Abstract
Alterations in gray and white matter have been well documented in individuals with multiple sclerosis. Severity and extent of such brain tissue damage have been associated with cognitive impairment, disease duration and neurological disability, making quantitative indices of tissue damage important markers of disease progression. In this study, we investigated the association between cardiorespiratory fitness and measures of gray matter atrophy and white matter integrity. Employing a voxel-based approach to analyses of gray matter and white matter, we specifically examined whether higher levels of fitness in multiple sclerosis participants were associated with preserved gray matter volume and integrity of white matter. We found a positive association between cardiorespiratory fitness and regional gray matter volumes and higher focal fractional anisotropy values. Statistical mapping revealed that higher levels of fitness were associated with greater gray matter volume in the midline cortical structures including the medial frontal gyrus, anterior cingulate cortex and the precuneus. Further, we also found increasing levels of fitness were associated with higher fractional anisotropy in the left thalamic radiation and right anterior corona radiata. Both preserved gray matter volume and white-matter tract integrity were associated with better performance on measures of processing speed. Taken together, these results suggest that fitness exerts a prophylactic influence on the cerebral atrophy observed early on preserving neuronal integrity in multiple sclerosis, thereby reducing long-term disability.
Keywords: Cortical atrophy, normal appearing gray matter, normal appearing white matter, cardiorespiratory fitness, processing speed, relapsing-remitting multiple sclerosis, neuroplasticity
1. INTRODUCTION
Multiple sclerosis (MS), a neurodegenerative, inflammatory disease is associated with focal areas of demyelination (lesions) in the central nervous system (Devins & Seland, 1987, Charil et al., 2007, Lester et al., 2007). These demyelinating lesions are accompanied with pathology in both normal appearing white matter (NAWM) and normal appearing gray matter (NAGM), suggesting that brain tissue damage in MS extends beyond the abnormal appearing white matter and significantly impacts multiple cortical structures (Amato et al., 2004, Bermel et al., 2002, Chard et al., 2004, Cifelli et al., 2002, De Stefano et al., 2003, Sailer et al., 2003, Prinster et al., 2006, Ceccarelli et al., 2008, Vrenken et al., 2006a, Roosendaal et al., 2009). Weak correlations between demyelinating lesions and neurological disability (Barkhof, 2002, Vrenken et al., 2006b), indicate that pathophysiological processes happening outside the white-matter lesions explain some of the variance in disease progression (De Stefano et al., 2001, Fisher et al., 2001, Chard et al., 2002). With advances in neuroimaging techniques and analyses, in vivo abnormalities of the NAWM and NAGM have been studied extensively (Miller et al., 2003, Filippi et al., 1995, Dehmeshki, et al., 2003, Cercignani et al., 2001, Werring et al., 1999, Chard et al., 2002, Vrenken et al., 2005, Ranjeva et al., 2006, Audoin et al., 2006), with research focusing on both the nature of pathology seen in GM and WM structures and their subsequent association with clinical and demographic factors.
Diffusion tensor imaging (DTI) is increasingly being used to detect disease related injury in the NAWM and provides information on the local properties of white matter tracts (Pierpaoli & Basser, 1996). Fractional anisotropy (FA), reflecting the preferential directionality of water diffusion, is the most commonly studied diffusion parameter and ranges from isotropic diffusion (FA value equal to zero) to anisotropic diffusion (FA value equal to 1). Reduced FA values have been reported in the WM lesions (Bammer et al., 2000, Tievsky et al., 1999) as well as the NAWM (Ciccarelli et al., 2001). Damage within the NAWM has been studied through many approaches such as ROI-based approaches (Ciccarelli et al., 2003, Hasan et al., 2005, Vrenken et al., 2006b), voxelwise approaches (Roosendaal et al., 2009, Dineen et al., 2008) and through histogram analyses (Rovaris et al., 2002a, Vrenken et al., 2006b). Voxelwise approaches, in contrast to the other approaches, have the advantage of investigating group differences in local structures in a more exploratory manner. Using Tract-Based Spatial Statistics (TBSS, Smith et al., 2006), a relatively new approach to conducting voxelwise statistical analyses of FA data, Roosendaal et al. (2009) reported differences in MS individuals relative to healthy controls in a number of brain regions including the corona radiata, fornices, inferior longitudinal fasciculus and optic radiation in both hemispheres, and parts of the corpus callosum. FA reductions in these tracts were associated with neurological disability and cognitive impairment, providing direct evidence for the impact of NAWM degeneration on the clinical progression of MS.
In addition to WM atrophy, MS is also characterized by significant neuronal loss in GM (Prinster et al., 2006, Chard et al., 2002, 2004, Dalton et al., 2004). Voxel-based morphometric analyses of GM structures have been employed in several studies, which provide evidence of a significant decline in global GM volume of MS participants (Audoin et al., 2006, Prinster et al., 2006, Ceccarelli et al., 2008). Decline in GM volume has been found to be associated with neurological disability as assessed through expanded disability status score (EDSS) and cognitive impairment (Amato et al., 2004; Benedict et al., 2004; Zivadinov et al., 2001, Morgen et al., 2006). Much recent work has also elucidated regional GM structures that show deterioration as a result of the neurodegenerative disease. These include the thalamus and the right lateral prefrontal cortices (Audoin et al., 2006), left prefrontal and temporal cortices (Prinster et al., 2006) and the right pre and post-central gyri (Ceccarelli et al., 2008). Thus, much of the recent research suggests that individuals with multiple sclerosis have a significant deterioration of both GM and WM structures that are not visible through conventional MRI and that deterioration in these cortical structures is associated with significant functional and cognitive limitations (Dineen et al., 2008). Lifestyle factors that moderate the decline of MS on cortical atrophy thus might help in slowing and even potentially reversing the disease progression in MS. One such factor that might potentially be associated with preserved GM or WM volumes or both is cardiorespiratory fitness.
Cardiorespiratory fitness, a physiological surrogate of physical activity is increasingly being recognized as having a neuroprotective effect on neurological disorders (White & Castellano, 2008). Both initial non-human animal research (Le Page et al., 1994, 1996) and cross-sectional human research (Prakash et al, 2007) provide promising evidence for the possible role of fitness in improving functioning in those with MS. Using functional magnetic resonance imaging, we have provided evidence for an association between increased levels of fitness and activation in the right middle frontal gyrus during performance on the Paced Visual Serial Addition Test (PVSAT), which in turn was associated with better behavioral performance (Prakash et al., 2007). These results provided the first evidence for the association of fitness levels with functional neuronal plasticity in MS patients. In the current study, we were interested in examining the association between cardiorespiratory fitness and structural loss as measured through voxel-based morphometric analysis of GM and tract-based spatial statistical analysis of diffusion tensor data.
We recruited relapsing-remitting MS participants and age, education and gender matched healthy controls for our study. We predicted that consistent with previous literature, we would firstly find differences in both global and regional GM volumes of MS participants relative to healthy controls. Cortical regions of GM loss would in turn be negatively associated with fitness, such that higher-fit MS participants would have less of a decline in these GM structures. Previous studies employing voxel-based approaches to analyses of gray matter decline have provided evidence for a reduction in the volume of midline cortical structures, such as the ACC and the medial frontal gyrus, neuronal regions that are responsible for interconnections between cortical and sub-cortical structures (Charil et al., 2007, Bendfeldt et al., 2009). Based on these results, we would expect that the volume of these cortical structures would be positively associated with fitness. In addition, we predicted that higher levels of fitness in our MS participants would also be associated with less reduction in FA values both globally and focally. Further given that the integrity of the white matter tracts and greater GM volumes have been associated with improved cognitive functioning, we predicted that structural areas that might show a preservation with increasing levels of fitness, will also be associated with improved cognitive functioning on measures of processing speed; tasks that often show significant deterioration in individuals with MS (Demaree et al., 1999, Henry & Beatty, 2006).
2. RESULTS
Neuropsychological Results
Differences in performance between healthy controls and MS participants on different neuropsychological measures were assessed by Student’s t-tests. For all the neuropsychological measures included in the study (Table II), we found that those with MS demonstrated significantly poor performance on the PASAT (t(34) = −2.02, p < 0.05), SDMT (t(34) = −3.66, p < 0.001) and the word list generation task (t(34) = −2.55, p <0.01). The results from SDMT and the word list generation task remained significant after correcting for multiple comparisons, however PASAT scores did not statistically differ between MS patients and healthy controls once we corrected for multiple comparisons. MS participants and healthy controls did not differ in their performance on the MMSE and K-BIT.
