SUMMARY
In addition to quantitative differences in morphogen signaling specifying distinct cell fates, the vector and slope of morphogen gradients can influence planar cell polarity (PCP) and growth [1–9]. The atypical cadherin Fat plays a central role in this process. Fat regulates PCP and growth through distinct downstream pathways, both of which appear to involve the establishment of molecular polarity within cells [reviewed in 10, 11]. Fat activity is regulated by the atypical cadherin Dachsous (Ds), and the protein kinase Four-jointed (Fj), which are expressed in opposing gradients in many tissues [12, 13]. Studies of their influence on Fat have implied that Fat is regulated by the vector and slope of the Fj and Ds expression gradients [2–9] but how this is achieved remained unknown. Here, we identify and characterize the molecular mechanism by which cells interpret a Fj expression gradient to polarize Fat activity. We demonstrate that Fj both promotes the ability of Fat to bind to its ligand Ds, and inhibits the ability of Ds to bind Fat. As a result these opposing Fj activities, the juxtaposition of cells differing in their levels of Fj expression results in asymmetric Fat-Ds binding. We also show that the influence of Fj on Fat is a direct consequence of Fat phosphorylation, and identify a phosphorylation site important for the stimulation of Fat-Ds binding by Fj. Our results define a molecular mechanism by which a morphogen gradient can drive the polarization of Fat activity to influence PCP and growth in developing tissues.
RESULTS
Fat and Ds are very large (5147 and 3503 amino acids, respectively) atypical cadherins [13, 14]. They influence growth by regulating the Hippo-Warts pathway, and they influence PCP through a distinct pathway that can impinge on canonical (Frizzled-dependent) PCP signaling [reviewed in 10, 11]. Genetic studies of their influence on both PCP and Hippo-Warts signaling have led to the inference that Fat functions as a transmembrane receptor and Ds as its transmembrane ligand [5, 15–18]; this inference has received further supported from the observation that Ds influences a Dco-mediated phosphorylation of the Fat cytoplasmic domain [19, 20]. Binding between Fat and Ds has not been demonstrated directly, but instead has been inferred from two classes of experiments. First, transfection of Fat and Ds into distinct populations of cultured Drosophila S2 cells causes these cells to adhere to each other [3]. Second, mutation or over-expression of Fat or Ds in patches of cells in imaginal discs can modulate the distribution of Ds or Fat on neighboring cells in a manner which suggests that Fat-Ds localization is influenced by intercellular binding [4, 9, 17, 21]. Mutation or over-expression of Fj can also modulate Fat and Ds localization [4, 9, 17, 21]. This observation, together with genetic studies that place Fj upstream of Fat in both PCP and Hippo-Warts signaling [5, 15–17, 22], and the determination that Fj is a Golgi-localized kinase that can directly phosphorylate particular cadherin domains of Fat and Ds [23], raised the possibility that Fj acts by modulating Fat-Ds binding, although other scenarios, such as effects on the trafficking or stability of Fat or Ds, could also be consistent with the published data.
Co-expression of Fj with Fat elevates Fat-Ds binding
In order to demonstrate direct binding between Fat and Ds proteins, and to establish an assay for characterizing the influence of Fj on this binding, we established a system for quantitatively measuring the effect of Fj on the Ds:Fat interaction. A stably transfected Drosophila S2 cell line (S2-Ds:AP cells) was generated that produced a secreted protein (Ds:AP) consisting of the entire Ds ectodomain fused to human placental alkaline phosphatase. Conditioned media from this cell line was concentrated and then incubated with stably transfected S2 cells expressing full length Fat (S2-Fat) or Fat and V5 epitope-tagged Fj (S2-Fat/Fj:V5). Binding sites for the Ds:AP protein on the surface of the S2-Fat and S2-Fat/Fj:V5 cells were determined by measuring cell-associated AP activity after washing to remove unbound Ds:AP.
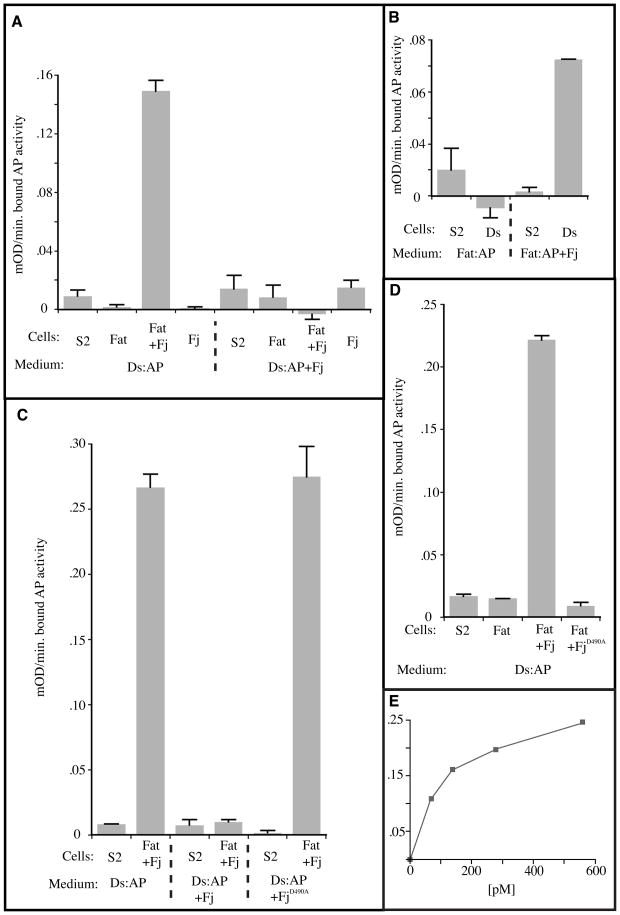
