Abstract
A diversity of immune tolerance mechanisms have evolved to protect normal tissues from immune damage. Immune regulatory cells are critical contributors to peripheral tolerance. These regulatory cells, exemplified by the CD4+Foxp3+ regulatory T (Treg) cells and a recently identified population named myeloid-derived suppressor cells (MDSCs), regulate immune responses and limiting immune-mediated pathology. In a chronic inflammatory setting, such as allograft-directed immunity, there may be a dynamic “crosstalk” between the innate and adaptive immunomodulatory mechanisms for an integrated control of immune damage. CTLA4-B7-based interaction between the two branches may function as a molecular “bridge” to facilitate such “crosstalk”. Understanding the interplays among Treg cells, innate suppressors and pathogenic effector T (Teff) cells will be critical in the future to assist in the development of therapeutic strategies to enhance and synergize physiological immunosuppressive elements in the innate and adaptive immune system. Successful development of localized strategies of regulatory cell therapies could circumvent the requirement for very high number of cells and decrease the risks associated with systemic immunosuppression. To realize the potential of innate and adaptive immune regulators for the still-elusive goal of immune tolerance induction, adoptive cell therapies may also need to be coupled with agents enhancing endogenous tolerance mechanisms.
Keywords: Immune tolerance, immunosuppression, regulatory cells, CTLA4, autoimmunity, transplant
Introduction
Multi-cellular organisms are equipped with an innate immune system that recognizes objects with a pattern distinct from their own tissues. Vertebrates have also evolved an adaptive immune system to fend off infectious agents with a fine specificity. Cell and organ transplants from genetically non-identical donors are recognized for their allogeneic “foreign” antigens by the host's immune system and rejected if anti-graft immune responses are not prevented or subdued. Since Medawar and colleagues demonstrated in 1953 that some mice treated with allogeneic cells at neonatal age tolerated skin allografts later (10), the hope for development of successful strategies for achieving acquired immune tolerance has dominated several decades of immune tolerance research with the objective to control alloimmunity and/or autoimmunity.
A variety of tolerance mechanisms have been discovered to be responsible for protection of the body's own tissue from immune injuries while effectively fighting pathogens. For T lymphocytes, these mechanisms include central tolerance (thymic deletion of autoreactive T cells). In transplantation, central tolerance may have contributed to the success of selected tolerance induction strategies aiming at the establishment of donor bone marrow chimerism (131). A critical mechanism of peripheral tolerance is dominant suppression by the Foxp3+ Treg cells (101), which is regarded with extraordinary potential by immune tolerance strategies. Nonetheless, a complex and synergistic interaction of several tolerance induction mechanisms may be required to prevent autoimmune damage in a normal individual (20), and may also be required for the induction of tolerance to transplanted organs, tissues or cells.
The CD4+ Foxp3+ Treg cell: a key suppressor in the adaptive immune system
Lymphocytes are the major effectors of adaptive immunity. Immunosuppression by lymphocytes, however, had remained an elusive and controversial phenomenon until Sakaguchi and Powrie independently identified inhibitory subsets of murine T cells expressing CD25 (100) or having a lower level of CD45RB (92). These two murine phenotypes overlap in a subset of CD4+CD25+ regulatory T cells that were later found by several studies to have a definitive marker, the Foxp3 transcription factor (101). Immunosuppressive activities have also been found in other lymphocyte subsets, including NK cells, NK T cells and B lymphocytes. Suppressive inflammatory cytokines, such as IL-10, can induce suppressive T cells without implicating Foxp3 expression, of which the Tr1 cell is a well known example (48). These various subsets of suppressive lymphocytes could play an important role within different contexts of inflammatory settings. Extensive studies have established Foxp3+ Treg cells as a key subset of regulatory cells that maintain homeostasis of the immune system, preventing the development of autoimmune responses. Lack of the Foxp3 gene causes a human autoimmune disorder, the Immunodysregulation, Polyendocrinopathy, Enteropathy, X linked (IPEX) Syndrome, characterized by chronic diarrhea, type 1 diabetes mellitus (T1DM), and inflammatory damages to other organs in early childhood (135). Mice with a null mutation of the Foxp3 gene have massive lymphoproliferation and severe inflammatory infiltration of the skin, liver and lung. Acute ablation of Treg cells in adult animals induced lethal autoimmune disorders (61). In a mouse model T1DM, absence of Foxp3 led to fulminant autoimmune destruction of pancreatic islet β cells in very young animals that would have otherwise been protected from diabetes for their life time (21). Evidence from extensive studies in the last decade has indicated that Treg cells have perhaps the most versatile suppressive function among immunoregulatory cells identified so far. The robust suppressive activity of Treg cells in various pre-clinical tests has led to an extraordinary expectation of Treg cell therapies for a variety of human immune disorders, for example, T1DM and transplantations.
