Abstract
Hippocampal neurogenesis can be regulated by extrinsic factors, such as exercise and antidepressants. While there is evidence that the serotonin re-uptake inhibitor (SSRI) fluoxetine (Prozac) enhances neurogenesis, the new dual serotonin/noradrenaline reuptake inhibitor (SNRI) duloxetine has not been evaluated in this context. In addition, it is unclear whether effects of antidepressants and running on cell genesis and behavior are of similar magnitude in mice. Here,we assessed neurogenesis and open field behavior in 2 month old female C57Bl/6 mice after 28 days of treatment with either fluoxetine (18 mg/kg), duloxetine (2, 6 or 18 mg/kg) or exercise. New cell survival, as measured by 5-bromo-2´-deoxyuridine (BrdU) labeled cells, was enhanced by 200% in the running group only. Both running and fluoxetine, but not duloxetine, increased the percentage of new cells that became neurons. In the open field test, animals treated with either drug spent less time in the center than controls and runners. In addition, fluoxetine treatment resulted in reduced locomotor activity. Together, these data not only show that the neurogenic response to exercise is much stronger than to antidepressants, but also imply a low likelihood that reported effects of these two drugs on anxiety are mediated by adult neurogenesis in C57Bl/6 mice.
Keywords: Neurogenesis, Duloxetine, Fluoxetine, Exercise, Hippocampus, Open Field
1. Introduction
Depression and anxiety disorders are common health problems with 10–20% lifetime prevalence (Wong and Licinio, 2001). Antidepressants like fluoxetine (Prozac) are prescribed to more than 40 million patients worldwide (Wong et al., 2005). The mechanism of antidepressant action, as well as the pathophysiology and etiology of depression, remain poorly understood (Nestler et al., 2002). The observed hippocampal atrophy in depressed patients (Czeh and Lucassen, 2007; Sheline et al., 2003) has raised the possibility that stress-induced reductions in adult hippocampal neurogenesis and structural plasticity may at least in part underlie the condition (Gould et al., 1997; Lemaire et al., 2000; McEwen, 1999; Sahay and Hen, 2007; Lucassen et al., 2010). Additional evidence in support of a link between adult neurogenesis and depression is that antidepressants promote newborn cell proliferation (Encinas et al., 2006; Wang et al., 2008) survival and neurogenesis (Malberg et al., 2000; Wang et al., 2008; Oomen et al., 2007). In addition, the 3–4 week therapeutic lag coincides with the maturation time-course of newly born neurons (Jacobs et al., 2000). Furthermore, some classes of antidepressants (tricyclics and SSRIs) reverse depression-like phenotypes in behavioral tests for anxiety, like the novelty suppressed feeding task (NSF (Santarelli et al., 2003). Moreover, selective deletion of hippocampal progenitors blocks the effects in the NSF, suggesting that neurogenesis is required for behavioral effects of antidepressants (Li et al., 2008; Santarelli et al., 2003).
Similar to pharmacological agents, exercise has anti-depressant and anxiolytic effects (Salmon, 2001). In fact, clinical data from humans shows that running and antidepressants have similar efficacy for treating major depressive disorder (Blumenthal et al., 2007). In the hippocampus, running increases trophic factor levels (Cotman and Berchtold, 2002; Neeper et al., 1995), angiogenesis (Van der Borght et al., 2009), dendritic spine density (Eadie et al., 2005) and synaptic plasticity (van Praag et al., 1999a). Specific to the dentate gyrus (DG) subfield of the hippocampus is a robust increase in neurogenesis with exercise (van Praag, 2008). Both running and antidepressants increase BDNF levels (Neeper et al., 1995; Russo-Neustadt et al., 2000), which is hypothesized to contribute significantly to neurogenesis and the regulation of mood (Bar, 2009). In addition, exercise elevates monoamine levels (Chaouloff, 1989) including the precursor for serotonin synthesis, tryptophan hydroxylase (Lim et al., 2001), which may mediate reported anti-depressant effects of exercise. Despite the similar behavioral and neurogenic effects of exercise and antidepressants, few direct quantitative and qualitative comparisons have been made so far (Engesser-Cesar et al., 2007; Russo-Neustadt et al., 2000). Neither has much attention been paid to such effects in females despite the fact that women are disproportionately susceptible to major depression and anxiety disorders and lifetime prevalence for depression is about twice as high in women than in men (Alexander, 2007; Ustun, 2000; Young, 1998).