Fj expression strongly enhanced the ability of Fat-expressing cells to bind Ds:AP (Fig. 1A). While Ds:AP binding by the S2-Fat/Fj:V5 cells was easily detected, the S2-Fat cells failed to exhibit detectable binding. The binding depended on the expression of Fat and appeared to be saturable with an apparent Kd of ~125 pM (Fig. 1E). These results indicate that Fj coexpression with Fat promotes formation of Ds:Fat complexes and suggest that Fj may regulate the ability of Fat to bind Ds. However, these data were also consistent with the possibility that Fj instead modulates the trafficking of Fat to the plasma membrane such that the S2-Fat/Fj:V5 cells have more Fat on their surface. In order to address this possibility, we established a reversed binding assay using stably transfected S2 cell lines that secreted the Fat ectodomain fused to alkaline phosphatase (Fat:AP) with or without concommitant Fj:V5 expression. Concentrated conditioned media from these two cell lines was incubated with S2 cells stably transfected to express full length Ds (S2-Ds cells). While binding of Fat:AP co-produced with Fj:V5 was readily apparent, equivalent amounts of Fat:AP made in the absence of Fj:V5 failed to detectably bind S2:Ds cells (Fig. 1B). Taken together, these experiments demonstrate that Fj increases the ability of Fat to bind Ds. Consistent with the idea that Fj acts on Fat via its kinase activity, expression of Fj in which an essential catalytic motif (DNE, [23], Supplementary Fig. S1) is mutated (to ANE or GGG) failed to promote the ability of Fat to bind Ds (Fig. 1C and data not shown).
Figure 1. Fj has a dual effect of Fat-Ds binding.
A-D) Histograms showing the results of binding of AP-tagged Fat or Ds proteins to cells expressing Fat or Ds. The histograms show the average of two replicate binding assays with bound AP activity expressed as milli-OD/min. Bars indicate the deviation between the replicates. To correct for endogenous cellular phosphatase activity, binding activity was reduced by the activity seen using control condition S2 media. The proteins expressed by the binding cells and the cells used to produce the conditioned media are indicated. A) Binding assays using Ds:AP conditioned media and cells. B) Binding assays using Fat:AP conditioned media and cells. A) The effects of Fj mutant on Ds binding to Fat. D) The effects of Fj mutant on Ft binding to Ds. E) Concentration dependence of Ds:AP binding to cells expressing Fat or Fat and Fj:V5. The concentration of Ds:AP is indicated below. Bound AP activity is given in mill-OD/min.
While Fj was required for detectable binding of Fat to Ds in these assays, the weaker phenotype of fj mutants in comparison to ds mutants, as well the ability of S2 cells expressing high levels of Fat and Ds to aggregate without exogenous Fj [3], implies that Fat does have some Ds-binding ability even without Fj. We attribute this difference to the more stringent requirements of our binding assays, which require individual Fat and Ds molecules to remain bound for an extended period and through many washing steps.
Co-expression of Fj with Ds impairs Fat-Ds binding
In order to examine whether Fj might also modify the ability of Ds to bind Fat, the S2-Ds:AP expressing cells were additionally transfected to express Fj:V5. The Fat binding activity of conditioned media produced by S2-Ds:AP/Fj:V5 cells was then compared to that of S2-Ds:AP cells. Fj expression had a profound effect on the ability of Ds to bind Ft. When media containing equivalent amounts of Ds:AP were compared, binding of the Ds:AP produced in the absence of Fj:V5 expression was easily detected. In contrast, Ds:AP produced in the presence of Fj:V5 had no detectable binding activity (Fig. 1A). These results indicate that Fj inhibits the ability of Ds to bind Fat. Similarly to Fj’s effect on Fat, the ability of Fj to regulate Ds depended on the DNE motif of Fj (Fig. 1D).
Fj has dual roles in regulating Fat-Ds binding in vivo
The experiments described above demonstrate that Fj regulates Ds:Fat dimer formation in two ways. One function of Fj is to increase the ability of Fat to bind Ds and thus promote the formation of Ds:Fat dimers when Fj is upregulated in the Fat-expressing cell. The second activity reduces the ability of Ds to bind Fat and thus inhibits the formation of Ds:Fat dimers when Fj is upregulated in the Ds-expressing cell. One prediction of these combined Fj activities is that a cell that strongly expresses Fj could draw the Ds of neighboring cells to their common border while failing to bind the neighboring cell’s Fat. We tested this by examining pupal wings containing clones of Fj-overexpressing cells.
