Abstract
Exercise has been shown to be potently neuroprotective in several neurodegenerative models, including 1-methyl-4 phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP) model of Parkinson’s disease (PD). In order to determine the critical duration of exercise necessary for DA neuroprotection, mice were allowed to run for either 1, 2 or 3 months prior to treatment with saline or MPTP. Quantification of DA neurons in the SNpc show that mice allowed to run unrestricted for 1 or 2 months lost significant numbers of neurons following MPTP administration as compared to saline treated mice; however, 3 months of exercise provided complete protection against MPTP-induced neurotoxicity. To determine the critical intensity of exercise for DA neuroprotection, mice were restricted in their running to either 1/3 or 2/3 that of the full running group for 3 months prior to treatment with saline or MPTP. Quantification of DA neurons in the SNpc show that mice whose running was restricted lost significant numbers of DA neurons due to MPTP toxicity; however, the 2/3 running group demonstrated partial protection. Neurochemical analyses of DA and its metabolites DOPAC and HVA show that exercise also functionally protects neurons from MPTP induced neurotoxicity. Proteomic analysis of SN and STR tissues indicates that 3 months of exercise induces changes in proteins related to energy regulation, cellular metabolism, the cytoskeleton, and intracellular signaling events. Taken together, these data indicate that exercise potently protects DA neurons from acute MPTP toxicity, suggesting that this simple lifestyle element may also confer significant protection against developing PD in humans.
INTRODUCTION
Parkinson’s disease (PD) is a progressive neurodegenerative disease characterized by resting tremor, slowness of movement, rigidity and postural instability (Olanow and Tatton, 1999). While great advances are being made in our understanding of the genetics and other risk factors underlying PD that may soon allow for the discovery and clinical use of preventative pharmaceuticals, at this time these do not exist (Mandel et al., 2003). The major pathology of PD is a progressive degeneration of the dopaminergic (DA) neurons located in the midbrain substantia nigra pars compacta (SNpc) resulting in the loss of SNpc afferents fibers that project to the striatum (STR). Concomitant to this is a significant reduction in the amount of dopamine released within the STR (Hornykiewicz, 1992). Since the surviving DA neurons are initially able to compensate for this loss, symptoms of PD often manifest only after approximately 60% of the SNpc neurons have died (German et al., 1989; Przedborski and Vila, 2001). Since the majority of PD patients are identified based on symptoms which typically manifest after much of the SNpc neurons have died, the effective application of neuroprotective strategies is limited to the time after symptoms have already appeared. Currently, most patients receive temporary relief of symptoms through agents such as L-Dopa; however, this treatment has been hypothesized to contribute to the progression of the disease (Fahn, 1996; Olanow, 1990), and virtually all patients eventually develop a tolerance to the drug, resulting in shorter effective time periods for symptom alleviation (Jankovic and Stacy, 2007; Muenter and Tyce, 1971; Zappia et al., 1999). As patients may live with PD for 15–20 years following the onset of symptoms (Korell and Tanner, 2004) this tolerance to pharmacologic interventions can rapidly limit effective therapeutic outcomes in these patients. Therefore, it is crucial to identify potential therapeutic interventions that delay or altogether prevent the progressive neurodegeneration underlying this disorder.
Administration of the neurotoxin 1-methyl-4 phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP) causes a specific loss of SNpc neurons that recapitulates the dopamine (DA) neuron loss seen in idiopathic PD (Langston et al., 1983; Smeyne and Jackson-Lewis, 2005). In the brain, MPTP is metabolized by glia using the enzyme MAO-B (Ransom et al., 1987), resulting in an unstable metabolite, MPDP, which further metabolizes to 1-methyl-4-phenyl 1-2, 3-dihydropyridium (MPP+) (Brooks et al., 1989). MPP+ is then released from the glia (Cui et al., 2009) and enters neurons via the dopamine transporter (DAT) where it interferes with Complex I respiration in the electron transport chain of the mitochondria (Jenner, 1991; Kopin, 1992) and creates further neuronal damage through the activation of reactive microglia (Gao et al., 2003;McGeer and McGeer, 2008; Teismann et al., 2003) and subsequent generation of free radicals (Zang and Misra, 1993). By one-week post administration of MPTP a significant loss of DA neurons is the SNpc is evident, along with a significant reduction of DA production in the terminal field within the striatum (Jackson-Lewis et al., 1995). Thus, MPTP- administration induces a DA neuron loss that mirrors the loss seen in end-stage PD.
One intervention that has been shown to reduce production of free radicals is exercise (Radak et al., 2007). Exercise appears to protect neurons against free radical damage through a number of processes including upregulation of trophic factors (Chen and Russo-Neustadt, 2009; Faherty et al., 2005; Neeper et al., 1995; Smith and Zigmond, 2003), dampening the production of free radicals (Bloomer et al., 2008; Radak et al., 2007) and modulation of excitatory amino acids in the brain (Guezennec et al., 1998; Mattson, 2008).
The aims of this paper were to elucidate the critical duration of exercise needed for neuroprotection against MPTP-induced neurotoxicity, and the possible mechanisms underlying any effect as shown by proteomic analysis. We show here that exercise potently protects neurons against MPTP-induced neurotoxicity in a manner that is dependent on the duration of the exercise period in terms of both the number of months exercised as well as the distance that was run daily. Proteomic analysis shows that exercise induces changes predominantly in metabolic and cytoskeletal related proteins within cells of the basal ganglia.
RESULTS
Exercise protects against MPTP-induced DA neurotoxicity
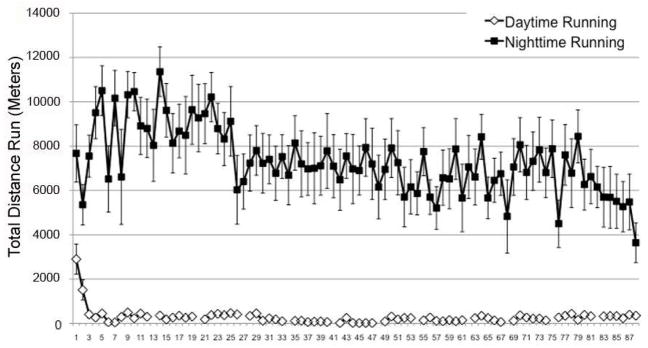
To determine the average amount of running that C57BL/6J mice performed over a 24-hour period, mice were allowed to run undisturbed for 3 months over which time their running activity was recorded (Figure 1). On average, C57BL/6J mice ran approximately 7.5km per 24 hour period (18,692.16 ±443.98 revolutions with a wheel having a diameter if 12.7 cm and 1 revolution equal to a distance of 0.4 meters). The majority of the activity occurred during the nighttime hours (7.2km), whereas only 3.9% of activity occurred during daytime hours (0.3 km).
Figure 1.
Pattern of average running activity for mice over the 90-day experimental period. Breaks in the Daytime Running data indicate days on which cages were changed; breaks in the nighttime running data are exclusions based on significant periods of missing values (>4h). Points represent the average running for mice (n=8) monitored either during the day or night period ± SEM.
To test the hypothesis that exercise is neuroprotective against MPTP-induced neurotoxicity, the number of DA neurons in the SNpc were quantified in animals kept in standard housing (SH) and compared to those allowed to exercise (EX) prior to administration of acute MPTP administration or saline.
First, to determine if the duration of exercise affected the degree of neuroprotection, animals were allowed access to unrestricted voluntary wheel running for either 1, 2 or 3 months (Figure 2A). After this exercise period, mice were transferred to individual caging for 1 day and then administered an acute regimen of MPTP (Boyd et al., 2007). Mice kept in SH for 3 months followed by administration of MPTP had 41.5% fewer DA neurons in the SNpc compared at day 7 post-injection to mice housed in SH and administered saline (p≤0.001). One month of unrestricted exercise conferred no protection against MPTP compared to mice in SH + MPTP. Mice that were allowed to run for 2 months followed by administration of MPTP lost significantly fewer neurons (16.6%) than mice that ran for one month prior to MPTP administration (p≤0.001), although this sparing of neurons was not complete. Mice that ran for the full 3 months lost the least number of DA neurons (9.2%), and were statistically no different than SH + Sal mice. Thus, our findings demonstrate that 3 months of voluntary and unrestricted exercise was necessary to fully protect against MPTP-induced SNpc DA neurodegeneration.
Figure 2.
(A) Exercise protects DA neurons in the SNpc against MPTP neurotoxicity. Mice kept in SH or that exercised for 1 month prior to MPTP administration had significantly fewer DA neurons in the SNpc as compared to SH+Sal. Mice that ran for at least 3 months were not significantly different in terms of neuron number as compared to SH+Sal. While mice that ran for 2 months lost a significant number of neurons compared to SH+Sal, they still lost significantly fewer DA neurons in the SNpc than those that had not been allowed to exercise. (B) Exercise-mediated protection against MPTP-induced cell death requires full running. Mice were allowed to run the average number of revolutions/24 hours (18,000), or 1/3 (6,000) or 2/3 (12,000) the amount of distance, for 3 months total. Mice that were kept in SH or whose running was restricted prior to MPTP administration lost significantly more neurons in the SNpc as compared to SH Sal (p<0.001); however, animals allowed to run unrestricted were not significantly different in terms of neuron number as compared to SH+Sal. Bars represent the average ± SEM; *p<0.05 as compared to SH+Sal; + = p<0.05 as compared to SH+MPTP.