Table II.
Neuropsychological results of MS participants and healthy controls (mean and standard error in parenthesis) are reported with the p-values from the independent t-tests conducted to examine differences between healthy controls and MS participants.
| Healthy Controls | MS patients | p-value | Cohen's d | |
|---|---|---|---|---|
| mMini Mental Status Examination | 55.8 (0.4) | 55.2 (0.3) | 0.25 | 0.39 |
| K-BIT (Verbal IQ) | 112.8 (2.2) | 109.9 (1.6) | 0.25 | 0.40 |
| Brief Repeatable Battery | ||||
| Selective Reminding Test | 46.9 (3.4) | 42.9 (1.7) | 0.27 | 0.36 |
| Spatial Recall Test | 24.8 (2.0) | 23.7 (1.3) | 0.64 | 0.46 |
| Paced Auditory Serial Addition Test | 49.7 (2.2) | 43.0 (2.3) | 0.04 | 0.67 |
| Symbol Digit Modalities Test | 61.2 (2.9) | 46.9 (2.5) | 0.001 | 1.24 |
| List Generation | 11.1 (0.7) | 8.9 (0.4) | 0.01 | 0.84 |
In order to examine associations with fitness, we conducted a series of partial correlations between the neuropsychological tests and cardiorespiratory fitness, after removing variance associated with age. As predicted, we found that higher levels of fitness were associated with higher scores on the composite measure of information processing speed (pr=0.46, p < 0.05, one-tailed).
Structural Imaging Results
Comparisons between healthy controls and MS participants
VBM findings
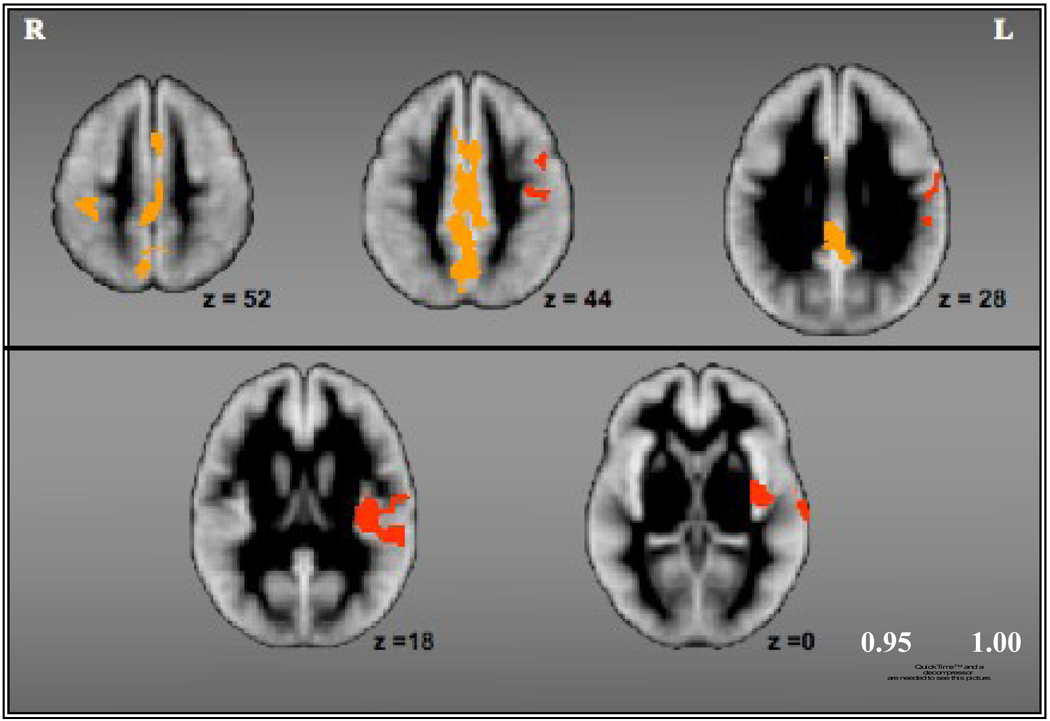
Comparing global GM volume between controls and MS participants, we found a significant difference between the two groups (F (1,32)=7.72, p <0.01), after controlling for age and ICV. These results confirm previous reports of a significant reduction in global GM volume of those with MS relative to controls (Audoin et al. 2006, Prinster et al., 2006, Chard et al., 2002). Further, consistent with previous research, we found a number of cortical areas that showed significant GM loss in MS participants as compared to healthy controls. Figure I and Table III show areas of significant GM loss in MS individuals relative to controls, after removing variance associated with age and ICV. Most of the clusters were located either in the left hemisphere, confirming previous findings of a preferential left-sided GM loss in multiple sclerosis or involved midline cortical structures such as the medial frontal gyrus, anterior cingulate cortex and the precuneus. GM loss in the right hemisphere was mostly noted in the post-central gyrus and the ACC. The controls did not show any areas of GM loss relative to MS participants.
Figure I.
Cortical areas that showed significant gray matter loss for MS participants relative to healthy controls.
Table III.
Cortical regions of significant gray matter loss in MS participants relative to healthy controls.
| Region | Hemisphere | X (MNI) | Y (MNI) | Z (MNI) |
|---|---|---|---|---|
| Anterior Cingulate Gyrus | R | 3 | 10 | 31 |
| Cingulate Gyrus | C | 0 | −12 | 52 |
| L | −9 | 4 | 57 | |
| Inferior Frontal Gyrus | L | −47 | 5 | 40 |
| Inferior Parietal Lobule | L | −60 | −29 | 31 |
| Medial Frontal Gyrus | C | 0 | −33 | 62 |
| Middle Temporal Gyrus | L | −52 | −13 | −5 |
| Postcentral Gyrus | L | −52 | −20 | 51 |
| R | 35 | −27 | 52 | |
| Precuneus | L | −1 | −67 | 49 |
| Superior Temporal Gyrus | L | −64 | −33 | 20 |
| Transverse Temporal Gyrus | L | −66 | −8 | 15 |
Tract-Based Spatial Statistics (TBSS) findings
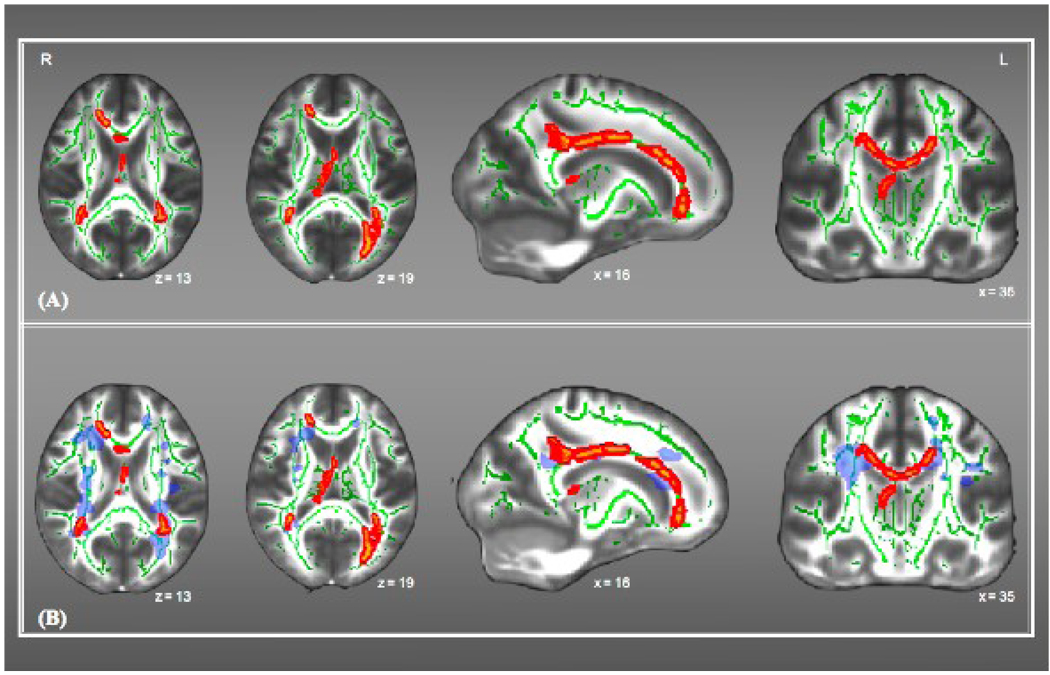
A significant difference was found between healthy controls and individuals with MS in the whole-brain TBSS skeleton mean FA, after removing variance associated with age (F(1,33)=19.42, p < 0.001). Regional differences in FA values were noted in the body and genu of the corpus callosum, bilateral posterior thalamic radiation, bilateral corona radiation, and left forceps major (Figure IIA). Further, we also found that some of the FA reductions were co-localized with the presence of focal lesions (Figure IIB), particularly bilateral posterior corona radiata and part of the genu of corpus callosum. However, consistent with previous work (Dineen et al., 2008) FA reductions were also seen in tracts where the probability of focal lesions was < 10%.
