Abstract
Objective
To evaluate pregnancy outcomes in normotensive second pregnancy following preeclampsia in first pregnancy.
Design
Population-based retrospective cohort study.
Setting
State of Missouri in the United States.
Sample
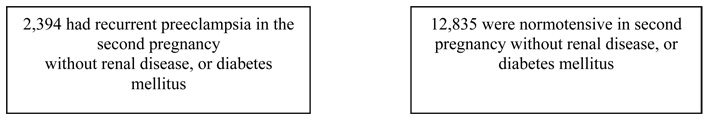
White European origin or African American women who delivered their first 2 non-anomalous singleton pregnancies between 20 and 44 weeks of gestation in Missouri, 1989–2005, without chronic hypertension, renal disease, or diabetes mellitus (n = 12,835).
Methods
Preeclampsia and delivery <= 34 weeks gestational age in first pregnancy was defined as early-onset preeclampsia whereas late-onset preeclampsia was preeclampsia with delivery > 34 weeks. Multivariate regression models were fit to estimate crude and adjusted odds ratios (aOR) and 95% confidence intervals (95% CI).
Main outcome measures
Preterm delivery, large and small for gestational age infant, Apgar scores at 5 minutes, fetal death, cesarean section, placental abruption
Results
Women with early-onset preeclampsia in first pregnancy were more likely to be younger, African American, recipients of Medicaid, unmarried, smokers. Despite a second normotensive pregnancy, women with early-onset preeclampsia in first pregnancy had greater odds of SGA, preterm births, fetal death, cesarean section and placental abruption in 2nd pregnancy, relative to women with late-onset preeclampsia after controlling for confounders. Moreover, maternal ethnic origin modified the association between early-onset preeclampsia in first pregnancy and preterm births in 2nd pregnancy. Having a history of early-onset preeclampsia reduces the odds of having a large for gestation infant in 2nd pregnancy.
Conclusion
A history of early-onset preeclampsia is associated with increased odds of adverse pregnancy outcomes despite a normotensive second pregnancy.
Keywords: Preeclampsia, Early-onset preeclampsia, Preterm birth, Small for gestational age, Fetal death, Cesarean section, Placental abruption
Introduction
Preeclampsia occurs in approximately 5% to 10% of nulliparous pregnancies1 and is among the top three causes of maternal mortality in both developed and developing countries2, 3 and one of the leading causes of maternal and fetal morbidity.4 Women who experience preeclampsia are at increased risk for adverse pregnancy outcomes, with its prognosis depending on the severity of the disease and the gestational age at the time of disease onset and at delivery.5 Most preeclampsia has an onset near term but approximately 10% of the cases have an early onset before 34 weeks of gestation.6 It is believed that early onset preeclampsia that requires preterm delivery has underlying pathology that differs and is more severe than late onset preeclampsia.7 Early-onset preeclampsia has been associated with worse perinatal outcomes such as small-for-gestational age infants, compared to preeclampsia that has onset at term.8, 9 Early onset of preeclampsia is also characterized by increased severity, including HELLP syndrome and placental abruption.9
Several studies have investigated the recurrence risk and subsequent pregnancy outcomes of women with a history of preeclampsia and eclampsia.9–11 However, few studies had examined outcomes of normotensive second pregnancy following preeclampsia. Even though the recurrence risk for preeclampsia is high, particularly among those with a history of early onset preeclampsia, it does not recur in about 80% of women with a history of preeclampsia.12 It has been observed that when preeclampsia does not reoccur in the second pregnancy, the overall obstetric outcome is favorable.13 Nonetheless, among the few studies that examined pregnancy outcomes in subsequent normotensive pregnancy following preeclampsia in first pregnancy, these findings were limited by inclusion of women with chronic hypertension, lack of statistical power, failure to control for potential confounders, or without classifying women by gestational age at delivery or early vs. late preeclampsia in first pregnancy.
Information regarding preeclampsia and its effects on subsequent pregnancy outcomes is essential in providing counseling to women with a history of preeclampsia and their caregivers to help them make important decisions pertaining to future pregnancy. Prior research has indicated that the mechanism of disease may be different in those pregnancies that are complicated by preeclampsia at term compared with those pregnancies that are preterm.7 In studying effect of preeclampsia on subsequent pregnancy outcomes, it is important to stratify women by gestational age at delivery in preeclampsia pregnancies. The objective of the present study was to investigate the association between early onset preeclampsia in first pregnancy and adverse maternal and perinatal outcomes in normotensive second pregnancy. The ethnic disparity in adverse maternal and fetal outcomes between African-American and women of White European origin has been recognized for decades and remains a major public health concern. Maternal ethnic origin has consistently been shown to be a contributing factor in maternal complications and adverse birth outcomes, including diabetes mellitus, preeclampsia, low birth weight infants, and preterm delivery.14–17 Therefore, we further evaluated whether maternal ethnic origin is an effect modifier. While racial group implies a specific genetic inheritance, ethnicity reflects culture and is therefore changeable. When analyzing groups such as a white European population versus an African-American population in the present study based on a U.S. popualtion, it is difficult to ascertain whether it is genetics or environment. In this study no attempt has been made to distinguish between the two effects in the role of maternal ethnic origin as an effect mofidier.