Treg cells in transplantation
Transplantation is a vital remedy in cases of organ failure or for the replacement of a defective physiologic function, such as insulin production in T1DM, but immune rejection of transplants has been a major hurdle. Potent immunosuppressive drugs are required to suppress rejection of the grafts. The indiscriminative immunosuppression often causes serious and sometimes life-threatening side-effects. It remains a goal to induce immune tolerance to transplants, so patients with transplants can achieve permanent acceptance of grafts in the absence of life-long immunosuppressive drugs. The discovery of Treg cells opens an exciting new venue for immune control of transplant rejection (138). Transplantation, on the other hand, offers one of the most appealing platforms for a Treg cell therapy trial for two reasons: 1) T lymphocytes play a major role in allotransplant rejection; 2) Treg cell therapy may be timed precisely to certain stages of graft rejection responses and the stages can be clinically managed with timing of surgery and a battery of immunosuppressive agents.
Preclinical studies with animal models of allotransplantation have indeed provided clear evidence to justify adoptive Treg cell therapies in clinical trials. Some trials have already begun (97,98). The preclinical efficacy of Treg cell therapy in transplantation experiments so far, however, has been limited to graft recipients with lymphopenic conditions, such as following irradiation or lymphodepletion treatments. It remains to be shown whether Treg cell therapies can be effective in transplantation settings in which the hosts are fully immunocompetent. Conceivably, Treg cell therapy would be difficult to succeed in such settings due to lack of “space” in a fully competent immune system which could make it challenging for adoptively transferred Treg cells to engraft and persist. However, host conditioning strategies that result in lymphopenia are well developed in the context of hematopoietic stem Cell Transplantation (HSCT) from allogeneic donors. In clinical HSCT, alloimmune responses of donor-derived T cells against recipient tissues cause graft-versus-host disease (GVHD), which is a major cause of morbidity and mortality. The protective role of Treg cells against GVHD was reported by Taylor et al (118) and subsequently confirmed by others (97,98). The field of prevention and treatment of GVHD provides perhaps the most convincing evidence for Treg-cell-based control of alloimmunity. Indeed, a pioneering clinical trial of Treg cell therapy in HSCT (97) is in progress with great anticipation for its possible implications for several immune-mediated conditions.
Even if lymphopenia is a requirement for engraftment of adoptive Treg cells, such therapy may still be applicable to the broader field of transplantation. Transient lymphopenia is an integral part of post-transplant physiopathological condition in several clinical settings besides HSCT. Lymphodepleting agents, such as ATG, anti-CD20 antibodies and Campath 1-H (anti-CD52 antibodies), are increasingly used. Lymphopenia is even observed and considered a requirement for success of immunosuppressive strategies in transplant recipients treated with agents that are not specifically designed for a primary lymphodepleting effect, such as the combination of rapamycin, FK506 and anti-IL2R antibodies (Edmonton Protocol) in islet transplant recipients (79).
Treg cells in T1DM
T1DM, also called insulin-dependent diabetes mellitus (IDDM), is mainly caused by T-cell mediated, spontaneous autoimmune destruction of the insulin-producing β cells in pancreatic islets. The critical features of IDDM in humans, especially the immunological aspects, are closely mimicked by the spontaneous diabetes in a murine model, the nonobese diabetic (NOD) mouse. Immunological research in T1DM has served as a flagship for the field of immune tolerance induction. Indeed, the discovery of Treg cells has led to an intensive focus on their preventive and therapeutic potentials for T1DM. The protective role of naturally-occurring Foxp3+ Treg (nTreg) cells has been unequivocally established in animal models of T1DM by many independent studies (107). T1DM is a major manifestation of IPEX, indicating that a lack of Treg cells causes a high probability of diabetes (135). However, IPEX cases are very rare. Most T1DM patients do not have a loss-of-function mutation in the Foxp3 gene. Actually, characterization with the Foxp3 marker for Treg cells refuted an earlier notion that there was a readily detectable Treg cell deficit in the general population of T1DM (16). In addition, there is no association of T1DM incidence with genetic polymorphisms at the Foxp3 locus in the general population (11). The upshot that Treg cells in most T1DM patients appear normal conveys a piece of mixed news. On one hand, the patients' own Treg cells, after in vitro expansion (70,93), should serve competently for potential therapeutic use; on the other hand, it raises doubt on the rationale for infusing more Treg cells into the patients if they do not have Treg cell deficit to begin with. Adding to the complexity, recent studies with peripheral blood samples of T1DM patients showed that the deficit of Treg-mediated in vitro immune suppression did not reside in Treg cells per se, but rather was due to resistance of pathogenic Teff cells from T1DM patients to Treg-cell-mediated suppression (65,103). These findings from in vitro human cell studies are consistent with in vitro and in vivo studies using the NOD mouse model of T1DM (26). In other words, although a clinical trial of Treg cell therapies for T1DM may well be encouraged by robust evidence from pre-clinical studies and logistic advances in expansion of Treg cells in vitro, Treg cell therapies alone may not meet the expectation of a “wonder cure” for most patients. Perhaps, a valuable lesson learned from the NOD model of T1DM is the diversity of immunological mechanisms that is required to enforce tolerance of pancreatic β cells. Besides the intrinsic “unruliness” of the pathogenic Teff cells, studies have demonstrated a flawed central tolerance as a root cause of the autoimmune diabetes in this model (75). Defective central tolerance may not be readily amenable with adoptive Treg cell therapies (75). In addition, alteration of innate immune cells may also contribute to the breakdown of immune tolerance in the NOD models, as suggested by many studies. Thus, a more effective outcome of immune tolerance therapy for T1DM may require a strategy that integrates the various tolerogenic elements in the immune system.