Figure II.
A & B - Displays clusters of group differences in FA values between MS participants and healthy controls. (A) Represents group differences in FA values overlaid on the mean skeleton image (green); (B) Represents group differences overlaid on the Lesion Probability Map (blue) and the mean skeleton image (green).
Correlations with Cardiorespiratory fitness
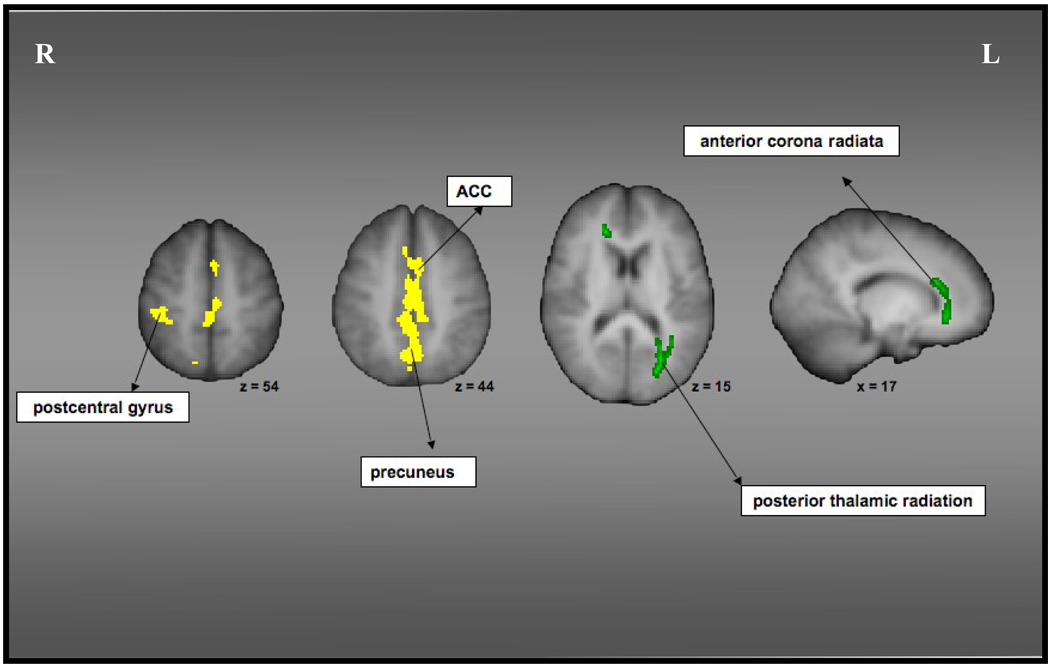
In order to examine the association of cardiorespiratory fitness with structural loss in multiple sclerosis, we performed partial correlations between fitness (V·O2peak) and the structural measures, after removing variance associated with nuisance variables. There were three main results of this analysis. Firstly, we found that after removing variance associated with age and ICV, lesion load volume was negatively correlated with increasing levels of cardiorespiratory fitness (pr = −0.44; p <0.05, one-tailed). These results thus suggest that increasing levels of cardiorespiratory fitness in MS participants is associated with less lesion load volume. Secondly, we also found significant correlations between fitness and GM volumes in the right postcentral gyrus (pr =0.45, p<0.05, one-tailed) and the midline cortical structures including the medial frontal gyrus, ACC and the precuneus (pr=0.45, p<0.05, one-tailed), suggesting that fitness is associated with preserved GM volume in cortical structures known to show significant deterioration as a result of this neurodegenerative disease (Figure III). Further, increased volume in both these structures within the MS sample were associated with better processing speed as indicated by the positive correlation between volume estimates and ZIPS (pr =0.46, p<0.05 for right post-central gyrus; pr=0.45, p<0.05 for the midline cortical structures).
Figure III.
Represents regions of GM loss (displayed in yellow) and FA reductions (displayed in green) in MS participants that are preserved with higher levels of cardiorespiratory fitness. Partial volume estimates in the GM structures and FA values in the white matter tracts correlated positively with fitness levels.
In addition to examining association with GM volume, we were also interested in investigating whether fitness levels were associated with greater integrity of the white matter tracts in multiple sclerosis. Removing variance associated with age, we found that increasing levels of fitness were associated with greater FA values in the left posterior thalamic radiation, including the optic radiation (pr=0.40, p<0.05, one-tailed) and the right anterior corona radiata, including the genu of the corpus callosum (pr=0.44, p<0.05, one-tailed; Figure III). The FA values in these tracts were also correlated with ZIPS (pr =0.41, p<0.05 for posterior thalamic radiation; pr=0.40, p<0.05 for the anterior corona radiata), thereby suggesting that higher integrity of the white matter tracts is in fact associated with better performance on measures of processing speed.
3. DISCUSSION
The results of this study provide evidence for an inverse association between cardiorespiratory fitness and brain tissue damage in individuals with multiple sclerosis. These results are exciting in that they suggest the possibility of preserved GM volume and intact tract integrity in the presence of a demyelinating disease. Given that higher levels of fitness in MS participants were also associated with higher scores on the composite measure of information processing speed (ZIPS), and better processing speed was in turn related to preserved structural measures (both GM volumes and WM tract integrity) suggests a clear triple association between fitness, cognitive functioning and GM and WM structures.
Consistent with previous research with MS participants (Chard et al., 2002, 2004, Dalton et al., 2004, Roosendaal et al., 2009, Dineen et al., 2008), we found that relative to healthy controls, MS participants demonstrated a decline in global GM volume and FA reductions in global mean skeleton. In addition, in line with a previous study employing VBM to assess regional differences in GM in persons with RRMS (Prinster et al., 2006), we found that MS participants demonstrated a reduction in the GM volume of the midline cortical structures and frontal and temporal regions. The midline cortical structures were also positively associated with fitness, suggesting that higher-fit MS participants demonstrated preserved GM volume in structures known to deteriorate as a result of the neurodegenerative process (Charil et al., 2007, Bendfeldt et al., 2009). Specifically, in MS there is a greater deterioration in the GM volumes of structures that are heavily interconnected with other regions (Charil et al., 2007, Bendfeldt et al., 2009), such as the ACC and the medial frontal gyrus. One possible reason for greater atrophy in structures with extensive cortico-cortical connections with other regions could be due to the indirect influence of distal lesions in the WM through processes of anterograde or Wallerian degeneration (Waller, 1850, Simon et al., 2000, Ciccarelli et al., 2003). The finding of preserved GM volume in these interconnected structures with increasing fitness suggests that aerobic fitness may play a key role in slowing the neurodegeneration caused by MS. Future intervention studies directly addressing this question will provide vital information on the causal relation between exercise and functioning in MS participants.
In addition to GM atrophy, increased levels of fitness were also inversely correlated with FA reductions in the white matter tracts. Using a relatively new approach to analysis of diffusion imaging data (TBSS, Smith et al., 2006), we were able to confirm previous reports of global and regional differences in the integrity of the white matter tracts in MS. MS participants showed a reduction in the integrity of the tracts involved in the NAWM, and though there was some degree of overlap with the lesion probability map, there was also a significant partial dissociation from the LPM providing further evidence that NAWM plays an important role in the pathological processes of MS (Zivadinov et al., 2001, Rovaris et al., 2002b, Dineen et al., 2008). Lifestyle factors that might be associated with sparing of the NAWM, thus need to be better understood in order to reduce the burden associated with the disease. Specifically, we found that higher levels of fitness were associated with higher FA values in the posterior thalamic radiation (including the optic radiation) and anterior corona radiata, including the genu of the corpus callosum. Both these tracts have been investigated in the MS literature and studies consistently report a reduction in the FA values of these tracts in MS participants relative to healthy controls (Roosendaal et al., 2009, Dineen et al., 2008). Posterior thalamic radiation, including the optic radiation involves projection from the lateral geniculate nucleus of the thalamus to the visual cortex (Mori et al., 2005). Disruptions in the integrity of these tracts may be causally related to problems of vision seen extensively in persons with MS (Roosendaal et al., 2009, Plant et al., 2008). Corpus callosum (CC) has been well studied in MS, with studies reporting reduced FA in CC (Ge et al., 2004, Coombs et al., 2004, Hasan et al., 2005), which in turn shows modest correlations with disease duration and neurological disability, suggesting that the reductions in the integrity of the white matter play an important role in the clinical progression of MS. Higher levels of fitness were associated with preserved integrity of both these tracts, as reflected by increase in FA values with increasing fitness, suggesting that fitness indeed might be an important variable playing a neuroprotective effect in the pathology of MS.