Methods
We conducted a population-based, retrospective cohort study of pregnancy outcomes in normotensive second pregnancy following preeclampsia in first pregnancy among women who reside in the State of Missouri. The study was based on data from the Missouri maternally linked cohort, which links sibling birth certificate data with the use of maternal identifiers. The database includes a large quantity of data regarding each birth that occur in the state, including parental demographic information, medical and obstetrical characteristics and complications, and neonatal status at birth. Details on the methods used for linking records of successive pregnancies, as well as the validation process of the linked reproductive histories have been described elsewhere.18 Briefly, degree of agreement across a set of common variables for two pregnancies (i.e., a “pair”) was used to calculate statistical weights.18 The pairs of pregnancies with the highest overall weights were selected based on the level of agreement and a priority indicator of variables (i.e., birth date, maternal name) with exact matches.18 The linkage rate was 93% for women born in Missouri. The Missouri vital record system is considered very reliable and one that has been adopted as a “gold standard” to validate other vital statistics data sets in the United States that involve matching and linking procedures.19 This research was reviewed by the Saint Louis University Institutional Review Board and was classified as exempt.
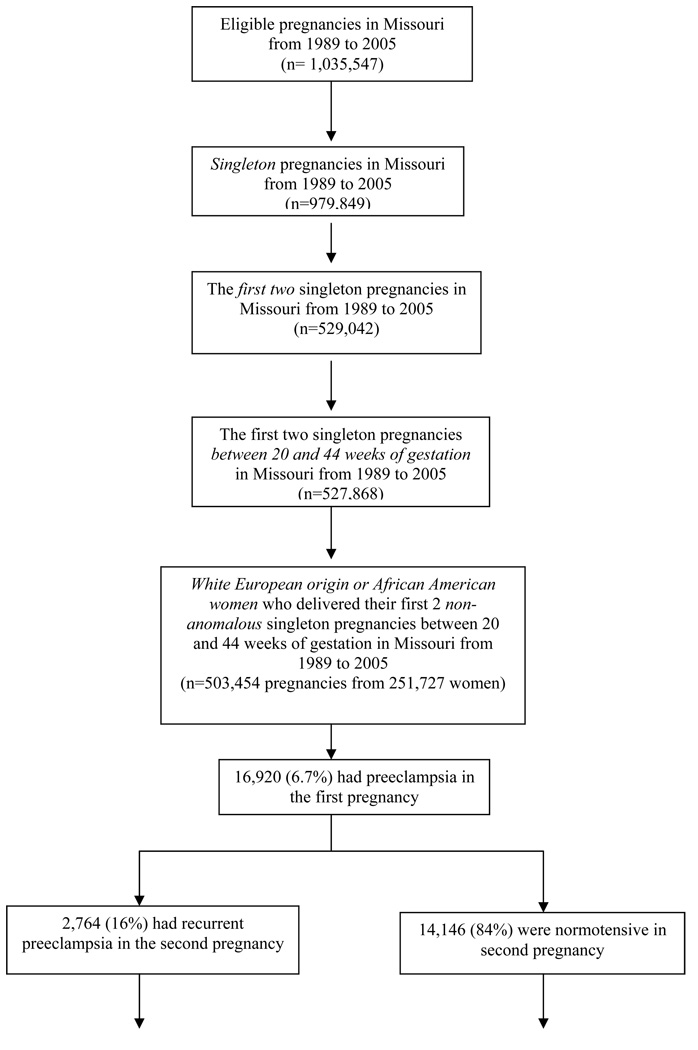
All women who had the condition “pregnancy-induced hypertension (preeclampsia)” or “eclampsia” checked for the first birth on the Missouri birth certificate were eligible for the present study. The entire cohort of Missouri maternally linked data, based on the old 1989 revision of birth certificates form, included all women who gave birth in the state between 1979 and 2005. Gestational age from the present study was based on the variable “clinical estimate of gestation” from the birth certificate because it is a more accurate reflection of gestational age at delivery than length of pregnancy calculated using the last menstrual period. However, the clinical estimated gestational age variable did not become a required field on the birth certificate until 1989. Therefore, we limited the analysis to the years from 1989 to 2005. Our study sample consists of women of White European origin or African American women who delivered their first 2 non-anomalous singleton pregnancies between 20 and 44 weeks of gestation in Missouri between January 1, 1989, and December 31, 2005. In our study sample, about 7% (n = 16,920) of women experienced preeclampsia in the first pregnancy and among those, eighty four percent of women (n=14,146) had a normotensive second pregnancy after having preeclampsia in the first pregnancy (Figure 1). In order to reduce heterogeneity of the study population, multiple gestation births were excluded due to its potential confounding effect on the outcomes of interest. Women with medical problems in second pregnancy such as chronic hypertension, renal disease, diabetes mellitus were also excluded. Specifically, nine percent (n = 1,313) of women with normotensive second pregnancy and 13% (n = 370) of women with recurrent preeclampsia were excluded due to medical problems such as chronic hypertension, renal disease, diabetes mellitus in second pregnancy. That results in an analytic sample of 12,835 women with history of preeclampsia in first pregnancy who had a normotensive second pregnancy (Figure 1).
Figure 1.