In addition to examining if duration of exercise was correlated with the amount of neurprotection, we also examined if the total daily distance run was also a critical variable to confer protection against MPTP-induced SNpc DA death, In this set of experiments, mice were allowed to run for 3 months at either 7.5 km (18,000 daily wheel revolutions) or 1/3 (2.4 km or 6000 wheel revolutions) or 2/3 (4.8 km or 12,000 wheel revolutions) of this amount (Figure 2B). Animals had free access to running wheels, but when the predetermined amount of running was reached, the system automatically locked each wheel. The wheels were then unlocked at the start of the next 24 hour light:dark cycle. We found that mice that were allowed to run the full 7.5km were completely protected from MPTP toxicity. Mice administered MPTP whose running was restricted to 1/3 of the average distance were not significantly different than MPTP treated animals that had not been allowed to exercise. However, mice that ran for 2/3 the average unrestricted distance did show a moderate levels of neuroprotection, although this was not complete (1/3 Run + MPTP = 35.6% loss, p<0.001; 2/3 Run+MPTP = 30.7% loss, p<0.001). Taken together, these data suggest that both duration of exercise and distance run/day are critical for inducing neuroprotection against MPTP-induced DA neurotoxicity.
Effects of Exercise on Dopamine, DOPAC and HVA
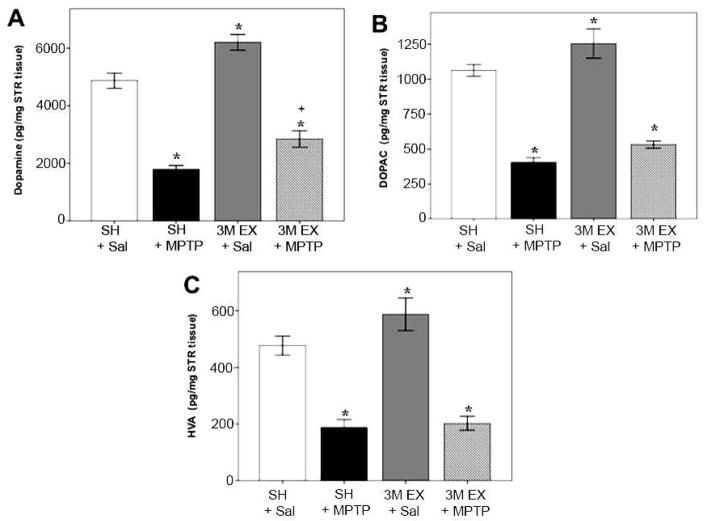
While we have shown that exercise can protect SNpc DA neurons from toxin-induced death, we also sought to determine if the remaining cells were functional, i.e. able to produce dopamine. We used HPLC/ED analysis to measure DA and its metabolites, DOPAC and HVA, in striatal tissue from mice that had been kept in either SH or exercise cages prior to administration of Sal or an acute regimen of MPTP (Figure 3). We found that 3 months of unrestricted exercise significantly increased DA (27%), DOPAC (17%), and HVA (50%) in the STR compared to mice kept in SH. Following administration of MPTP, we found that DA and its metabolites were significantly decreased in the STR regardless of housing condition. However, unlike the complete SNpc neuronal protection that we observed following 3 months of voluntary exercise, we only observed a partial, albeit significant, protection against DA loss (18% greater following exercise, p≤.01). It is interesting to note that exercise did not provide protection against MPTP toxicity for measures of DOPAC and HVA, suggesting that the protective effects are specific for DA production, but not for DA turnover.
Figure 3.
Exercise functionally protects DA neurons in the SNpc against MPTP neurotoxicity. HPLC/ED analysis of DA and the metabolites DOPAC and HVAC in the STR of SH or Ex mice administered either Sal or MPTP. Exercise significantly increased the measures of DA and its metabolites in the STR. Mice that exercised for 3 months administered MPTP had significantly lower measures of DA and its metabolites in the STR as compared to SH+Sal. However, Ex+MPTP mice lost significantly less DA in the STR compared to those kept in SH+MPTP. Bars represent the average ± SEM; *p<0.05 as compared to SH Sal; + = p<0.05 as compared to SH+MPTP.
Proteomic analysis
To investigate changes in proteins that may be mediating the observed neuroprotection, we used 2D gel electrophoresis followed by mass spectrometry to analyze differences in the proteome that occurred following 1 and 3 months of unrestricted voluntary exercise. Although the proteins that can be identified by this method are limited by size, abundance and isoelectric focusing range (Willis et al., 2005), this method can identify proteins that may contribute to the pathways involved in neuroprotection. For this analysis, tissues from SH animals were designated as the reference gel against which spots from Ex animal brains were compared. Results of the pairwise analysis of the volume of matched spots are reported in terms of fold-increase/decrease. Proteins whose expression was altered by at least 1.5-fold were compared for significance using a paired t-test with p<0.05 considered significant (Table 1). The majority of these protein changes occurred in the SN while a smaller number were observed in the STR.
Table 1.
Protein changes identified by 2D-Gel electrophoresis following exercise in substantia nigra and striatum
| Accession # | Timepoint | Brain Region | p-value | Change | |
|---|---|---|---|---|---|
| Channels and Receptors | |||||
| Voltage-dependent anion channel 2 (VDAC2) | 37514858 | 3M | SN | 0.003 | Dec 1.30 in Ex |
| Cytoskeletal Proteins and Assembly Regulators | |||||
| Cofilin-1 | 74354727 | 1M | SN | 0.012 | Inc 1.63 in Ex |
| Profilin 1 | 42476144 | 3M | SN | 0.032 | Inc 1.5 in Ex |
| Vesicle Trafficking and Neurotransmitter Release | |||||
| N-ethylmaleimide sensitive fusion protein | 31543349 | 1M | SN | 0.042 | Inc 1.55 in Con |
| Energy Metabolism | |||||
| NADH dehydrogenase (ubiquinone) | 2170402 | 1M | SN | 0.028 | Inc 1.60 in Con |
| ATP synthase, H+ transporting, mitochondrial | 50345982 | 1M | SN | 0.019 | Inc. 1.72 in Ex |
| Enol Coenzyme A hydratase, short chain 1 (Echs1) | 29789289 | 3M | SN | 0.023 | Dec 2.1 in Ex |
| Amino Acid Transport and Metabolism | |||||
| Apoa1 | 61402210 | 1M | SN | 0.004 | Inc. 1.48 in Ex |
| Isovaleryl coenzyme A dehydrogenase | 9789985 | 1M | SN | 0.001 | Inc 1.77 in Ex |
| Dihydropteridine reductase | 21312520 | 1M | SN | 0.021 | Inc 1.55 in Ex |
| Protein-L-isoaspartate (D-aspartate) O-methyltransferase-1 | 56961640 | 3M | STR | 0.035 | Inc 1.71 in Ex |
| Argininosuccinate synthetase | 6996911 | 3M | SN | 0.036 | Inc 1.78 in Ex |
| Cytoplasmic Signaling Molecules | |||||
| Hippocalcin | 747652 | 3M | SN | 0.012 | Inc 1.9 in Ex |
| Post-Transcription Modification Factors | |||||
| Chaperonin subunit 2 (beta) | 13938629 | 2M | STR | 0.049 | Dec 2.0 in Ex |
| Other | |||||
| Arl3-Gdp | 12084691 | 1M | SN | 0.002 | Inc 1.85 in Ex |
| Brain lipid-binding protein (BLBP) | 13540630 | 3M | SN | 0.014 | Inc 2.0 in Ex |
At 3 months, we found differences in both the gross expression level (7 proteins) and phophorylation state (7 proteins) of identified proteins. Many of the proteins that were significantly altered by 3 months of exercising were in pathways that govern energy regulation and cellular metabolism, including alterations in the phosphorylation state of NADH dehydrogenase, Enol Coenzyme A hydratase (Echs1), which is essential to the metabolism of fatty acids (Nagi et al., 1988), creatine kinase, which functions in the creation of energy reserves for rapid regeneration of ATP and is involved in maintaining structural integrity of mitochondria (Qin et al., 1998) and aldolase C, an enzyme in the glycolytic pathway (Villar-Palasi and Larner, 1970). We also noted changes in the phosphorylation state of proteins known to affect cellular metabolism including sepiapterin reductase, an enzyme required for tetrahydrobiopterin (BH4) biosynthesis (Levine et al., 1990) and glutamate oxaloacetate transaminase 1 (Got1) (Reshef et al., 2003; von Deimling et al., 1988). Changes in the phosphorylation state of the vesicle release and trafficking protein N-ethylmaleimide sensitive fusion attachment protein alpha (Andreeva et al., 2006) were also noted.