Our results of a positive association between fitness and GM and WM structures compliments and extends the growing literature on the benefits of fitness on neuronal plasticity (Colcombe & Kramer et al., 2003, Colcombe et al., 2003, 2004, Kramer & Erickson 2007, Prakash et al., 2007). Much of this work conducted with healthy older adults provides evidence for the beneficial effect of exercise training on brain structure (Colcombe et al., 2006) and function (Colcombe et al., 2004) of older adults. Research with nonhumans has provided further support for the prophylactic influence of exercise training (van Praag et al., 1999; 2005, Cotman and Berchtold, 2002). In rodents, exercise has shown to induce a series of molecular and cellular cascade of events such as cell proliferation, synaptic plasticity (Black et al., 1990, Kleim et al., 2002, Swain et al., 2003, Farmer et al., 2004; Christie et al., 2008) and an increase in the production of nerve-growth factors like brain derived neurotrophic factor (BDNF, Vaynman et al., 2004), insulin-like growth factor-1 (IGF-1, Trejo et al., 2001, Lopez-Lopez et al., 2004) and vascular endothelial growth factor (VEGF, Fabel et al., 2003), providing elucidation on the possible mechanisms through which exercise might promote neuroplasticity. In fact animal work with experimental autoimmune encephalomyelitis (EAE), the animal model of MS, suggests that treatment with IGF-1 significantly reduces the number and area of demyelinating lesions in the spinal cord (Yao et al., 1995). There is also evidence of a reduction in serum BDNF during relapses in MS individuals relative to episodes of remission (Azoulay et al., 2005), which in turn is known to increase after a single bout of light exercise (Gold et al., 2003) as well as with chronic exercise (Cotman & Berchtold, 2002). Taken together these findings thus suggest that exercise might be associated with a sparing of brain tissue in individuals with MS, possibly as a result of increase in the nerve growth factors such as BDNF and IGF-1. Our results are cross-sectional in nature and thus do not go beyond implying an association between increased fitness levels and preserved GM volume and WM integrity. They do, however, lay the foundation for further exploring the causal effects of a fitness training intervention on the cognitive and brain functioning of individuals with MS.
An important limitation of our study, as acknowledged above, is the cross-sectional design, which prohibits us from making causal inferences about the effect of fitness on structural differences seen in MS individuals relative to healthy controls. Though the two groups of participants were matched for age, education and gender and all statistical analyses conducted within the MS cohort were controlled for age and intracranial volume, there might be other variables such as depression experienced by the participant; or other dietary variables etc. that might have an influence on the results. A randomized clinical trial examining the influence of fitness training on cortical atrophy would help to better characterize the role of exercise in MS without the presence of confounding variables. Further, it is important to note here that MS patients with lower disability scores are also better able to maintain their cardiorespiratory fitness and as a result of which may show improved cognitive functioning and preserved neuronal integrity. Another limitation of the study was the rather homogeneous sample of MS participants that was recruited for the current study, which in turn restricts the generalizations that can be made from the results of the study. We included only relapsing remitting MS female participants because of pragmatic concerns. It would be important to explore this relationship in a much more heterogeneous and a much larger sample with different disease types and greater severity of neurological disability to examine for some variables that might moderate the relation between fitness and preserved structural integrity.
Lastly, to avoid misclassification of T2 lesions as gray matter during VBM, we masked out the lesions independently so as to not contaminate estimation of GM changes in individuals with MS relative to healthy controls. Further we also included a GM mask in our analyses to avoid including pixels with a low probability of belonging to the GM, so that unbiased estimates of GM volumes in the two groups could be made. We also included a stringent threshold for multiple comparisons in both VBM and TBSS analyses, thereby reducing the likelihood of false positive results. However, brain segmentation techniques are complicated in patient populations that are characterized by WM hyperintensities because of a difference in tissue signal intensity and presence of microscopic lesions that might not be detected in T2 images. Given that our between group results are compatible with those found in previous studies employing voxelwise analyses (Prinster et al., 2006, Roosendaal et al., 2009), suggests that we were able to successfully mask out the effects of lesion volume on GM classification to investigate between group differences and association with cardiorespiratory fitness.
To summarize, the results of our study provide evidence for an association between aerobic fitness levels and sparing of regional gray matter volume and white matter tract integrity of NAWM in MS. Structural decline in MS, independent of demyelinating lesions, is now recognized as an important and defining feature of the neurodegenerative disease. The finding of spared brain tissue in the presence of the degenerative disease makes an important contribution to the MS literature and can have potentially significant implications for individuals diagnosed with MS.
4. METHODS
Participants
Twenty-one females diagnosed with definite relapsing-remitting MS (Poser et al., 1983) and fifteen age and education-matched healthy female controls were recruited for the current study. Participants were recruited by advertising in the local newspapers and using the database maintained by one of the authors of the study. Table I provides the descriptive characteristics of MS individuals and healthy controls. All participants were screened for any contraindications for participating in an MR study. MS participants were excluded from the study if they met any one of the following criteria: lack of consent from their primary care physician, or an inability to participate in an MR study. Further, during the initial screening, participants were also given a health history questionnaire in which questions were asked about history of different psychiatric disorders, other neurological disorders, head injury and substance abuse or dependence. Participants were excluded from the study if they reported any history of psychiatric disorders other than depression. Two of the twenty-one participants were currently on anti-depressants but none of the participants met DSM-IVTR diagnosis of a mood disorder. None of the participants endorsed any items related to head injury or other neurological disorders. The visual acuity of all participants was screened, with corrective lenses provided in order to achieve visual acuity of at least 20/30. The University of Illinois Institutional Review Board approved the study, and all participants provided informed consent.
Table I.
Descriptive characteristics of MS participants and healthy controls. Standard error presented in parentheses with the exception of EDSS for which the range of neurological disability is provided.
| Healthy Controls | MS patients | |
|---|---|---|
| Sample Size | 15 | 21 |
| Age | 45.8 (1.8) | 44.2 (1.9) |
| Education | 16.5 (0.5) | 15.6 (0.4) |
| Disease Duration (years) | na | 7.3 (0.1) |
| Mean EDSS (range) | na | 2.2 (0–6) |
| WM Lesion Load (cm3) | na | 1.92 (0.6) |
Neuropsychological Tests
All participants underwent a basic screening using the modified mini-mental status examination (mMMSE, highest score = 57; Stern et al., 1987), and the verbal portion of the Kaufman-Brief Intelligence Test to assess crystallized general intelligence (Kaufman & Kaufman, 1990). To further evaluate the cognitive status of participants, we also administered Rao’s Brief Repeatable Battery (BRB) of neuropsychological tests. The BRB includes five subtests: the Selective Reminding Test (SRT), a measure of verbal learning and delayed recall of a 12 paired word list; the Spatial Recall Test which measures visuo-spatial learning & delayed recall; the Symbol Digit Modalities Test (SDMT), which is a measure of sustained attention, working memory, and information processing speed; and the Word List Generation (WLG), a verbal fluency test and the Paced Auditory Serial Addition Test (PASAT), a test of sustained attention, working memory and information processing speed.