Outcomes of interest for the present study include preterm births, small- for-gestational-age infant (SGA), large for gestation age (LGA), low Apgar scores at 5 minutes (<7), fetal death, cesarean section, and placental abruption in the second pregnancy. Preterm birth as defined by the World Health Organization is delivery at less than 37 weeks gestational age.20 We focused our analysis on those preterm births occurring at less than 35 weeks in order to avoid borderline gestational ages, which are more prone to misclassification bias, and to identify the population of infants born at the earliest gestations when prognoses are often poor. SGA and LGA were measured as birth weight below the 10th percentile and above the 90th percentile, respectively, for gestational age and ethnicity, with the United States population serving as the reference for fetal growth.21 In accordance with WHO definition of fetal death, cases were defined as those pregnancy that had a gestational age of 22 completed weeks with a fetal weight ≥ 500 g.22 The cesarean section outcome referred to primary elective and emergency cesarean section as indicated on the birth certificate for the second pregnancy after a vaginal delivery in the first prengnacy. Preeclampsia and delivery at or before 34 weeks gestational age in first pregnancy was defined as early-onset preeclampsia whereas late-onset preeclampsia was preeclampsia with delivery after 34 weeks.23
Factors that may be associated with early-onset preeclampsia and outcomes of interest were evaluated as potential confounders. Data for the following maternal demographic and lifestyle variables from the second pregnancy were obtained from the birth certificate: maternal age, ethnic origin (White European origin or African American), marital status (single or married), smoking during pregnancy (yes or no), Medicaid use (yes or no), pre-pregnancy body mass index (BMI), and inter-pregnancy interval. For easier parameter estimate interpretation, maternal age was mean centered.24 BMI, calculated as weight (kilograms) per height (square meters), was categorized as underweight (BMI, < 18.5 kg/m2), normal weight (BMI, 18.5 – 24.9 kg/m2), overweight (BMI, 25 – 29.9 kg/m2), and obese (BMI, ≥ 30 kg/m2). Inter-pregnancy interval was calculated as the time (in years) from the first birth until conception of the second pregnancy (estimated from clinical gestational age) and was categorized as < 1, 1–2, 2+ to 4, and > 4 years.
Statistical analysis
Differences in sample characteristics by gestational timing of preeclampsia in the first pregnancy were assessed by using the Pearson Chi-square test (χ2) for categorical variables and t-test for continuous variables. Multivariate binary logistic regression models were used to estimate the odds of SGA, LGA, preterm delivery, low Apgar scores, fetal death, and placental abruption in the second pregnancy. A Poisson regression model with robust error variance was constructed to estimate relative risk and 95% confidence intervals (95% CI) for cesarean section to avoid the pitfall of using the odds ratio to estimate the risk ratio when the outcome is common (greater than 10%).25 The estimated relative risk and 95% confidence interval for cesarean section was further verified with a negative log-binomial regression model for common outcome. 25,26 To reduce the bias in the estimation of risk, potential confounders were included in the multivariate analysis, including maternal age, marital status, smoking during pregnancy, Medicaid use, BMI, and inter-pregnancy interval. To evaluate if maternal ethnic origin is an effect modifier, the Wald test was used to test if the regression coefficient of the product term of gestational timing of preeclampsia in first pregnancy and maternal ethnic origin is statistically different from zero. All tests were 2 tailed and p < .05 was considered significant. All statistical analyses were performed with STATA (version 10.0, STATA Corp, College Station, TX).
Results
The characteristics of study participants are summarized by gestational timing of preeclampsia in the first pregnancy in Table 1. Compared to women with late-onset preeclampsia in first pregnancy, those who had a history of early onset preeclampsia were younger and more likely to be African American, smoke during pregnancy, and Medicaid recipients, unmarried, and have infants with shorter inter-pregnancy interval and lower mean birth weight. We also compared the normotensive second pregnancy outcomes by early or late onset of preeclampsia in the first pregnancy and the results are detailed in Table 2. Women who experienced early onset preeclampsia in first pregnancy were less likely to have larger for gestational age infants but more likely to have infants who were SGA, premature, had greater number of fetal death, cesarean section, and placental abruption in the second normotensive pregnnacy, compared to those who had late onset preeclampsia in first pregnancy.
TABLE 1.
Characteristics of study sample by gestational timing of preeclampsia in first pregnancy (n = 12,835).
| Preeclampsia (1st pregnancy)* |
|||
|---|---|---|---|
| Characteristics | Early onset n (%) |
Late onset n (%) |
p- value** |
| Maternal age, mean (SD) | 25.6 (5.32) | 26.0 (5.2) | < 0.01 |
| < 20 (years) | 121 (11.7) | 1,049 (8.9) | 0.03 |
| 20–29 | 657 (63.5) | 7,666 (65.0) | |
| 30–34 | 191 (18.5) | 2,317 (19.6) | |
| 35–39 | 60 (5.8) | 675 (5.7) | |
| ≥ 40 | 5 (0.5) | 94 (0.8) | |
| Maternal ethnic origin | < 0.01 | ||
| White European origin | 795 (76.9) | 10,018 (84.9) | |
| African American | 239 (23.1) | 1,783 (15.1) | |
| Smoking during pregnancy | 0.04 | ||
| No | 843 (81.69) | 9,913 (84.1) | |
| Yes | 189(18.3) | 1,871 (15.9) | |
| Pre-pregnancy BMI | < 0.01 | ||
| Underweight | 51 (5.2) | 358 (3.1) | |
| Normal | 433 (43.9) | 4,502 (39.2) | |
| Overweight | 226 (22.9) | 2,972 (25.9) | |
| Obese | 276 (28.0) | 3,644 (31.8) | |
| Medicaid use | < 0.01 | ||
| No | 547 (53.1) | 7,087 (60.2) | |
| Yes | 483(46.9) | 4,686 (39.8) | |
| Marital status | < 0.01 | ||
| Married | 668 (64.6) | 8,595(72.9) | |
| Unmarried | 366 (35.4) | 3,202 (27.1) | |
| Inter-pregnancy interval | < 0.01 | ||
| < 1 year | 253 (24.5) | 2,460 (20.9) | |
| 1 to 2 years | 278 (26.9) | 3,608 (30.6) | |
| 2+ to 4 years | 331 (32.1) | 3,800 (32.3) | |
| > 4 years | 170 (16.5) | 1,915 (16.3) | |
| Mean birth weight in grams (SD) | 3,086 (708.5) | 3,417 (558.5) | < 0.01 |
Preeclampsia and delivery at or before 34 weeks gestational age in first pregnancy was defined as early-onset preeclampsia whereas late-onset preeclampsia was preeclampsia with delivery after 34 weeks.