Therefore, we compared effects of two antidepressants and running on neurogenesis and open field behavior, as a test for anxiety in female mice. Specifically, the SSRI fluoxetine and the more potent, new antidepressant duloxetine (Bymaster et al., 2005) were evaluated in a chronic four week dosing paradigm and compared to exercise. These two compounds differ in inhibitory constants (Ki) and pharmacokinetics; Duloxetine e.g., is a 4-fold more potent inhibitor of serotonin receptors and preferentially partitions to the brain, while fluoxetine has low blood-brain-barrier penetration (Bymaster et al., 2005). By comparing both fluoxetine and duloxetine, the norepinepherine reuptake inhibition (NRI) pharmacology present in duloxetine could be evaluated. To study potential effects on neurogenesis, the 18 mg/kg duloxetine dose was compared to a previously published 18 mg/kg fluoxetine dose (Santarelli et al., 2003), in addition to a 10-fold lower dose (2 mg/kg), and a middle dose (6 mg/kg). Here we show, that in contrast to running, neither the dual-pharmacology anti-depressant Duloxetine nor fluoxetine improved new cell survival whereas fluoxetine did enhance neuronal differentiation of the newly generated cells. These findings suggest a low likelihood that psychotropic effects of SSRI and SNRI compounds are mediated by adult neurogenesis.
2. Results
2.1 Behavior
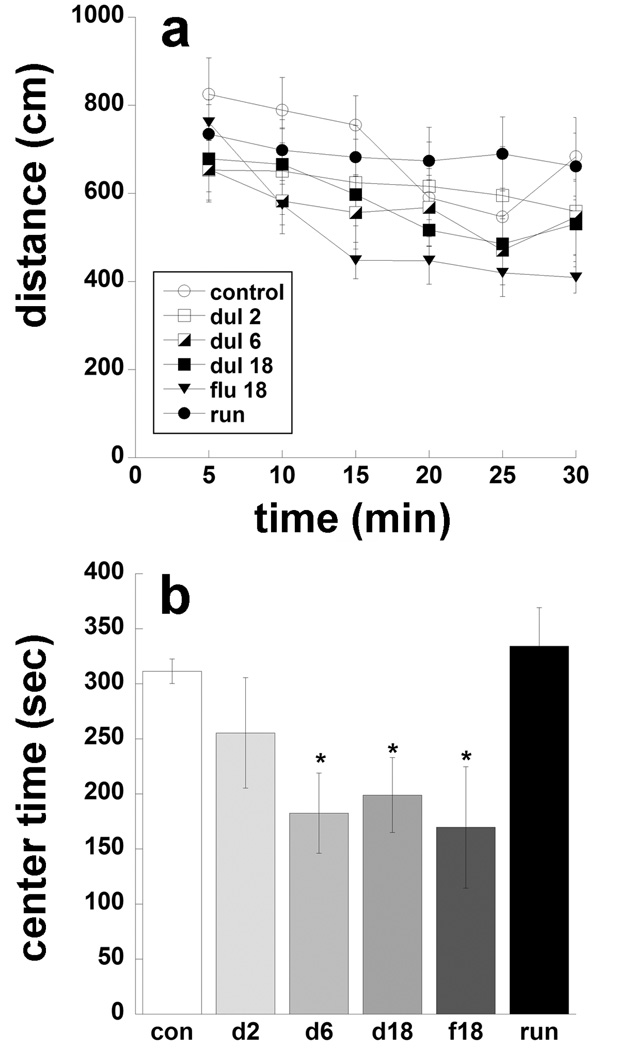
All of the mice where individually housed (n=6 per group) at the start of the experiment with one group assigned to running wheel cages. The average distance run per day was 4.5 ± 0.7 km. Twenty-one days after the start of the experiment mice were tested in open field arenas to evaluate exploratory behavior and anxiety between 08:00 and 11:00 hrs (it should be noted that there was a 14–17 hr interval between behavioral testing and injections, which were given shortly before lights out at 18:00 hrs). Analysis of variance (ANOVA) with repeated measures over time (30 min total, divided into 5 min bins) revealed that there was a significant interaction between distance traveled over time and group (F(5,25) =1.61, p<0.044). Specific post-hoc comparisons showed that the fluoxetine treated mice had significantly reduced overall locomotor activity as compared to controls (p<0.047), and a strong non-significant trend as compared to runners (p=0.057) (Fig. 1a). Furthermore, to assess anxiety levels, the time spent in the center of the open field was measured. One way ANOVA (Group x Center time) revealed a significant difference between the groups (F(5,30) = 3.08, p<0.02). Post-hoc comparisons showed that fluoxetine-treated mice (p<0.016) as well as both the duloxetine 6 mg/kg and 18 mg/kg groups (p<0.05) spent less time in the center than controls and runners (Fig. 1b). Thus, the open field data suggest that motor activity is reduced by fluoxetine and that neither fluoxetine nor duloxetine has anxiolytic effects in female C57Bl/6 mice (Figure 1).
