Abstract
The subventricular zone (SVZ) is a principal site of adult neurogenesis and appears to participate in the brain’s response to injury. Thus, measures that enhance SVZ neurogenesis may have a role in treatment of neurological disease. To better characterize SVZ cells and identify potential targets for therapeutic intervention, we studied electrophysiological properties of SVZ cells in adult mouse brain slices using patch-clamp techniques. Electrophysiology was correlated with immunohistochemical phenotype by injecting cells with lucifer yellow and by studying transgenic mice carrying green fluorescent protein under control of the doublecortin (DCX) or glial fibrillary acidic protein (GFAP) promoter. We identified five types of cells in the adult mouse SVZ: type 1 cells, with 4-aminopyridine (4-AP)/tetraethylammonium (TEA)-sensitive and CdCl2-sensitive inward currents; type 2 cells, with Ca2+-sensitive K+ and both 4-AP/TEA-sensitive and -insensitive currents; type 3 cells, with 4-AP/TEA-sensitive and -insensitive and small Na+ currents; type 4 cells, with slowly activating, large linear outward current and sustained outward current without fast-inactivating component; and type 5 cells, with a large outward rectifying current with a fast inactivating component. Type 2 and 3 cells expressed DCX, types 4 and 5 cells expressed GFAP, and type 1 cells expressed neither. We propose that SVZ neurogenesis involves a progression of electrophysiological cell phenotypes from types 4 and 5 cells (astrocytes) to type 1 cells (neuronal progenitors) to types 2 and 3 cells (nascent neurons), and that drugs acting on. ion channels expressed during neurogenesis might promote therapeutic neurogenesis in the injured brain.
Keywords: doublecortin, potassium channels, sodium channels, neurogenesis, stroke
1. Introduction
The subventricular zone (SVZ) is a major site of neurogenesis in adult mammals (Lois and Alvarez-Buylla 1993; Luskin 1993). SVZ neurogenesis contributes to olfactory function in some species, and can also respond to cerebral (e.g., ischemic or epileptic) injury with increased production of new neurons (Arvidsson et al. 2002; Jin et al. 2001; Parent et al. 2002a; Parent et al. 2002b; Zhang et al. 2001). In some cases, these new neurons have been shown to migrate to the lesion site (Arvidsson et al. 2002; Jin et al. 2003; Parent et al. 2002b; Zhang et al. 2003), where they may participate in functional recovery (Thored et al. 2006; Yamashita et al. 2006). Adult SVZ neurogenesis is also stimulated by a variety of drugs (Chen et al. 2003; Zhang et al. 2002), suggesting that administration of such drugs might improve clinical outcome in neurological disorders like stroke.
Understanding the detailed organization of the SVZ may be important for developing drugs to target specific cell subpopulations and, therefore, specific neuropathological states. By analogy to the hematopoietic system, where lineage-specific growth factors can be used to replete different bone-marrow cell types (Kaushansky 2006), it may be possible to devise treatments to replace neural cells that are preferentially lost in particular diseases. Existing evidence (reviewed in (Marshall et al. 2003)) points to heterogeneity of the SVZ with respect to embryonic origin (Young et al. 2007), cytoarchitecture and cell composition (Doetsch et al. 1997; Mercier et al. 2002; Ponti et al. 2006), signaling pathways (Haskell and LaMantia 2005), gene expression (Redmond et al. 1996) and cell migration routes (Suzuki and Goldman 2003). According to one widely used classification scheme (Doetsch et al. 1997), the SVZ contains five principal cell types: type B cells (astrocytes), some of which give rise to type C cells (transit-amplifying neuronal precursors), which, in turn, generate type-A cells (neuroblasts that migrate in the rostral migratory stream); as well as D cells (tanycytes) and E cells (ependyma).
Several studies have also explored the electrophysiological properties of SVZ-derived cells in dissociated cell culture, documenting the presence of A-type K+ (KA) (Scheffler et al. 2005; Stewart et al. 1999), delayed rectifying K+ (KDR) (Scheffler et al. 2005; Stewart et al. 1999), inward-rectifying K+ (KIR) (Yasuda et al. 2008), Ca2+-dependent K+ (KCa) (Stewart et al. 1999), tetrodotoxin-sensitive Na+ (Stewart et al. 1999), and GABAA receptor-gated Cl− (Stewart et al. 2002) currents, as well as GABA-mediated inhibitory (Scheffler et al. 2005) and glutamate-induced excitatory (Liu et al. 1999) responses. In some cases, differences in current-voltage (I–V) responses to step currents have led to the classification of SVZ cells into three types: type I (neurons), with outward rectification; type II (glia), with linear I–V responses; and type 3 (undifferentiated cells), with both outward and inward rectification (Liu et al. 1999; Whittemore et al. 1999).