The p-value for a chi-square test for categorical variables and for a t-test for continuous variables.
TABEL 2.
Normotensive Second pregnancy outcomes by gestational age of preeclampsia in first pregnancy (n = 12,835).
| Preeclampsia status in first pregnancy |
|||
|---|---|---|---|
| Perinatal outcomes in second pregnancy | Early onset n (%) |
Late onset n (%) |
p-value* |
| Small for gestational age† | 144 (14.1) | 894 (7.6) | < 0.01 |
| Large for gestational age† | 82 (8.1) | 1,477 (12.6) | < 0.01 |
| Preterm delivery (< 35 weeks) † | 97 (9.6) | 349 (3.0) | < 0.01 |
| Low Apgar scores (< 7 at 5 min) | 16 (1.6) | 124 (1.1) | 0.13 |
| Fetal death | 10 (1.0) | 48 (0.4) | 0.01 |
| Cesarean section** | 63 (14.4) | 712 (9.0) | < 0.01 |
| Placental abruption | 24 (2.3) | 91(0.8) | < 0.01 |
The p-value for a chi-square test.
among women who had a vaginal delivery in first pregnancy
Restricted to live births
Because of the differences in the distribution of risk factors between the two study groups by gestational timing of preeclampsia in the first pregnancy, the analyses were adjusted for variables potentially associated with outcomes of interest. After controlling for confounding, having a history of early onset preeclampsia in the first pregnancy increased the odds of SGA by 75% but reduced the odds by 36% for LGA in a normotensive second pregnancy, compared to women who had a late onset preeclampsia in the first pregnancy (Table 3). Furthermore, the odds of having preterm birth in second normotensive pregnancy were 3 times as much as those who had a late onset preeclampsia in first pregnancy. For fetal death in normotensive second pregnancy, our findings showed that women who had early onset preeclampsia in the first pregnancy had more than 2 times the odds of those who had late onset preeclampsia in first pregnancy. Furthermore, having early onset preeclampsia in first pregnancy increased the odds of placental abruption by 140% in a normotensive second pregnancy, after controlling for covariates. To reduce the heterogeneity of our study sample in the analysis of outcomes in the second pregnancy, we further estimated the odds of the first occurrence of the outcomes of interest in the second pregnancy and obtained similar results. Specifically, having early onset preeclampsia in first pregnancy yields an adjusted odds ratio of 1.94 (95% CI: 1.48, 2.53) for SGA, 0.73 (95% CI: 0.56, 0.94) for LGA, 1.28 (95% CI: 0.68, 2.40) for low apgar scores, 2.22 (95% CI: 1.07, 4.60) for fetal death, and 2.26 (95% CI: 1.33, 3.83) for placental abruption in the normotensive second pregnancy (results not shown).
TABLE 3.
Odds ratios for normotensive second pregnancy outcomes among women who experienced early onset preeclampsia in first pregnancy, Missouri, 1989–2005 (n = 12,835)
| Perinatal outcomes in second pregnancy | cOR (95% CI) ** | aOR (95% CI)*, ** |
|---|---|---|
| Small for gestational age ‡ | 2.00 (1.65, 2.41) | 1.75 (1.43, 2.15) |
| Large for gestational age‡ | 0.61 (0.48,0.77) | 0.64 (0.50, 0.81) |
| Preterm delivery (< 35 weeks) ‡ | 3.43(2.71, 4.34) | 3.00 (2.35, 3.85) |
| Low Apgar scores (< 7 at 5 min) | 1.50 (0.89, 2.53) | 1.31 (0.75, 2.29) |
| Fetal death | 2.40 (1.21, 4.75) | 2.43 (1.21, 4.89) |
| Cesarean section† | 1.60 (1.26, 2.04) | 1.65 (1.29, 2.10) |
| Placental abruption | 3.06 (1.94, 4.82) | 2.40 (1.46, 3.94) |
analysis adjusted for maternal age, ethnic origin, marital status, smoking during pregnancy, Medicaid use, BMI, and inter-inter-pregnancy interval
adjusted odds ratios and 95% confidence interval
relative risk estimated by Poisson regression model with a robust error variance among women who had vaginal delivery in first pregnancy
restricted to live births
Table 4 details results of our analysis on whether the effect of gestational age of preeclampsia on adverse maternal and fetal outcomes was modified by maternal ethnic origin, and we detected a significant additive interaction. The effect of gestational timing of preeclampsia in first pregnancy on preterm delivery in second normotensive pregnancy varied by maternal ethnic origin (interaction term p value = 0.04). Having a history of early onset preeclampsia alone carried a more than 3 fold increased odds of preterm delivery in normotensive second pregnancy relative to late onset preeclampsia (Table 4). Being African American alone carried a 2.43-fold increased odds of preterm delivery. Being African American with a history of early onset preeclampsia carried a markedly increased odd of preterm delivery, with adjusted odds ratios of 5.45, albeit this is a weak positive (i.e., synergistic) interaction on the additive scale since the observed joint odds ratio is slightly greater than the expected joint odds ratio of 5.14 (=3.71 + 2.43 − 1).27
TABLE 4.