Figure 1. Locomotor activity in the open field.
a. Mice on the SSRI fluoxetine traveled a smaller distance than controls (p<0.047) or runners (p<0.057). b. Mice on fluoxetine (p<0.016) and duloxetine (6 and 18 mg/kg), (p<0.05) spent significantly less time in the center and more time in the periphery of the maze than controls and runners. N=6 mice per group, error bars indicate SEM. *p<0.05.
2.2 Survival and phenotype of newborn cells
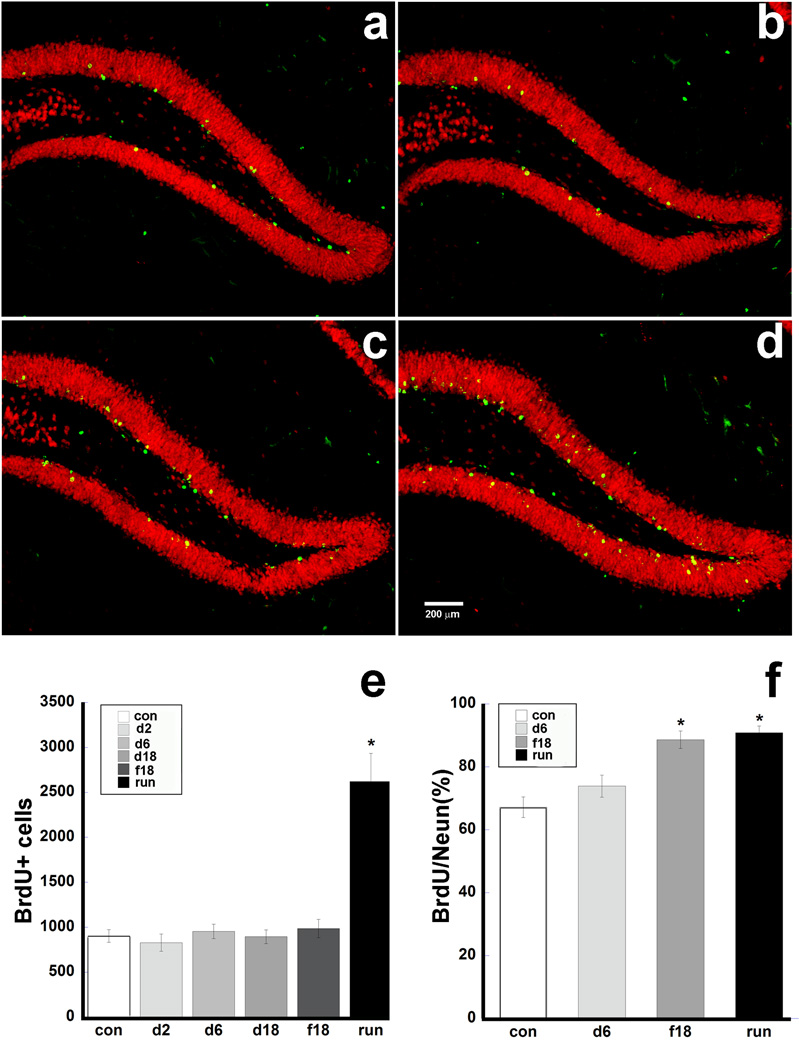
Mice were injected with bromodeoxyuridine (BrdU) for four days (75 mg/kg) to label dividing cells. BrdU is a thymidine analog incorporated into the DNA of dividing cells, which allows BrdU containing nuclei in the DG to be identified at later survival times by means of immunohistochemistry. BrdU–labeled cells were counted in DG granule cell layer and hilus in 6 equidistant sections (240 µm between sections starting from the rostral dentate gyrus) per subject. One way ANOVA showed that there was a significant difference between the groups (F(5,30) = 21.87, p<0.0001). Specific comparison revealed that runners (2621±312 BrdU+ cells) had a significantly higher number of cells as compared to all other groups (control: 902+69.71; fluoxetine: 985+102; duloxetine: 2 mg/kg, 828±95; 6 mg/kg, 953±80; 18 mg/kg, 894±76 BrdU+ cells; p<0.0001) (Fig. 2e).
Figure 2. The effects of running and antidepressants on cell survival and neurogenesis.