Cardiorespiratory Fitness Assessment
V·O2peak measures were assessed for each of the participant in order to determine aerobic endurance capacity. Participants performed an incremental exercise test on an electronically braked cycle ergometer to measure peak oxygen consumption (V·O2peak). Initially, participants were fitted to the cycle ergometer. The participants were then provided with standardized, tape-recorded instructions for correctly using the overall perceived exertion (Borg 6–20 scale; 4) scales. An investigator described the maximal exercise test procedures to all participants. After inserting a mouthpiece for collecting expired gases, the participants performed a 5-min warm-up at 0 W, and then the power output continuously increased at a rate of 15 W-min−1 until the participant reached volitional fatigue. Using an open-circuit spirometry system, ventilation (V.·E), oxygen consumption (V·O2), carbon dioxide production (V·CO2), and respiratory exchange ratio (RER) were measured every 20 seconds. Heart rate, rating of perceived exertion, and power output were recorded during the last 10 seconds of every min during the test of peak oxygen consumption. V·O2peak which was subsequently used for all analyses of cardiorespiratory fitness was defined as the highest recorded V·O2 value when two of three criteria were satisfied: (1) RER ≥1.10; (2) peak heart rate within 10 beats·min−1 of age-predicted maximum (i.e., ~1 SD); or (3) peak rating of perceived exertion ≥18.
MRI Acquisition
Participants were scanned in a 3T Siemens Allegra head-only scanner. High-resolution structural images were collected for each participant using a 3D MPRAGE (Magnetization Prepared Rapid Gradient Echo Imaging) protocol with 144 contiguous axial slices (TE/TR/TI 3.87/1800/900 ms), collected in an ascending fashion parallel to the anterior and posterior commissures using a spoiled gradient sequence (256×256mm FOV; 1.3mm thick slices, with a 1.3×1.3 mm2 in-plane resolution). To identify white-matter lesions, we acquired 38 T2-weighted axial slices (TE/TR 96/7050 ms) with a field of view of 220 × 220; 4 mm thick slices and a 1.3×1.3 mm2 in-plane resolution.
To examine the anisotropy values of white matter, all participants underwent axial single-shot sequence diffusion-weighted imaging (TE/TR 94/4200 ms) with diffusion gradients in 12 different spatial directions. The images had a field of view of 240 × 240 mm2, resulting in voxel sizes of 1.8 × 1.8 mm2 . The b values were 0 and 1000 s/ mm2 and we acquired 28 axially oriented slices.
Image Analyses
Lesion Load Analysis
MS lesions were outlined on T2-weighted images warped to the MPRAGE space, to create co-registered 3D lesion masks. AMIRA 3.1.1 (Mercury Computer Systems Inc; Kappos et al., 2006) was used to mark hyperintense WM lesions and subsequently calculate WM lesion volumes. The calculated WM lesion volume was then correlated with cardiorespiratory fitness in order to examine the association between the two variables.
There were two additional goals for the lesion load analysis: firstly, automated segmentation techniques such as the one used in the current study (FAST) for voxel-based morphometric analysis of GM data, often misclassify WM lesions as GM (Sanfilipo et al., 2005). In order to avoid MS lesion misclassification during optimized VBM (described below), we used the co-registered lesion masks, generated using AMIRA to zero out the MS lesions on the MPRAGE volumes. In other words, MPRAGE images of all MS participants were weighted by the corresponding 3D MS lesions masks and the voxels within lesions were not used for subsequent GM volume analyses, thus ensuring that all segmented GM volumes in fact reflected GM and not WM hyperintensities. Secondly, in order to examine whether regions of significant FA reduction co-occurred primarily with the focal lesions, we created a mean lesion probability map (LPM). This was done by firstly warping the MPRAGE images to standard space and then using the transformation matrix created in the first step to normalize the co-registered lesion masks to standard space. These registered lesion masks from all MS participants were then added to create a single map, which was averaged using FSLmaths tools to create a probability map, with values ranging from 0 to 1. This probability map was thresholded at 0.1, thus showing voxels in which 10% of the subjects had a lesion present. The lesion probability map was subsequently used as an overlay to examine co-localization of FA reductions with focal lesions.
Optimized Voxel-Based Morphometry (VBM)
High-resolution T1-weighted MPRAGE images were acquired to assess global regional volumetric changes in gray matter (GM) in individuals with multiple sclerosis relative to controls and to assess association with cardiorespiratory fitness. MPRAGE images of MS participants before VBM analysis were masked by 3D lesion masks to avoid misclassification of tissue types (see details on this under Lesion Load Analysis reported below). The structural data was analyzed using an optimized VBM approach (Ashburner & Friston, 2000; Good et al., 2001) using FSL-VBM, available as part of FMRIB’s software library (FSL Version 4.0, Smith, 2004). Full details of this technique have been discussed in many articles (for example, see Ashburner & Friston, 2000; Good et al., 2001) and here, we briefly outline the steps that we took in order to assess changes in regional GM volume.
We first created a study-specific template using both 15 healthy controls and randomly selected 15 MS participants to reduce systematic registration biases. The study-specific template was created by: (a) removing the skull from each of the MPRAGE volumes using BET (Smith et al., 2002a), (b) segmenting the brain extracted images into GM, WM and CSF probability maps, (c) normalizing the segmented GM probability map of each participant to MNI152 standard space using the affine registration tool FLIRT (Jenkinson et al., 2001, 2002), (d) creating an average of these registered images; and (e) spatially smoothing the average image with a 10-mm (FWHM) Gaussian kernel to create a study-specific template. The native GM probability maps of each participant were subsequently registered to this study-specific template using non-linear registration; modulated to incorporate the point-wise volume expansion or contraction induced by the transformation by dividing with the Jacobian of the warp field and finally spatially smoothed with an isotropic Gaussian kernel with a sigma of 7-mm FWHM to be then used as an input for voxelwise GLM statistical analyses.
In order to adjust the regional GM volumes for differences in height, we calculated intra cranial volume (ICV) for each of the participant using the cross-sectional version of the Structural Imaging Evaluation of Normalized Atrophy (SIENAx; Smith et al., 2002b). ICV was then used as a nuisance variable along with age for all subsequent statistical analyses with GM volumes.
Anisotropy Measurement
The diffusion-weighted images were preprocessed using FMRIB’s Diffusion Toolbox (FDT). The raw data was first corrected for head movements and eddy current distortion. The tensor model was then fitted to these preprocessed diffusion images to generate FA images, which were subsequently used as inputs for Tract Based Spatial Statistics (TBSS, Smith et al., 2006), a technique used to conduct voxelwise analysis of multi-subject diffusion tensor data.
The TBSS pipeline involved warping the subjects’ FA images to a target space using non-linear registration tool FNIRT (Andersson, 2007 a, b). We used the target space provided by FMRIB”s software library - FMRIB58_FA; a high-resolution average of 58 well-aligned good quality FA images from healthy male and female participants. This was followed by an affine registration of the target to the MNI standard space, thereby resulting in (a) non-linear transformation of each subjects’ FA to target space; and (b) affine transformation of the target to the MNI space. These two transformations were then applied to warp each subject’s FA image into standard space to create standardized FA maps. This was followed by averaging the standardized FA maps to create a mean FA image of all participants and subsequently thinned to produce a mean FA skeleton representing the centers of all tracts common to the group. For each of the subjects, a distance map was calculated from this mean skeletonized image to estimate the maximum FA value at each point in the skeleton. This distance map was finally used for subsequent voxelwise statistics.
Statistical Analyses
Neuropsychological data was firstly analyzed by conducting independent samples t-test between MS participants and healthy controls. Individuals with MS often exhibit deficits on tasks involving processing speed, with many studies reporting deficits in processing speed to be one of the core cognitive deficits observed in this population (Demaree et al., 1999). In order to examine association of neuropsychological scores with fitness and GM and WM focal regions, we created an information processing speed composite score, in order to provide a reliable measure of this construct, by merging performance on the PASAT, SDMT and the WLG (ZIPS), all three of which are known to measure processing speed abilities. We then conducted a series of partial correlations, after removing the variance associated with age to examine whether higher levels of fitness and preserved GM and WM structures were associated with better speed of processing in the MS participants.
Statistical analyses with the structural data were firstly conducted to examine differences in regional GM volumes and FA between healthy controls and MS participants and secondly, to examine the association between cardiorespiratory fitness and GM volume and white matter integrity as assessed through FA.
Between-group differences in GM volume and FA were examined using a nonparametric approach within a general linear model with age and ICV as covariates. Specifically, we used FSL’s Randomise tool for permutation based inferencing, which uses Monte Carlo permutation testing where n=1000, to generate the random permutations. Group comparisons were performed by directly comparing the GM volume and FA maps of Healthy Controls > MS participants, representing a contrast that would provide information on areas of GM loss in MS participants and MS participants> Healthy Controls, representing a contrast that would provide information on areas of GM loss in healthy controls. The final statistical images were corrected for multiple comparisons by using cluster-based thresholding of t=2.33 and a corrected cluster size significance level of p < 0.05.
