Abstract
Dietary omega-3 fatty acid (i.e. docosohexaenoic acid (DHA)) and exercise are gaining recognition for supporting brain function under normal and challenging conditions. Here we evaluate the possibility that the interaction of DHA and exercise can involve specific elements of the synaptic plasma membrane. We found that voluntary exercise potentiated the effects of a 12-day DHA dietary supplementation regimen on increasing the levels of syntaxin 3 (STX-3) and the growth-associated protein (GAP-43) in the adult rat hippocampus region. STX-3 is a synaptic membrane-bound protein involved in the effects of DHA on membrane expansion. The DHA diet and exercise also elevated levels of the NMDA receptor subunit NR2B, which is important for synaptic function underlying learning and memory. The actions of exercise and DHA dietary supplementation reflected on enhanced learning performance in the Morris water maze as learning ability was associated with higher levels of STX-3 and NR2B. The overall findings reveal a mechanism by which exercise can interact with the function of DHA dietary enrichment to elevate the capacity of the adult brain for axonal growth, synaptic plasticity, and cognitive function.
Keywords: Omega-3 fatty acid, Voluntary exercise, Syntaxin, Synaptic membrane, Hippocampus
1. Introduction
The omega-3 fatty acid, docosahexaenoic acid (22:6n-3, DHA), is critical for CNS development and is regarded essential for brain's structure and function (Gomez-Pinilla, 2008). DHA supplementation has been shown to enhance hippocampal-dependent learning and memory (Holguin et al., 2008; Wu et al., 2008) in rodents, and to reduce the incidence of mood disorders in humans (Gomez-Pinilla, 2008; Jacka et al., 2004).
The actions of DHA on several neural processes have been identified in vitro or in vivo such as promoting growth of hippocampal neurons, reducing inflammation, and improving signal transduction and neurotransmission (Calderon and Kim, 2004; Horrocks and Farooqui, 2004; Stillwell et al., 2005). DHA is an important constituent of neuronal membrane phospholipids in the brain, reaching up to 17% of the total fatty acids (Horrocks and Farooqui, 2004; Salem et al., 2001). The flexibility of DHA within the lipid bilayer provides cell membranes with the fluidity (Hashimoto et al., 2006; Suzuki et al., 1998) required for proper functioning during axonal and synaptic growth (Teague et al., 2002). Although DHA is critical for brain function, the brain and body are inefficient at synthesizing DHA (Kim, 2007). This has suggested to us that the brain may have intrinsic strategies to preserve membrane DHA. Given that exercise is a crucial component of daily living, we have initiated studies to determine the influence of exercise on molecular mechanisms associated with the metabolism and function of DHA in the hippocampus.
Like DHA dietary supplementation, exercise influences synaptic function and plasticity (O'Callaghan et al., 2007; Vaynman and Gomez-Pinilla, 2005; Wu et al., 2008). Exercise acts on specific molecular systems that control axonal growth and synaptic plasticity, which are also modulated by a DHA diet (Ding et al., 2006; Farmer et al., 2004; Vaynman et al., 2006; Wu et al., 2008), such as growth-associated protein 43 (GAP-43) (Chytrova et al., 2008; Gomez-Pinilla et al., 2002). Additionally, exercise can affect the NR2B subunit of the NMDA receptor, which is implicated in positively modulating synaptic growth and plasticity (Loftis and Janowsky, 2003; Xu et al., 2005). Illustrating the effects of exercise on NMDA function, it has been shown that the application of NR2B subunit antagonists abolishes the effects of exercise on receptor-dependent LTP in the mouse dentate gyrus (Vasuta et al., 2007). Like exercise, a DHA-enriched diet has been shown to increase NR2B levels in association with improved cognitive performance in rodents (Calon et al., 2005; Dyall et al., 2007; Mesches et al., 2004). Accordingly, the study of NR2B seems important to understanding how the DHA diet and exercise may interact to modulate cognitive function.
Plasma membrane syntaxin-3 (STX-3) has the advantage of relating DHA and synaptic membrane function. STX-3 is present in synaptic membranes (Curtis et al., 2008) and in neuronal growth cones (Darios and Davletov, 2006). DHA dietary supplementation elevates synaptic STX-3 levels (Cansev and Wurtman, 2007), and the action of DHA on promoting neurite outgrowth and membrane expansion has been shown to rely on STX-3 (Darios and Davletov, 2006). Proper levels of membrane-bound DHA are crucial to maintaining membrane fluidity and neuronal signaling, with strong implications for mental health (Bazan, 2003; Rao et al., 2007). Accordingly, our goal is to understand how DHA can support synaptic plasticity and cognitive abilities, and how exercise plays into these events.
2. Results
2.1. DHA dietary supplementation contributes to the effects of exercise on synaptic plasma membrane-associated proteins
Encouraged by findings in vitro that DHA promotes synaptic membrane expansion through activation of STX-3 (Darios and Davletov, 2006), we sought to determine whether exercise could enhance the action of DHA on hippocampal levels of STX-3. The DHA diet (150% of control, p<0.01) or exercise (126% of control, p<0.01) produced a significant increase in levels of STX-3 (Fig. 1A). Interestingly, the combination of DHA diet and exercise regimens resulted in elevated up-regulation of STX-3 levels (186% of control, P<0.01) beyond either DHA-Sed or RD-Exc values (Fig. 1A). A two-way ANOVA analysis (diet vs. behavior) indicated the effects of diet or behavior on syntaxin 3 (diet: F1,23=32.639, p<0.001; behavior: F1,23=95.128, p<0.001, and an interaction of diet×behavior: F1,23=25.819, p<0.001). Neither the DHA diet nor exercise altered the expression of syntaxin-1 (STX-1), which belongs to the same syntaxin family of plasma membrane proteins (data not shown). Since exercise increased levels of STX-3, we examined a possible association between the running distance and STX-3 levels. A linear regression analysis of individual samples showed a positive correlation between the amount of exercise and levels of STX-3 for DHA-Exc (r=0.897, P<0.01) (Fig. 1B) and RD-Exc (r=0.959, P<0.01, data not shown). On the other hand, there was no correlation between exercise and STX-1 in DHA-Exc (r=−0.300, P>0.05) or RD-Exc (r=0.011, P>0.05, data not shown).