Representative photomicrographs (a. control, b. duloxetine 6 mg/kg, c. fluoxetine 18 mg/kg, d. runner) of the SGZ demonstrate the location of BrdU labeled cells (green) within the contour of the DG labeled with NeuN (red). e. Running mice had a more than 2-fold increase in the number of surviving BrdU labeled cells. f. Fluoxetine and running both significantly increased the percentage of BrdU cells co-localizing with the neuronal marker (NeuN). N=6 mice per group, error bars indicate SEM. *p<0.003.
In order to establish whether BrdU+ cells had matured to become granule neurons in the DG we co-labeled these cells with mature neuronal nuclei marker (NeuN). Sections were double-labeled with primary antibodies for BrdU and NeuN and incubated with species-specific secondary antibodies conjugated to Alexa-488 and Cy5 fluorophore dyes, respectively. Z-stack images were obtained on a spinning-disk confocal microscope and neurons in the DG were analyzed for the presence of both markers (Fig. 2a–d). Since only the 6 mg/ kg dose of duloxetine showed a trend toward increased survival of BrdU+ cells, this was the only duloxetine group to be evaluated for cell fate analysis. One way ANOVA showed a significant difference in the percentage of BrdU/NeuN positive cells between the groups (F(5,30)=21.87, p<0.0001). Specific comparisons showed that both fluoxetine (88.6 ± 2.78%; p<0.003) and running (90.87 ±2.15%; p<0.0006) significantly increased the population of BrdU/NeuN-positive cells as compared to controls (67.15 ± 3.27%), whereas duloxetine (73.88 ± 3.49%) had no effect (Fig. 2f).
3. Discussion
We studied female C57Bl6 mice to compare effects of chronic antidepressant treatment and exercise on parameters of anxiety and neurogenesis. In the open field, the SSRI fluoxetine reduced distance traveled indicating that locomotor activity was suppressed. Surprisingly, both duloxetine (at doses of 6 and 18 mg/kg) and fluoxetine (18 mg/kg) reduced the amount of time spent in the center of the open field. Regarding new cell survival, only runners had a significant increase in BrdU+ cells as compared to control animals. However, both exercise and fluoxetine enhanced neuronal differentiation. The SNRI duloxetine affected neither cell survival nor neurogenesis. These findings suggested that only some classes of antidepressants can exert a mild neurogenic effect which notably does not correlate with reduced anxiety in the open field.
Our study was carried out in female mice as little is known on the neurogenic effects of antidepressants and running in females and as women are disproportionately susceptible to major depression and anxiety disorders. Lifetime prevalence for depression is about 21% in women and 13% in men in the United States (Alexander, 2007; Ustun, 2000; Young, 1998) while significant gender differences have been documented; girls are twice as likely as boys to have experienced an anxiety disorder by age 6 (Anderson et al., 1987; Lewinsohn et al., 1998). Separate epidemiological studies have further identified that women are at higher risk for developing anxiety disorders than men (Angst and Dobler-Mikola, 1985; Bruce et al., 2005).
Interestingly, in the female C57Bl/6 mice there was no effect of fluoxetine on new cell survival. This finding differs from the initial report in male rats (Malberg et al., 2000), but is consistent with research by others in female rats (Hodes et al., 2009a). In addition, previous findings indicate that the neurogenic affects of anti-depressants are hinged upon age. There is evidence that enhanced survival occurs upon administration of fluoxetine in mice that are less than 3 months-old, but that aging abolishes this effect on new cell survival (Couillard-Despres et al., 2009; Navailles et al., 2008). Our study with young mice however, does not show increased BrdU labeling in the C57Bl/6 strain, similar to another recent study in this strain (David et al., 2009) and research in rats (Hodes et al., 2009a).
A side by side comparison of inbred mouse strains found that responses to fluoxetine were dependent on inherent predisposition for serotonin-induced neurogenesis (Miller et al., 2008). Separate experiments indicated that chronic fluoxetine administration did not alter hippocampal neurogenesis in BALB/cJ mice (Huang et al., 2008) or in Sprague-Dawley rats (Cowen et al., 2008). Thus, the effect of antidepressants on cell genesis may become apparent only under disease conditions or in more anxious mouse strains (Santarelli et al., 2003; David et al., 2009). The outcome of the present study and of work by others indicates that anti-depressants have varying neurogenic effects that depend on the mouse strain, gender, age and pharmacology of the antidepressant. Running, on the other hand, has a potent positive influence on neurogenesis regardless of the gender or strain of the animals (van Praag, 2008).
Upon testing in the open field, runners did not differ from controls with regard to distance traveled and the amount of time spent in the center of the field. As running is considered anxiolytic it may be expected that runners would adapt more quickly to the open field and would spend more time in the center. However, in mice apparently gender differences exist in this regard. Male mice show better adaptation and more center entries after two weeks of voluntary wheel running (Salam et al., 2009). Consistent with the results of the present study, in an experiment in which mice ran for 3 months, female runners did not differ in open field behavior from controls (Pietropaolo et al., 2008).