In addition to changes in phosphorylation state, we also noted 7 proteins whose abundance was altered. These include energy regulation and cellular metabolism proteins Echs1, and Arginiosuccinate synthetase, an enzyme involved in the production of nitric oxide (Zhang WY, et al., 2000). In addition, the protein repair enzyme protein-L-isoaspartate (D-aspartate) O-methyltranserase 1 (Clarke, 2003; Zhu et al., 2006) was also identified. We also noted changes in hippocalcin, a protein involved in intracellular signaling events triggered by Ca+2 changes (O’Callaghan et al., 2003) and voltage dependent anion channel 2 (VDAC2) that is located on the outer membrane of mitochondria and functions to regulate much of the activity of these organelles (see Lemaster, JJ 2007 for review). We also noted changes in cytoskeletal element Profilin, which regulates actin polymerization (Witke W et al. 1998) and brain lipid-binding protein (BLBP), which is involved in neuronal migration and differentiation (Feng et al., 1994)
Discussion
In a previous study (Faherty et al., 2005) it was shown that environmental enrichment and exercise for 3 months was able to confer neuroprotection against MPTP-induced parkinsonism. In these studies, C57BL/6 mice that were allowed to run unrestricted for 3 months prior to MPTP administration had statistically identical numbers of SNpc DA neurons compared to animals administered saline; however, when they were allowed to run for 3 months but had their daily distance restricted, or were only allowed to run for shorter periods of time, these animals lost significant numbers of SNpc DA neurons. This suggests that of all the components of the enriched environment, including increased social interactons, introduction of environemnal novelty and exercise, the exercise component is alone sufficient to protect SNpc DA neurons from oxidative insult resulting from mitochondrial inhibition (Smeyne and Jackson-Lewis, 2005). In another study that examined potential beneficial effects of exercise in an MPTP paradigm, that using chronic administration of MPTP and probenecid (10 injections given over 5 weeks), behavioral deficits in spontaneous movement, balance and gait were eliminated by treadmill running of 40 minutes per day beginning one week prior to injections and continuing for a total of 12 weeks (Pothakos et al., 2009). It is important to note, however, that while those mice were protected against behavioral deficits, this shorter amount of exercise did not protect against DA neuron loss in the SN.
Studies using other models of chemically-induced parkinsonsim have shown that exercise can alter both the neurodegenerative as well as behavioral consequences of these agents. For example, rats allowed to run for one week prior to and 2 weeks following 6-hydroxydopamine (6-OHDA) administration into the medial forebrain bundle displayed significantly fewer apomorphine-induced rotations, suggesting that SNpc DA neurons had been spared in the exercising rats (Mabandla et al., 2004). In another study, rats treated with a low dose of 6-OHDA that had use of the affected forelimb restricted showed increased neurochemical loss in the STR, indicating that exercise after the onset of neurodegeneration may prevent continued cell loss (Anstrom et al., 2007; Tillerson et al., 2003). What is interesting about these later studies is that far less time of exercise was necessary to protect these animals than was necessary in this study (between 2–3 months of sustained voluntary exercise). It may be that exercises such as activities such as vibrissae-induced limb placing are specifically directed at the motor system that degenerates in Parkinson’s disease (Anstrom et al., 2007; Poulton and Muir, 2005), while running wheels take longer to confer protection as this mode of exercise is more generalized.
Thus, using our generalized exercise model, we find that the duration is crucial to protect DA neurons against death caused by acute MPTP-intoxication. In terms of distance, we find that smaller amounts of exercise can provide a partial protection against MPTP-induced free-radical insult (Obata, 2002; Smeyne and Jackson-Lewis, 2005). Animals whose running was restricted to 2/3 of total distance lost significant numbers of neurons in the SNpc compared to saline treated mice. However, these animals still had significantly greater numbers of DA neurons compared to mice kept in SH +MPTP, suggesting that this level of daily running provides some neuroprotection. In terms of time, we find that a partial, but significant, protection occurred starting about 2 months into the exercise regimen. Examination of the distribution of protection in these animals showed that about half of the 2M Ex + MPTP mice only lost ~10% of the neurons in the SNpc, similar to the statistically non-significant loss seen in animals that ran for the full 3 months. However, the other half of the mice had a less complete neuron sparing, losing amounts similar to that seen in SH+MPTP mice. This suggests that any changes that have occurred to confer protection may stabilize in and around the 2 month time point
Although we observed complete protection against MPTP-induced neuronal death of SNpc DA neurons following 3 months of unrestricted exercise and moderate protection with lower amounts, we did not see similar levels of protection when examining DA levels in the striatum. This lack of terminal DA sparing is consistent with the work of Petzinger et al. (2007) (Petzinger et al., 2007) who have shown a lack of DA sparing in animals exposed to exercise 5 days prior to and 23 days after MPTP administration. This dichotomy in protection suggests that there may separate mechanisms that protect against cell death compared to those that keep spared DA neurons continuing to properly function. We have previously shown that exercise does not alter the entry of MPP+ into the brain (Faherty et al., 2005), and thus it is possible that the interference with Complex I lowers cellular energetics enough to prevent the upstream production of DA at the point where tyrosine hydroxylase converts L-tyrosine to dihydroxyphenylalanine (DOPA) (Cooper et al., 2002). It is also possible that MPP+ lowers the ability for these neurons to transport the DA contained in vesicles within the cell body to its striatal terminals (Croft et al., 2005; Van der Kloot, 2003), as we continue to see robust TH-immunostaining in SNpc DA soma (Faherty et al., 2005). It is also possible that the uptake of MPP+ through the DAT results in formation of hydrogen peroxide and nitrosylated proteins that damage the DA terminals without causing cell death.
While we report neuronal sparing following MPTP administration using our free access exercise regimen, others have reported that exercise only provides behavioral sparing without protection of SNpc neurons (Fisher et al., 2004; O’Dell et al., 2007; Pothakos et al., 2009; Steiner et al., 2006). Several explanations can be proffered to account for this dichotomous type of protection including increased functional capacity of remaining DA neurons by compensatory mechanisms within spared neurons, insufficient time for restoration of the DA phenotype (in these cases expression of TH) in injured neurons (Fornai et al., 2000; Nishi et al., 1989; Tillerson et al., 2003), differences in strain (Hamre et al., 1999; Muthane et al., 1994; Sedelis et al., 2000), species (Terzioglu and Galter, 2008), and age of the experimental animals used in these studies (Tatton et al., 1992), differences in the experimental toxin used to generate the lesion (Smith and Zigmond, 2003), and/or differences in the exercise regimens (Cohen et al., 2003; Mabandla et al., 2004; Petzinger et al., 2007).
In this study we have identified exercise as an initiator for neuroprotection to systemic administration of MPTP, it is critical to understand the mechanism by which this occurs. Previous studies of neuroprotection have shown that exercise can induce growth factor expression (Cotman and Berchtold, 2002; Faherty et al., 2005; Siamilis et al., 2009; Smith and Zigmond, 2003) as well as inhibition of anti-apoptotic genes (Sandri et al., 1997; Um et al., 2008). It has also been demonstrated that exercise alters expression of the dopamine transporter, which allows entry of MPP+ into the cell (Del Zompo et al., 1993; Gainetdinov et al., 1997), as well as the vesicular monoamine transporter, VMAT2, which sequesters MPP+ (Gainetdinov et al., 1998) such that the VMAT2:DAT is increased (Faherty et al., 2005). This increase in sequestration versus entry suggests that intracellular handling of toxins may be an important component in our observed neuroprotection (Miller et al., 1999).
In terms of growth factors, both we, and others, have shown the mRNA and protein for these genes are increased following exercise (Faherty et al., 2005; O’Callaghan et al., 2009; Rasmussen et al., 2009). In the hippocampus it has also been shown that these changes only occur only if the exercise is sustained and vigorous (Cechetti et al., 2008). As the observed neuroprotective changes in BDNF occurred in juvenile mice (Faherty et al., 2005) and because BDNF is required for the beneficial effects of exercise in other models (Chytrova et al., 2008; Griesbach et al., 2009; Ploughman et al., 2009; Sandrow-Feinberg et al., 2009) we expected that changes in BDNF (or other neurotrophins) would have been identified in our proteomic analysis. However, we were not able to identify any growth factor “spots” in our analyses, likely due to bias in proteomics for only identifying high abundance proteins.
Our proteomics analysis revealed a number of changes in protein levels, including those shown to play a role in pathways thought to confer improved resiliency to cells faced with toxic insults including energy metabolism, glycolysis, amino acid transport and neurotransmitter trafficking, each of which could contribute to the neuroprotective pathway induced by exercise. Although we observed these exercise-induced changes in SN and striatum, many of these proteins changes also occur in other regions of the brain (Ding et al., 2006) suggesting that these responses are global but may function differently depending on anatomical context. In terms of energy metabolism, we observe changes NAPH dehydrogenase, a key component of Complex I in the mitochondrial electron transport chain. Alterations in expression of this protein can be correlated to enhanced activity of complex I as well as stimulation of mitochondrial respiration supported by NAD-linked substrates (Scacco et al., 2000); both of which can lead to increased protection against stimuli that induce cell death (Garcia et al., 2005). We also find a significant increase in Arginiosuccinate synthetase, the rate-limiting enzyme in the metabolic pathway leading from L-citrulline to L-arginine, which functions as a substrate in the production of nitric oxide synthases (Kawahara et al., 2001). It has been suggested that at least one isoform of NOS, nNOS, can be neuroprotective (Scorziello et al., 2007), although the mechanism for this protection has not been elucidated. One possibility is that exercise induces a transient ischemia (Win et al., 2005); an event shown to induce this enzyme (Bizzoco et al., 2007).