Fewer studies have examined the electrophysiology of SVZ cells in brain slices in situ, which permits neuronal progenitor cells (NPCs) to be distinguished from other cell types based on location in relation to the ventricular wall, cell morphology, and immunostaining. However, these studies have shown that NPCs exhibit depolarized resting membrane potential and high input resistance; express KCa and KDR but not inward K+ currents; fail to generate action potentials; and depolarize in response to GABA (Liu et al. 2005; Wang et al. 2003a; b).
The electrophysiological properties of SVZ cells are likely to be important not only for classifying cells, but also for understanding their function and ultimately, perhaps, for NPC-based therapy. For example both K+ (Liebau et al. 2006; Yasuda et al. 2008) and L-type Ca2+ (D’Ascenzo et al. 2006) channels regulate the proliferation and differentiation of NPCs, and ion channels also mediate some of the effects of growth factors involved in neurogenesis (Blum and Konnerth 2005).
In the present study, we investigated the electrophysiological properties of SVZ cells in adult mouse brain slices. Electrophysiology was correlated with immunohistochemical phenotype by injecting lucifer yellow into cells after recording, and by studying transgenic mice carrying green fluorescent protein (GFP) under the control of either the doublecortin (DCX) or glial fibrillary acidic protein (GFAP) promoter. Here we report our findings regarding the electrophysiological heterogeneity of SVZ cells.
2. Results
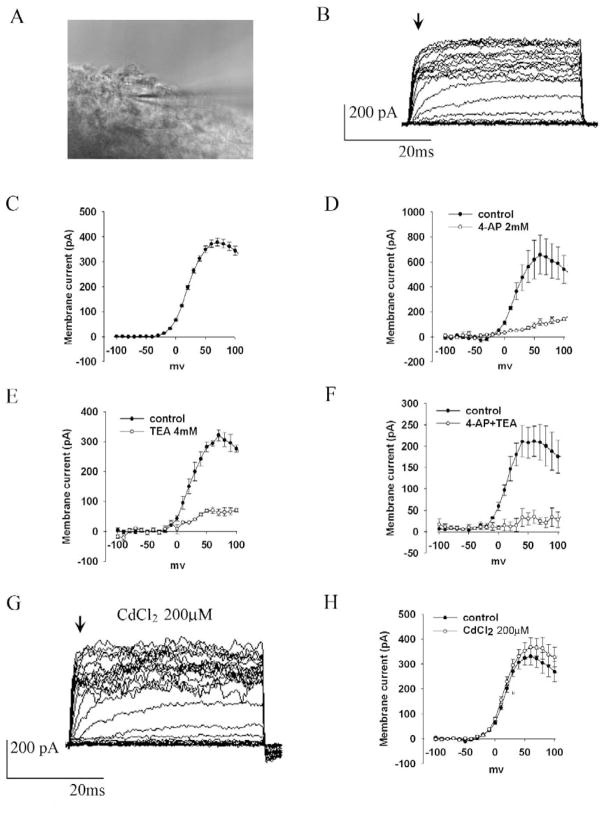
Whole-cell patch-clamp was used to explore the electrophysiological properties of SVZ cells from adult mouse brain (Figure 1A). In the initial set of experiments, a total of 225 cells were recorded from, all of which displayed outward currents. Among these, 86 cells (38%) showed an outward delayed rectifier current (Figure 1B), which was rapidly activated at approximately −20 mV, reached a peak at +70 mV, and then decreased (Figure 1C). These outward currents were partially blocked by the K+ channel blocker 4-aminopyridine (4-AP, 2 mM) (Figure 1D) or tetraethylammonium (TEA, 4mM) (Figure 1E), and were completely blocked when 4-AP and TEA were applied together (Figure 1F), suggesting that they were delayed rectifier K+ currents (KDR). Cd2+ (CdCl2, 200 μM) slightly increased the outward current (Figure 1G–H) suggesting that there is also a small Cd2+-sensitive inward current. We designated these cells type 1 cells. To determine their immunohistochemical properties, Lucifer yellow was injected into type 1 cells after recording, and immunocytochemistry was performed using antibodies against NSC protein markers and GFAP. We found that type 1 cells expressed neither DCX, nor GFAP, nor nestin.
Fig. 1. Type 1 SVZ cells express 4-AP- and TEA-sensitive K+ currents and CdCl2-sensitive inward currents.