Fig. 1.
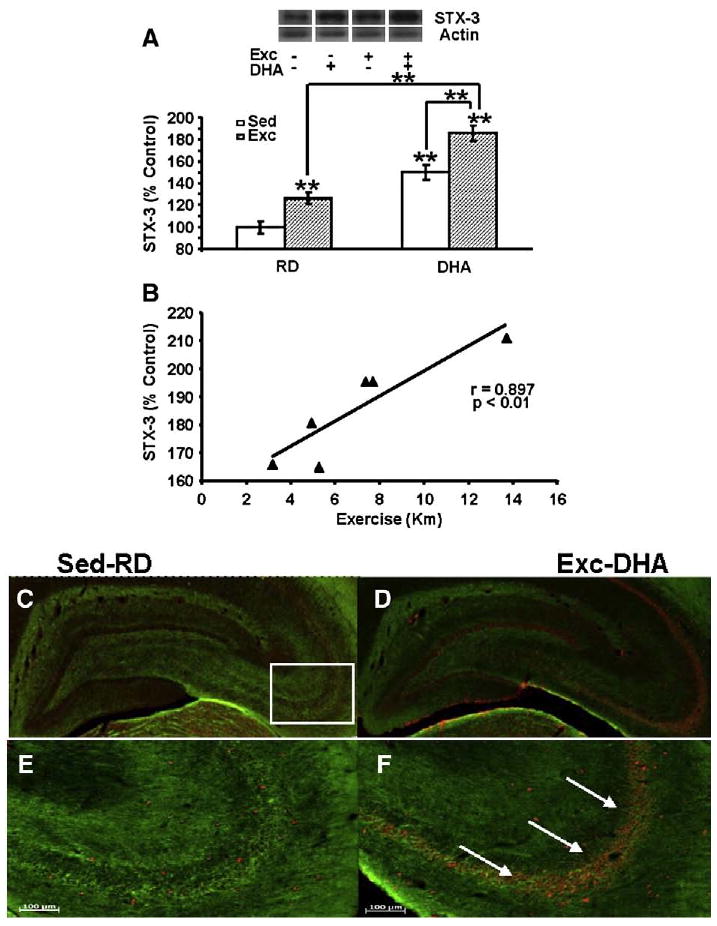
Exercise enhanced the effects of DHA dietary supplementation on STX-3, a protein implicated with the action of DHA on synaptic membrane (A). The values were converted to percent of RD-Sed controls (each value represents the mean latency±SEM, two-way ANOVA, **P<0.01). (B) The levels of STX-3 changed proportionally to the amount of exercise in animals receiving DHA diet (r=0.897, p<0.01). (C–F) Immunofluorescence for STX-3 in coronal sections of the hippocampus shows STX-3 (red, Cy3 secondary antibody) in RD-Sed controls (C, E) and animals receiving combined exercise and diet treatment (DHA-Exc; D, F). (D) and (F) are high magnification photomicrographs (of D and F). A marked increase in STX-3 immunofluorescence was shown in the DHA-Exc group (white arrows, F). Myelinated axons were labeled using immunofluorescence for myelin-associated glycoprotein (green, FITC secondary antibody). RD-Sed: regular diet-sedentary; RD-Exc: regular diet-exercise; DHA-Sed: DHA diet-sedentary; DHA-Exc: DHA diet-exercise.
Furthermore, we analyzed the effects of DHA diet and exercise on the phenotypic expression of STX-3 using immunofluorescence with a Cy3 secondary antibody in coronal sections of the hippocampus (Figs. 1C–F). STX-3/Cy3 labeling was apparent in neuronal cells that were distributed sparsely in the various subfields of the hippocampus of RD-Sed control rats (Figs. 1C, E). The co-application of the DHA diet and exercise greatly increased STX-3/Cy3 immunofluorescence, depicted as a qualitative increase in the density in Cy3-labeled cells in the hippocampal subregions CA1–CA4 and dentate gyrus (white arrows, Figs. 1D, F).
2.2. DHA dietary supplementation enhances the effects of exercise on the levels of the synaptic plasticity protein NR2B and the growth-associated protein GAP-43
Given the involvement of the NR2B subunit of the NMDA receptor in synaptic plasticity and learning, we assessed the effects of the DHA diet and exercise on hippocampal levels of NR2B. The DHA diet resulted in a significant increase of NR2B levels to 145% of RD-Sed control group (P<0.01; Fig. 2A). Exercise alone also elevated NR2B levels to 123% compared to the controls (P<0.05; Fig. 2A). The simultaneous application of the DHA diet and exercise regimens produced a greater increase in NR2B levels (169% of control, P<0.01) compared to either DHA-Sed or RD-Exc animals (Fig. 2A). A two-way ANOVA analysis (diet vs. behavior) indicated the effects of diet or behavior on NR2B (diet: F1,23=39.084, p<0.001; behavior: F1,23=9.871, p<0.01, and an interaction of diet×behavior: F1,23=0.001, p>0.05). The results also showed a positive correlation between levels of NR2B and STX-3 (r=0.878, P<0.05, Fig. 2B) following the combined application of DHA diet and exercise (DHA-Exc).
Fig. 2.
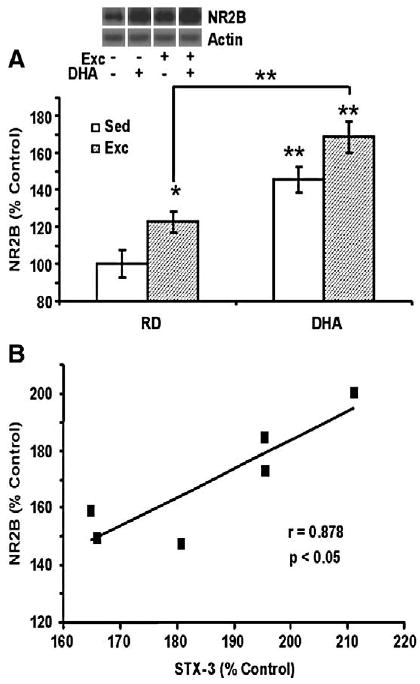
DHA dietary supplementation and exercise affected synaptic plasticity markers in hippocampus. (A) The DHA diet and exercise elevated levels of NR2B while the combination of both resulted in greater NR2B levels (mean± SEM, ANOVA, *P<0.05, **P<0.01). (B) NR2B levels changed in proportion to STX-3 levels for individual rats receiving the DHA diet and exercise combination (r=0.878, p<0.05). Levels of STX-3 and NR2B values are expressed as a percent of RD-Sed controls.