Multivariate adjusted interaction between gestational age of preeclampsia in first pregnancy and maternal ethnic origin on preterm delivery in normotensive second pregnancy, Missouri, 1989–2005 (n = 12,835)
| Preterm delivery |
||
|---|---|---|
| Gestational timing of preeclampsia in first pregnancy by maternal ethnic origin |
aOR†‡ | 95% CI† |
| Non-Hispanic White and late onset | 1.0 | Reference |
| Early onset of preeclampsia only | 3.71 | 2.81, 4.90 |
| African American only | 2.43 | 1.86, 3.17 |
| Early onset preeclampsia and African American | 5.45 | 3.62, 8.21 |
adjusted odds ratios and 95% confidence interval
the multivariate model includes gestational timing of preeclampsia in first pregnancy, maternal age, ethnic origin, marital status, smoking during pregnancy, Medicaid use, BMI, and inter-inter-pregnancy interval, gestational timing of preeclampsia in first pregnancy and maternal ethnic origin product term
Discussion
In our study, we evaluated the perinatal outcomes in normotensive second pregnancy of women with early onset preeclampsia in first pregnancy. Despite a normotensive second pregnancy, we observed that having a history of early onset preeclampsia, as compared to late onset in first pregnancy, increased odds of SGA, preterm birth, fetal death, cesarean section, and placental abruption, after controlling for confounders. It is noteworthy that in the second normotensive pregnancies of women with late-onset preeclampsia, the incidence of small for gestational age babies was less than those with early-onset preeclampsia (7.6% vs. 14.1%) whereas the incidence of large gestational age babies was greater (12.6% vs. 8.1%). These findings support the hypothesis that preeclampsia is an etiologically heterogeneous disorder with two subtypes: a late-onset preeclampsia with normal fetal growth denoting normal placental function and an early-onset type with fetal growth restriction implying placental dysfunction. In the early-onset subtype, it has been hypothesized that placental hypoperfusion is caused by shallow invasion of fetal trophoblast in early pregnancy, leading to fetal growth restriction in early-onset preeclampsia.28 And decreased perfusion of the feto-placental unit decrease fetal size, even before the appearance of the defining criteria of preeclampsia (hypertension and proteinuria).29 The higher rate of large for gestational age infants in late-onset preeclampsia in the present study may suggest that placental dysfunction is absent or plays only a minor role in late-onset preeclamptic pregnancies. The excess of large for gestational age infants could be explained by increased cardiac output in late-onset preeclamptic pregnancies as late-onset preeclampsia is believed to be a maternal reaction to more than averagely rapid fetal growth, secondary to impaired placental perfusion and abnormal placentation.30, 31
Our findings also indicated that maternal ethnic origin modified the association between early onset preeclampsia in first pregnancy and risk of preterm delivery in normotensive second pregnancy. Specifically, the risk of preterm birth is even more pronounced among African American women who had a history of early onset preeclampsia, relative to Caucasian women who had late onset preeclampsia (Table 4). Numerous studies have documented that African American women are at increased risk for preterm labor and preterm delivery.15 Our findings showed that risk of preterm delivery is markedly increased among African American when the maternal obstetric risk factor is superimposed with early onset preeclampsia in first pregnancy.
Our results are similar to those from prior research. In a hospital-based sample, Lain and associates compared second pregnancy outcomes among 130 women with and 6148 women without preeclampsia in their first pregnancies who all had second pregnancies without preeclampsia. They reported that women with early onset preeclampsia delivered earlier in their second nonpreeclamptic pregnancy compared to women with late onset preeclampsia or no preeclampsia in the first pregnancy.23 However, findings from Lain et al were limited by lack of adjustment of important confounders such as body mass index, pregnancy interval, and smoking during pregnancy. In another study, Makkonen et al reported that infants of mothers with preeclampsia in the first pregnancy had increased risk of admission to a neonatal unit and intrauterine fetal death in the normotensive second pregnancy, compared to secundagravid women without previous preeclamptic history.13 On the contrary, they also observed that a history of preeclampsia has no significant effects on infant birthweight, fetal distress, or prematurity rate.13 The differences in findings may be attributed to that women in Makkonen et al were not stratified by gestational timing of preeclampsia which could mask the underline risk of a prior early onset preeclampsia on pregnancy outcomes. In other study, Sibai and associates observed that women with preeclampsia in first pregnancy had a significantly higher incidence of placental abruption, perinatal mortality, fetal growth retardation, and premature delivery in their subsequent pregnancies than the normotensive control group.5 However, preeclampsia status in subsequent pregnancies were not reported in the study. Findings from Sibai et al were also limited by incorrect statistical modeling for clustered data which underestimates the standard error of risk estimate.