(A) Image of patch pipette in SVZ of mouse brain slice. (B) Representative traces of membrane currents recorded from type 1 cells. (C) Mean I-V curves of steady-state outward currents in type 1 cells (n=22). (D) Mean I–V curves of steady-state outward currents under control conditions and in the presence of 2 mM 4-AP in type 1 cells (n=7). (E) Mean I–V curves of steady-state outward currents under control conditions and in the presence of 4 mM TEA in type 1 cells (n=7). (F) Mean I–V curves of steady-state outward currents under control conditions and in the presence of both 2 mM 4-AP and 4 mM TEA (n=5) in type 1 cells. (G) Outward currents in type 1 cells are increased in the presence of 200 μM CdCl2. (H) Mean I–V curves of steady-state outward currents before and after application of 200 μM CdCl2 in type 1 cells (n=5).
Another 107 cells (48%) displayed an outward delayed rectifier current that activated at about −20 mV and increased during the recording (Figure 2A–B). These outward currents were partially blocked by 4-AP (2 mM) (Figure 2C) or TEA (4 mM) (Figure 2D). However, blockade was observed only between −20 mV and +120 mV, above which range currents were insensitive to these agents (Figure 2E–F). The outward currents were also partially blocked by CdCl2 (200 μm) (Figure 2G–H), suggesting the presence of Ca2+-sensitive K+ currents (KCa) in these (type 2) cells. When Lucifer yellow was injected into type 2 cells after recording and immunocytochemistry was performed, the cells were found to express DCX, but not GFAP or nestin.
Fig. 2. Type 2 SVZ cells display 4-AP- and TEA-sensitive and -insensitive outward K+ currents, and Ca2+-sensitive K+ currents.
(A) Representative traces of outward currents recorded from type 2 cells. (B) Mean I–V curves of steady-state outward currents in type 2 cells (n=24). (C) Mean I–V curves of steady-state outward currents under control conditions and in the presence of 2 mM 4-AP in type 2 cells (n=7). (D) Mean I–V curves of steady-state outward currents under control conditions and in the presence of 4 mM TEA in type 2 cells (n=7). (E) 4-AP- and TEA-insensitive currents in type 2 cells (F) Mean I-V curves of steady-state outward currents under control conditions and in the presence of both 2 mM 4-AP and 4 mM TEA in type 2 cells (n=5). (G) Outward currents in type 2 cells are decreased in the presence of 200 μM CdCl2. (H) Mean I–V curves of steady-state outward currents before and after the application of 200 μM CdCl2 in type 2 cells (n=5).
An additional 23 cells (10%) exhibited inward membrane currents that were activated at -20 mV, peaked at −10 mV and reversed near 0 mV (Figure 3A). This inward current was blocked completely in the presence of 0.5 μM tetrodotoxin (TTX) (Figure 3B), but the Na+ conductance was apparently too low to generate fast action potentials. An outward delayed rectifier current was also observed, which increased continuously until the end of the recording (Figure 3C). TEA (4 mM), 4-AP (2 mM), or both partly inhibited outward current between 0 and +120 mV, but had no effect on inward current (Figure 3D–F). Outward current was not altered in the presence of 200 μM CdCl2 (Figure 3G–H). Thus, these (type 3) cells exhibited small Na+ currents and 4-AP- and TEA-sensitive and insensitive outward currents, but no Ca2+-sensitive K+ currents. Immunostaining showed that type 3 cells expressed DCX. To confirm the association of type 2 and type 3 electrophysiology with NSC marker expression, whole-cell patch-clamp recording was performed on brain slices from transgenic mice carrying the GFP gene under the control of the DCX promoter. We found that DCX-GFP-positive cells (n=17) displayed the electrophysiological properties of type 2 and 3 cells, as described above.
Fig. 3. Type 3 SVZ cells show 4-AP- and TEA-sensitive and -insensitive K+ currents and small Na+ currents, but no Ca2+-sensitive K+ currents.
(A) Representative traces of outward currents and small transient inward currents recorded from type 3 cells. Inward currents are activated at −20 mV, peak at −10 mV and reverse near 0 mV; outward currents increase continuously until the end of recording. (B) Small transient inward currents are completely inhibited in the presence of 0.5 μM TTX. (C) Mean I–V curves of steady-state outward currents in type 3 cells (n=23). (D) Mean I–V curves of steady-state outward currents under control conditions and in the presence of 2 mM 4-AP in type 3 cells (n=5). (E) Mean I–V curves of steady-state outward currents under control conditions and in the presence of 4 mM TEA in type 3 cells (n=7). (F) Mean I–V curves of 4-AP- and TEA-sensitive and insensitive outward currents in type 3 cells (n=5); inward currents are unaffected by 4-AP and TEA. (G) Outward currents in the presence of 200 μM CdCl2 in type 3 cells. (H) Mean I–V curves of steady-state outward currents before and after application of 200 μM CdCl2 in type 3 cells (n=6).