Analysis of the effects of antidepressants on open field behavior revealed differences between the SNRI duloxetine and the SSRI fluoxetine. Adaptation to the open field was not changed by treatment with duloxetine. This is consistent with other reports that duloxetine does not affect locomotor activity (Prinssen et al., 2006). Fluoxetine treated mice, on the other hand, showed reduced locomotion in the open field. This finding is in contrast to several reports using a similar dose range showing that fluoxetine increased activity (Brocco et al., 2002; Prinssen et al., 2006). Other researchers, however, report that a lower dose of fluoxetine (6 mg/kg) reduces the amount of voluntary wheel running (Weber et al., 2009) and that a 10 mg/kg dose reduces distance traveled in the open field (Dulawa et al., 2004). In addition, treatment with fluoxetine as well as duloxetine at the 6 and 18 mg/kg doses resulted in less time in the center of the field than controls and runners. These findings may be specific to the C57Bl/6 strain as an increase in center time has been reported with fluoxetine but only in the more anxious Balb/C mouse strain (Dulawa et al., 2004).
Consistent with previous reports, 4 weeks of voluntary wheel running resulted in a robust enhancement in the survival of newly born cells in the DG of the hippocampus as well as an increase in neurogenesis. This finding has been replicated in different mouse strains, ages and exercise paradigms (van Praag, 2008; van Praag et al., 1999b). The antidepressants, on the other hand, appear to have minimal neurogenic effects. The potent SNRI Duloxetine while effective in human patients, did not change cell survival or neurogenesis at any of the doses tested. It is possible that this anti-depressant has an entirely different effect on the factors mediating neurogenesis when compared to fluoxetine. While administration of duloxetine has been associated with a significant increase in BDNF mRNA levels in frontal cortex (Calabrese et al., 2007), in the hippocampus only the synaptic compartment shows a change (Molteni et al., 2009). Fluoxetine, on the other hand results in a significant upregulation of BDNF protein in the hippocampus (Hodes et al., 2009b) and was shown to increase neuronal differentiation in the present study, consistent with some previous research in mice (David et al., 2009; Navailles et al., 2008), but not with research in rats (Malberg et al., 2000; Hodes et al., 2009a), indicating there may be species differences in this regard (Snyder et al., 2009).
In summary, the present study shows that exercise has a robust effect on hippocampal neurogenesis, whereas of the two antidepressants tested only fluoxetine enhanced neuronal differentiation. In addition, antidepressant treated mice spent less time in the center of the open field. These findings suggest there is no close association between the neurogenic and anxiolytic effects of these antidepressants in female C57Bl/6 mice.
4. Experimental Procedures
4.1 Mice
Female C57Bl6J mice (5 weeks old) were purchased from the Jackson Lab (Bar Harbor, ME). The mice were maintained on a standard NIH-07 diet (Harlan-Tekland, Indianapolis, IN) with free access to water during a 12-hour light/12-hour dark cycle. Two weeks after arrival the animals were housed individually and randomly assigned to control (con), Fluoxetine 18mg/kg (flu 18), and Duloxetine 2/6/18 mg/kg (dul 2/6/18) or running (run) groups. Anti-depressants were obtained from Toronto Research Chemicals (TRC Inc, North York, Ontario, Canada), dissolved in sterile saline, and administered once-daily by subcutaneous injection. Control animals received sterile saline only, injections were performed daily shortly before lights out at 18:00hrs. The running mice were housed with a running wheel and distance run was recorded daily (Clocklab, Coulborn Instruments, Whitehall, PA).
In order to analyze newborn cells, BrdU (75 mg/kg) was injected intraperiotoneally for the first 4 days. Twenty eight days later, animals were deeply anesthetized by isoflurane inhalation and perfused with phosphate buffered saline. Animals were decapitated and brains were immediately removed. The right hemisphere was washed and placed in 4% paraformaldehyde for 7 days, followed by equilibration in 30% sucrose. Tissue was sectioned coronally (40 µm) on a freezing microtome (Thermo-Fisher) and stored at −20°C in cryoprotectant solution. The left hemisphere was dissected and frozen on dry ice for biochemical analysis in a separate study. All animal procedures were done in accordance with guidelines approved by the National Institute of Health Animal Care and Use Committee.