Early onset preeclampsia is often defined as a syndrome of first pregnancies and its underline cause largely unknown.32, 33 It has been suggested that early onset and late onset preeclampsia should be regarded as different forms of disease.33 Egbor and associates evaluated morphometric placental villous and vascular abnormalities in early and late onset preeclampsia. It was observed that late onset preeclampsia had a minimal influence on placental villous and vascular morphology (i.e. reduced stem vein volume) compared with gestational-age-matched controls.34 In contrast, early-onset preeclampsia was associated with placental dysfunction marked by a reduction in placental weights, volume or the intervillous space, terminal villous volumes and surface area.34 In another study, Moldenhauser and colleagues studied placental lesions according to gestational age at delivery. The study found that the rate of placental lesions were higher the earlier the gestational age at the time of delivery, compared with normotensive control subjects.7 It is not clear whether the implantation and placental abnormality recurs and affects fetal birth weight and length of gestation despite a normotensive second pregnancy.
Prior research has shown that early onset preeclampsia in first pregnancy has long term health implications to the mother. After preeclamptic pregnancies, metabolic syndrome markers often remain elevated, as does cardiovascular risks.33 Women followed up after preeclampsia also had higher levels of 8-isoprostane, a marker of oxidative stress, and plasma von Willebrand factor, a marker of endothelial dysfunction.33, 35 Women with history of early onset preeclampsia seen 6 months to 20 years postpartum have also been noted to have increased risk of later hypertension, heart disease, and associated metabolic disturbances, including higher insulin levels and reduced endothelial functions, as compared with women with uncomplicated pregnancies.5, 33, 36–38 In turn, cardiac disease, chronic hypertension, and diabetes are significant maternal chronic medical risk factors associated with preterm birth, low birth weight, and infant mortality.39 In our study, women who had recurrent preeclampsia had higher rate of chronic hypertension, renal disease, or diabetes mellitus relative to those who were normotensive in the second pregnancy (Figure 1).
Some methodological limitations of this study need to be considered in interpreting the study findings. They include the potential for inaccurate reporting, residual confounding by socioeconomic and other maternal characteristics, the lack of information regarding the diagnosis and severity of preeclampsia, and misclassification of medical and obstetrical conditions. However, a prior validation study has indicated that the reporting rate of preeclampsia on birth certificates with a check-box format (such as that used in Missouri) is fairly good, ranging from 85% to 97% when compared with risks based on hospital discharge data.40 In addition, the generalizability of this study is limited to Caucasian American and African American women who reside in Missouri or other populations with similar demographics and characteristics of Missouri women. Furthermore, potential confounding due to changes in the management around treating women during and after preeclampsia in the 16 year of study period could not be excluded. The strength of this study rests in classifying preeclampsia in first pregnancy by gestational age at delivery as early onset or late onset, its use of a large population-based sample of women with preeclampsia in first pregnancy, and the availability of information on many potential confounders that may affect risk of adverse pregnancy outcomes in second pregnancy. The large sample size provided the study adequate statistical power to detect significant associations and increased precision in the risk estimates. To the authors’ knowledge, the present study is the first to examine the role of maternal ethnic origin in the association between early onset preeclampsia and pregnancy outcomes in subsequent normotensive pregnancies. Our results indicated presence of health disparity in risk of preterm delivery in normotensive second pregnancy among women with a history of early onset preeclampsia.
Conclusion
We have shown that women with normotensive second preeclampsia following early onset preeclampsia in first pregnancy were at increased risk of adverse maternal and fetal outcomes compared to women with late onset preeclampsia in first pregnancy. The magnitude of risk for these conditions also varied by maternal ethnic origin, with worse prognosis associated with African American women with earlier onset preeclampsia in first pregnancy. It is increasingly accepted that early onset and late onset preeclampsia may be of different underlying etiology and our findings lend support to this theory. Our findings have clinical relevance for the management of subsequent pregnancy for women with a history of preeclampsia. Given the increased odds of SGA in a normotensive second pregnancy after having early onset preecampsia in the first pregnancy, obstetricians should consider an ultrasound to evaluate fetal growth, in addition to clinical follow up in the second pregnancy. Increased awareness of the association between early onset preeclampsia in first pregnancy and adverse maternal and fetal outcomes in subsequent nomotensive pregnancies is needed among health care professionals to optimize maternal and fetal outcome.
Acknowledgements
The authors acknowlege and appreciate the Missouri Deparment of Health and Senior Services, Secition of Public Health Practice and Administrative Support as the original source of the data. The analysis, interpretations, and conclusions in the present study are those of the author and not the Missouri Department of Health and Senior Services, Secition of Public Health Practice and Administrative Support.
Funding
This study was supported by KL2 Multidisciplinary Clinical Research Career Development Program Scholar award from the National Institute of Health.
Footnotes
Disclosure of interests
We have no conflicts of interest to declare.
Contribution to authorship
J.J.C wrote the study protocol, performed the analyses, and wrote the first draft of the manuscript. L.J. M. and G.A. M. all made substantial contributions to the study design, interpretation of results, and manuscript revision.
Details of ethics approval
This research was reviewed by the Saint Louis University Institutional Review Board and was classified as exempt.