We also found that 9 SVZ cells (4%) displayed larger outward currents compared to those in type 1–3 cells. Immunostaining showed that these cells expressed GFAP. Therefore, whole-cell patch clamp recording was performed on brain slices from transgenic mice that express GFP under the control of the GFAP promoter. We found that most (17/24) GFAP-GFP-positive cells displayed large, but slowly activating and sustained outward currents without a fast inactivating component (Type 4 cells), whereas a minority (7/24) showed a large outward rectifier current with a fast inactivating component (Type 5 cells) (Figure 4). No GFAP-GFP-positive cells displayed inward currents. TEA (4 mM) reduced the amplitude of the outward currents by ~50% in both types of cells (Figure 5). Similarly, the outward currents were sensitive to 4-AP and CdCl2 (Figure 6).
Fig. 4. Electrophysiological properties of GFAP-GFP-positive (type 4 and 5) SVZ cells.
(A) Representative current traces show two subtypes of outward current in GFAP-positive SVZ cells. Holding potential was −75 mV and test potentials were from −140 mV to +140 mV, with 20 mV increments, for 400 ms. Most cells (type 4 cells) show slowly activating and non-inactivating outward current (a). The remainder (type 5 cells) show fast inactivating current with a small steady-state component (b). Arrows indicate time points at which steady-state currents were measured. (B) Mean I–V relationships obtained from group a (type 4) and group b (type 5) cells. Steady state outward current in group a (type 4) cells continues to increase with increasing depolarization, whereas that in group b (type 5) cells plateaus at about +60 mV. Inset, GFP-positive cells in SVZ of brain slice from GFAP-GFP transgenic mouse.
Fig. 5. Effects of channel blockers on outward current in type 4 cells.
(A) TEA (4 mM) decreased steady-state outward currents in type 4 cells. Representative current traces (a) and mean I-V curves of steady-state outward currents (b) are shown (n= 6). (B) 4-AP (2 mM) also reduced steady–state outward currents in type 4 cells. Representative current traces (a) and mean I–V curves of steady-state outward currents (b) are shown (n= 4). (C) CdCl2 reduced steady-state outward currents in type 4 cells. Representative current traces (a) and mean I–V curves of steady-state outward currents (b) are shown (n= 4). Holding potential was −75 mV and voltage steps were applied from −140 mV to +140 mV with 20 mV increments. Arrows indicate time points at which steady-state currents were measured.
Fig. 6. Effects of channel blockers on outward current in type 5 cells.
(A) TEA (4 mM) decreased steady-state and the transient peak outward currents in type 5 cells. Representative current traces (a) and mean I–V curves of steady-state outward currents (b) are shown (n=4). (B) 4-AP (2 mM) also blocks fast inactivating outward currents in type 5 cells. Representative current traces (a) and mean I–V curves of steady-state outward currents (b, n= 4). Holding potential was −75 mV and voltage steps were applied from −140 mV to +140 mV with 20 mV increments. Arrows indicate time points at which steady-state currents were measured.
All tested SVZ cells were excited with depolarizing inward current pulses. Spikes were found at the beginning of the pulse when the depolarizing inward current was increased from 0.12 nA to 0.16 nA (Figure 7A). The spikes were not affected in the presence of 0.5 μM TTX, suggesting that these spikes were not Na+ current-dependent (Figure 7B). In contrast, the spikes disappeared completely in the presence of 4-AP in extracellular solution and Cs2+ in internal solution, suggesting they were K+ current-dependent (Figure 7C). The electrophysiological properties and morphological characteristics of the type 1–5 cells in the SVZ in adult brain are summarized in Table 1.
Fig. 7. Type 1–5 cells SVZ cells fail to produce action potentials in response to depolarizing current.
(A) A few of spikes are observed at the beginning of pulses in all cell types when depolarizing inward currents are increased from 0.12 nA to 0.16 nA. (B) Spikes are not altered in the presence of 0.5 μM TTX. (C) Spikes are completely blocked in the presence of 2 mM 4-AP (out-solution) and Cs2+ (inter-solution), suggesting that spikes are K+ current-dependent.
Table 1.