Proteins involved in fatty acid metabolism have also been shown to provide protection against oxidative insult in the brain. We found changes in expression of two members of this class: Echs1 and Brain Lipid Binding Protein (BLBP) also known as fatty acid binding protein 7 (FABP7). Echs1 localizes to the mitochondrial matrix where it functions in the second step of the mitochondrial fatty acid beta-oxidation pathway (Janssen et al., 1997) to catalyze the hydration of 2-trans-enoyl-coenzyme A (CoA) intermediates to L-3-hydroxyacyl-CoAs (Mursula, 2002). BLBP, a brain brain-specific member of the lipid-binding protein family (Feng et al., 1994), is thought to involve regulation of fatty acid uptake and intracellular transport as well as modify expression of transcription factors including the nuclear receptor PPAR (peroxisome proliferator-activated receptor) (Chmurzynska, 2006), an effect which offers neuroprotection against free radical damage (Wang et al., 2009; Yu et al., 2008; Zhao et al., 2009), which is thought to be the mechanism for MPTP-induced toxicity (Anantharam et al., 2007; Yokoyama et al., 2008).
It is important to note our observed changes in energy metabolism, as defects in mitochondrial pathways have been implicated in the pathogenesis of PD (see Bueler, 2009, for review). NADH-quinone oxiodoreductase, an enzyme in the mitochondrial respiratory complex I, is reduced in patients with PD (Schapira et al., 1990). Mutations in a gene coding for mitochondrial DNA (mtDNA) were found in one family with PD (Swerdlow et al., 1998), and mtDNA deletions are more common in the SN of PD patients (Bender et al., 2006). We show here, along with other published research (Ding et al., 2006) that exercise induces alterations in these energy pathways, and these alterations may underlie observed exercise-induced neuroprotection in the MPTP model of parkinsonism. Thus, exercise-induced increases in energy regulation of neurons may strengthen the ability of neurons to withstand a broad range of toxic insults.
In humans, aerobic fitness has been found to confer substantial health benefits beyond that of just cardiovascular health to encompass brain health as well. Aerobic training prevents the normal age-related decline in the gray matter of the frontal, parietal and temporal cortices (Colcombe et al., 2003) and improves the cognitive functions of these brain regions during aging (Colcombe et al., 2004; Lautenschlager et al., 2008). Even short periods of exercise have been shown to improve performance on certain cognitive tasks in aged humans (Chodzko-Zajko and Moore, 1994; Emery et al., 1998; Hawkins et al., 1992). Importantly, it has been shown that vigorous physical activity early in adulthood significantly decreases the risk of PD in men by 50% (Chen et al., 2005). Therefore, when taken into consideration with the larger body of research, our work suggests that daily exercise that is sustained over a long period of time is potently neuroprotective, and will likely prove effective in not only in protecting against age related declines in cognition, but also for protecting against PD and other neurodegenerative disorders in which exposure to oxidative stress destroys neurons.
MATERIALS AND METHODS
Animals and Experimental Conditions
All mice used in this study were C57BL/6J mice (Jackson Laboratory, Bar Harbor, Maine) that were between 4 and 6 weeks of age at the onset of the experiment. Due to housing constraints, females were used in these experiments; however, we have previously reported that there was no difference in MPTP-induced neurodegeneration between male and female C57BL/6J mice (Hamre et al., 1999). To minimize the influence of age, all mice were between 4 and 6 months of age at the time of collection for experimental analysis. All mice were maintained in a temperature-controlled environment with free access to food and water and kept on a 12-h light/dark cycle from 7AM to 7PM each day. All animal procedures were in compliance with St. Jude Children’s Hospital Institutional guidelines and were approved by the SJCRH Institutional Animal Care and Use Committee.
For the Exercise (Ex) condition, exercise “Wheel Cages” from Lafayette Instruments (Lafayette, IN, Model 80820) were used to monitor individual mouse activity. At all times each mouse in Ex housing had access to an exercise wheel. For standard housing (SH), mice were kept in standard mouse cages for the full 3 month duration of the experiment. For all experiments, mice in the MPTP condition were transferred to SH on the day they were administered i.p. injections of MPTP (Sigma, St. Louis, MO) at 4 × 20 mg/kg at 2-h intervals in a 5-ml/kg volume or vehicle (sterile saline). This protocol has been well characterized in our lab and is routinely used in our studies (Boyd et al., 2007; Jackson-Lewis et al., 1995).
The numbers of experimental animals in this study were: Standard housing animals treated with saline (SH+Sal; n=8); Standard housing mice administered MPTP (SH+MPTP; n=10); mice that ran for 1 month prior to MPTP administration (1M Ex+MPTP; n=7); 2 months exercise prior to MPTP administration (2M Ex+MPTP; n=6); 3 months running prior to MPTP administration (3M Ex+MPTP; n=5).
To examine the effects of restricted running on neuroprotection, the running wheel in each cage was immobilized for either 1/3 (6000 revolutions or 2.4 km) or 2/3 (12,000 revolutions or 4.8 km) of average activity levels (18,000 revolutions per 24 hour cycle) as determined in pilot studies. Numbers for each experimental group for these studies are as follows: SH+Sal and SH+MPTP same as above; 1/3 of activity levels +MPTP (1/3 Run+MPTP; n=5); 2/3 of activity levels +MPTP (2/3 Run+MPTP; n=4); Full activity levels +MPTP (Full run+MPTP; n=6).
To ensure continuity between experiments by minimizing possible seasonal changes in running patterns, animals in the neuroprotection and restricted running experiments were run simultaneously. Thus, for each 3-month experiment the monitored cages housed animals that were either running for a pre-determined duration, or whose running was restricted daily. In addition, control animals (SH+Sal) were included for each run. The experiments were repeated 3 times over the course of a year, and the data was compiled.
For HPLC, (n=4 for all groups), animals were kept either in SH or allowed to exercise for 3 months. One half each of SH and 3 months exercise animals were injected with sterile saline, all other animals were administered MPTP as described above.
For proteomic analyses (n=3 for all groups) animals were kept in either SH, or allowed to exercise for 1, 2 or 3 months.
Immunohistochemistry
Seven days following MPTP administration animals were anesthetized with tribromoethanol (250 mg/kg (i.p.)) and transcardially perfused with 0.1 M phosphate buffered saline (PBS; pH7.4) followed by 4% paraformaldehyde. This time point corresponds to maximal SNpc cell loss due to MPTP toxicity (Boyd et al., 2007; Jackson-Lewis et al., 1995). Brains were removed, post-fixed overnight, and then paraffin embedded. Brains were subsequently sectioned at 5 μm, and then mounted onto SuperFrost Plus Slides (Fisher Scientific; Atlanta, GA). Standard immunhistochemical techniques were used to identify cells positve for tyrosine hydroxylase (TH) in the SNpc. Slides were deparaffinized in xylenes and rehydrated in graded alcohols followed by soaking in Tris-buffered saline (TBS; pH7.4). Following blocking of nonspecific staining by incubation in blocking buffer (0.5% BSA, 0.3% Triton, 5% normal goat serum, in TBS) sections were then stained with a polyclonal antibody specific for TH (1:250 in blocking buffer; Pel Freez, Rogers, AR) by incubation overnight at 4°C. Following 3 washes in TBS, sections were visualized using a peroxidase-anti-peroxidase system (Vector Laboratories, CA) followed by deposition of diaminobenzidine (DAB; DAB Immunoperoxidase Substrate kit; Vector Laboratories; Burlingame, CA). Slides were then counterstained with Neutral Red, dehydrated through a graded series of alcohol, mounted in Permount and coverslipped.
DA Cell Quantification and Analysis
Dopaminergic neurons in the SNpc were quantified using methods-based sterological (Baquet et al., 2009). Briefly, to ensure that all neurons in the SNpc were counted, all sections were counterstained with Neutral Red. TH positive DA neurons in the SNpc were quantified and the numbers of cells were adjusted for volume using measurements of the size of cell nuclei.
Neurochemical Analysis of Dopamine
Dopamine and its metabolites were quantified using high-pressure liquid chromatography with electrochemical detection (HPLC-ED). For HPLC analysis, mice were anesthetized with tribromoethanol (250 mg/kg (i.p.) seven days following MPTP administration, and the brain tissue was dissected, weighed and homogenized in 0.3N perchloric acid for the analysis by HPLC/ED method as previously described.