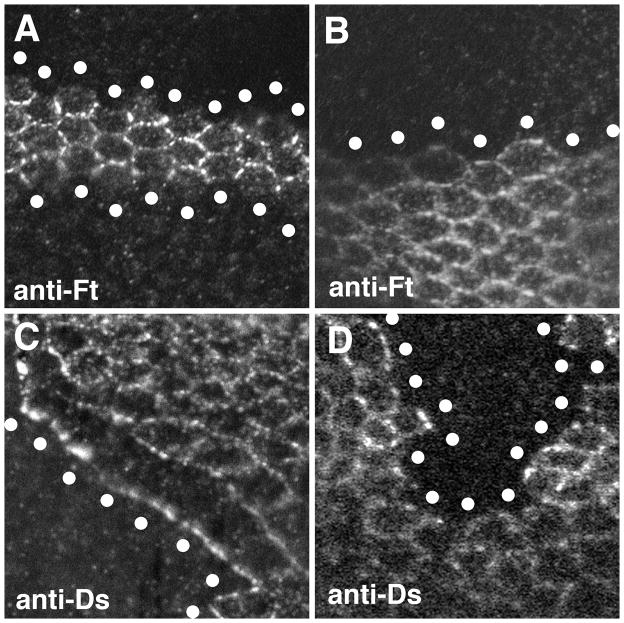
Prior experiments examining Fat or Ds relocalization induced by Fj could not clearly define the influence of Fj because confocal microscopy does not have sufficient resolution to distinguish the respective contributions of two neighboring cells to protein staining at the membrane interface between them. Thus, it was not possible to know whether Fat or Ds protein at the edge of a Fj-expressing clone comes from the Fj-expressing cell, its neighbor, or both cells [4, 9, 17, 21]. To bypass this, clones of marked cells both overexpressing Fj and lacking Fat function were generated in pupal wing discs, and examined for Fat localization. Since the Fj-expressing cells lack Fat, the extent of Fat accumulation along the clone border reflects the ability of Ds made in the presence of Fj overexpression to recruit Fat from the neighboring wild-type cells. We found that Fat failed to localize to the clone border, thus confirming that Fj overexpression reduces the ability of Ds to bind Fat in vivo (Fig. 2A,B). In a second experiment, the localization of Ds was examined in clones of cells overexpressing Fj and lacking Ds. In this case, the ability of Ds to accumulate along the clone border is an indication of the ability of Fat produced by the Fj-expressing cells to bind Ds from the bordering wild-type cells. Examination of these wings showed that the Ds in the adjacent wild-type cells was drawn preferentially to the clone border, thus indicating that Fat produced in the presence of high levels of Fj expression has an enhanced ability to bind Ds (Fig. 2C,D). These results confirm that the effects of Fj on Fat-Ds binding defined in the cell-based binding assays described above represent its effects on Fat-Ds binding in vivo.
Figure 2. Effect of Fj on Fat-Ds localization.
Confocal images of pupal wing discs stained with either anti-Fat or anti-Ds as indicated. In each panel, the approximate center of the mutant cells at the edge of the clone are indicated by the white circles. The images are oriented with distal towards the right. A) Two clones of fat cells over-expressing Fj. Fat in the wild-type cells immediately adjacent to the Fj over-expressing cells fails to accumulate along the border with the Fj over-expressing cells. B) A clone of fat cells that do not overexpress Fj. In the absence of Fj over-expression, Fat is still present at the clone border. C) A clone of ds cells over-expressing Fj. Note that Ds in the adjacent wild-type cells is preferentially drawn to the border of the Fj over-expressing cells. This can be seen by the reduced Ds staining at the cell-cell boundaries between the wild-type cells that border the Fj over-expressing cells. D) A clone of ds cells that do not over-express Fj. No effect on Ds localization is seen in the adjacent wild-type cells. Similar effects on Fat and Ds localization can be observed on all sides of Fj over-expressing clones. The genotypes are A) hsFLP/+; TubGal80, FRT40A/ftG-rv, FRT40A; Tub-Gal4/UAS-Fj, B) hsFLP/+; P[Ubi-GFP]2L, FRT40A/ftG-rv, FRT40A, C) hsFLP/+; TubGal80, FRT40A/ds38k, FRT40A; Tub-Gal4/UAS-Fj and D) hsFLP/+; P[Ubi-GFP]2L, FRT40A/ds38k, FRT40A.
Fat-Ds binding mediated by N-terminal cadherin domains
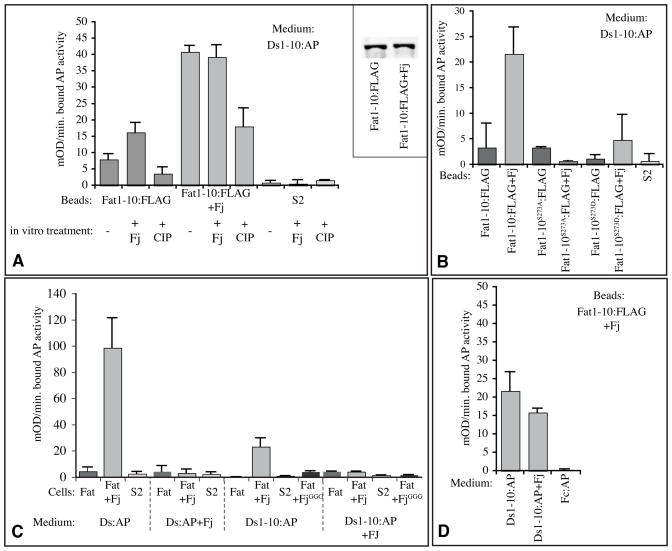
To investigate the molecular mechanism by which Fj modulates Fat-Ds binding, we sought to establish an in vitro binding assay with purified components. Fragments of Fat including portions of the extracellular cadherin domains were labeled with a C-terminal FLAG epitope-tag, expressed in S2 cells, and then purified from conditioned medium using anti-FLAG affinity beads. These beads were then assayed for their ability to bind secreted, AP-tagged Ds polypeptides. Because we could not express and purify sufficient quantities of soluble, full length extracellular domains of Fat and Ds polypeptides for these experiments, we investigated whether smaller (~125 kD) N-terminal fragments of Fat and Ds, comprising the first 10 cadherin domains of each protein (Ft1-10:FLAG and Ds1-10:AP) could bind to each other. Indeed, a modest but reproducible binding of Ds1-10:AP to Fat1-10:FLAG could be detected, and the binding of Ds1-10:AP to Fat1-10:FLAG was increased several fold when Fat1-10:FLAG was purified from cells co-transfected to express Fj (Fig. 3A,B). The success of this assay established three important points:
Figure 3. Molecular characterization of the influence of Fj on Fat-Ds binding.