Electrophysiological and immunocytochemical properties of SVZ cells
| Type1 | Type 2 | Type 3 | Type 4 | Type 5 | |
|---|---|---|---|---|---|
| Currents | KDR | KDR KCa2+ |
KDR Na+ |
KDR KCa2+ |
KDR KA-like |
| DCX expression | − | + | + | − | − |
| GFAP expression | − | − | − | + | + |
3. Discussion
In the present study, we examined electrophysiological membrane properties of SVZ cells in adult mouse brain slices using the whole-cell patch clamp technique. Five types of cells were found. Type 1 cells displayed 4-AP/TEA-sensitive (KDR) and CdCl2-sensitive inward currents. Type 2 cells exhibited Ca2+-sensitive K+ (KCa) and both 4-AP/TEA-sensitive (KDR) and 4-AP/TEA-insensitive currents. Type 3 cells showed 4-AP/TEA-sensitive (KDR) and 4-AP/TEA-insensitive, as well as small Na+ currents. Type 4 cells had a slowly activating, large linear outward current and a sustained outward current without fast-inactivating component. Type 5 cells had a large outward rectifying current with a fast inactivating component.
We also performed immunostaining in an effort to correlate electrophysiology with immunochemical phenotypes of these cells, and found that type 1 cells express neither DCX nor GFAP, type 2–3 cells express DCX, and type 4–5 cells express GFAP. These results were further confirmed using transgenic mice expressing GFP under the control of either the DCX or GFAP promoter. Previous immunohistochemical findings regarding the cellular composition of the SVZ led to the hypothesis that GFAP-positive astrocytes (type B cells) give rise to GFAP- and neuronal marker-negative precursors (type C cells) and thence neuronal marker-positive neuroblasts (Doetsch et al. 1997). By analogy, we propose that neurogenesis in the adult SVZ proceeds from electrophysiologically defined type 4 or 5 cells (astrocytes) → type 1 cells (NPCs) → type 2 cells (early differentiating neurons) → type 3 cells (later differentiating neurons).
The previous study most similar to ours also involved recording from SVZ cells in mouse brain slices, but electrophysiological properties of individual cells were not correlated with the expression of cell-type marker proteins (Wang et al. 2003a). Nevertheless, the results of the two studies are substantially in agreement. Specifically, both found KDR, KCa and Na currents, but not spontaneous action potentials. These properties, and the fact that the region recorded from in the previous study contained predominantly class III β-tubulin-immunoreactive cells, suggests that the main cell type studied corresponds to our type 3 cells.
Electrical activity, including that mediated through ion channels, appears to have an important role in neuronal development, influencing cell proliferation, migration, differentiation, synapse formation and survival (Spitzer 2006). With respect to neuronal differentiation in particular, ion currents have been implicated in ion channel expression, acquisition of neurotransmitter phenotype, and axonal and dendritic growth (Spitzer 2006). Although most supporting evidence for these observations comes from systems other than the adult SVZ, many parallels have been observed between embryonic and adult (Esposito et al. 2005) and between hippocampal and SVZ (Ming and Song 2005) neurogenesis. It is, therefore, likely that in SVZ neurogenesis as well, electrophysiological character is linked to cell fate.
How might the electrophysiology of NPCs be exploited to enhance endogenous neurogenesis for therapeutic purposes? K+ channel antagonists, including 4-AP and TEA, inhibit depolarization-induced neurogenesis in NPC cultures from Bax-deficient mice (Shi et al. 2007), raising the possibility that K+ channel activators might enhance neurogenesis. L-type Ca2+ channel agonists increase, whereas antagonists reduce, the percentage of neurons in cultures of rat hippocampal (Deisseroth et al. 2004) or mouse cortical (D’Ascenzo et al. 2006) NPCs, and antagonists also block ischemia-induced neurogenesis in mouse SVZ and hippocampus (Luo et al. 2005), suggesting that L-channel agonists might also be useful for promoting neurogenesis in vivo. Activation of ligand-gated channels, such as GABAA (Tozuka et al. 2005) and NMDA receptor-gated glutamate (Deisseroth et al. 2004) channels, likewise promotes hippocampal neurogenesis, so drugs that interact with these channels could stimulate SVZ neurogenesis as well.