References
- 1.Sibai BM, Ewell M, Levine RJ, et al. The Calcium for Preeclampsia Prevention (CPEP) Study Group. Risk factors associated with preeclampsia in healthy nulliparous women. Am J Obstet Gynecol. 1997 Nov;177(5):1003–1010. doi: 10.1016/s0002-9378(97)70004-8. [DOI] [PubMed] [Google Scholar]
- 2.Chang J, Elam-Evans LD, Berg CJ, et al. Pregnancy-related mortality surveillance--United States, 1991--1999. MMWR Surveill Summ. 2003 Feb 21;52(2):1–8. [PubMed] [Google Scholar]
- 3.Ujah IA, Aisien OA, Mutihir JT, Vanderjagt DJ, Glew RH, Uguru VE. Factors contributing to maternal mortality in north-central Nigeria: a seventeen-year review. Afr J Reprod Health. 2005 Dec;9(3):27–40. [PubMed] [Google Scholar]
- 4.Waterstone M, Bewley S, Wolfe C. Incidence and predictors of severe obstetric morbidity: case-control study. Bmj. 2001 May 5;322(7294):1089–1093. doi: 10.1136/bmj.322.7294.1089. discussion 1093–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sibai BM, el-Nazer A, Gonzalez-Ruiz A. Severe preeclampsia-eclampsia in young primigravid women: subsequent pregnancy outcome and remote prognosis. Am J Obstet Gynecol. 1986 Nov;155(5):1011–1016. doi: 10.1016/0002-9378(86)90336-4. [DOI] [PubMed] [Google Scholar]
- 6.Lain KY, Roberts JM. Contemporary concepts of the pathogenesis and management of preeclampsia. Jama. 2002 Jun 26;287(24):3183–3186. doi: 10.1001/jama.287.24.3183. [DOI] [PubMed] [Google Scholar]
- 7.Moldenhauer JS, Stanek J, Warshak C, Khoury J, Sibai B. The frequency and severity of placental findings in women with preeclampsia are gestational age dependent. Am J Obstet Gynecol. 2003 Oct;189(4):1173–1177. doi: 10.1067/s0002-9378(03)00576-3. [DOI] [PubMed] [Google Scholar]
- 8.Sibai BM. Hypertension in pregnancy. In: Gabbe SG, Niebyl JR, Simpson JL, editors. Obstetrics normal and problem pregnancies. New York: Churchill Livingstone; 1996. pp. 935–996. [Google Scholar]
- 9.van Rijn BB, Hoeks LB, Bots ML, Franx A, Bruinse HW. Outcomes of subsequent pregnancy after first pregnancy with early-onset preeclampsia. Am J Obstet Gynecol. 2006 Sep;195(3):723–728. doi: 10.1016/j.ajog.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 10.Ananth CV. Epidemiologic approaches for studying recurrent pregnancy outcomes: challenges and implications for research. Semin Perinatol. 2007 Jun;31(3):196–201. doi: 10.1053/j.semperi.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Mendilcioglu I, Trak B, Uner M, Umit S, Kucukosmanoglu M. Recurrent preeclampsia and perinatal outcome: a study of women with recurrent preeclampsia compared with women with preeclampsia who remained normotensive during their prior pregnancies. Acta Obstet Gynecol Scand. 2004 Nov;83(11):1044–1048. doi: 10.1111/j.0001-6349.2004.00424.x. [DOI] [PubMed] [Google Scholar]
- 12.Caritis S, Sibai B, Hauth J, et al. Low-dose aspirin to prevent preeclampsia in women at high risk. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 1998 Mar 12;338(11):701–705. doi: 10.1056/NEJM199803123381101. [DOI] [PubMed] [Google Scholar]
- 13.Makkonen N, Heinonen S, Kirkinen P. Obstetric prognosis in second pregnancy after preeclampsia in first pregnancy. Hypertens Pregnancy. 2000;19(2):173–181. doi: 10.1081/prg-100100133. [DOI] [PubMed] [Google Scholar]
- 14.Gregory KD, Korst LM. Age and racial/ethnic differences in maternal, fetal, and placental conditions in laboring patients. Am J Obstet Gynecol. 2003 Jun;188(6):1602–1606. doi: 10.1067/mob.2003.391. discussion 1606–1608. [DOI] [PubMed] [Google Scholar]
- 15.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown HL, Chireau MV, Jallah Y, Howard D. The "Hispanic paradox": an investigation of racial disparity in pregnancy outcomes at a tertiary care medical center. Am J Obstet Gynecol. 2007 Aug;197(2):e191–e197. doi: 10.1016/j.ajog.2007.04.036. 197 discussion 197 e197–199. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka M, Jaamaa G, Kaiser M, et al. Racial disparity in hypertensive disorders of pregnancy in New York State: a 10-year longitudinal population-based study. Am J Public Health. 2007 Jan;97(1):163–170. doi: 10.2105/AJPH.2005.068577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herman AA, McCarthy BJ, Bakewell JM, et al. Data linkage methods used in maternally-linked birth and infant death surveillance data sets from the United States (Georgia, Missouri, Utah and Washington State), Israel, Norway, Scotland and Western Australia. Paediatr Perinat Epidemiol. 1997 Jan;11 Suppl 1:5–22. doi: 10.1046/j.1365-3016.11.s1.11.x. [DOI] [PubMed] [Google Scholar]