2.3. Association between plasticity markers and learning performance after DHA dietary supplementation and exercise
We have performed studies to determine how an additive effect of the diet and exercise on learning could relate to the assessed properties of the membrane. We first tested the effects of the DHA diet and exercise on the learning performance by measuring the time taken to locate the hidden platform in the MWM using a challenging 2-trial-per-day, 5-day paradigm. As previously shown (Gomez-Pinilla, 2008; Wu et al., 2008), we found that the latency to locate the platform was decreased in all three experimental groups (RD-Exc, DHA-Sed, DHA-Exc) compared to control animals (RD-Sed). We assessed the learning speed in the MWM by measuring the slope of the escape latency value across the five days of learning (Fig. 3A), as the latency slope has been shown to provide a reliable index of the learning speed throughout the test period (Gomez-Pinilla et al., 2008). All three experimental groups (RD-Exc, DHA-Sed and DHA-Exc) showed significantly increased rate of learning compared to the control group (P<0.01; Fig. 3A). A two-way ANOVA analysis (diet vs. behavior) indicated the effects of diet or behavior on the slope of learning (diet: F1,23=8.715, p<0.01; behavior: F1,23=4.463, p<0.05, and an interaction of diet×behavior: F1,23=0.740, p>0.400).
Fig. 3.
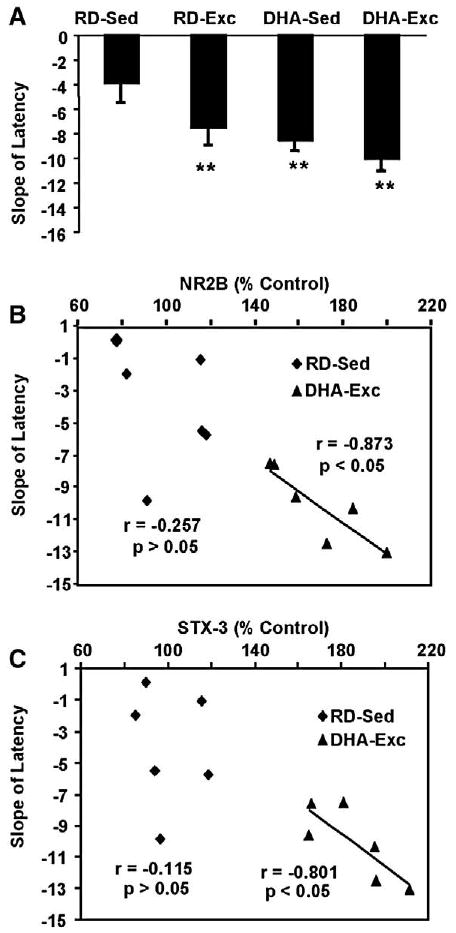
Association between plasticity markers and learning performance after DHA dietary supplementation and exercise. The effects of exercise and DHA dietary supplementation on the Morris water maze test over the five consecutive days of training were reflected in changes in the learning speed (slope of the latency, A) to locate the hidden platform. We measured the slope of the escape latency across the five days of learning, and each value represents the mean slope latency±SEM (ANOVA, **P<0.01). A correlation analysis revealed an association between the learning speed and levels of NR2B (B) and syntaxin 3 (C) in the DHA-Exc group but not in the RD-Sed group. RD-Sed: regular diet-sedentary; RD-Exc: regular diet-exercise; DHA-Sed: DHA diet-sedentary; DHA-Exc: DHA diet-exercise.
To shed light on a possible involvement of NR2B or syntaxin proteins on cognitive function in our paradigm, we assessed the correlation between learning speed in the MWM with levels of NR2B. The results showed an association between the learning speed and NR2B levels following co-application of DHA diet and exercise (DHA-Exc, r=−0.873, P<0.05), but not in the control group (RD-Sed, r=−0.257, P>0.05) (Fig. 3B). The results also showed an association between the learning speed and levels of STX-3 following the combined DHA diet and exercise regimens (DHA-Exc, r= −0.801, P<0.05), but not in the control group (RD-Sed, r= −0.115, P>0.05; Fig. 3C). We found no correlation between the learning speed and levels of STX-1 in DHA-Exc (r=0.465, P>0.05) or RD-Sed animals (r=0.349, P>0.05; data not shown).
Given that GAP-43 has been implicated on axonal remodeling and learning and memory, we also evaluated the possibility that exercise and the DHA diet could contribute to elevate the capacity of the brain for plasticity. The results showed that exercise (128%, P<0.01) and the DHA diet (119%, P<0.05 Fig. 4A) increased GAP-43 levels compared to the control group. Exercise potentiated the elevation of GPA-43 that occurred after DHA treatments, such that the DHA-Exc group reached 153% of control values (P<0.01, Fig. 4A). A two-way ANOVA analysis (diet vs. behavior) indicated the effects of diet or behavior on GAP-43 (diet: F1,23=13.795, p<0.001; behavior: F1,23=26.893, p< 0.001, and an interaction of diet×behavior: F1,23=0.243, p>0.05). Furthermore, a linear regression analysis of individual samples showed a positive correlation between the amount of exercise and levels of GAP-43 for both DHA-Exc (r=0.908, P<0.01) (Fig. 4B) and RD-Exc (r=0.953, P<0.01) groups (data not shown).
Fig. 4.