4.2 Open Field Arenas
Animals from all 6 groups were randomized and tested on 2 consecutive days in an open field arena (27.3 × 27.3 cm, height 20.3 cm) (Med Associates Inc., Georgia, VT). Animals were placed in the center of the arena at the beginning of the testing paradigm and were left undisturbed for 30 minutes. All testing occurred between 08:00 and 11:00 hrs on the day of testing, approximately 14–17 hours after the animals had been injected with compounds or saline. The center zone was defined as a 10.2 cm square equidistant from the peripheral walls. Each arena had a black floor and walls where x–y movements are monitored by two sets of pulsed-modulated infrared photobeams. This tracking system records data directly to a networked computer and measures ambulatory counts, entries into defined zones, distance traveled, and time spent in each zone (Med Associates Inc., Georgia, VT).
4.3 Bromodeoxyuridine immunohistochemistry and cell counts
A one in six series of free floating sections (40 µm) was washed in TBS and pre-incubated with 0.6% H2O2 for 30 minutes. After rinsing, the sections were incubated in 2N HCl at 37°C for 30 minutes to denature DNA then neutralized in 0.1 M Borate buffer at RT. After thorough washing, the sections were blocked with TBS++ (3% Donkey Serum-0.05 M TBS, 0.5% Triton-X 100) for 30 min at room temperature and incubated with rat anti-BrdU (1: 200, Accurate Chemical Westbury NY) overnight at 4° C. Thereafter, the sections were washed and immersed in biotin-SP-conjugated donkey anti-rat IgG (1: 250, Jackson ImmunoResearch, West Grove, PA) followed by 2 hrs in ABC reagent (1:1000, Vestastain Elite; Vector Laboratories, Burlingame, CA). The sections were then incubated with the substrate 3, 3’-Diaminobenzidine (D4418, Sigma, St. Louis, MO) for 5 min to visualize the cells that had incorporated BrdU. BrdU-positive cells were counted in a one-in-six series of sections (240 µm apart) through a 20X objective (Olympus, BX51) throughout 6 sections per animal starting at the rostro-caudal extent of the granule cell layer.
4.4 Double immunofluorescence for cell fate analysis
Free floating sections (1:6 series) were simultaneously incubated with primary antibodies against BrdU (1: 100 Accurate Chemical Westbury NY) and NeuN (1:100 Millipore, Billerica, MA). Antibodies were diluted in TBS++ and then sections were incubated for 48 hr at 4° C. After rinses in TBS++ and TBS sections were co-incubated with donkey ant-rat Alexa Fluor 488 (1:250, Molecular Probes, Carlsbad, CA) and donkey anti-mouse Cy3 fluorophore dyes (1:250, Jackson ImmunoResearch, West Grove, PA) for 2 hours at RT. Z-stacked images of cells in the granule cell layer were imaged on an Olympus IX81 spinning disk confocal microscope (Olympus, Center Valley, PA). At least 50 cells in the DG of each mouse were analyzed for double-labeling between BrdU and the neuronal marker NeuN. Ratio of BrdU-positive cells colabeling with NeuN was determined.
Acknowledgements
This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging. MWM and PJL are further supported by the European Union (NEURAD Graduate Program). PJL is supported by the International Stichting Alzheimer Onderzoek (ISAO) and the Netherlands Brain Foundation. We thank Dr. Nigel Greig for helpful discussions and Linda Kitabayashi for her expertise in figure preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander JL. Quest for timely detection and treatment of women with depression. J Manag Care Pharm. 2007;13:S3–S11. doi: 10.18553/jmcp.2007.13.9-a.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JC, et al. DSM-III disorders in preadolescent children. Prevalence in a large sample from the general population. Arch Gen Psychiatry. 1987;44:69–76. doi: 10.1001/archpsyc.1987.01800130081010. [DOI] [PubMed] [Google Scholar]
- Angst J, Dobler-Mikola A. The Zurich Study. V. Anxiety and phobia in young adults. Eur Arch Psychiatry Neurol Sci. 1985;235:171–178. doi: 10.1007/BF00380989. [DOI] [PubMed] [Google Scholar]
- Bar M. A cognitive neuroscience hypothesis of mood and depression. Trends Cogn Sci. 2009;13:456–463. doi: 10.1016/j.tics.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal JA, et al. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med. 2007;69:587–596. doi: 10.1097/PSY.0b013e318148c19a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocco M, et al. Induction of hyperlocomotion in mice exposed to a novel environment by inhibition of serotonin reuptake. A pharmacological characterization of diverse classes of antidepressant agents. Pharmacol Biochem Behav. 2002;71:667–680. doi: 10.1016/s0091-3057(01)00701-8. [DOI] [PubMed] [Google Scholar]
