamus. Respir Physiol & Neurobiol. 2007;158:30–38. doi: 10.1016/j.resp.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Kanagy NL, Walker BR, Nelin LD. Role of endothelin in intermittent hypoxia-induced hypertension. Hypertension. 2001;37(part 2):511–515. doi: 10.1161/01.hyp.37.2.511. [DOI] [PubMed] [Google Scholar]
- 16.Kurokawa K, Yamada H, Ochi J. Topographical distribution of neurons containing endothelin type A receptor in the rat brain. The Journal of Comparative Neurology. 1997;389:348–360. doi: 10.1002/(sici)1096-9861(19971215)389:2<348::aid-cne11>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 17.Liu J-L, Murakami H, Zucker IH. Angiotensin II-nitric oxide interaction on sympathetic outflow in conscious rabbits. Circ Res. 1998;82:496–502. doi: 10.1161/01.res.82.4.496. [DOI] [PubMed] [Google Scholar]
- 18.Liu J-L, Zucker IH. Regulation of sympathetic nerve activity in heart failure: a role for nitric oxide and angiotensin II. Circ Res. 1999;84:417–423. doi: 10.1161/01.res.84.4.417. [DOI] [PubMed] [Google Scholar]
- 19.Ma R, Zucker IH, Wang W. Reduced NO enhances the central gain of cardiac sympathetic afferent reflex in dogs with heart failure. Am J of Physiol Heart Circ Physiol. 1999;276:H19–H26. doi: 10.1152/ajpheart.1999.276.1.H19. [DOI] [PubMed] [Google Scholar]
- 20.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th ed. San Diego CA: Academic Press; 1998. [Google Scholar]
- 21.Patel KP. Role of paraventricular nucleus in mediating sympathetic outflow in heart failure. Heart Fail Rev. 2000;5:73–86. doi: 10.1023/A:1009850224802. [DOI] [PubMed] [Google Scholar]
- 22.Peppard PE, Young T, Palta M, Skatrud J. Prospective Study of the Association between Sleep-Disordered Breathing and Hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 23.Rey S, Corthorn J, Chacon C, Iturriaga R. Expression and Immunolocalization of Endothelin Peptides and Its Receptors, ETA AND ETB, in the Carotid Body Exposed to Chronic Intermittent Hypoxia. J Histochem Cytochem. 2006a;55:167–174. doi: 10.1369/jhc.6A7079.2006. [DOI] [PubMed] [Google Scholar]
- 24.Rey S, Del Rio R, Iturriaga R. Contribution of endothelin-1 to the enhanced carotid body chemosensory responses induced by chronic intermittent hypoxia. Brain Res. 2006b;1086:152–159. doi: 10.1016/j.brainres.2006.02.082. [DOI] [PubMed] [Google Scholar]
- 25.Rossi NF, Chen H. PVN lesions prevent the endothelin 1-induced increase in arterial pressure and vasopressin. Am J of Physiol Endocrinol Metab. 2001;280:E349–E356. doi: 10.1152/ajpendo.2001.280.2.E349. [DOI] [PubMed] [Google Scholar]
- 26.Rossi NF, O'Leary DS, Chen H. Mechanisms of centrally administered ET-1-induced increases in systemic arterial pressure and AVP secretion. Am J of Physiol Endocrinol Metab. 1997;272:E126–E132. doi: 10.1152/ajpendo.1997.272.1.E126. [DOI] [PubMed] [Google Scholar]
- 27.Rossi NF, O'Leary DS, Woodbury D, Chen H. Endothelin-1 in hypertension in the baroreflex-intact SHR: a role independent from vasopressin release. Am Jof Physiol Endocrinol Metab. 2000;279:E18–E24. doi: 10.1152/ajpendo.2000.279.1.E18. [DOI] [PubMed] [Google Scholar]
- 28.Wang H, Huang BS, Ganten D, Leenen FHH. Prevention of sympathetic and cardiac dysfunction after myocardial infarction in transgenic rats deficient in brain angiotensinogen. Circ Res. 2004;94:843–849. doi: 10.1161/01.res.0000120864.21172.5a. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Liu XF, Cornish KG, Zucker IH, Patel KP. Effects of nNOS antisense in the paraventricular nucleus on blood pressure and heart rate in rats with heart failure. Am J of Physiol Heart Circ Physiol. 2005;288:H205–H213. doi: 10.1152/ajpheart.00497.2004. [DOI] [PubMed] [Google Scholar]
- 30.Zhang K, Mayhan WG, Patel KP. Nitric oxide within the paraventricular nucleus mediates changes in renal sympathetic nerve activity. Am J of Physiol Regul Integr Comp Physiol. 1997;273:R864–R872. doi: 10.1152/ajpregu.1997.273.3.R864. [DOI] [PubMed] [Google Scholar]
- 31.Zucker IH. Brain angiotensin II: New insights into its role in sympathetic regulation. Circ Res. 2002;90:503–505. doi: 10.1161/01.res.0000014287.96335.21. [DOI] [PubMed] [Google Scholar]
- 32.Zucker IH, Schultz HD, Li YF, Wang Y, Wang W, Patel KP. The origin of sympathetic outflow in heart failure: the roles of angiotensin II and nitric oxide. Prog Biophys Mol Biol. 2004;84:217–232. doi: 10.1016/j.pbiomolbio.2003.11.010. [DOI] [PubMed] [Google Scholar]