Associations of GM volume and FA with cardiorespiratory fitness were conducted using a cluster-based approach. The clusters identified through the Controls>MS patients contrast were used to pull out mean partial volume estimates and mean FA values from the VBM and DTI analyses respectively. Partial GM volume estimates were then examined for their association with cardiorespiratory fitness via partial correlations, after removing variance associated with age and ICV. For DTI findings, associations were again examined with fitness using partial correlations, after removing the variance associated with age.
Rendering
All cortical renderings were performed using FSLVIEW, part of FMRIB’s software library. For VBM data, group statistical maps were mapped onto the study-specific template registered to MNI space. For Figure 1, clusters of GM loss are presented as 1 – p-value images, such that a value of 1 represents an area that is most significant. The image was thresholded at p<0.05 and thus only cortical regions that showed a statistically significant reduction at p<0.05 were included in the results. All VBM images are presented in radiological convention. DTI findings were mapped onto mean FA skeleton, a representation of the standard-space voxels that were tested in the multi-subject statistics. The mean FA skeleton was then mapped onto MNI space.
ACKNOWLEDGMENTS
We would like to thank the National Institute on Aging [RO1 AG25667, RO1 AG25032] for supporting this research. We would also like to thank Nancy Dodge and Holly Tracy and for their help in data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Andersson JLR, Jenkinson M, Smith S. Non-linear optimisation. 2007a FMRIB technical report TR07JA1 from www.fmrib.ox.ac.uk/analysis/techrep.
- Andersson JLR, Jenkinson M, Smith S. Non-linear optimisation. 2007b FMRIB technical report TR07JA1 from www.fmrib.ox.ac.uk/analysis/techrep.
- Amato MP, Bartolozzi ML, Zipoli V, Portaccio E, Mortilla M, Guidi L, Siracusa G, Sorbi S, Federico A, De Stefano N. Neocortical volume decrease in relapsing-remitting MS patients with mild cognitive impairment. Neurology. 2004;63:89–93. doi: 10.1212/01.wnl.0000129544.79539.d5. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston K. Voxel-based morphometry - The methods. NeuroImage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Audoin B, Davies GR, Finisku L, Chard DT, Thompson A, Miller DH. Localization of grey matter atrophy in early RRMS: A longitudinal study. Journal of Neurology. 2006;253:1495–1501. doi: 10.1007/s00415-006-0264-2. [DOI] [PubMed] [Google Scholar]
- Azoulay D, Vachapova V, Shihman B, Miler A, Karni A. Lower brain-derived neurotrophic factor in serum of relapsing remitting MS: Reversal by glatiramer acetate. Journal of Neuroimmunology. 2005;167:215–218. doi: 10.1016/j.jneuroim.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Bammer R, Augustin M, Strasser-Fuchs S, Seifert T, Kapeller P, Stollberger R, Ebner F, Hartung HP, Fazekas F. Magnetic resonance diffusion tensor imaging for characterizing diffuse and focal white matter abnormalities in multiple sclerosis. Magn. Reson. Med. 2000;44:583–591. doi: 10.1002/1522-2594(200010)44:4<583::aid-mrm12>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Barkhof C. The clinico-radiological paradox in multiple sclerosis revisited. Current Opinion in Neurology. 2002;15:239–245. doi: 10.1097/00019052-200206000-00003. [DOI] [PubMed] [Google Scholar]
- Bendfeldt K, Kuster P, Traud S, Egger H, Winklhofer S, Mueller-Lenke N, Naegelin Y, Gass A, Kappos L, Matthews PM. Association of regional gray matter volume loss and progression of white matter lesions in multiple sclerosis — A longitudinal voxel-based morphometry study. NeuroImage. 2009;45(1):60–67. doi: 10.1016/j.neuroimage.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Benedict RH, Carone DA, Bakshi R. Correlating brain atrophy with cognitive dysfunction, mood disturbances, and personality disorder in multiple sclerosis. J. Neuroimaging. 2004;14:36S–45S. doi: 10.1177/1051228404266267. [DOI] [PubMed] [Google Scholar]
- Bermel RA, Bakshi R, Tjoa C, Puli SR, Jacobs L. Bicaudate ratio as a magnetic resonance imaging marker of brain atrophy in multiple sclerosis. Arch. Neurol. 2002;59:275–280. doi: 10.1001/archneur.59.2.275. [DOI] [PubMed] [Google Scholar]
- Black JE, et al. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc. Natl. Acad. Sci. U. S. A. 1990;87:5568–5572. doi: 10.1073/pnas.87.14.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cercignani M, Bozzali M, Iannucci G, Comi G, Filippi M. Magnetisation transfer ratio and mean diffusivity of normal appearing white and gray matter from patients with multiple sclerosis. J Neurol Neurosurg Psychiatry. 2001;70:311–317. doi: 10.1136/jnnp.70.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chard DT, Griffin CM, Parker GJM, Kapoor R, Thompson AJ, Miller DH. Brain atrophy in clinically early relapsing-remitting multiple sclerosis. Brain. 2002;125:327–337. doi: 10.1093/brain/awf025. [DOI] [PubMed] [Google Scholar]
- Chard DT, Griffin CM, Rashid W, Davies GR, Altmann DR, Kapoor R, Barker GJ, Thompson AJ, Miller DH. Progressive gray matter atrophy in clinically early relapsing-remitting multiple sclerosis. Mult. Scler. 2004;10:387–391. doi: 10.1191/1352458504ms1050oa. [DOI] [PubMed] [Google Scholar]
- Cifelli A, Arridge M, Jezzard P, Esiri MM, Palace J, Matthews PM. Thalamic neurodegeneration in multiple sclerosis. Ann. Neurol. 2002;52:650–653. doi: 10.1002/ana.10326. [DOI] [PubMed] [Google Scholar]
- Charil A, Dagher A, Lerch JP, Zijdenbos AP, Worsley KJ, Evans AC. Focal cortical atrophy in multiple sclerosis: Relation to lesion load and disability. NeuroImage. 2007;34:509–517. doi: 10.1016/j.neuroimage.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Christie BR, Eadie BD, Kannangara TS, Robillard JM, Shin J, Titterness AK. Exercising our brains: how physical activity impacts synaptic plasticity in the dentate gyrus. Neuromolecular Med. 2008;10:47–58. doi: 10.1007/s12017-008-8033-2. [DOI] [PubMed] [Google Scholar]
- Ciccarelli O, Werring DJ, Wheeler-Kingshott CA, Barker GJ, Parker GJ, Thompson AJ, et al. Investigation of MS normal-appearing brain using diffusion tensor MRI with clinical correlations. Neurology. 2001;56:926–933. doi: 10.1212/wnl.56.7.926. [DOI] [PubMed] [Google Scholar]
- Ciccarelli O, Werring DJ, Barker GJ, Griffin CM, Wheeler-Kingshott CA, Miller DH, et al. A study of the mechanisms of normal- appearing white matter damage in multiple sclerosis using diffusion tensor imaging—Evidence of Wallerian degeneration. J. Neurol. 2003;250:867–877. doi: 10.1007/s00415-003-0992-5. [DOI] [PubMed] [Google Scholar]
- Ciccarelli O, Rocca MA, Pagani E, Colombo B, Martinelli V, Comi G, Filippi M. A voxel-based morphometry study of gray matter loss in MS patients with different clinical phenotypes. NeuroImage. 2008;42:315–322. doi: 10.1016/j.neuroimage.2008.04.173. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Raz N, Webb GA, Cohen NJ, Kramer AF. Aerobic Fitness Reduces Brain Tissue Loss in Aging Humans. J. Gerentol. 2003;58(2):176–180. doi: 10.1093/gerona/58.2.m176. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF. Fitness effects on the cognitive function of older adults: A Meta-Analytic study. Psychol. Sci. 2003;14(2):125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, Webb A, Jerome GJ, Marquez DX, Elavsky S. Cardiovascular fitness, cortical plasticyt, and aging. Proc Natl Acad Sci USA. 2004;101:3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- Coombs BD, Best A, Brown MS, Miller DE, Corboy J, Baier M, Simon JH. Multiple sclerosis pathology in the normal and abnormal appearing white matter of the corpus callosum by diffusion tensor imaging. Multiple Sclerosis. 2004;10:392–397. doi: 10.1191/1352458504ms1053oa. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Dalton CM, Chard DT, Davies GR, Miszkiel KA, Altmann DR, Fernando K, Plant GT, Thompson AJ, Miller DH. Early development of multiple sclerosis is associated with progressive gray matter atrophy in patients presenting with clinically isolated syndromes. Brain. 2004;127:1101–1107. doi: 10.1093/brain/awh126. [DOI] [PubMed] [Google Scholar]