The signal from the electrochemical detector was recorded with an electronic data station (model SS420x, Scientific Software, Inc), were determined by comparing the areas and heights of sample peaks with those from serial dilutions of the standard curve. The statistical significance was assessed by one-way analysis of variance. Concentration of dopamine and its metabolites were expressed in pg/mg tissue wt (mean ± SEM; n = 5–7) over the entire sampling period for each batch of experiment. A probability (p) value of 0.05 or less was considered statistically significant.
2-D Gel Electrophoresis and Analysis
For proteomic analyses (n=3 for each of the groups) animals were kept in either SH, or allowed to exercise for 1, 2 or 3 months, after which time mice were anesthetized with tribromoethanol (250 mg/kg (i.p.), and the striatum (STR) and substantia nigra (SN) were removed by dissection from excised brains and used for analyses. Tissues were lysed in a buffer containing 8 M urea, 2 M thiourea, 2% CHAPS, 0.3% (w/v) Bio-Lyte ampholytes from BioRad Laboratories, Hercules, CA., (usually pH range 3–10), 50 mM dithiothreitol, a trace of bromophenol blue, and a protease inhibitor cocktail (one mini tablet of Pefabloc SC, Roche Applied Science, Indianapolis, IN). Protein concentrations were quantified (PlusOne 2-D quantitation kit; Amersham Pharmacia Biotech) and equalized to 200 ug. Samples were centrifuged at 100,000 g for 1 hour at RT to remove particulates that may interfere with protein separation.
Isoelectric focusing was performed with immobilized pH gradient (IPG) strips from Bio-Rad Laboratories and processed for 2D gel–electrophoresis as previously described (Galea et al., 2006).
Images of 2-D gels were acquired with a Fuji FLA-5100 imager using 16-bit resolution, and files were saved in .img format. Image analysis was performed using the Progenesis Workstation package from Nonlinear Dynamics Ltd., Newcastle-upon-Tyne, U.K. This package contains routines for automated spot detection, electropherogram matching, and comparative quantitation. Results of pair-wise image comparisons in which one gel is designated as the reference and the other as the test sample are reported in terms of fold-increase/decrease in the normalized volume of matched spots. For all analyses, tissues from SH animals were the reference against which the corresponding time-matched exercise tissues were compared. For example, STR from 3M Ex mice were compared against the reference gel generated from 3M SH STR, and so forth. For all comparisons p<0.05 and a fold change of ± 1.5 was considered significant.
In addition, during the analysis several spots were identified that either shifted positions, or had a twin spot which was present in one condition but not in the other. Because in most cases these twin spots were identified as the same proteins in both the spots, these spots were assumed to be post-translationally modified proteins. The most common modification showing this kind of shifting pattern is phosphorylation. Phosphorylated proteins typically have a different isoelectric point than the native proteins and thus they shift horizontally on the gel, while there is usually very little change in vertical mobility of these spots. In contrast, glycosylated proteins shift both horizontally and vertically on a gel. Because we were interested in identifying proteins that were modified due to exercise, spots that had shifted in position, or that had twin spots present in only one condition, were also included for identification.
Proteins of interest were excised either manually, or with a ProPic Spot Picker from Genomic Solutions Inc, Ann Arbor, MI. In-gel digestion was then performed with sequencing-grade, modified trypsin supplied frozen by Promega Corp., Madison WI. Peptides released from gel plugs were then extracted, purified using C18 ZipTips from (Millipore Corp., Bedford MA), and spotted onto MALDI targets for identification using mass spectrometry.
Mass spectrometric analysis was performed using a Model 4700 Proteomics Analyzer from Applied Biosystems (Foster City, CA). This instrument employs matrix-assisted laser desorption/ionization (MALDI), in conjunction with tandem time-of-flight (TOF) mass analyzers. The digest was introduced into the instrument in a crystalline matrix of α-cyano-4-hydroxycinnamic acid also containing 2 mM Ammonium citrate to suppress ionization of matrix clusters. Database searches were performed with Applied Biosystem’s GPS explorer software, which uses the Mascot search engine. Protein assignments are made on the basis of both MS and MS/MS spectra. At all stages of post-electrophoretic sample processing, rigorous procedures were employed to minimize sample contamination.
Statistical Analyses
Statistical analyses in each of the experiments were done using ANOVA followed by LSD post-hoc comparisons (SPSS v16.0 software), and statistical significance was set to p<0.05.
Acknowledgments
We thank Matt Hatler and Justin Griner for their help with preparing tissues for DA neuron estimation, and Kiran Kodali for his assistance with 2D gel electrophoresis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Anantharam V, Kaul S, Song C, Kanthasamy A, Kanthasamy AG. Pharmacological inhibition of neuronal NADPH oxidase protects against 1-methyl-4-phenylpyridinium (MPP+)-induced oxidative stress and apoptosis in mesencephalic dopaminergic neuronal cells. Neurotoxicology. 2007;28:988–97. doi: 10.1016/j.neuro.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreeva AV, Kutuzov MA, Voyno-Yasenetskaya TA. A ubiquitous membrane fusion protein alpha SNAP: a potential therapeutic target for cancer, diabetes and neurological disorders? Expert Opin Ther Targets. 2006;10:723–33. doi: 10.1517/14728222.10.5.723. [DOI] [PubMed] [Google Scholar]
- Anstrom KK, Schallert T, Woodlee MT, Shattuck A, Roberts DC. Repetitive vibrissae-elicited forelimb placing before and immediately after unilateral 6-hydroxydopamine improves outcome in a model of Parkinson’s disease. Behav Brain Res. 2007;179:183–91. doi: 10.1016/j.bbr.2007.01.028. [DOI] [PubMed] [Google Scholar]
- Baquet ZC, Williams D, Brody J, Smeyne RJ. A comparison of model-based (2D) and design-based (3D) stereological methods for estimating cell number in the substantia nigra pars compacta (SNpc) of the C57BL/6J Mouse. Neuroscience. 2009;161:1082–1090. doi: 10.1016/j.neuroscience.2009.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender A, Krishnan KJ, Morris CM, Taylor GA, Reeve AK, Perry RH, Jaros E, Hersheson JS, Betts J, Klopstock T, Taylor RW, Turnbull DM. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet. 2006;38:515–7. doi: 10.1038/ng1769. [DOI] [PubMed] [Google Scholar]
- Bizzoco E, Faussone-Pellegrini MS, Vannucchi MG. Activated microglia cells express argininosuccinate synthetase and argininosuccinate lyase in the rat brain after transient ischemia. Exp Neurol. 2007;208:100–9. doi: 10.1016/j.expneurol.2007.07.018. [DOI] [PubMed] [Google Scholar]
- Bloomer RJ, Schilling BK, Karlage RE, Ledoux MS, Pfeiffer RF, Callegari J. Effect of resistance training on blood oxidative stress in Parkinson disease. Med Sci Sports Exerc. 2008;40:1385–9. doi: 10.1249/MSS.0b013e31816f1550. [DOI] [PubMed] [Google Scholar]
- Boyd JD, Jang H, Shepherd KR, Faherty C, Slack S, Jiao Y, Smeyne RJ. Response to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) differs in mouse strains and reveals a divergence in JNK signaling and COX-2 induction prior to loss of neurons in the substantia nigra pars compacta. Brain Res. 2007;1175:107–16. doi: 10.1016/j.brainres.2007.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks WJ, Jarvis MF, Wagner GC. Astrocytes as a primary locus for the conversion MPTP into MPP+ J Neural Transm. 1989;76:1–12. doi: 10.1007/BF01244987. [DOI] [PubMed] [Google Scholar]