- Bruce SE, et al. Influence of psychiatric comorbidity on recovery and recurrence in generalized anxiety disorder, social phobia, and panic disorder: a 12-year prospective study. Am J Psychiatry. 2005;162:1179–1187. doi: 10.1176/appi.ajp.162.6.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bymaster FP, et al. The dual transporter inhibitor duloxetine: a review of its preclinical pharmacology, pharmacokinetic profile, and clinical results in depression. Curr Pharm Des. 2005;11:1475–1493. doi: 10.2174/1381612053764805. [DOI] [PubMed] [Google Scholar]
- Calabrese F, et al. Chronic duloxetine treatment induces specific changes in the expression of BDNF transcripts and in the subcellular localization of the neurotrophin protein. Neuropsychopharmacology. 2007;32:2351–2359. doi: 10.1038/sj.npp.1301360. [DOI] [PubMed] [Google Scholar]
- Chaouloff F. Physical exercise and brain monoamines: a review. Acta Physiol Scand. 1989;137:1–13. doi: 10.1111/j.1748-1716.1989.tb08715.x. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Couillard-Despres S, et al. Ageing abolishes the effects of fluoxetine on neurogenesis. Mol Psychiatry. 2009;14:856–864. doi: 10.1038/mp.2008.147. [DOI] [PubMed] [Google Scholar]
- Cowen DS, et al. Age-dependent decline in hippocampal neurogenesis is not altered by chronic treatment with fluoxetine. Brain Res. 2008;1228:14–19. doi: 10.1016/j.brainres.2008.06.059. [DOI] [PubMed] [Google Scholar]
- Czeh B, Lucassen PJ. What causes the hippocampal volume decrease in depression? Are neurogenesis, glial changes and apoptosis implicated? Eur Arch Psychiatry Clin Neurosci. 2007;257:250–260. doi: 10.1007/s00406-007-0728-0. [DOI] [PubMed] [Google Scholar]
- David DJ, et al. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62:479–493. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulawa SC, et al. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology. 2004;29:1321–1330. doi: 10.1038/sj.npp.1300433. [DOI] [PubMed] [Google Scholar]
- Eadie BD, et al. Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. J Comp Neurol. 2005;486:39–47. doi: 10.1002/cne.20493. [DOI] [PubMed] [Google Scholar]
- Engesser-Cesar C, et al. Wheel running and fluoxetine antidepressant treatment have differential effects in the hippocampus and the spinal cord. Neuroscience. 2007;144:1033–1044. doi: 10.1016/j.neuroscience.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Gould E, et al. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes GE, Yang L, Van Kooy J, Santollo J, Shors TJ. Prozac during puberty: distinctive effects on neurogenesis as a function of age and sex. Neuroscience. 2009a;163(2):609–617. doi: 10.1016/j.neuroscience.2009.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes GE, et al. Sex Specific Effects of Chronic Fluoxetine Treatment on Neuroplasticity and Pharmacokinetics in Mice. J Pharmacol Exp Ther. 2009b;332(1):266–273. doi: 10.1124/jpet.109.158717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GJ, et al. Chronic fluoxetine treatment alters behavior, but not adult hippocampal neurogenesis, in BALB/cJ mice. Mol Psychiatry. 2008;13:119–121. doi: 10.1038/sj.mp.4002104. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, et al. Adult brain neurogenesis and psychiatry: a novel theory of depression. Mol Psychiatry. 2000;5:262–269. doi: 10.1038/sj.mp.4000712. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, et al. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire V, et al. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc Natl Acad Sci U S A. 2000;97:11032–11037. doi: 10.1073/pnas.97.20.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn PM, et al. Gender differences in anxiety disorders and anxiety symptoms in adolescents. J Abnorm Psychol. 1998;107:109–117. doi: 10.1037//0021-843x.107.1.109. [DOI] [PubMed] [Google Scholar]
- Li Y, et al. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron. 2008;59:399–412. doi: 10.1016/j.neuron.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim BV, et al. Caffeine inhibits exercise-induced increase in tryptophan hydroxylase expression in dorsal and median raphe of Sprague-Dawley rats. Neurosci Lett. 2001;308:25–28. doi: 10.1016/s0304-3940(01)01980-2. [DOI] [PubMed] [Google Scholar]
- Lucassen PJ, et al. Regulation of neurogenesis by stress, sleep, exercise and inflammation; implications for depression and antidepressant action. European NeuroPsychoPharmacology. 2010;vol 20(Issue 1):1–17. doi: 10.1016/j.euroneuro.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Malberg JE, et al. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- Miller BH, et al. Genetic regulation of behavioral and neuronal responses to fluoxetine. Neuropsychopharmacology. 2008;33:1312–1322. doi: 10.1038/sj.npp.1301497. [DOI] [PubMed] [Google Scholar]