- 19.Ananth CV, Peltier MR, Chavez MR, Kirby RS, Getahun D, Vintzileos AM. Recurrence of ischemic placental disease. Obstet Gynecol. 2007 Jul;110(1):128–133. doi: 10.1097/01.AOG.0000266983.77458.71. [DOI] [PubMed] [Google Scholar]
- 20.Kramer MS, Goulet L, Lydon J, et al. Socio-economic disparities in preterm birth: causal pathways and mechanisms. Paediatr Perinat Epidemiol. 2001 Jul;15 Suppl 2:104–123. doi: 10.1046/j.1365-3016.2001.00012.x. [DOI] [PubMed] [Google Scholar]
- 21.Alexander GR, Kogan MD, Himes JH. 1994–1996 U.S. singleton birth weight percentiles for gestational age by race, Hispanic origin, and gender. Matern Child Health J. 1999 Dec;3(4):225–231. doi: 10.1023/a:1022381506823. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization. The OBSQUID project: quality development in perinatal care-final report. WHO Reg Publ Europe Service. 1995
- 23.Lain KY, Krohn MA, Roberts JM. Second pregnancy outcomes following preeclampsia in a first pregnancy. Hypertens Pregnancy. 2005;24(2):159–169. doi: 10.1081/PRG-200059869. [DOI] [PubMed] [Google Scholar]
- 24.Singer JD, Willett JB. Applied longitudinal data analysis: modeling change and event occurrence. Oxford, NY: Oxford University Press; 2003. [Google Scholar]
- 25.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004 Apr 1;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 26.Spiegelman D, Hertzmark E. Easy SAS Calculations for Risk or Prevalence Ratios and Differences. Am. J. Epidemiol. 2005;162(3):199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 27.Szklo M, Nieto EJ. Epidemiology. Beyond the basics. 2nd ed. Gaithersburg, MD: Jones and Bartlett Publishers; 2007. [Google Scholar]
- 28.Brosens I, Dixon HG, Robertson WB. Fetal growth retardation and the arteries of the placental bed. Br J Obstet Gynaecol. 1977 Sep;84(9):656–663. doi: 10.1111/j.1471-0528.1977.tb12676.x. [DOI] [PubMed] [Google Scholar]
- 29.Long PA, Abell DA, Beischer NA. Fetal growth retardation and pre-eclampsia. Br J Obstet Gynaecol. 1980 Jan;87(1):13–18. doi: 10.1111/j.1471-0528.1980.tb04419.x. [DOI] [PubMed] [Google Scholar]
- 30.Easterling TR, Benedetti TJ, Schmucker BC, Millard SP. Maternal hemodynamics in normal and preeclamptic pregnancies: a longitudinal study. Obstet Gynecol. 1990 Dec;76(6):1061–1069. [PubMed] [Google Scholar]
- 31.Obed S, Patience A. Birth weight and ponderal index in pre-eclampsia: a comparative study. Ghana Med J. 2006 Mar;40(1):8–13. [PMC free article] [PubMed] [Google Scholar]
- 32.Luo ZC, An N, Xu HR, Larante A, Audibert F, Fraser WD. The effects and mechanisms of primiparity on the risk of pre-eclampsia: a systematic review. Paediatr Perinat Epidemiol. 2007 Jul;21 Suppl 1:36–45. doi: 10.1111/j.1365-3016.2007.00836.x. [DOI] [PubMed] [Google Scholar]
- 33.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005 Feb 26–Mar 4;365(9461):785–799. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 34.Egbor M, Ansari T, Morris N, Green CJ, Sibbons PD. Morphometric placental villous and vascular abnormalities in early- and late-onset pre-eclampsia with and without fetal growth restriction. Bjog. 2006 May;113(5):580–589. doi: 10.1111/j.1471-0528.2006.00882.x. [DOI] [PubMed] [Google Scholar]
- 35.Ness RB, Sibai BM. Shared and disparate components of the pathophysiologies of fetal growth restriction and preeclampsia. Am J Obstet Gynecol. 2006 Jul;195(1):40–49. doi: 10.1016/j.ajog.2005.07.049. [DOI] [PubMed] [Google Scholar]
- 36.Powers RW, Evans RW, Majors AK, et al. Plasma homocysteine concentration is increased in preeclampsia and is associated with evidence of endothelial activation. Am J Obstet Gynecol. 1998 Dec;179(6 Pt 1):1605–1611. doi: 10.1016/s0002-9378(98)70033-x. [DOI] [PubMed] [Google Scholar]
- 37.Dekker GA, de Vries JI, Doelitzsch PM, et al. Underlying disorders associated with severe early-onset preeclampsia. Am J Obstet Gynecol. 1995 Oct;173(4):1042–1048. doi: 10.1016/0002-9378(95)91324-6. [DOI] [PubMed] [Google Scholar]
- 38.Pouta A, Hartikainen AL, Sovio U, et al. Manifestations of metabolic syndrome after hypertensive pregnancy. Hypertension. 2004 Apr;43(4):825–831. doi: 10.1161/01.HYP.0000120122.39231.88. [DOI] [PubMed] [Google Scholar]
- 39.Graham J, Zhang L, Schwalberg R. Association of maternal chronic disease and negative birth outcomes in a non-Hispanic Black-White Mississippi birth cohort. Public Health Nurs. 2007 Jul–Aug;24(4):311–317. doi: 10.1111/j.1525-1446.2007.00639.x. [DOI] [PubMed] [Google Scholar]
- 40.Frost F, Starzyk P, George S, McLaughlin JF. Birth complication reporting: the effect of birth certificate design. Am J Public Health. 1984 May;74(5):505–506. doi: 10.2105/ajph.74.5.505. [DOI] [PMC free article] [PubMed] [Google Scholar]