Histograms show the results (averages of from two to four replicate experiments) of binding of AP-tagged Ds proteins, with bound AP activity expressed as milli-OD/min. Error bars indicate standard deviation. A) In vitro Fat-Ds binding assays, using conditioned medium from cells expressing Ds1-10:AP (2400 mOD/min) and beads loaded with Fat1-10:FLAG from S2 cells, Fat1-10:FLAG from S2 cells co-transfected to express Fj, or conditioned medium from S2 cells transfected with empty vector, as indicated. Where indicated, beads were phosphorylated in vitro with Fj or treated with phosphatase. For simplicity of display, the “−” samples here display averages from the mock treated (no enzyme) kinase and phosphatase experiments. Inset shows the results of Western blotting (anti-FLAG) on Fat1-10:FLAG from S2 cells without or with (+Fj) Fj co-expression to show that the amounts of Fat1-10 are similar. B) In vitro Fat-Ds binding assays, using conditioned medium from cells expressing Ds1-10:AP (2400 mOD/min) and beads loaded with Fat1-10:FLAG from S2 cells with or without Fj co-transfection, or conditioned medium from S2 cells, as indicated. Where indicated by superscripts, Fat1-10:FLAG with point mutations in the Fj site in Cadherin domain 3 were used. C) Cell based binding assays, using conditioned medium from cells expressing full length DS:AP or Ds1-10:AP (1200 mOD/min), with or without Fj co-expression, as indicated. Cells were transfected to express full length Fat, Fat and Fj together, Fat and kinase-dead Fj (FjGGG), or empty vector (S2). D) In vitro Fat-Ds binding assays, using conditioned medium from cells expressing Ds1-10:AP (2400 mOD/min) with or without Fj co-expression, or as a control Fc:AP, as indicated, and beads loaded with Fat1-10:FLAG from S2 cells co-transfected to express Fj.
First, binding between Fat and Ds is mediated by the N-terminal thirds of their extracellular domains (Fat and Ds contain 34 and 27 cadherin domains, respectively). Binding of Ds1-10:AP to full length Fat in a cell-based assay was lower than binding of full length Ds:AP (Fig. 3C), which might reflect an influence of C-terminal cadherin domains on the folding, structure, or stability of N-terminal cadherin domains. Second, the observation that Fj modulates binding between Fat1-10 and Ds1-10, stimulating it when expressed on the Fat side, and inhibiting it when expressed on the Ds side, indicates that sites sufficient for the Fj-mediated modulation of binding are contained within these ten N-terminal cadherin domains. Third, the observation that binding could be detected with an affinity purified Fat polypeptide implies that Fat does not require additional co-factors to bind to Ds. However, when we affinity purified Ds1-10:AP (using C-terminal V5 or 6xHis tags), its Fat-binding activity was lost. Thus, although we suspect that Ds structure was simply not stable through purification, we cannot exclude the possibility that Ds requires association with one or more co-factors for Fat binding. We also found that while inhibition of Ds1-10:AP binding when co-expressed with Fj could be observed in cell-based assays, this inhibition was barely detectable using in vitro assays (Fig. 3D).
Fj regulates Fat binding to Ds by directly phosphorylating cadherin domain 3
Since Fj is a kinase, and its kinase activity is required for its biological activity [23], and its influence on Fat-Ds binding (Figs. 1,3) one could infer that it modulates binding by phosphorylating its substrates. A subset of the cadherin domains of Fat and Ds are phosphorylated by Fj [23], but since all of the experiments described above involve in vivo co-expression of Fj, we could not formally exclude the possibility that other, as yet unidentified Fj substrates contribute to its ability to modulate Fat-Ds binding. Moreover, experiments involving in vivo phosphorylation by Fj leave open the question of whether direct phosphorylation of Fat or Ds cadherin domains is sufficient to modulate Fat-Ds binding, or whether instead this phosphorylation serves as a precursor to subsequent modifications that actually modulate binding.
To address these questions, we investigated the consequences of in vitro phosphorylation. Since, as noted above, we were unable to purify Ds molecules that retained binding activity, this analysis was restricted to investigations of the influence of Fj on Fat. Fat1-10:FLAG was affinity purified on anti-FLAG beads from conditioned medium of cells that did not express exogenous Fj. This Fat1-10:FLAG was then phosphorylated in vitro using affinity purified Fj, ATP, and buffer. Comparison of the Ds1-10:AP binding-activity of this in vitro phosphorylated Fat1-10:FLAG to mock treated Fat1-10:FLAG (incubated in the absence of Fj or ATP) established that in vitro phosphorylation of Fat1-10:FLAG enhanced its binding to Ds (Fig. 3A). In complementary experiments, we assayed the consequences of in vitro removal of phosphates from Fat1-10:FLAG. For these experiments, Fat1-10:FLAG was purified from Fj-expressing cells, and then incubated either with Antarctic phosphatase (AnP) or Calf intestinal alkaline phosphatase (CIP). Phosphatase treatment of Fat1-10:FLAG reduced its ability to bind Ds1-10:AP (Fig. 3A). Together, these observations establish that the simple presence or absence of phosphate groups on Cadherin domains of Fat is sufficient to modulate its binding to Ds. Since the modulation of binding observed using in vitro modifications was weaker than that achieved by in vivo modifications, its conceivable that other factors contribute to the effectiveness of Fj modification, but clearly they are not required. The observations that Fat-Ds binding can be modulated by in vitro modification provides independent support for the conclusion from reversed binding experiments that Fj does not enhance Fat-Ds binding by modulating Fat trafficking or stability in vivo, and also establishes that rather than acting as a precursor to subsequent post-translational modifications or recruitment of co-factors, phosphorylation alone can act directly to modulate Fat-Ds binding.