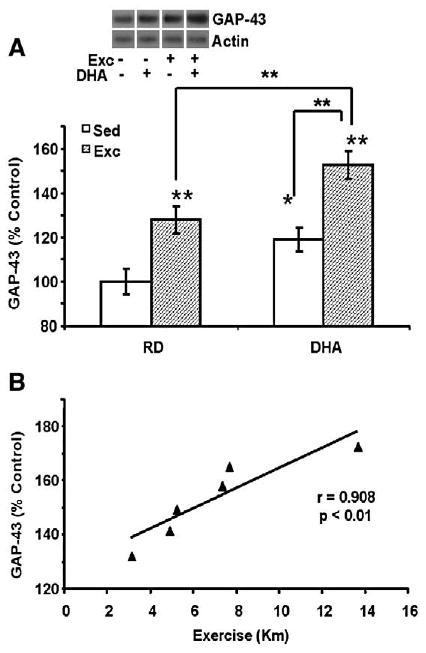
(A) The separate applications of exercise or the DHA diet elevated levels of GAP-43 while the concurrent application of both elevated GPA-43 levels further. Each value represents the mean latency±SEM (two-way ANOVA, *p<0.05, **p<0.01). (B) Levels of GAP-43 changed in proportion to the total amount of exercise in animals receiving DHA diet and exercise combined treatment (r=0.908, p<0.01).
3. Discussion
In agreement with the preferential distribution of DHA in plasma membranes, here we report that DHA dietary supplementation may affect synaptic plasticity and cognitive function by involving select proteins, which are integral components of synaptic membranes. In particular, we have found that DHA influences hippocampal STX-3, a plasma membrane-bound protein associated with the action of DHA on cell membrane expansion (Cansev and Wurtman, 2007; Darios and Davletov, 2006). We have also found that the application of exercise concurrent to the DHA diet resulted in a greater elevation of STX-3. The overall evidence seems to indicate that exercise can influence DHA function on synaptic plasticity and cognitive function by interacting with molecular systems that stabilize DHA to plasma membrane.
3.1. Exercise influences the DHA action on plasma membrane proteins
We have evaluated the capacity of exercise to work together with DHA to modulate synaptic plasticity and cognition (Gomez-Pinilla, 2008; Wu et al., 2008). Our results demonstrate that voluntary exercise enhances the effects of DHA dietary supplementation to elevate hippocampal levels of STX-3 (Fig. 1A) but not STX-1. It has been shown that plasma membrane STX-3 can act in response to local increases in DHA by trafficking to the trans-Golgi network via vesicular cycling (Band and Kuismanen, 2005). The fact that neither the DHA diet nor exercise altered STX-1 levels argues in favor of the specificity of STX-3. Along this line of thought, conversely to STX-3, STX-1 does not seem to affect neurite outgrowth (Darios and Davletov, 2006; Shirasu et al., 2000); neither does the omega-3 fatty acid deficiency affect hippocampal STX-1 (Pongrac et al., 2007). Our results suggest a potential mechanism by which the DHA diet and exercise can promote neurite outgrowth by using STX-3. In additional support for a role of diet and exercise on neurite outgrowth, voluntary exercise alone or in combination with DHA supplementation increased levels of the axonal growth-associated molecule, GAP-43. Previous studies have documented a potential link between DHA and neurite outgrowth, i.e., DHA administration has resulted in increase of hippocampal GAP-43 mRNA (Calderon and Kim, 2004; Furuya et al., 2002), and growth of the neurite branches (Cao et al., 2005). Our results showed that the protein levels of STX-3 (Fig. 1B) and GAP-43 (Fig. 4B) changed in proportion to the amount of exercise for individual animals, which had received DHA dietary supplementation in addition to exercise. Given that the feeding remained steady throughout the experiment, it is likely that exercise was a crucial factor for the reported influence of DHA on STX-3 and GAP-43 hippocampal levels.
3.2. Implications of exercise and DHA diet on hippocampal plasticity and cognition
Consistent with the proposed role of DHA and exercise in learning and memory, our results show that both DHA diet and exercise significantly increased protein levels of the NMDA receptor subunit NR2B (Fig. 2A). When we tested the learning performance in the MWM, we found that NR2B levels correlated with the MWM performance, with animals exposed to the DHA diet and exercise showing the highest NR2B levels. These findings are in agreement with other studies reporting higher NR2B levels in the rodent hippocampus in association with improved learning and memory (Tang et al., 1999; Xu et al., 2005). NR2B is abundantly expressed in growth cones of growing neurites and is a critical component of molecular signaling pathways regulating synaptic growth and plasticity (Loftis and Janowsky, 2003; Nakazawa et al., 2004).
It has been reported that dietary omega-3 fatty acid depletion leads to a significant NR2B decrease (Calon et al., 2005) while omega-3 fatty acids enrichment results in NR2B elevation (Dyall et al., 2007). Similarly, voluntary exercise up-regulates hippocampal NR2B mRNA and enhances long-term potentiation (Farmer et al., 2004; van Praag et al., 1999). Treatment with a specific NR2B antagonist abolishes this exercise-induced enhancement of LTP in the mouse dentate gyrus (Vasuta et al., 2007). Our findings additionally provide novel evidence that DHA diet and exercise can have additive effects on NR2B with potential effects on hippocampal plasticity and cognitive function. Beside the influence of diet and exercise on levels of NR2B receptors, they can indirectly affect the function of NR2B and other receptors through their membrane interactions (Fig. 5). As discussed above, the flexibility of the membrane is crucial for the function of embedded receptors and signal transduction (Hashimoto et al., 2006). Accordingly, it is possible the influence of the DHA diet and exercise can contribute to the performance of NMDA and other receptors important for synaptic function. It is also significant that the effects of the diet and exercise also were expressed on levels of GAP-43, which has been associated with neurite growth expansion and learning and memory.
Fig. 5.