- Cechetti F, Fochesatto C, Scopel D, Nardin P, Goncalves CA, Netto CA, Siqueira IR. Effect of a neuroprotective exercise protocol on oxidative state and BDNF levels in the rat hippocampus. Brain Res. 2008;1188:182–8. doi: 10.1016/j.brainres.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Chen H, Zhang SM, Schwarzschild MA, Hernan MA, Ascherio A. Physical activity and the risk of Parkinson disease. Neurology. 2005;64:664–9. doi: 10.1212/01.WNL.0000151960.28687.93. [DOI] [PubMed] [Google Scholar]
- Chen MJ, Russo-Neustadt AA. Running exercise-induced up-regulation of hippocampal brain-derived neurotrophic factor is CREB-dependent. Hippocampus. 2009 doi: 10.1002/hipo.20579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmurzynska A. The multigene family of fatty acid-binding proteins (FABPs): function, structure and polymorphism. J Appl Genet. 2006;47:39–48. doi: 10.1007/BF03194597. [DOI] [PubMed] [Google Scholar]
- Chodzko-Zajko WJ, Moore KA. Physical fitness and cognitive functioning in aging. Exerc Sport Sci Rev. 1994;22:195–220. [PubMed] [Google Scholar]
- Chytrova G, Ying Z, Gomez-Pinilla F. Exercise normalizes levels of MAG and Nogo-A growth inhibitors after brain trauma. Eur J Neurosci. 2008;27:1–11. doi: 10.1111/j.1460-9568.2007.05982.x. [DOI] [PubMed] [Google Scholar]
- Clarke S. Aging as war between chemical and biochemical processes: protein methylation and the recognition of age-damaged proteins for repair. Ageing Res Rev. 2003;2:263–85. doi: 10.1016/s1568-1637(03)00011-4. [DOI] [PubMed] [Google Scholar]
- Cohen AD, Tillerson JL, Smith AD, Schallert T, Zigmond MJ. Neuroprotective effects of prior limb use in 6-hydroxydopamine-treated rats: possible role of GDNF. J Neurochem. 2003;85:299–305. doi: 10.1046/j.1471-4159.2003.01657.x. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Raz N, Webb AG, Cohen NJ, McAuley E, Kramer AF. Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol A Biol Sci Med Sci. 2003;58:176–80. doi: 10.1093/gerona/58.2.m176. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, Webb A, Jerome GJ, Marquez DX, Elavsky S. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci U S A. 2004;101:3316–21. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JR, Bloom FE, Roth RH. The Biochemical Basis of Neuropharmacology. Oxford University Press; New York: 2002. [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Croft BG, Fortin GD, Corera AT, Edwards RH, Beaudet A, Trudeau LE, Fon EA. Normal biogenesis and cycling of empty synaptic vesicles in dopamine neurons of vesicular monoamine transporter 2 knockout mice. Mol Biol Cell. 2005;16:306–15. doi: 10.1091/mbc.E04-07-0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui M, Aras R, Christian WV, Rappold PM, Hatwar M, Panza J, Jackson-Lewis V, Javitch JA, Ballatori N, Przedborski S, Tieu K. The organic cation transporter-3 is a pivotal modulator of neurodegeneration in the nigrostriatal dopaminergic pathway. Proc Natl Acad Sci U S A. 2009;106:8043–8. doi: 10.1073/pnas.0900358106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Zompo M, Piccardi MP, Ruiu S, Quartu M, Gessa GL, Vaccari A. Selective MPP+ uptake into synaptic dopamine vesicles: possible involvement in MPTP neurotoxicity. British Journal Of Pharmacology. 1993;109:411–4. doi: 10.1111/j.1476-5381.1993.tb13584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Q, Vaynman S, Souda P, Whitelegge JP, Gomez-Pinilla F. Exercise affects energy metabolism and neural plasticity-related proteins in the hippocampus as revealed by proteomic analysis. Eur J Neurosci. 2006;24:1265–76. doi: 10.1111/j.1460-9568.2006.05026.x. [DOI] [PubMed] [Google Scholar]
- Emery CF, Schein RL, Hauck ER, MacIntyre NR. Psychological and cognitive outcomes of a randomized trial of exercise among patients with chronic obstructive pulmonary disease. Health Psychol. 1998;17:232–40. doi: 10.1037//0278-6133.17.3.232. [DOI] [PubMed] [Google Scholar]
- Faherty CJ, Raviie Shepherd K, Herasimtschuk A, Smeyne RJ. Environmental enrichment in adulthood eliminates neuronal death in experimental Parkinsonism. Brain Res Mol Brain Res. 2005;134:170–9. doi: 10.1016/j.molbrainres.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Fahn S. Is levodopa toxic? Neurology. 1996;47:S184–95. doi: 10.1212/wnl.47.6_suppl_3.184s. [DOI] [PubMed] [Google Scholar]
- Feng L, Hatten ME, Heintz N. Brain lipid-binding protein (BLBP): a novel signaling system in the developing mammalian CNS. Neuron. 1994;12:895–908. doi: 10.1016/0896-6273(94)90341-7. [DOI] [PubMed] [Google Scholar]
- Fisher BE, Petzinger GM, Nixon K, Hogg E, Bremmer S, Meshul CK, Jakowec MW. Exercise-induced behavioral recovery and neuroplasticity in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse basal ganglia. J Neurosci Res. 2004;77:378–90. doi: 10.1002/jnr.20162. [DOI] [PubMed] [Google Scholar]
- Fornai F, Battaglia G, Gesi M, Giorgi FS, Orzi F, Nicoletti F, Ruggieri S. Time-course and dose-response study on the effects of chronic L-DOPA administration on striatal dopamine levels and dopamine transporter following MPTP toxicity. Brain Res. 2000;887:110–7. doi: 10.1016/s0006-8993(00)02999-1. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Fumagalli F, Jones SR, Caron MG. Dopamine transporter is required for in vivo MPTP neurotoxicity: evidence from mice lacking the transporter. J Neurochem. 1997;69:1322–1325. doi: 10.1046/j.1471-4159.1997.69031322.x. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Fumagalli F, Wang YM, Jones SR, Levey AI, Miller GW, Caron MG. Increased MPTP neurotoxicity in vesicular monoamine transporter 2 heterozygote knockout mice. J Neurochem. 1998;70:1973–8. doi: 10.1046/j.1471-4159.1998.70051973.x. [DOI] [PubMed] [Google Scholar]
- Galea CA, Pagala VR, Obenauer JC, Park CG, Slaughter CA, Kriwacki RW. Proteomic studies of the intrinsically unstructured mammalian proteome. J Proteome Res. 2006;5:2839–48. doi: 10.1021/pr060328c. [DOI] [PubMed] [Google Scholar]
- Gao HM, Liu B, Zhang W, Hong JS. Critical role of microglial NADPH oxidase-derived free radicals in the in vitro MPTP model of Parkinson’s disease. Faseb J. 2003;17:1954–6. doi: 10.1096/fj.03-0109fje. [DOI] [PubMed] [Google Scholar]
- Garcia O, Almeida A, Massieu L, Bolanos JP. Increased mitochondrial respiration maintains the mitochondrial membrane potential and promotes survival of cerebellar neurons in an endogenous model of glutamate receptor activation. J Neurochem. 2005;92:183–90. doi: 10.1111/j.1471-4159.2004.02851.x. [DOI] [PubMed] [Google Scholar]
- German DC, Manaye K, Smith WK, Woodward DJ, Saper CB. Midbrain dopaminergic cell loss in Parkinson’s disease: computer visualization. Annals of Neurology. 1989;26:507–14. doi: 10.1002/ana.410260403. [DOI] [PubMed] [Google Scholar]
- Griesbach GS, Hovda DA, Gomez-Pinilla F. Exercise-induced improvement in cognitive performance after traumatic brain injury in rats is dependent on BDNF activation. Brain Res. 2009;1288:105–15. doi: 10.1016/j.brainres.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guezennec CY, Abdelmalki A, Serrurier B, Merino D, Bigard X, Berthelot M, Pierard C, Peres M. Effects of prolonged exercise on brain ammonia and amino acids. Int J Sports Med. 1998;19:323–7. doi: 10.1055/s-2007-971925. [DOI] [PubMed] [Google Scholar]
- Hamre K, Tharp R, Poon K, Xiong X, Smeyne RJ. Differential strain susceptibility following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) administration acts in an autosomal dominant fashion: quantitative analysis in seven strains of Mus musculus. Brain Res. 1999;828:91–103. doi: 10.1016/s0006-8993(99)01273-1. [DOI] [PubMed] [Google Scholar]
- Hawkins HL, Kramer AF, Capaldi D. Aging, exercise, and attention. Psychol Aging. 1992;7:643–53. doi: 10.1037//0882-7974.7.4.643. [DOI] [PubMed] [Google Scholar]
- Hornykiewicz O. Mechanisms of neuronal loss in Parkinson’s disease: a neuroanatomical-biochemical perspective. Clinical Neurology & Neurosurgery. 1992;94(Suppl):S9–11. doi: 10.1016/0303-8467(92)90008-q. [DOI] [PubMed] [Google Scholar]
- Jackson-Lewis V, Jakowec M, Burke RE, Przedborski S. Time course and morphology of dopaminergic neuronal death caused by the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neurodegeneration. 1995;4:257–69. doi: 10.1016/1055-8330(95)90015-2. [DOI] [PubMed] [Google Scholar]