In prior studies we mapped several phosphorylation sites on Fat and Ds, and identified the minimal requirements for a Fj phosphorylation site, consisting of a Ser or Thr residue at the seventh residue of a cadherin domain [23]. Among the three Fj sites within the first ten cadherin domains of Fat, only the site in cadherin domain 3 is highly conserved among vertebrate and invertebrate Fat homologues (Supplementary Fig. S2). To investigate the functional significance of this site, we first mutated it within Fat1-10:FLAG by changing the conserved Ser residue to either Ala or Asp. The Ser-to-Ala mutation (S273A) completely blocked the ability of Fj to enhance Fat-Ds binding in our in vitro assay (Fig. 3B). This observation implies that the enhancement of Fat-Ds binding is dependent upon phosphorylation of this single Ser residue in cadherin domain 3. In some cases the effects of phosphorylation on proteins can be partially mimicked by replacement of Ser residues with Asp residues, however this was not the case for Fj-mediated phosphorylation of Fat, because the binding of Fat1-10:FLAG with the Ser-to-Asp mutation was not significantly different from that of Fat with the Ser to Ala mutation. To investigate requirements for Ser273 phosphorylation in the context of full length Fat, we employed the cell-based binding assay. Mutation of Ser273 reduced, but did not abolish, the ability of Fj to promote Fat-Ds binding (Supplementary Fig. 3). This confirms that Ser273 makes contribution to Fj modulation of Fat-Ds binding, but at the same time implies that binding interactions of full length Fat and Ds may be more complex, with contributions from multiple Fj phosphorylation sites.
DISCUSSION
The induction of distinct cell fates in response to quantitatively distinct levels of morphogen signaling is a classic paradigm for developmental patterning, and has been well studied [24]. There is also evidence that the vector and slope of morphogen gradients can be interpreted by cells, and used to direct planar cell polarity (PCP) and growth [1–8], but the molecular mechanisms by which this occurs have remained poorly understood. The transmembrane receptor Fat is regulated by the graded expression of Fj and Ds [4–9]. PCP is evidenced in the polarization of cellular structures and cellular behaviors. In some instances, Fat influences PCP through the Fz-PCP pathway, and polarizes the localization of core PCP proteins [reviewed in 10]. Fat regulates growth through the Hippo-Warts pathway [reviewed in 11], and its effects on Warts are dependent upon Dachs, which is polarized within cells in a Fat-dependent manner, and can be considered a direct read-out of Fat activity [16, 21]. Thus, for both the Fat-PCP and Fat-Warts pathways, the Ds and Fj gradients appear to act by polarizing Fat activity within cells, but the molecular mechanism by which cells interpret these gradients to polarize Fat activity was unknown. In this report, we have demonstrated that Fj regulates the binding of the Fat receptor to its ligand Ds. Importantly, Fj regulates Fat-Ds binding in two ways. Fj acts directly on Fat to promote its binding to Ds, while also acting on Ds to inhibit its interaction with Fat. As described below, these dual, opposing, influences of Fj on Fat-Ds binding provide a molecular explanation for how the slope and vector of a gradient can be interpreted to establish polarity within cells with high fidelity and irrespective of absolute concentration.
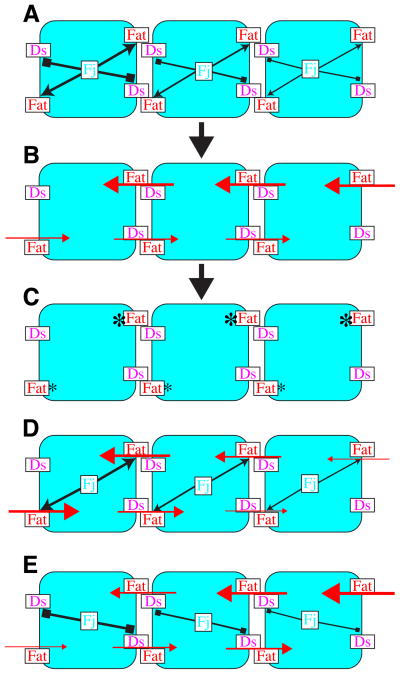
For any cell at any point within a Fj expression gradient, Fj-mediated phosphorylation will both enhance the ability of Fat in that cell to bind Ds on neighboring cells, and inhibit the ability of Ds in that cell to bind to Fat on neighboring cells (Fig. 4A). If a neighboring cell is at a higher point in the Fj expression gradient, then Fj-mediated phosphorylation will have a relatively greater effect both in enhancing the ability of Fat to bind Ds, and in inhibiting the ability of Ds to bind Fat. The net consequence of the dual effects of Fj then, is that any cell at a relatively higher point in the Fj gradient is both better at receiving a Fat signal and worse at sending a Fat signal (ie, Ds) than its neighbor at a lower point in the Fj gradient (Fig. 4B). Thus, given uniform Fat and Ds, the dual action of Fj on Fat and Ds is expected to polarize each and every boundary between cells that express different levels of Fj, with relatively higher Fat activity in the cell with higher Fj. Importantly, a similar process is expected to occur on the opposite side of a cell, but there, the cell that had higher Fj expression now confronts a cell with even higher Fj expression, resulting again in lower Fat activity within the cell with less Fj and higher Fat activity within the cell with more Fj. When applied across a tissue where Fj is expressed in a gradient, the mechanism we identify should thus lead to the polarization of Fat activity within every cell (Fig. 4C), with the direction of polarization reflecting the vector of the Fj gradient, and the magnitude of polarization reflecting its slope. We propose that these initial polarizations in Fat activity could then be amplified by subsequent steps to generate the overt asymmetries in the distribution of core PCP proteins and Dachs.