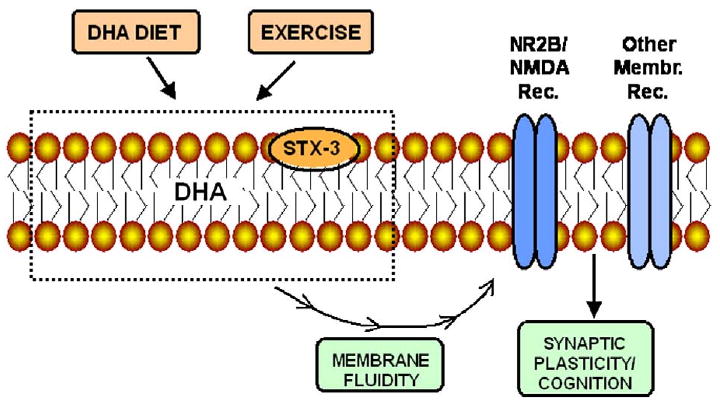
Schematic diagram depicting the effects of the DHA diet and exercise on key elements involved in the maintenance of the synaptic membrane. Dietary DHA and exercise can influence contents of membrane DHA and STX-3 with subsequent effects on membrane stability and fluidity. The function of membrane embedded receptors such as NR2B depends on the fluidity of the membrane affecting synaptic function and learning and memory.
3.3. Conclusions and clinical implications
We have found that exercise can complement the action of DHA dietary supplementation on the modulation of molecular systems important for the maintenance of plasma membranes. Membrane stability and fluidity are fundamental for synaptic function and processing of higher order information. Our results suggest that the DHA diet and exercise may help to maintain synaptic and cognitive function by supporting membrane stability. DHA deficiency has been associated with the incidence of various mental diseases such as depression and schizophrenia (Gomez-Pinilla, 2008), and DHA dietary supplementation can reduce the effects of brain trauma in rodents (Wu et al., 2004). Low consumption of omega-3 fatty acids may increase the risk of getting Alzheimer's disease (Corrigan et al., 1998; Tully et al., 2003), while their high consumption could do the opposite (Barberger-Gateau et al., 2002; Morris et al., 2003). Omega-3 fatty acid dietary supplementation may also decrease the incidence or improve the clinical outcome of patients with other neurode-generative disorders such as multiple sclerosis (Nordvik et al., 2000; Weinstock-Guttman et al., 2005), Parkinson's disease (de Lau et al., 2005) and Huntington's disease (Murck and Manku, 2007; Vaddadi et al., 2002). Our results also suggest that exercise is a crucial modulator of the efficacy of dietary factors on brain function. Accordingly, the overall evidence indicates that DHA diet and exercise is a powerful strategy that can be applied to alleviate numerous neurological disorders.
4. Experimental procedures
The experiments were performed in accordance with the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals. All animals were continually monitored and all procedures were approved by the UCLA Chancellor's Animal Research Committee.
4.1. DHA diet and exercise paradigms
Adult male Sprague–Dawley rats (n=24, Charles River Laboratories, Inc., Wilmington, MA, USA), approximately 2 months of age (200–240 g), were housed in standard polyethylene cages in an environmentally controlled room (22–24 °C) with a 12 h light/dark cycle. The rats were randomly divided into 4 groups: (1) RD (regular diet)-Sed (sedentary), (2) RD-Exc (exercise), (3) DHA-Sed, and (4) DHA-Exc; RD-Sed group was regarded as a control. After acclimatization for 1 week on standard rat chow, the rats were exposed to voluntary exercise or a sedentary condition, while subgroups of each were exposed to a DHA-enriched diet (1.25% DHA) or a regular diet for 12 days. The diets, fed ad libitum, were provided in powder. The regular diet was the standard rat chow with a ratio of omega-6/omega-3 at 6:1 (#5001, PMI Nutrition), total fat: 4.5%; arachidonic acid: <0.01%; calorie: 4.07 kcal/gm. The rats were initially omega-3 sufficient by being maintained on the standard rat chow (0.05% DHA), and DHA was supplemented in the regular diet with a ratio of omega-6/omega-3 at 1:1 (1.25% DHA, 0.25% EPA, Nordic Naturals). The rats were allowed to exercise ad libitum in individual cages with an unlimited access to a running wheel (diameter=31.8 cm, width=10.0 cm). The running wheel rotated freely against a resistance of 100 g attached to a receiver that monitored revolutions every hour (VitalViewer Data Acquisition System software, Mini Mitter, Sunriver, OR, USA). Animals were sacrificed by decapitation the morning after the last running period. The fresh tissue containing the hippocampus was dissected, frozen on dry ice and stored at −70 °C until further use.
4.2. Tissue preparation and protein determination
The hippocampi were rapidly dissected out upon decapitation. Tissue was collected into 1.5 mL Eppendorf tubes, immediately frozen on dry ice and stored at −70 °C. Hippocampi from the left side of the brain were homogenized in a freshly prepared lysis buffer (137 mM NaCl, 20 mM Tris–HCl pH 8.0, 1% NP-40, 10% glycerol, 1 mM phenylmethylsulfonyl fluoride, 10 μg/mL aprotinin, 1 μg/mL leupeptin and 0.5 mM sodium vanadate). Homogenates were centrifuged at 12,000 rpm for 20 min to remove insoluble material. The supernatants were collected into clean 1.5 mL tubes, frozen on dry ice and stored at −70 °C. The total protein concentration of hippocampal homogenates was determined with a MicroBCA kit (Pierce, Rockford, IL, USA), using BSA as a standard.
4.3. Western blot
Relative levels of STX-3, STX-1, NR2B, and GAP-43 were analyzed by Western blot. Equal amounts (25 μg) of protein samples were separated by electrophoresis on 8–15% polyacrylamide gels and electrotransferred to an Immobilon-P transfer membrane (Millipore, Bedford, MA, USA). Nonspecific binding sites were blocked with 2% BSA in TBS buffer with 0.1% Tween-20 (pH 7.6). Membranes were incubated with the following primary antibodies: anti-STX-3 (1:5000, Abcam), anti-STX-1 (1:2000, Santa Cruz Biotechnology), anti-NR2B (1:1000; Upstate), anti-GAP-43 (1:2000; Santa Cruz Biotechnology), and anti-actin (1:2000; Santa Cruz Biotechnology) followed by anti-rabbit IgG horseradish peroxidase conjugate for STX-3, STX-1 and NR2B, or anti-goat IgG horseradish peroxidase conjugate for GAP-43 and actin (Santa Cruz Biotechnology). After rinsing with buffer (0.1% Tween-20 in TBS), the immunocomplexes were visualized by chemiluminescence using the Amersham ECL Plus Western Blotting Detection kit (GE Healthcare Bio-Sciences, Piscataway, NJ, USA) according to the manufacturer's instructions. The film signals were digitally scanned using a HP Scanner (HP Scanjet 3970) and quantified with NIH Image software, normalized for actin levels.