- Dehmeshki J, Chard DT, Leary SM, et al. The normal appearing gray matter in primary progressive multiple sclerosis: a magnetisation transfer imaging study. J Neurol. 2003;250:67–74. doi: 10.1007/s00415-003-0955-x. [DOI] [PubMed] [Google Scholar]
- Demaree HA, DeLuca J, Gaudino EA, Diamond BJ. Speed of information processing as a key deficit in multiple sclerosis: implications for rehabilitation. Journal of Neurology, Neurosurgery & Psychiatry. 1999;67(5):661–663. doi: 10.1136/jnnp.67.5.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dineen RA, Viliasaar J, Hlina J, Bradshaw CM, Morgan PS, Constantinescu CS, Auer DP. Disconnection as a mechanism for cognitive dysfunction in multiple sclerosis. Brain. 2008;275:1–11. doi: 10.1093/brain/awn275. [DOI] [PubMed] [Google Scholar]
- DeStefano N, Narayanan S, Francis GS, Arnaoutelis R, Tartaglia MC, Antel J, Mathews PM, Arnold DL. Evidence of axonal damage in the early stages of multiple sclerosis and its relevance to disability. Archives of Neurology. 2001;58:65–70. doi: 10.1001/archneur.58.1.65. [DOI] [PubMed] [Google Scholar]
- DeStefano N, Matthews PM, Filippi M, Agosta F, De Luca M, Bartolozzi ML, Guidi L, Ghezzi A, Montanari E, Cifelli A, Federico A, Smith SM. Evidence of early cortical atrophy in MS: relevance to white matter changes and disability. Neurology. 2003;60:1157–1162. doi: 10.1212/01.wnl.0000055926.69643.03. [DOI] [PubMed] [Google Scholar]
- Devins GM, Seland TP. Emotional impact of multiple sclerosis: Recent findings and suggestions for future research. Psychological Bulletin. 1987;101:363–375. [PubMed] [Google Scholar]
- Fabel K, et al. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur. J. Neurosci. 2003;18:2803–2812. doi: 10.1111/j.1460-9568.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience. 2004;124:71–79. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Filippi M, Campi A, Dousset V, et al. A magnetization transfer imaging study of normal- appearing white matter in multiple sclerosis. Neurology. 1995;45:478–482. doi: 10.1212/wnl.45.3.478. [DOI] [PubMed] [Google Scholar]
- Fisher E, Rudick RA, Cutter G, Baier M, Miller D, Weinstock-Gutman B, Mass MK, Dougherty DS, Simonian NA. Relationship between brain atrophy and disability: an 8 year follow up study of multiple sclerosis patients. Multiple Sclerosis. 2000;6:373–377. doi: 10.1177/135245850000600602. [DOI] [PubMed] [Google Scholar]
- Ge Y, Law M, Johnson G, Herbert J, Babb JS, Mannon LJ, et al. Preferential occult injury of corpus callosum in multiple sclerosis measured by diffusion tensor imaging. J. Magn. Reson. Imaging. 2004;20:1–7. doi: 10.1002/jmri.20083. [DOI] [PubMed] [Google Scholar]
- Gold SM, Schulz K-H, Hartmann S, et al. Basal serum levels and reactivity of nerve growth factor and brain-derived neurotrophic factor to standardized acute exercise in multiple sclerosis and controls. J Neuroimmunol. 2003;138:99–105. doi: 10.1016/s0165-5728(03)00121-8. [DOI] [PubMed] [Google Scholar]
- Good C, Johnsrude I, Ashburner J, Henson R, Friston K, Frackowiak R. A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage. 2001;14(1):21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Grigsby J, Ayarbe S, Kravcisin N, Busenbark D. Working memory impairment among persons with chronic-progressive multiple sclerosis. Journal of Neurology. 1994;241:125–131. doi: 10.1007/BF00868338. [DOI] [PubMed] [Google Scholar]
- Hasan KM, Gupta RK, Santos RM, Wolinsky JS, Narayana PA. Diffusion tensor fractional anisotropy of the normal-appearing seven segments of the corpus callosum in healthy adults and relapsing-remitting multiple sclerosis patients. J. Magn. Reson. Imaging. 2005;21:735–743. doi: 10.1002/jmri.20296. [DOI] [PubMed] [Google Scholar]
- Henry JD, Beatty WW. Verbal fluency deficits in multiple sclerosis. Neuropsychologia. 2006;44:1166–1174. doi: 10.1016/j.neuropsychologia.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith SM. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5(2):143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister PR, Brady JM, Smith SM. Improved optimisation for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Kappos L, Antel J, Comi G, Montalban X, O'Connor P, Polman CH, Haas T, Korn AA, Karlsson G, Radue EW. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N. Engl. J. Med. 2006;355:1124–1140. doi: 10.1056/NEJMoa052643. [DOI] [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL. Kaufman brief intelligence test: Manual. Circle Pines, MN: American Guidance Service; 1990. [Google Scholar]
- Kleim JA, et al. Exercise induces angiogenesis but does not alter movement representations within rat motor cortex. Brain Res. 2002;934:1–6. doi: 10.1016/s0006-8993(02)02239-4. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Erickson KI. Capitalizing on cortical plasticity: influence of physical activity on cognition and brain function. Trends Cogn Sci. 2007;11:342–348. doi: 10.1016/j.tics.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Le Page C, Ferry A, Rieu M. Effect of muscular exercise on chronic relapsing experimental autoimmune encephalomyelitis. J Appl Physiol. 1994;77:2341–2347. doi: 10.1152/jappl.1994.77.5.2341. [DOI] [PubMed] [Google Scholar]
- Le Page C, Bourdoulous S, Beraud E, et al. Effect of physical exercise on adoptive experimental auto-immune encephalo-myelitis in rats. Eur J Appl Physiol Occup Physiol. 1996;73:130–135. doi: 10.1007/BF00262821. [DOI] [PubMed] [Google Scholar]
- Lester K, Stepleman L, Hughes M. The association of illness severity, self-reported cognitive impairment, and perceived illness management with depression and anxiety in a multiple sclerosis clinic population. Journal of Behavioral Medicine. 2007;30:177–186. doi: 10.1007/s10865-007-9095-6. [DOI] [PubMed] [Google Scholar]
- Lopez-Lopez C, et al. Insulin-like growth factor I is required for vessel remodeling in the adult brain. Proc. Natl. Acad. Sci. U. S. A. 2004;101:9833–9838. doi: 10.1073/pnas.0400337101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DH, Thompson AJ, Filippi M. Magnetic resonance studies of abnormalities in the normal appearing white matter and gray matter in multiple sclerosis. J Neurol. 2003;250:1407–1419. doi: 10.1007/s00415-003-0243-9. [DOI] [PubMed] [Google Scholar]
- Morgen K, Sammer G, Courtney SM, Wolters T, Melchior H, Blecker CR, Oschmann P, Kaps M, Vaitl D. Evidence for a direct association between cortical atrophy and cognitive impairment in relapsing-remitting MS. NeuroImage. 2006;30:891–898. doi: 10.1016/j.neuroimage.2005.10.032. [DOI] [PubMed] [Google Scholar]
- Mori S, Wakana S, Nagae-Poetscher LM, van Zijl PCM. MRI atlas of human white matter. Elsevier, B.V.: Amsterdam, The Netherlands; 2005. [Google Scholar]
- Nagels G, Geentjens L, Kos D, Vleugels L, D'hooghe MB, Asch PV, Deyn PP. Paced visual serial addition test in multiple sclerosis. NeuroImage. 2005;107:218–222. doi: 10.1016/j.clineuro.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Pagani E, Rocca MA, Gallo A, Rovaris M, Martinelli V, Comi G, Filippi M. Regional brain atrophy evolves differently in patients with multiple sclerosis according to clinical phenotype. AJNR Am. J. Neuroradiol. 2005;26:341–346. [PMC free article] [PubMed] [Google Scholar]