Figure 4. Schematic model for polarization of Fat activity in response to a Fj gradient.
Rounded rectangles represent cells at different points in an Fj expression gradient, illustrated by different levels of shading. A) Fj acts cell autonomously to decrease the Fat-binding activity of Ds (block arrows) and increase the Ds-binding activity of Fat (pointed arrows). B) As a consequence of these different binding activities, strong signaling (large red arrows) occurs from cells with lower Fj to cells with higher Fj, and weak signaling (small red arrows) occurs from cells with higher Fj to cells with lower Fj C) The action of this mechanism at each cell boundary results in polarized Fat activity (asterisks) within each cell. D) If Fj were only to act on Fat, Fat activity would vary across the tissue, but would not be polarized within indivdual cells. E) If Fj were only to act on Ds, cells would have to be able to discriminate both between relatively high levels of Fat (right) and between relatively low levels of Fat (left) in order to polarize. See text for details.
The consequences of the dual action of Fj are evident if one considers what would happen if Fj had only one of its two activities. If Fj were only to act on Fat, then in every cell, Fat would confront an equally active Ds on both the high and low side of the Fj gradient (Fig. 4D). The result would be that Fat activity would vary across the tissue, with high Fat activation in regions where Fj was high and low Fat activity in regions where Fj was low. However, while Fat signaling would differ between cells, individual cells would fail to polarize Fat activity internally. If Fj were only to act on Ds, then Fat activity within cells could still be polarized (Fig. 4E). However, both Fat activity, and the efficiency of this polarization, could vary across the tissue, as at low Fj concentrations, in order to polarize, a cell would have to make distinctions between two high levels of Fat activity, whereas at high Fj levels a cell would have to make distinctions between two low levels of Fat activity. The consequence of the dual action of Fj then, is not only to make the polarization process more efficient, by affecting both Fat and Ds, but also to uncouple it from the absolute concentration of Fj (assuming that the degree to which Fj enhances and inhibits Fat’s and Ds’s respective abilities to bind each other is comparable). In addition, we note that the dual action of Fj suggests that even though Fat activity is polarized within cells, the absolute amount of Fat activation within cells could be relatively constant across a tissue with graded Fj, which would not be achieved if Fj acted only on Fat or only on Ds (Fig. 4).
In describing this mechanism for how cells interpret an Fj gradient, we have made the simplifying assumption that Ds expression is uniform. In reality, Fj and Ds are generally expressed in opposing gradients [12, 13]. The predicted effect of the opposing Ds gradient would be to strengthen the Fj-driven polarization of Fat activity. Consistent with this idea, the information provided by the Ds and Fj gradients is often partially redundant. For example, the correct polarization of ommatidia in the eye relies on the Ds and Fj gradients, but as long as one of them is expressed in a gradient over 90% of ommatidia polarize correctly [2].
EXPERIMENTAL PROCEDURES
DNA Constructs and Stable Cell Lines
Fat1-10:FLAG and Ds1-10:FLAG have been described previously [23]. Ds1-10:AP was constructed by replacing the C-terminal FLAG tag of Ds1-10:FLAG with the human placental alkaline phosphatase coding region from Dl:AP [25]. The Fat and Ds expression plasmids, pMT-Fat and pMT-Ds, were generated by cloning expression constructs of pUAS-Fat and pUAS-Ds in pMTHisV5 (Invitrogen)[2]. These were further modified by fusing the human alkaline phosphatase coding region of pAP-Tag2 at the end of the extracellular domains to generate pMT-Fat:AP and pMT-Ds:AP[26]. These four constructs were then further modified by the inclusion of a copia-neo cassette. Stable S2 cell lines expressing Fat, Fat:AP, Ds or Ds:AP were established after Ca2PO4 mediated transfection followed by selection with M3 media containing G418 (1mg/ml) and single cell cloning. For cells co-expressing Fj:V5, these lines were retransfected with pMT-Fj:V5hygro, which contains the entire Fj coding region fused to the V5 epitope as well as a copia-hygro cassette, and selected with hygromycin (200μg/ml). Sequences of these constructs are available on request.