4.4. Immunofluorescence
Additional rats from all of the four groups (n=8) were injected with a lethal dose of Nembutal (75 mg/kg i.p.), then intracardially perfused with PBS (pH 7.4) followed by 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) and 20% sucrose in 4% paraformaldehyde. Serial coronal brain sections (25 μμm) were cut on a cryostat, collected free-floating in anti-freeze solution and stored at −20 °C before processing for immunofluorescence. Tissue sections were rinsed three times for 10 min with PBS buffer on a plate shaker and nonspecific binding sites were blocked for 1 h at room temperature in PBS with 1% BSA and 0.3% Triton X-100. The sections were incubated overnight at 4 °C and then for 1 h at room temperature in a solution containing the primary antibody (rabbit-anti-STX-3, 1:150, Sigma; mouse-anti-MAG, 1:500, Chemicon International) diluted in PBS with 1% BSA and 0.1% Triton X-100. After washing three times for 10 min with PBS buffer and 0.1% Triton X-100, the sections were kept in dark and incubated for 1 h in a solution containing the secondary antibody (Cy 3-conjugated goat-anti-rabbit IgG, 1:500, Jackson ImmunoResearch; Fluorescein (FITC)-conjugated goat-anti-mouse IgG, 1:500, Jackson ImmunoResearch) diluted in PBS with 1% BSA and 0.1% Triton X-100. The sections were then rinsed in the dark three times for 10 min in a washing solution, mounted on microscope slides, coverslipped with fluorescence protecting solution and stored in the dark at 4 °C. The immunofluorescence analysis was performed using a Zeiss microscope (Zeiss Imager.Z1, Carl MicroImaging). Control sections were incubated in PBS with 1% BSA and 0.1% Triton X-100 without the primary antibody. No staining was observed in cell structures of control sections.
4.5. Morris water maze (MWM)
After one week on the diet or exercise, the rats were tested in the MWM for their learning ability as previously described (Wu et al., 2004). Briefly, the rats were trained in the water maze for 5 days with 2 consecutive trials per day. The animals were placed into the tank facing the wall from one of the equally spaced starting locations that were randomly changed every trial. Each trial lasted until the rat found the platform or for a max of 60 s. If the rat failed to find the platform in the allocated time, it was gently placed on the platform. At the end of each trial, the animals were allowed to rest on the platform for 60 s. The escape latencies to find the platform were recorded.
4.6. Statistical analyses
The mean protein levels were calculated for each group (n=6 rats per group). All statistical analyses were done by statistic software SPSS 16.0. A level of 5% probability was considered significant. The results were expressed as the mean percent of control values and represent the mean ±standard error of the mean (SEM). MWM and protein data were analyzed by two-way ANOVA [(behavior: Sedentary vs. Exercise) and (diet: Regular Diet vs. DHA)]. Interaction effects were further analyzed by performing means comparisons, and desired contrast weights were specified. Post hoc analyses were conducted using Bonferroni comparisons.
A linear regression analysis was performed on individual samples to evaluate association between variables (running distance or water maze times with protein levels).
Acknowledgments
This study was supported by the National Institutes of Health award NS500465.
Abbreviations
- DHA
docosahexaenoic acid
- DHA-Exc
DHA diet-exercise
- DHA-Sed
DHA diet-sedentary
- GAP-43
growth-associated protein 43
- MWM
Morris water maze
- RD-Exc
regular diet-exercise
- RD-Sed
regular diet-sedentary
- STX-1
syntaxin-1
- STX-3
syntaxin-3
References
- Band AM, Kuismanen E. Localization of plasma membrane t-SNAREs syntaxin 2 and 3 in intracellular compartments. BMC Cell Biol. 2005;6:26. doi: 10.1186/1471-2121-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberger-Gateau P, Letenneur L, Deschamps V, Peres K, Dartigues JF, Renaud S. Fish, meat, and risk of dementia: cohort study. Bmj. 2002;325:932–933. doi: 10.1136/bmj.325.7370.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan NG. Synaptic lipid signaling: significance of polyunsaturated fatty acids and platelet-activating factor. J Lipid Res. 2003;44:2221–2233. doi: 10.1194/jlr.R300013-JLR200. [DOI] [PubMed] [Google Scholar]
- Calderon F, Kim HY. Docosahexaenoic acid promotes neurite growth in hippocampal neurons. J Neurochem. 2004;90:979–988. doi: 10.1111/j.1471-4159.2004.02520.x. [DOI] [PubMed] [Google Scholar]
- Calon F, Lim GP, Morihara T, Yang F, Ubeda O, Salem N, Jr, Frautschy SA, Cole GM. Dietary n-3 polyunsaturated fatty acid depletion activates caspases and decreases NMDA receptors in the brain of a transgenic mouse model of Alzheimer's disease. Eur J Neurosci. 2005;22:617–626. doi: 10.1111/j.1460-9568.2005.04253.x. [DOI] [PubMed] [Google Scholar]
- Cansev M, Wurtman RJ. Chronic administration of docosahexaenoic acid or eicosapentaenoic acid, but not arachidonic acid, alone or in combination with uridine, increases brain phosphatide and synaptic protein levels in gerbils. Neuroscience. 2007;148:421–431. doi: 10.1016/j.neuroscience.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao D, Xue R, Xu J, Liu Z. Effects of docosahexaenoic acid on the survival and neurite outgrowth of rat cortical neurons in primary cultures. J Nutr Biochem. 2005;16:538–546. doi: 10.1016/j.jnutbio.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Chytrova G, Ying Z, Gomez-Pinilla F. Exercise normalizes levels of MAG and Nogo-A growth inhibitors after brain trauma. Eur J Neurosci. 2008;27:1–11. doi: 10.1111/j.1460-9568.2007.05982.x. [DOI] [PubMed] [Google Scholar]