4. Experimental Procedure
4.1. Brain slices
Transverse, 250 μm-thick brain slices were prepared from postnatal day 14 (P14) to P180 CD-1 mice. Animals were anesthetized with chloral hydrate and decapitated. Brains were removed and incubated at 28°C for 30 min in ice-cold sucros e slicing artificial CSF (ACSF) containing (in mM): 85 NaCl, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 25 glucose, 75 sucrose, 0.5 CaCl2, and 4 MgCl2, and equilibrated with 95% O2 and 5% CO2. Sucrose slicing solution was replaced by standard ACSF containing (in mM): 125 NaCl, 2.4 KCl, 1.2 NaH2PO4, 25 NaHCO3, 25 glucose, 1 CaCl2, and 2 MgCl2, supplemented with 10 μM D, L-APV and 100 μM kynurenate. A single brain slice was transferred to a recording chamber and submerged in continuously flowing ACSF, which differed from that described above in that it contained 2 mM CaCl2 and 1 mM MgCl2.
4.2. Electrophysiological recording
Recordings were obtained using the whole cell patch-clamp technique. Electrodes were pulled from thick-walled borosilicate glass on a Flaming/Brown P-97 pipette puller (Sutter Instruments, Novato, CA). Pipettes had resistances of 4–5 MΩ when filled with an intracellular solution containing 140 mM K-gluconate, 0.1 mM CaCl2, 2 mM MgCl2, 1 mM EGTA, 2 mM ATP•K2, 0.1 mM GTP•Na3 and 10 mM HEPES (pH adjusted to 7.25 with KOH). Whole-cell recordings were performed using an Axopatch 200B amplifier, and current signals were low-pass filtered at 2 kHz and digitized online at 5–50 kHz using a Digidata 1322A digitizing board interfaced a computer (Axon Instruments, Foster City, CA). Data were analyzed with pClamp 9.2 software. Action potentials were elicited by applying depolarizing current pulses (100 ms) ranging from −0.06 to 0.16 nA. Membrane currents were recorded with voltage step from −120 to +170 mV, 10-mV step (holding potential −70 mV) and −140 to +140 mV, 20-mV step (holding potential −75 mV). Mean I–V curves were calculated by averaging the individual I–V curves obtained from each cell in the study. All recordings were performed at room temperature.
4.3. Immunocytochemistry
After recording, slices were transferred to fixation medium containing 4% paraformaldehyde in PBS. Slices were washed three times with PBS for 1 hr and incubated in blocking solution (2% goat serum, 0.1% Triton X-100, 1% bovine serum albumin in PBS) for 1 hr at room temperature and then treated overnight at 4°C with primary antibodies. Immunocytochemistry was performed as described previously (Jin et al. 2004). Primary antibodies were: guinea pig polyclonal anti-DCX (Santa Cruz Biotechnology Inc.; 1:500), rabbit polyclonal anti-GFAP (Sigma-Aldrich; 1:400), rabbit polyclonal anti-β III tubulin (Covance; 1:1000) and mouse monoclonal anti-nestin (Chemicon; 1:200). Secondary antibodies were Alexa Fluro 488-, or 594- or 647-conjugated donkey anti-mouse, or anti-rabbit or anti-goat IgG (Molecular Probes; 1:200-500). Fluorescence signals were detected using an LSM 510 NLO Confocal Scanning System mounted on an Axiovert 200 inverted microscope (Carl Zeiss Ltd) equipped with a two-photon Chameleon laser (Coherent Inc.). Controls included omitting or preabsorbing primary or omitting secondary antibody.
Acknowledgments
This work was supported by NIH grant AG21980 (to K.J.) and NS44921 (to D.A.G.)