Cell-based Binding Assays
The binding assays for Fig. 1 and Supplementary Fig. S3 were conducted as previously described for suspension cells with the following modifications: 1) 100μl of tenfold concentrated cells were used per assay, 2) 2mM CaCl2 and 1mM MgCl2 were added to all binding and washing solutions and 3) binding was performed in 1 ml for 1h at 20°[27]. Ds and Fat expression with induced by the addition of 1mM CuSO4 for 24h. Conditioned media for these binding assays was prepared by approximately 20-fold concentration (using Macrosep-300 concentrator, Pall Corporation) of media from Fat:AP or Ds:AP expressing cells that were induced for 48h with 1mM CuSO4. Binding activity was calculated after subtraction of the activity measured using media conditioned by control S2 cells. Cell-based binding assays for Fig. 3 were conducted essentially as described previously for Notch-Delta binding assays [25]. 8 μg of DNA (3 μg of pUAST-fat or empty vector, 3 μg pMT-Gal4, 2 μg empty vector or pMT-fj) was transiently transfected into S2 cells, and expression induced with 0.7 mM CuSO4 for 2 days, 24h after transfection. Cells were washed with TBS, and resuspended in TBS at 107 cells/mL. 0.3 mL cells were incubated with 0.3mL of conditioned media from cells expressing Ds-AP or Ds1-10:AP (1200 mOD/min AP activity) at room temperature for 1.5 h with middle agitation. Cells were washed in TBS, and lysed in 50 μL lysis buffer (10 mM Tris, pH 8.0, 1% Triton-X 100) at 4°C for 30 min. 25 μL lysate was then incubated with 25 μL AP substrate in reaction buffer (1 M diethanolamine, 5 mM MgCl2, 6.25 mM p-nitrophenyl phosphate) at 37°C for 30 min, reactions were stopped by addition of 50 μL 1 M NaOH, and substrate production assayed by spectrophotometry at 405 nm.
In Vitro Binding Assays
Purification of Fat:FLAG fragments, in vitro Fj kinase reactions, and in vitro phosphatase reactions, were performed essentially as described previously [23]. In brief, conditioned media from cells expressing Fat1-10:FLAG was incubated with EZview Red ANTI-FLAG M2 Affinity agarose beads (Sigma) overnight at 4°C. Beads were collected by gentle centrifugation, and washed 5 times with TBS. Binding assays were performed essentially as described for Notch-Delta binding [25]. In brief, 0.1 μg Fat-1-10:FLAG on beads was incubated with 300 μL Ds1-10:AP conditioned medium (2400 mOD/min) for 1.5 hr at room temperature. Beads were washed in TBS, and binding quantified by assaying AP activity. Fj phosphorylation of cadherin domains was performed in 10 μL reactions, using 1mM ATP, 50 mM Tris (pH 7.0), 10 mM MnCl2, 10 mM CaCl2, 0.1% BSA, purified secreted Fj:V5 (10 ng, 0.17 pmol), and purified Ft-1-10-3xFLAG (0.1 μg, on beads), at 37°C for 1 hour with mild agitation. Beads were then washed four times in TBS, and then used for in vitro binding. Phosphatase treatment of cadherin domains was performed using Antarctic Phosphatase reaction buffer or CIP buffer and 5 units of phosphatase at 37°C for 1 hour. Beads were then washed four times in TBS, and used for in vitro binding.
Immunocytochemistry and genetics
Pupal wing discs were stained after fixation in PBS containing 4% paraformaldehyde and 0.1% Triton X-100 with rabbit anti-GFP (Molecular Probes )and either rat anti-Fat (1:1000) or anti-Ds (1:5000) as described previously[4] using FITC conjugated anti-rabbit and Cy3-conjugated anti-rat antibodies (Jackson Immunochemicals). Clones were induced by 1h heat shock at 37° at 48–72 h of development.
HIGHLIGHTS.
We show, using in vivo, cell based, and in vitro binding assays, that the Golgi-localized cadherin domain kinase Fj enhances the ability of Fat to bind to Ds, and inhibits the ability of Ds to bind Fat.
Using an in vitro binding assay, we demonstrate that direct phosphorylation of Fat by Fj is necessary and sufficient to modulate its binding to Ds, and we identify a unique Fj phosphorylation site important for this effect.
The dual, opposing influences of Fj on Fat-Ds binding provide an explanation for how a molecular gradient is interpreted to polarize cells within a tissue.
Supplementary Material
Acknowledgments
Research in KDIs lab was supported by the HHMI and NIH grant GM078620. MAS was supported by NIH grant GM069923.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rogulja D, Irvine KD. Regulation of cell proliferation by a morphogen gradient. Cell. 2005;123:449–461. doi: 10.1016/j.cell.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 2.Simon MA. Planar cell polarity in the Drosophila eye is directed by graded Four-jointed and Dachsous expression. Development. 2004;131:6175–6184. doi: 10.1242/dev.01550. [DOI] [PubMed] [Google Scholar]
- 3.Matakatsu H, Blair SS. Interactions between Fat and Dachsous and the regulation of planar cell polarity in the Drosophila wing. Development. 2004;131:3785–3794. doi: 10.1242/dev.01254. [DOI] [PubMed] [Google Scholar]
- 4.Ma D, Yang CH, McNeill H, Simon MA, Axelrod JD. Fidelity in planar cell polarity signalling. Nature. 2003;421:543–547. doi: 10.1038/nature01366. [DOI] [PubMed] [Google Scholar]
- 5.Yang C, Axelrod JD, Simon MA. Regulation of Frizzled by Fat-like Cadherins during Planar Polarity Signaling in the Drosophila Compound Eye. Cell. 2002;108:675–688. doi: 10.1016/s0092-8674(02)00658-x. [DOI] [PubMed] [Google Scholar]