- Jankovic J, Stacy M. Medical management of levodopa-associated motor complications in patients with Parkinson’s disease. CNS Drugs. 2007;21:677–92. doi: 10.2165/00023210-200721080-00005. [DOI] [PubMed] [Google Scholar]
- Janssen U, Davis EM, Le Beau MM, Stoffel W. Human mitochondrial enoyl-CoA hydratase gene (ECHS1): structural organization and assignment to chromosome 10q26.2–q26.3. Genomics. 1997;40:470–5. doi: 10.1006/geno.1996.4597. [DOI] [PubMed] [Google Scholar]
- Jenner P. Oxidative stress as a cause of Parkinson’s disease. Acta Neurol Scand Suppl. 1991;136:6–15. doi: 10.1111/j.1600-0404.1991.tb05013.x. [DOI] [PubMed] [Google Scholar]
- Kawahara K, Gotoh T, Oyadomari S, Kajizono M, Kuniyasu A, Ohsawa K, Imai Y, Kohsaka S, Nakayama H, Mori M. Co-induction of argininosuccinate synthetase, cationic amino acid transporter-2, and nitric oxide synthase in activated murine microglial cells. Brain Res Mol Brain Res. 2001;90:165–73. doi: 10.1016/s0169-328x(01)00100-0. [DOI] [PubMed] [Google Scholar]
- Kopin IJ. Features of the dopaminergic neurotoxin MPTP. Ann. N.Y. Acad. Sci. 1992;648:96–104. doi: 10.1111/j.1749-6632.1992.tb24527.x. [DOI] [PubMed] [Google Scholar]
- Korell M, Tanner CM. Epidemiology of Parkinson’s Disease: An Overview. In: Ebadi M, Pfeiffer RF, editors. Parkinson’s Disease. CRC Press; Boca Raton: 2004. pp. 39–50. [Google Scholar]
- Langston JW, Ballard P, Tetrud JW, Irwin I. Chromic parkinsonism in humans due to a product of merperidine-analog synthesis. Science. 1983;219:979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- Lautenschlager NT, Cox KL, Flicker L, Foster JK, van Bockxmeer FM, Xiao J, Greenop KR, Almeida OP. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. Jama. 2008;300:1027–37. doi: 10.1001/jama.300.9.1027. [DOI] [PubMed] [Google Scholar]
- Levine RA, Kapatos G, Kaufman S, Milstien S. Immunological evidence for the requirement of sepiapterin reductase for tetrahydrobiopterin biosynthesis in brain. J Neurochem. 1990;54:1218–24. doi: 10.1111/j.1471-4159.1990.tb01951.x. [DOI] [PubMed] [Google Scholar]
- Mabandla M, Kellaway L, St Clair Gibson A, Russell VA. Voluntary running provides neuroprotection in rats after 6-hydroxydopamine injection into the medial forebrain bundle. Metab Brain Dis. 2004;19:43–50. doi: 10.1023/b:mebr.0000027416.13070.c3. [DOI] [PubMed] [Google Scholar]
- Mandel S, Grunblatt E, Riederer P, Gerlach M, Levites Y, Youdim MB. Neuroprotective strategies in Parkinson’s disease: an update on progress. CNS Drugs. 2003;17:729–62. doi: 10.2165/00023210-200317100-00004. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Glutamate and neurotrophic factors in neuronal plasticity and disease. Ann N Y Acad Sci. 2008;1144:97–112. doi: 10.1196/annals.1418.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. Glial reactions in Parkinson’s disease. Mov Disord. 2008;23:474–83. doi: 10.1002/mds.21751. [DOI] [PubMed] [Google Scholar]
- Miller GW, Gainetdinov RR, Levey AI, Caron MG. Dopamine transporters and neuronal injury. Trends Pharmacol Sci. 1999;20:424–9. doi: 10.1016/s0165-6147(99)01379-6. [DOI] [PubMed] [Google Scholar]
- Muenter MD, Tyce GM. L-dopa therapy of Parkinson’s disease: plasma L-dopa concentration, therapeutic response, and side effects. Mayo Clin Proc. 1971;46:231–9. [PubMed] [Google Scholar]
- Mursula A. Department of Biochemistry, Vol. PhD. University of Oulu; Oulu: 2002. Δ3-Δ2-Enoyl-CoA isomerase from the yeast Saccharomyces cerevisiae: Molecular and structural characterization. [Google Scholar]
- Muthane U, Ramsay KA, Jiang H, Jackson-Lewis V, Donaldson D, Fernando S, Ferreira M, Przedborski S. Differences in nigral neuron number and sensitivity to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in C57/bl and CD-1 mice. Exp Neurol. 1994;126:195–204. doi: 10.1006/exnr.1994.1058. [DOI] [PubMed] [Google Scholar]
- Nagi MN, Cook L, Laguna JC, Cinti DL. Dual action of 2-decynoyl coenzyme A: inhibitor of hepatic mitochondrial trans-2-enoyl coenzyme A reductase and peroxisomal bifunctional protein and substrate for the mitochondrial beta-oxidation system. Arch Biochem Biophys. 1988;267:1–12. doi: 10.1016/0003-9861(88)90001-x. [DOI] [PubMed] [Google Scholar]
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature. 1995;373:109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- Nishi K, Kondo T, Narabayashi H. Difference in recovery patterns of striatal dopamine content, tyrosine hydroxylase activity and total biopterin content after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) administration: a comparison of young and older mice. Brain Res. 1989;489:157–62. doi: 10.1016/0006-8993(89)90018-8. [DOI] [PubMed] [Google Scholar]
- O’Callaghan DW, Tepikin AV, Burgoyne RD. Dynamics and calcium sensitivity of the Ca2+/myristoyl switch protein hippocalcin in living cells. J Cell Biol. 2003;163:715–21. doi: 10.1083/jcb.200306042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Callaghan RM, Griffin EW, Kelly AM. Long-term treadmill exposure protects against age-related neurodegenerative change in the rat hippocampus. Hippocampus. 2009 doi: 10.1002/hipo.20591. [DOI] [PubMed] [Google Scholar]
- O’Dell SJ, Gross NB, Fricks AN, Casiano BD, Nguyen TB, Marshall JF. Running wheel exercise enhances recovery from nigrostriatal dopamine injury without inducing neuroprotection. Neuroscience. 2007;144:1141–51. doi: 10.1016/j.neuroscience.2006.10.042. [DOI] [PubMed] [Google Scholar]
- Obata T. Dopamine efflux by MPTP and hydroxyl radical generation. Journal of Neural Transmission. 2002;109:1159–1180. doi: 10.1007/s00702-001-0683-2. [DOI] [PubMed] [Google Scholar]
- Olanow CW. Oxidation reactions in Parkinson’s disease. Neurology. 1990;40(Suppl 3):32–37. [PubMed] [Google Scholar]
- Olanow CW, Tatton WG. Etiology and pathogenesis of Parkinson’s disease. Ann Revi Neurosci. 1999;22:123–44. doi: 10.1146/annurev.neuro.22.1.123. [DOI] [PubMed] [Google Scholar]
- Petzinger GM, Walsh JP, Akopian G, Hogg E, Abernathy A, Arevalo P, Turnquist P, Vuckovic M, Fisher BE, Togasaki DM, Jakowec MW. Effects of treadmill exercise on dopaminergic transmission in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. J Neurosci. 2007;27:5291–300. doi: 10.1523/JNEUROSCI.1069-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploughman M, Windle V, MacLellan CL, White N, Dore JJ, Corbett D. Brain-derived neurotrophic factor contributes to recovery of skilled reaching after focal ischemia in rats. Stroke. 2009;40:1490–5. doi: 10.1161/STROKEAHA.108.531806. [DOI] [PubMed] [Google Scholar]
- Pothakos K, Kurz MJ, Lau YS. Restorative effect of endurance exercise on behavioral deficits in the chronic mouse model of Parkinson’s disease with severe neurodegeneration. BMC Neurosci. 2009;10:6. doi: 10.1186/1471-2202-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulton NP, Muir GD. Treadmill training ameliorates dopamine loss but not behavioral deficits in hemi-parkinsonian rats. Exp Neurol. 2005;193:181–97. doi: 10.1016/j.expneurol.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Przedborski S, Vila M. The last decade in Parkinson’s disease research. Basic sciences. Adv Neurol. 2001;86:177–86. [PubMed] [Google Scholar]
- Qin W, Khuchua Z, Cheng J, Boero J, Payne RM, Strauss AW. Molecular characterization of the creatine kinases and some historical perspectives. Mol Cell Biochem. 1998;184:153–67. [PubMed] [Google Scholar]
- Radak Z, Kumagai S, Taylor AW, Naito H, Goto S. Effects of exercise on brain function: role of free radicals. Appl Physiol Nutr Metab. 2007;32:942–6. doi: 10.1139/H07-081. [DOI] [PubMed] [Google Scholar]
- Ransom BR, Kunis DM, Irwin I, Langston JW. Astrocytes convert the parkinsonism inducing neurotoxin, MPTP, to its active metabolite, MPP+ Neurosci Lett. 1987;75:323–8. doi: 10.1016/0304-3940(87)90543-x. [DOI] [PubMed] [Google Scholar]
- Rasmussen P, Brassard P, Adser H, Pedersen MV, Leick L, Hart E, Secher NH, Pedersen BK, Pilegaard H. Evidence for a release of BDNF from the brain during exercise. Exp Physiol. 2009 doi: 10.1113/expphysiol.2009.048512. [DOI] [PubMed] [Google Scholar]