- Pierpaoli P, Basser P. Toward a quantitive assessment of diffusion anisotropy. Magn. Reson. Med. 1996;36:893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- Plant GT. Optic neuritis and multiple sclerosis. Current Opinions in Neurology. 2008;21:16–21. doi: 10.1097/WCO.0b013e3282f419ca. [DOI] [PubMed] [Google Scholar]
- Poser CM, Paty DW, Scheinberg L, McDonald WI, Davis FA, Ebers GC. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Annals of Neurol. 1983;13:227–231. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- Prakash RS, Snook EM, Erickson KI, Colcombe SJ, Voss MW, Motl RW, Kramer AF. Cardiorespiratory Fitness: A Predictor of Cortical Plasticity in Multiple Sclerosis. NeuroImage. 2007;38:1238–1244. doi: 10.1016/j.neuroimage.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Prakash RS, Snook EM, Lewis JM, Motl RW, Kramer AF. Cognitive impairments in relapsing remitting multiple sclerosis: A meta-analysis. Mult Scler. 2008;14(9):1250–1261. doi: 10.1177/1352458508095004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinster A, Quarantelli M, Orefice G, Lanzillo R, Brunetti A, Mollica C, Salvatore E, Morra VB, Coppola G, Vacca G, Alfano B, Salvatore M. Gray matter loss in relapsing-remitting multiple sclerosis: a voxel-based morphometry study. NeuroImage. 2006;29:859–867. doi: 10.1016/j.neuroimage.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Ranjeva J-P, Audoin B, Au Dong MV, Confort-Gouny S, Malikova I, Viout P, Soulier E, Pelletier J, Cozzone PJ. Structural and functional surrogates of cognitive impairment at the very early stage of multiple sclerosis. Journal of the Neurological Sciences. 2006;245:161–167. doi: 10.1016/j.jns.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Roosendaal SD, Geurts JJ, Vrenken H, Hulst HE, Cover KS, Castelijns JA, Pouwels PJ, Barkhof F. Regional DTI differences in multiple sclerosis patients. NeuroImage. 2009;44(4):1397–1403. doi: 10.1016/j.neuroimage.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Rovaris M, Bozzali M, Iannucci G, et al. Assessment of normal-appearing white and gray matter in patients with primary progressive multiple sclerosis: a diffusion-tensor magnetic resonance imaging study. Arch Neurol. 2002a;59:1406–1412. doi: 10.1001/archneur.59.9.1406. [DOI] [PubMed] [Google Scholar]
- Rovaris M, Iannucci G, Falautano M, Possa F, Martinelli V, Comi G, et al. Cognitive dysfunction in patients with mildly disabling relapsing-remitting multiple sclerosis: an exploratory study with diffusion tensor. J Neurol Sci. 2002b;195:103–109. doi: 10.1016/s0022-510x(01)00690-6. [DOI] [PubMed] [Google Scholar]
- Sailer M, Fischl B, Salat D, Tempelmann C, Schonfeld MA, Busa E, Bodammer N, Heinze HJ, Dale A. Focal thinning of the cerebral cortex in multiple sclerosis. Brain. 2003;126:1734–1744. doi: 10.1093/brain/awg175. [DOI] [PubMed] [Google Scholar]
- Sanfilipo MP, Benedict RB, Sharma J, Weinstock-Guttman B, Bakshi R. The relationship between whole brain volume and disability in multiple sclerosis: A comparison of normalized gray vs. white matter with misclassification correction. NeuroImage. 2005;26:1068–1077. doi: 10.1016/j.neuroimage.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Sepulcre J, Sastre-Garriga J, Cercignani M, Ingle GT, Miller DH, Thompson AJ. Regional gray matter atrophy in early primary progressive multiple sclerosis: a voxel-based morphometry study. Arch. Neurol. 2006;63:1175–1180. doi: 10.1001/archneur.63.8.1175. [DOI] [PubMed] [Google Scholar]
- Simon JH, Kinkel RP, Jacobs L, Bub L, Simonian N. A Wallerian degeneration pattern in patients at risk for MS. Neurology. 2000;54:1155–1160. doi: 10.1212/wnl.54.5.1155. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002a;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Zhang Y, Jenkinson M, Chen J, Matthews PM, Federico A, De Stefano N. Accurate, robust and automated longitudinal and cross-sectional brain change analysis. NeuroImage. 2002b;17(1):479–489. doi: 10.1006/nimg.2002.1040. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich JW, Beckmann CF, Behrens TEJ, Johansen-Berg, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy R, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(S1):208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TEJ. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;3:1487–1487. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Stern Y, Sano M, Paulsen J, Mayeux R. Modified Mini-Mental State Examination: Validity and Reliability. Neurology. 1987;37:179. [Google Scholar]
- Swain RA, et al. Prolonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the rat. Neuroscience. 2003;117:1037–1046. doi: 10.1016/s0306-4522(02)00664-4. [DOI] [PubMed] [Google Scholar]
- Tievsky AL, Ptak T, Farkas J. Investigation of apparent diffusion coefficient and diffusion tensor anisotropy in acute and chronic multiple sclerosis lesions. AJNR Am. J. Neuroradiol. 1999;20:1491–1499. [PMC free article] [PubMed] [Google Scholar]
- Trejo JL, et al. Circulating insulin-like growth factor mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J. Neurosci. 2001;21:1628–1634. doi: 10.1523/JNEUROSCI.21-05-01628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neuroscience. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20:2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- Vrenken H, Barkhof F, Uitdehaag BM, Castelijns JA, Polman CH, Pouwels PJ. MR spectroscopic evidence for glial increase but not for neuro-axonal damage in MS normal- appearing white matter. Magn Reson Med. 2005;53:256–266. doi: 10.1002/mrm.20366. [DOI] [PubMed] [Google Scholar]
- Vrenken H, Petra JW, Pouwels P, Geurts J, Knol DL, Polman CH, Barkhof F, Castelijns JA. Altered Diffusion Tensor in Multiple Sclerosis Normal-Appearing Brain Tissue: Cortical Diffusion Changes Seem Related to Clinical Deterioration. Journal of Magnetic Resonance Imaging. 2006a;23:628–636. doi: 10.1002/jmri.20564. [DOI] [PubMed] [Google Scholar]
- Vrenken H, Petra JW, Pouwels P, Geurts J, Knol DL, Polman CH, Barkhof F, Castelijns JA. Whole-Brain T1 Mapping in Multiple Sclerosis: Global Changes of Normal-appearing Gray and White Matter. Radiology. 2006b;240:811–820. doi: 10.1148/radiol.2403050569. [DOI] [PubMed] [Google Scholar]
- Waller AV. Experiments on the section of the glossopharayngeal and hyoglossal nerves of the frog, and observations of the alterations produced thereby in the structure of the primitive fiber. Philos Trans R Soc Lond. 1850;140:423–429. [Google Scholar]
- Werring DJ, Clark CA, Barker GJ, Thompson AJ, Miller DH. Diffusion tensor imaging of lesions and normal-appearing white matter in multiple sclerosis. Neurology. 1999;52:1626–1632. doi: 10.1212/wnl.52.8.1626. [DOI] [PubMed] [Google Scholar]
- White LJ, Castellano V. Exercise and Brain Health –L Implications for Multiple Sclerosis. Part 1 – Neuronal Growth Factors. Sports Medicine. 2008;38(2):91–100. doi: 10.2165/00007256-200838020-00001. [DOI] [PubMed] [Google Scholar]
- Yao D, Liu X, Hudson L, et al. Insulin-like growth factor I treatment reduces demyelination and up-regulates gene expression of myelin-related proteins in experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 1995;92:6190–6194. doi: 10.1073/pnas.92.13.6190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivadinov R, Sepcic J, Nasuelli D, De Masi R, Bragadin LM, Tommasi MA, Zambito-Marsala S, Moretti R, Bratina A, Ukmar M, Pozzi-Mucelli RS, Grop A, Cazzato G, Zorzon M. A longitudinal study of brain atrophy and cognitivedisturbances in the early phase of relapsing – remitting multiple sclerosis. J. Neurol. Neurosurg. Psychiatry. 2001;70:773–780. doi: 10.1136/jnnp.70.6.773. [DOI] [PMC free article] [PubMed] [Google Scholar]