- 6.Casal J, Struhl G, Lawrence P. Developmental compartments and planar polarity in Drosophila. Curr Biol. 2002;12:1189–1198. doi: 10.1016/s0960-9822(02)00974-0. [DOI] [PubMed] [Google Scholar]
- 7.Willecke M, Hamaratoglu F, Sansores-Garcia L, Tao C, Halder G. Boundaries of Dachsous Cadherin activity modulate the Hippo signaling pathway to induce cell proliferation. Proc Natl Acad Sci U S A. 2008;105:14897–14902. doi: 10.1073/pnas.0805201105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rogulja D, Rauskolb C, Irvine KD. Morphogen control of wing growth through the Fat signaling pathway. Dev Cell. 2008;15:309–321. doi: 10.1016/j.devcel.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strutt H, Strutt D. Nonautonomous planar polarity patterning in Drosophila: dishevelled-independent functions of frizzled. Dev Cell. 2002;3:851–863. doi: 10.1016/s1534-5807(02)00363-5. [DOI] [PubMed] [Google Scholar]
- 10.Strutt D. The planar polarity pathway. Curr Biol. 2008;18:R898–902. doi: 10.1016/j.cub.2008.07.055. [DOI] [PubMed] [Google Scholar]
- 11.Reddy BV, Irvine KD. The Fat and Warts signaling pathways: new insights into their regulation, mechanism and conservation. Development. 2008;135:2827–2838. doi: 10.1242/dev.020974. [DOI] [PubMed] [Google Scholar]
- 12.Villano JL, Katz FN. four-jointed is required for intermediate growth in the proximal-distal axis in Drosophila. Development. 1995;121:2767–2777. doi: 10.1242/dev.121.9.2767. [DOI] [PubMed] [Google Scholar]
- 13.Clark HF, Brentrup D, Schneitz K, Bieber A, Goodman C, Noll M. Dachsous encodes a member of the cadherin superfamily that controls imaginal disc morphogenesis in Drosophila. Genes Dev. 1995;9:1530–1542. doi: 10.1101/gad.9.12.1530. [DOI] [PubMed] [Google Scholar]
- 14.Mahoney PA, Weber U, Onofrechuk P, Biessmann H, Bryant PJ, Goodman CS. The fat tumor suppressor gene in Drosophila encodes a novel member of the cadherin gene superfamily. Cell. 1991;67:853–868. doi: 10.1016/0092-8674(91)90359-7. [DOI] [PubMed] [Google Scholar]
- 15.Casal J, Lawrence PA, Struhl G. Two separate molecular systems, Dachsous/Fat and Starry night/Frizzled, act independently to confer planar cell polarity. Development (Cambridge, England) 2006;133:4561–4572. doi: 10.1242/dev.02641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho E, Feng Y, Rauskolb C, Maitra S, Fehon R, Irvine KD. Delineation of a Fat tumor suppressor pathway. Nat Genet. 2006;38:1142–1150. doi: 10.1038/ng1887. [DOI] [PubMed] [Google Scholar]
- 17.Cho E, Irvine KD. Action of fat, four-jointed, dachsous and dachs in distal-to-proximal wing signaling. Development. 2004;131:4489–4500. doi: 10.1242/dev.01315. [DOI] [PubMed] [Google Scholar]
- 18.Matakatsu H, Blair SS. Separating the adhesive and signaling functions of the Fat and Dachsous protocadherins. Development. 2006;133:2315–2324. doi: 10.1242/dev.02401. [DOI] [PubMed] [Google Scholar]
- 19.Sopko R, Silva E, Clayton L, Gardano L, Barrios-Rodiles M, Wrana J, Varelas X, Arbouzova NI, Shaw S, Saburi S, Matakatsu H, et al. Phosphorylation of the tumor suppressor fat is regulated by its ligand Dachsous and the kinase discs overgrown. Curr Biol. 2009;19:1112–1117. doi: 10.1016/j.cub.2009.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng Y, Irvine KD. Processing and phosphorylation of the Fat receptor. Proc Natl Acad Sci U S A. 2009;106:11989–11994. doi: 10.1073/pnas.0811540106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mao Y, Rauskolb C, Cho E, Hu WL, Hayter H, Minihan G, Katz FN, Irvine KD. Dachs: an unconventional myosin that functions downstream of Fat to regulate growth, affinity and gene expression in Drosophila. Development. 2006;133:2539–2551. doi: 10.1242/dev.02427. [DOI] [PubMed] [Google Scholar]
- 22.Strutt H, Mundy J, Hofstra K, Strutt D. Cleavage and secretion is not required for Four-jointed function in Drosophila patterning. Development. 2004;131:881–890. doi: 10.1242/dev.00996. [DOI] [PubMed] [Google Scholar]
- 23.Ishikawa HO, Takeuchi H, Haltiwanger RS, Irvine KD. Four-jointed is a Golgi kinase that phosphorylates a subset of cadherin domains. Science. 2008;321:401–404. doi: 10.1126/science.1158159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ashe HL, Briscoe J. The interpretation of morphogen gradients. Development. 2006;133:385–394. doi: 10.1242/dev.02238. [DOI] [PubMed] [Google Scholar]
- 25.Xu A, Haines N, Dlugosz M, Rana NA, Takeuchi H, Haltiwanger RS, Irvine KD. In vitro reconstitution of the modulation of Drosophila Notch-ligand binding by Fringe. J Biol Chem. 2007;282:35153–35162. doi: 10.1074/jbc.M707040200. [DOI] [PubMed] [Google Scholar]
- 26.Flanagan JG, Cheng HJ, Feldheim DA, Hattori M, Lu Q, Vanderhaeghen P. Alkaline phosphatase fusions of ligands or receptors as in situ probes for staining of cells, tissues, and embryos. Methods in enzymology. 2000;327:19–35. doi: 10.1016/s0076-6879(00)27264-9. [DOI] [PubMed] [Google Scholar]
- 27.Flanagan JG, Cheng HJ. Alkaline phosphatase fusion proteins for molecular characterization and cloning of receptors and their ligands. Methods in enzymology. 2000;327:198–210. doi: 10.1016/s0076-6879(00)27277-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.