- Corrigan FM, Horrobin DF, Skinner ER, Besson JA, Cooper MB. Abnormal content of n-6 and n-3 long-chain unsaturated fatty acids in the phosphoglycerides and cholesterol esters of parahippocampal cortex from Alzheimer's disease patients and its relationship to acetyl CoA content. Int J Biochem Cell Biol. 1998;30:197–207. doi: 10.1016/s1357-2725(97)00125-8. [DOI] [PubMed] [Google Scholar]
- Curtis LB, Doneske B, Liu X, Thaller C, McNew JA, Janz R. Syntaxin 3b is a t-SNARE specific for ribbon synapses of the retina. J Comp Neurol. 2008;510:550–559. doi: 10.1002/cne.21806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darios F, Davletov B. Omega-3 and omega-6 fatty acids stimulate cell membrane expansion by acting on syntaxin 3. Nature. 2006;440:813–817. doi: 10.1038/nature04598. [DOI] [PubMed] [Google Scholar]
- de Lau LM, Bornebroek M, Witteman JC, Hofman A, Koudstaal PJ, Breteler MM. Dietary fatty acids and the risk of Parkinson disease: the Rotterdam study. Neurology. 2005;64:2040–2045. doi: 10.1212/01.WNL.0000166038.67153.9F. [DOI] [PubMed] [Google Scholar]
- Ding Q, Vaynman S, Souda P, Whitelegge JP, Gomez-Pinilla F. Exercise affects energy metabolism and neural plasticity-related proteins in the hippocampus as revealed by proteomic analysis. Eur J Neurosci. 2006;24:1265–1276. doi: 10.1111/j.1460-9568.2006.05026.x. [DOI] [PubMed] [Google Scholar]
- Dyall SC, Michael GJ, Whelpton R, Scott AG, Michael-Titus AT. Dietary enrichment with omega-3 polyunsaturated fatty acids reverses age-related decreases in the GluR2 and NR2B glutamate receptor subunits in rat forebrain. Neurobiol Aging. 2007;28:424–439. doi: 10.1016/j.neurobiolaging.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague–Dawley rats in vivo. Neuroscience. 2004;124:71–79. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Furuya H, Watanabe T, Sugioka Y, Inagaki Y, Okazaki I. Effect of ethanol and docosahexaenoic acid on nerve growth factor-induced neurite formation and neuron specific growth-associated protein gene expression in PC12 cells. Nihon Arukoru Yakubutsu Igakkai Zasshi. 2002;37:513–522. [PubMed] [Google Scholar]
- Gomez-Pinilla F. Brain foods: the effects of nutrients on brain function. Nat Rev Neurosci. 2008;9:568–578. doi: 10.1038/nrn2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Ying Z, Roy RR, Molteni R, Edgerton VR. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J Neurophysiol. 2002;88:2187–2195. doi: 10.1152/jn.00152.2002. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Vaynman S, Ying Z. Brain-derived neurotrophic factor functions as a metabotrophin to mediate the effects of exercise on cognition. Eur J Neurosci. 2008;28:2278–2287. doi: 10.1111/j.1460-9568.2008.06524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M, Hossain S, Shimada T, Shido O. Docosahexaenoic acid-induced protective effect against impaired learning in amyloid beta-infused rats is associated with increased synaptosomal membrane fluidity. Clin Exp Pharmacol Physiol. 2006;33:934–939. doi: 10.1111/j.1440-1681.2006.04467.x. [DOI] [PubMed] [Google Scholar]
- Holguin S, Huang Y, Liu J, Wurtman R. Chronic administration of DHA and UMP improves the impaired memory of environmentally impoverished rats. Behav Brain Res. 2008;191:11–16. doi: 10.1016/j.bbr.2008.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrocks LA, Farooqui AA. Docosahexaenoic acid in the diet: its importance in maintenance and restoration of neural membrane function. Prostaglandins Leukot Essent Fatty Acids. 2004;70:361–372. doi: 10.1016/j.plefa.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Jacka FN, Pasco JA, Henry MJ, Kotowicz MA, Nicholson GC, Berk M. Dietary omega-3 fatty acids and depression in a community sample. Nutr Neurosci. 2004;7:101–106. doi: 10.1080/10284150410001710438. [DOI] [PubMed] [Google Scholar]
- Kim HY. Novel metabolism of docosahexaenoic acid in neural cells. J Biol Chem. 2007;282:18661–18665. doi: 10.1074/jbc.R700015200. [DOI] [PubMed] [Google Scholar]
- Loftis JM, Janowsky A. The N-methyl-D-aspartate receptor subunit NR2B: localization, functional properties, regulation, and clinical implications. Pharmacol Ther. 2003;97:55–85. doi: 10.1016/s0163-7258(02)00302-9. [DOI] [PubMed] [Google Scholar]
- Mesches MH, Gemma C, Veng LM, Allgeier C, Young DA, Browning MD, Bickford PC. Sulindac improves memory and increases NMDA receptor subunits in aged Fischer 344 rats. Neurobiol Aging. 2004;25:315–324. doi: 10.1016/S0197-4580(03)00116-7. [DOI] [PubMed] [Google Scholar]
- Morris MC, Evans DA, Bienias JL, Tangney CC, Bennett DA, Wilson RS, Aggarwal N, Schneider J. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch Neurol. 2003;60:940–946. doi: 10.1001/archneur.60.7.940. [DOI] [PubMed] [Google Scholar]
- Murck H, Manku M. Ethyl-EPA in Huntington disease: potentially relevant mechanism of action. Brain Res Bull. 2007;72:159–164. doi: 10.1016/j.brainresbull.2006.10.020. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, McHugh TJ, Wilson MA, Tonegawa S. NMDA receptors, place cells and hippocampal spatial memory. Nat Rev Neurosci. 2004;5:361–372. doi: 10.1038/nrn1385. [DOI] [PubMed] [Google Scholar]