- Reshef L, Olswang Y, Cassuto H, Blum B, Croniger CM, Kalhan SC, Tilghman SM, Hanson RW. Glyceroneogenesis and the triglyceride/fatty acid cycle. J Biol Chem. 2003;278:30413–6. doi: 10.1074/jbc.R300017200. [DOI] [PubMed] [Google Scholar]
- Sandri M, Podhorska-Okolow M, Geromel V, Rizzi C, Arslan P, Franceschi C, Carraro U. Exercise induces myonuclear ubiquitination and apoptosis in dystrophin-deficient muscle of mice. J Neuropathol Exp Neurol. 1997;56:45–57. doi: 10.1097/00005072-199701000-00005. [DOI] [PubMed] [Google Scholar]
- Sandrow-Feinberg HR, Izzi J, Shumsky JS, Zhukareva V, Houle JD. Forced exercise as a rehabilitation strategy after unilateral cervical spinal cord contusion injury. J Neurotrauma. 2009;26:721–31. doi: 10.1089/neu.2008.0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scacco S, Vergari R, Scarpulla RC, Technikova-Dobrova Z, Sardanelli A, Lambo R, Lorusso V, Papa S. cAMP-dependent phosphorylation of the nuclear encoded 18-kDa (IP) subunit of respiratory complex I and activation of the complex in serum-starved mouse fibroblast cultures. J Biol Chem. 2000;275:17578–82. doi: 10.1074/jbc.M001174200. [DOI] [PubMed] [Google Scholar]
- Schapira AH, Cooper JM, Dexter D, Clark JB, Jenner P, Marsden CD. Mitochondrial complex I deficiency in Parkinson’s disease. J Neurochem. 1990;54:823–7. doi: 10.1111/j.1471-4159.1990.tb02325.x. [DOI] [PubMed] [Google Scholar]
- Scorziello A, Santillo M, Adornetto A, Dell’aversano C, Sirabella R, Damiano S, Canzoniero LM, Renzo GF, Annunziato L. NO-induced neuroprotection in ischemic preconditioning stimulates mitochondrial Mn-SOD activity and expression via Ras/ERK1/2 pathway. J Neurochem. 2007;103:1472–80. doi: 10.1111/j.1471-4159.2007.04845.x. [DOI] [PubMed] [Google Scholar]
- Sedelis M, Hofele K, Auburger GW, Morgan S, Huston JP, Schwarting RK. Evidence for resistance to MPTP in C57BL/6 × BALB/c F1 hybrids as compared with their progenitor strains. Neuroreport. 2000;11:1093–6. doi: 10.1097/00001756-200004070-00037. [DOI] [PubMed] [Google Scholar]
- Siamilis S, Jakus J, Nyakas C, Costa A, Mihalik B, Falus A, Radak Z. The effect of exercise and oxidant-antioxidant intervention on the levels of neurotrophins and free radicals in spinal cord of rats. Spinal Cord. 2009;47:453–7. doi: 10.1038/sc.2008.125. [DOI] [PubMed] [Google Scholar]
- Smeyne RJ, Jackson-Lewis V. The MPTP model of Parkinson’s disease. Brain Res Mol Brain Res. 2005;134:57–66. doi: 10.1016/j.molbrainres.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Smith AD, Zigmond MJ. Can the brain be protected through exercise? Lessons from an animal model of parkinsonism. Exp Neurol. 2003;184:31–9. doi: 10.1016/j.expneurol.2003.08.017. [DOI] [PubMed] [Google Scholar]
- Steiner B, Winter C, Hosman K, Siebert E, Kempermann G, Petrus DS, Kupsch A. Enriched environment induces cellular plasticity in the adult substantia nigra and improves motor behavior function in the 6-OHDA rat model of Parkinson’s disease. Exp Neurol. 2006;199:291–300. doi: 10.1016/j.expneurol.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH, Parks JK, Davis JN, 2nd, Cassarino DS, Trimmer PA, Currie LJ, Dougherty J, Bridges WS, Bennett JP, Jr, Wooten GF, Parker WD. Matrilineal inheritance of complex I dysfunction in a multigenerational Parkinson’s disease family. Ann Neurol. 1998;44:873–81. doi: 10.1002/ana.410440605. [DOI] [PubMed] [Google Scholar]
- Tatton WG, Greenwood CE, Seniuk NA, Salo PT. Interactions between MPTP-induced and age-related neuronal death in a murine model of Parkinson’s disease. Can J Neurol Sci. 1992;19:124–33. [PubMed] [Google Scholar]
- Teismann P, Tieu K, Cohen O, Choi DK, Wu DC, Marks D, Vila M, Jackson-Lewis V, Przedborski S. Pathogenic role of glial cells in Parkinson’s disease. Mov Disord. 2003;18:121–9. doi: 10.1002/mds.10332. [DOI] [PubMed] [Google Scholar]
- Terzioglu M, Galter D. Parkinson’s disease: genetic versus toxin-induced rodent models. Febs J. 2008;275:1384–91. doi: 10.1111/j.1742-4658.2008.06302.x. [DOI] [PubMed] [Google Scholar]
- Tillerson JL, Caudle WM, Reveron ME, Miller GW. Exercise induces behavioral recovery and attenuates neurochemical deficits in rodent models of Parkinson’s disease. Neuroscience. 2003;119:899–911. doi: 10.1016/s0306-4522(03)00096-4. [DOI] [PubMed] [Google Scholar]
- Um HS, Kang EB, Leem YH, Cho IH, Yang CH, Chae KR, Hwang DY, Cho JY. Exercise training acts as a therapeutic strategy for reduction of the pathogenic phenotypes for Alzheimer’s disease in an NSE/APPsw-transgenic model. Int J Mol Med. 2008;22:529–39. [PubMed] [Google Scholar]
- Van der Kloot W. Loading and recycling of synaptic vesicles in the Torpedo electric organ and the vertebrate neuromuscular junction. Prog Neurobiol. 2003;71:269–303. doi: 10.1016/j.pneurobio.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Villar-Palasi C, Larner J. Glycogen metabolism and glycolytic enzymes. Annu Rev Biochem. 1970;39:639–72. doi: 10.1146/annurev.bi.39.070170.003231. [DOI] [PubMed] [Google Scholar]
- von Deimling OH, Gaa A, Simon MM. Esterase-18 (ES-18) of the house mouse (Mus musculus): biochemical characterization and genetics of an allozyme system linked to chromosome 19. Biochem Genet. 1988;26:617–29. doi: 10.1007/BF02399606. [DOI] [PubMed] [Google Scholar]
- Wang CH, Lee WJ, Ghanta VK, Wang WT, Cheng SY, Hsueh CM. Molecules involve in the self-protection of neurons against glucose-oxygen-serum deprivation (GOSD)-induced cell damage. Brain Res Bull. 2009;79:169–76. doi: 10.1016/j.brainresbull.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Willis D, Li KW, Zheng JQ, Chang JH, Smit A, Kelly T, Merianda TT, Sylvester J, van Minnen J, Twiss JL. Differential transport and local translation of cytoskeletal, injury-response, and neurodegeneration protein mRNAs in axons. J Neurosci. 2005;25:778–91. doi: 10.1523/JNEUROSCI.4235-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Win HK, Chang SM, Raizner M, Shah G, Al Basky F, Desai U, Plana JC, Mahmarian JJ, Quinones MA, Zoghbi WA. Percent change in B-type natriuretic peptide levels during treadmill exercise as a screening test for exercise-induced myocardial ischemia. Am Heart J. 2005;150:695–700. doi: 10.1016/j.ahj.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Yokoyama H, Kuroiwa H, Yano R, Araki T. Targeting reactive oxygen species, reactive nitrogen species and inflammation in MPTP neurotoxicity and Parkinson’s disease. Neurol Sci. 2008;29:293–301. doi: 10.1007/s10072-008-0986-2. [DOI] [PubMed] [Google Scholar]
- Yu X, Shao XG, Sun H, Li YN, Yang J, Deng YC, Huang YG. Activation of cerebral peroxisome proliferator-activated receptors gamma exerts neuroprotection by inhibiting oxidative stress following pilocarpine-induced status epilepticus. Brain Res. 2008;1200:146–58. doi: 10.1016/j.brainres.2008.01.047. [DOI] [PubMed] [Google Scholar]
- Zang LY, Misra HP. Generation of reactive oxygen species during the monoamine oxidase catalyzed oxidation of the neurotoxicant, 1-methyl-4-phenyl-1,2,3,6-tetra hydropyridine. J. Biol. Chem. 1993;268:16504–16512. [PubMed] [Google Scholar]
- Zappia M, Oliveri RL, Montesanti R, Rizzo M, Bosco D, Plastino M, Crescibene L, Bastone L, Aguglia U, Gambardella A, Quattrone A. Loss of long-duration response to levodopa over time in PD: implications for wearing-off. Neurology. 1999;52:763–7. doi: 10.1212/wnl.52.4.763. [DOI] [PubMed] [Google Scholar]
- Zhao X, Strong R, Zhang J, Sun G, Tsien JZ, Cui Z, Grotta JC, Aronowski J. Neuronal PPARgamma deficiency increases susceptibility to brain damage after cerebral ischemia. J Neurosci. 2009;29:6186–95. doi: 10.1523/JNEUROSCI.5857-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JX, Doyle HA, Mamula MJ, Aswad DW. Protein repair in the brain, proteomic analysis of endogenous substrates for protein L-isoaspartyl methyltransferase in mouse brain. J Biol Chem. 2006;281:33802–13. doi: 10.1074/jbc.M606958200. [DOI] [PubMed] [Google Scholar]