Abbreviations
- 4-AP
4-aminopyridine
- ACSF
artificial CSF
- DCX
doublecortin
- GFAP
glial fibrillary acidic protein
- GFP
green fluorescent protein
- NPCs
neuronal progenitor cells
- SVZ
subventricular zone
- TEA
tetraethylammonium
- TTX
tetrodotoxin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Blum R, Konnerth A. Neurotrophin-mediated rapid signaling in the central nervous system: mechanisms and functions. Physiology (Bethesda) 2005;20:70–78. doi: 10.1152/physiol.00042.2004. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang ZG, Li Y, Wang Y, Wang L, Jiang H, Zhang C, Lu M, Katakowski M, Feldkamp CS, Chopp M. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol. 2003;53:743–751. doi: 10.1002/ana.10555. [DOI] [PubMed] [Google Scholar]
- D’Ascenzo M, Piacentini R, Casalbore P, Budoni M, Pallini R, Azzena GB, Grassi C. Role of L-type Ca2+ channels in neural stem/progenitor cell differentiation. Eur J Neurosci. 2006;23:935–944. doi: 10.1111/j.1460-9568.2006.04628.x. [DOI] [PubMed] [Google Scholar]
- Deisseroth K, Singla S, Toda H, Monje M, Palmer TD, Malenka RC. Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron. 2004;42:535–552. doi: 10.1016/s0896-6273(04)00266-1. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito MS, Piatti VC, Laplagne DA, Morgenstern NA, Ferrari CC, Pitossi FJ, Schinder AF. Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J Neurosci. 2005;25:10074–10086. doi: 10.1523/JNEUROSCI.3114-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskell GT, LaMantia AS. Retinoic acid signaling identifies a distinct precursor population in the developing and adult forebrain. J Neurosci. 2005;25:7636–7647. doi: 10.1523/JNEUROSCI.0485-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, Greenberg DA. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci USA. 2001;98:4710–4715. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Peel AL, Mao XO, Xie L, Cottrell BA, Henshall DC, Greenberg DA. Increased hippocampal neurogenesis in Alzheimer’s disease. Proc Natl Acad Sci USA. 2004;101:343–347. doi: 10.1073/pnas.2634794100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Sun Y, Xie L, Peel A, Mao XO, Batteur S, Greenberg DA. Directed migration of neuronal precursors into the ischemic cerebral cortex and striatum. Mol Cell Neurosci. 2003;24:171–189. doi: 10.1016/s1044-7431(03)00159-3. [DOI] [PubMed] [Google Scholar]
- Kaushansky K. Lineage-specific hematopoietic growth factors. N Engl J Med. 2006;354:2034–2045. doi: 10.1056/NEJMra052706. [DOI] [PubMed] [Google Scholar]
- Liebau S, Propper C, Bockers T, Lehmann-Horn F, Storch A, Grissmer S, Wittekindt OH. Selective blockage of Kv1.3 and Kv3.1 channels increases neural progenitor cell proliferation. J Neurochem. 2006;99:426–437. doi: 10.1111/j.1471-4159.2006.03967.x. [DOI] [PubMed] [Google Scholar]
- Liu RH, Morassutti DJ, Whittemore SR, Sosnowski JS, Magnuson DS. Electrophysiological properties of mitogen-expanded adult rat spinal cord and subventricular zone neural precursor cells. Exp Neurol. 1999;158:143–154. doi: 10.1006/exnr.1999.7078. [DOI] [PubMed] [Google Scholar]
- Liu X, Wang Q, Haydar TF, Bordey A. Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors. Nat Neurosci. 2005;8:1179–1187. doi: 10.1038/nn1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. Proc Natl Acad Sci USA. 1993;90:2074–2077. doi: 10.1073/pnas.90.5.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo CX, Zhu XJ, Zhang AX, Wang W, Yang XM, Liu SH, Han X, Sun J, Zhang SG, Lu Y, Zhu DY. Blockade of L-type voltage-gated Ca channel inhibits ischemia-induced neurogenesis by down-regulating iNOS expression in adult mouse. J Neurochem. 2005;94:1077–1086. doi: 10.1111/j.1471-4159.2005.03262.x. [DOI] [PubMed] [Google Scholar]
- Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- Marshall CA, Suzuki SO, Goldman JE. Gliogenic and neurogenic progenitors of the subventricular zone: who are they, where did they come from, and where are they going? Glia. 2003;43:52–61. doi: 10.1002/glia.10213. [DOI] [PubMed] [Google Scholar]
- Mercier F, Kitasako JT, Hatton GI. Anatomy of the brain neurogenic zones revisited: fractones and the fibroblast/macrophage network. J Comp Neurol. 2002;451:170–188. doi: 10.1002/cne.10342. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- Parent JM, Valentin VV, Lowenstein DH. Prolonged seizures increase proliferating neuroblasts in the adult rat subventricular zone-olfactory bulb pathway. J Neurosci. 2002a;22:3174, 3173–3188. doi: 10.1523/JNEUROSCI.22-08-03174.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002b;52:802–813. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- Ponti G, Aimar P, Bonfanti L. Cellular composition and cytoarchitecture of the rabbit subventricular zone and its extensions in the forebrain. J Comp Neurol. 2006;498:491–507. doi: 10.1002/cne.21043. [DOI] [PubMed] [Google Scholar]