- Molteni R, et al. Acute stress responsiveness of the neurotrophin BDNF in the rat hippocampus is modulated by chronic treatment with the antidepressant duloxetine. Neuropsychopharmacology. 2009;34:1523–1532. doi: 10.1038/npp.2008.208. [DOI] [PubMed] [Google Scholar]
- Navailles S, et al. Antidepressant drug-induced stimulation of mouse hippocampal neurogenesis is age-dependent and altered by early life stress. J Comp Neurol. 2008;509:372–381. doi: 10.1002/cne.21775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeper SA, et al. Exercise and brain neurotrophins. Nature. 1995;373:109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, et al. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- Oomen CA, et al. Brief treatment with the glucocorticoid receptor antagonist mifepristone normalizes the reduction in neurogenesis after chronic stress. Eur. J. Neurosci. 2007;26:3395–3401. doi: 10.1111/j.1460-9568.2007.05972.x. [DOI] [PubMed] [Google Scholar]
- Pietropaolo S, et al. The impact of voluntary exercise on mental health in rodents: a neuroplasticity perspective. Behav Brain Res. 2008;192:42–60. doi: 10.1016/j.bbr.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Prinssen EP, et al. The effects of serotonin reuptake inhibitors on locomotor activity in gerbils. Pharmacol Biochem Behav. 2006;85:44–49. doi: 10.1016/j.pbb.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Russo-Neustadt AA, et al. Physical activity and antidepressant treatment potentiate the expression of specific brain-derived neurotrophic factor transcripts in the rat hippocampus. Neuroscience. 2000;101:305–312. doi: 10.1016/s0306-4522(00)00349-3. [DOI] [PubMed] [Google Scholar]
- Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10:1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- Salam JN, et al. Voluntary exercise in C57 mice is anxiolytic across several measures of anxiety. Behav Brain Res. 2009;197:31–40. doi: 10.1016/j.bbr.2008.07.036. [DOI] [PubMed] [Google Scholar]
- Salmon P. Effects of physical exercise on anxiety, depression, and sensitivity to stress: a unifying theory. Clin Psychol Rev. 2001;21:33–61. doi: 10.1016/s0272-7358(99)00032-x. [DOI] [PubMed] [Google Scholar]
- Santarelli L, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Sheline YI, et al. Untreated depression and hippocampal volume loss. Am J Psychiatry. 2003;160:1516–1518. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Choe JS, Clifford MA, Jeurling SI, Hurley P, Brown A, Kamhi JF, Cameron HA. Adult-born hippocampal neurons are more numerous, faster maturing, and more involved in behavior in rats than in mice. J Neurosci. 2009;29(46):14484–14495. doi: 10.1523/JNEUROSCI.1768-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ustun TB. Cross-national epidemiology of depression and gender. J Gend Specif Med. 2000;3:54–58. [PubMed] [Google Scholar]
- Van der Borght K, et al. Physical exercise leads to rapid adaptations in hippocampal vasculature: temporal dynamics and relationship to cell proliferation and neurogenesis. Hippocampus. 2009;19:928–936. doi: 10.1002/hipo.20545. [DOI] [PubMed] [Google Scholar]
- van Praag H. Neurogenesis and exercise: past and future directions. Neuromolecular Med. 2008;10:128–140. doi: 10.1007/s12017-008-8028-z. [DOI] [PubMed] [Google Scholar]
- van Praag H, et al. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999a;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, et al. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999b;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Wang JW, et al. Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J Neurosci. 2008;28:1374–1384. doi: 10.1523/JNEUROSCI.3632-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M, et al. Running wheel activity is sensitive to acute treatment with selective inhibitors for either serotonin or norepinephrine reuptake. Psychopharmacology (Berl) 2009;203:753–762. doi: 10.1007/s00213-008-1420-4. [DOI] [PubMed] [Google Scholar]
- Wong DT, et al. Case history: the discovery of fluoxetine hydrochloride (Prozac) Nat Rev Drug Discov. 2005;4:764–774. doi: 10.1038/nrd1821. [DOI] [PubMed] [Google Scholar]
- Wong ML, Licinio J. Research and treatment approaches to depression. Nat Rev Neurosci. 2001;2:343–351. doi: 10.1038/35072566. [DOI] [PubMed] [Google Scholar]
- Young EA. Sex differences and the HPA axis: implications for psychiatric disease. J Gend Specif Med. 1998;1:21–27. [PubMed] [Google Scholar]