- Nordvik I, Myhr KM, Nyland H, Bjerve KS. Effect of dietary advice and n-3 supplementation in newly diagnosed MS patients. Acta Neurol Scand. 2000;102:143–149. doi: 10.1034/j.1600-0404.2000.102003143.x. [DOI] [PubMed] [Google Scholar]
- O'Callaghan RM, Ohle R, Kelly AM. The effects of forced exercise on hippocampal plasticity in the rat: a comparison of LTP, spatial- and non-spatial learning. Behav Brain Res. 2007;176:362–366. doi: 10.1016/j.bbr.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Pongrac JL, Slack PJ, Innis SM. Dietary polyunsaturated fat that is low in (n-3) and high in (n-6) fatty acids alters the SNARE protein complex and nitrosylation in rat hippocampus. J Nutr. 2007;137:1852–1856. doi: 10.1093/jn/137.8.1852. [DOI] [PubMed] [Google Scholar]
- Rao JS, Ertley RN, DeMar JC, Jr, Rapoport SI, Bazinet RP, Lee HJ. Dietary n-3 PUFA deprivation alters expression of enzymes of the arachidonic and docosahexaenoic acid cascades in rat frontal cortex. Mol Psychiatry. 2007;12:151–157. doi: 10.1038/sj.mp.4001887. [DOI] [PubMed] [Google Scholar]
- Salem N, Jr, Litman B, Kim HY, Gawrisch K. Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids. 2001;36:945–959. doi: 10.1007/s11745-001-0805-6. [DOI] [PubMed] [Google Scholar]
- Shirasu M, Kimura K, Kataoka M, Takahashi M, Okajima S, Kawaguchi S, Hirasawa Y, Ide C, Mizoguchi A. VAMP-2 promotes neurite elongation and SNAP-25A increases neurite sprouting in PC12 cells. Neurosci Res. 2000;37:265–275. doi: 10.1016/s0168-0102(00)00125-5. [DOI] [PubMed] [Google Scholar]
- Stillwell W, Shaikh SR, Zerouga M, Siddiqui R, Wassall SR. Docosahexaenoic acid affects cell signaling by altering lipid rafts. Reprod Nutr Dev. 2005;45:559–579. doi: 10.1051/rnd:2005046. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Park SJ, Tamura M, Ando S. Effect of the long-term feeding of dietary lipids on the learning ability, fatty acid composition of brain stem phospholipids and synaptic membrane fluidity in adult mice: a comparison of sardine oil diet with palm oil diet. Mech Ageing Dev. 1998;101:119–128. doi: 10.1016/s0047-6374(97)00169-3. [DOI] [PubMed] [Google Scholar]
- Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G, Tsien JZ. Genetic enhancement of learning and memory in mice. Nature. 1999;401:63–69. doi: 10.1038/43432. see comments. [DOI] [PubMed] [Google Scholar]
- Teague WE, Fuller NL, Rand RP, Gawrisch K. Polyunsaturated lipids in membrane fusion events. Cell Mol Biol Lett. 2002;7:262–264. [PubMed] [Google Scholar]
- Tully AM, Roche HM, Doyle R, Fallon C, Bruce I, Lawlor B, Coakley D, Gibney MJ. Low serum cholesteryl ester-docosahexaenoic acid levels in Alzheimer's disease: a case-control study. Br J Nutr. 2003;89:483–489. doi: 10.1079/BJN2002804. [DOI] [PubMed] [Google Scholar]
- Vaddadi KS, Soosai E, Chiu E, Dingjan P. A randomised, placebo-controlled, double blind study of treatment of Huntington's disease with unsaturated fatty acids. Neuroreport. 2002;13:29–33. doi: 10.1097/00001756-200201210-00011. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasuta C, Caunt C, James R, Samadi S, Schibuk E, Kannangara T, Titterness AK, Christie BR. Effects of exercise on NMDA receptor subunit contributions to bidirectional synaptic plasticity in the mouse dentate gyrus. Hippocampus. 2007;17:1201–1208. doi: 10.1002/hipo.20349. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Gomez-Pinilla F. License to run: exercise impacts functional plasticity in the intact and injured central nervous system by using neurotrophins. Neurorehabil Neural Repair. 2005;19:283–295. doi: 10.1177/1545968305280753. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Wu A, Gomez-Pinilla F. Coupling energy metabolism with a mechanism to support brain-derived neurotrophic factor-mediated synaptic plasticity. Neuroscience. 2006;139:1221–1234. doi: 10.1016/j.neuroscience.2006.01.062. [DOI] [PubMed] [Google Scholar]
- Weinstock-Guttman B, Baier M, Park Y, Feichter J, Lee-Kwen P, Gallagher E, Venkatraman J, Meksawan K, Deinehert S, Pendergast D, Awad AB, Ramanathan M, Munschauer F, Rudick R. Low fat dietary intervention with omega-3 fatty acid supplementation in multiple sclerosis patients. Prostaglandins Leukot Essent Fatty Acids. 2005;73:397–404. doi: 10.1016/j.plefa.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Wu A, Ying Z, Gómez-Pinilla F. Dietary omega-3 fatty acids normalize BDNF levels, reduce oxidative damage, and counteract learning disability after traumatic brain injury in rats. J Neurotrauma. 2004;21:1457–1467. doi: 10.1089/neu.2004.21.1457. [DOI] [PubMed] [Google Scholar]
- Wu A, Ying Z, Gomez-Pinilla F. Docosahexaenoic acid dietary supplementation enhances the effects of exercise on synaptic plasticity and cognition. Neuroscience. 2008;155:751–759. doi: 10.1016/j.neuroscience.2008.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu SJ, Chen Z, Zhu LJ, Shen HQ, Luo JH. Visual recognition memory is related to basic expression level of NMDA receptor NR1/NR2B subtype in hippocampus and striatum of rats. Acta Pharmacol Sin. 2005;26:177–180. doi: 10.1111/j.1745-7254.2005.00532.x. [DOI] [PubMed] [Google Scholar]