- Redmond L, Hockfield S, Morabito MA. The divergent homeobox gene PBX1 is expressed in the postnatal subventricular zone and interneurons of the olfactory bulb. J Neurosci. 1996;16:2972–2982. doi: 10.1523/JNEUROSCI.16-09-02972.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffler B, Walton NM, Lin DD, Goetz AK, Enikolopov G, Roper SN, Steindler DA. Phenotypic and functional characterization of adult brain neuropoiesis. Proc Natl Acad Sci USA. 2005;102:9353–9358. doi: 10.1073/pnas.0503965102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Miles DK, Orr BA, Massa SM, Kernie SG. Injury-induced neurogenesis in Bax-deficient mice: evidence for regulation by voltage-gated potassium channels. Eur J Neurosci. 2007;25:3499–3512. doi: 10.1111/j.1460-9568.2007.05624.x. [DOI] [PubMed] [Google Scholar]
- Spitzer NC. Electrical activity in early neuronal development. Nature. 2006;444:707–712. doi: 10.1038/nature05300. [DOI] [PubMed] [Google Scholar]
- Stewart RR, Hoge GJ, Zigova T, Luskin MB. Neural progenitor cells of the neonatal rat anterior subventricular zone express functional GABA(A) receptors. J Neurobiol. 2002;50:305–322. doi: 10.1002/neu.10038. [DOI] [PubMed] [Google Scholar]
- Stewart RR, Zigova T, Luskin MB. Potassium currents in precursor cells isolated from the anterior subventricular zone of the neonatal rat forebrain. J Neurophysiol. 1999;81:95–102. doi: 10.1152/jn.1999.81.1.95. [DOI] [PubMed] [Google Scholar]
- Suzuki SO, Goldman JE. Multiple cell populations in the early postnatal subventricular zone take distinct migratory pathways: a dynamic study of glial and neuronal progenitor migration. J Neurosci. 2003;23:4240–4250. doi: 10.1523/JNEUROSCI.23-10-04240.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thored P, Arvidsson A, Cacci E, Ahlenius H, Kallur T, Darsalia V, Ekdahl CT, Kokaia Z, Lindvall O. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells. 2006;24:739–747. doi: 10.1634/stemcells.2005-0281. [DOI] [PubMed] [Google Scholar]
- Tozuka Y, Fukuda S, Namba T, Seki T, Hisatsune T. GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron. 2005;47:803–815. doi: 10.1016/j.neuron.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Wang DD, Krueger DD, Bordey A. Biophysical properties and ionic signature of neuronal progenitors of the postnatal subventricular zone in situ. J Neurophysiol. 2003a;90:2291–2302. doi: 10.1152/jn.01116.2002. [DOI] [PubMed] [Google Scholar]
- Wang DD, Krueger DD, Bordey A. GABA depolarizes neuronal progenitors of the postnatal subventricular zone via GABAA receptor activation. J Physiol. 2003b;550:785–800. doi: 10.1113/jphysiol.2003.042572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittemore SR, Morassutti DJ, Walters WM, Liu RH, Magnuson DS. Mitogen and substrate differentially affect the lineage restriction of adult rat subventricular zone neural precursor cell populations. Exp Cell Res. 1999;252:75–95. doi: 10.1006/excr.1999.4621. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Ninomiya M, Hernandez Acosta P, Garcia-Verdugo JM, Sunabori T, Sakaguchi M, Adachi K, Kojima T, Hirota Y, Kawase T, Araki N, Abe K, Okano H, Sawamoto K. Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum. J Neurosci. 2006;26:6627–6636. doi: 10.1523/JNEUROSCI.0149-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda T, Bartlett PF, Adams DJ. K(ir) and K(v) channels regulate electrical properties and proliferation of adult neural precursor cells. Mol Cell Neurosci. 2008;37:284–297. doi: 10.1016/j.mcn.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Young KM, Fogarty M, Kessaris N, Richardson WD. Subventricular zone stem cells are heterogeneous with respect to their embryonic origins and neurogenic fates in the adult olfactory bulb. J Neurosci. 2007;27:8286–8296. doi: 10.1523/JNEUROSCI.0476-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Wang Y, Zhang L, Zhang Z, Tsang W, Lu M, Chopp M. Sildenafil (Viagra) induces neurogenesis and promotes functional recovery after stroke in rats. Stroke. 2002;33:2675–2680. doi: 10.1161/01.str.0000034399.95249.59. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Zhang L, Zhang ZG, Morris D, Jiang Q, Wang L, Zhang LJ, Chopp M. Migration and differentiation of adult rat subventricular zone progenitor cells transplanted into the adult rat striatum. Neuroscience. 2003;116:373–382. doi: 10.1016/s0306-4522(02)00696-6. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Zhang ZG, Zhang L, Chopp M. Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience. 2001;105:33–41. doi: 10.1016/s0306-4522(01)00117-8. [DOI] [PubMed] [Google Scholar]