MDSCs as innate Immunomodulators
Cells derived from myeloid hematopoiesis, including granulocytes, monocytes (macrophages, MΦ) and dendritic cells (DCs) are critical constituents of the innate immune system. Tumor immunologists have long discovered the phenomenon of immunosuppression by cells from myeloid origins. Among the pioneers are Lopez and colleagues, who discovered suppressive activity of macrophages in tumor bearing animals (37). Suppressive macrophages were also identified in human cancer tissues (62). Their immunosuppressive function was shown to be mediated, at least partially, by B7-H4. B7-H4 is a recently discovered member in the co-stimulatory family but functions as an inhibitory molecule suppressing immune responses. It plays a distinct role in modulating both adaptive and innate immunity (142). A novel agent, human B7-H4-Ig fusion protein, showed potential in suppressing in vitro responses of human T cells to β cell antigens (88).
DCs, well known for their capacity to elicit immunity, also play a substantial role in the physiological process of immune tolerance induction. DCs refer to a heterogeneous population of professional antigen presenting cells (APCs) derived from bone marrow (113). The “two-signal' hypothesis states that lymphocyte activation requires not only APCs to present antigen-specific signals T cells, but also proper quantity and quality of co-stimulatory signals (13,68). The expression of co-stimulatory molecules by DCs is tightly controlled, with immature DCs lacking sufficient co-stimulatory molecules serving to enforce immune tolerance under a physiological steady state. Inflammatory signals elicited by infections or injuries cause maturation of DCs with a high level expression of co-stimulatory molecules and thus can elicit immunity. Some members of the co-stimulatory family negatively regulate T cell responses and are called co-inhibitors. An unbalanced presentation of co-inhibitory signals by DCs could also lead to suppression of adverse immune responses such as that in transplant rejection (124). Thus, DCs uploaded with specific antigens can be manipulated to be immunogenic to fight cancer and infectious agents, or tolerogenic to specifically turn off transplant immunity and autoimmunity (43,80,112). Although a tolerogenic DC (tDC) is not specific to a DC subset nor restricted to the immature state of the cell, immature or maturation-resistant DCs are more likely to be tolerogenic (80). Indeed, in vitro derived immature DCs with low expression of B7 co-stimulatory molecules protected allogeneic heart transplants in mice (36). The graft-protecting effect could be mediated by tDCs from host (40) or donor origins (71). Cell therapies with tDCs remain an appealing concept, particularly with its potential to propagate other tolerogenic elements in the adaptive and innate immune system (80). However, a robust protocol to control DC generation, proliferation and differentiation remains to be developed, likely awaiting a better understanding of the DC biology (42). The risk of terminal differentiation of immature DC into mature DC and thus exacerbating transplant rejection and autoimmunity may also be a substantial issue, especially in patients at the per-transplantation period with surgery-induced milieu of inflammatory signals.
MDSC — new suppressors increasingly attract attention
In mouse models of chronic inflammations, especially under tumor-bearing settings, a heterogeneous population marked by cell surface expression of both Gr1 and CD11b, now known collectively as MDSCs (38), undergo massive expansion. The immunosuppressive function of MDSCs is best characterized in tumor-bearing animals. Evidence also exists for their function in human patients with cancer (39,87). Unlike Treg cells which can be defined by Foxp3 expression (in mice), MDSCs are not a defined cellular lineage/subset, but instead an inflammation-induced collection of heterogeneous immature myeloid cells with common markers and immunosuppressive activity. Nonetheless, evidence for the suppressive function of MDSCs in a number of inflammatory settings (39,87) does suggest that they may constitute a type of myeloid regulatory cell or innate suppressor.
CD11b+Gr1+ myeloid cells represent a major population in the bone marrow, reflecting a continuous physiological process of myelopoiesis. They normally develop into mature myeloid cells, including granulocytes, macrophages and DCs. Under chronic inflammatory settings such as in tumor-bearing hosts, the cells with these immature phenotypes accumulate abnormally in the periphery including in the spleen and peripheral blood. Furthermore, the chronic inflammatory signals also activate these cells to produce arginase 1 to deplete arginine, so T cells in the vicinity would be “starved” and thus suppressed. Alternatively, activated MDSCs may produce inducible nitric oxide synthase (iNOS) and reactive oxygen species (ROS) to inhibited T cell signaling (39,87). Although the spleen is the peripheral site that MDSCs abound in tumor-bearing mice, the cells accumulate at the non-lymphoid sites of inflammation as well. For example, Ilkovitch and Lopez (55) recently discovered that MDSCs accumulated in the liver to an extent comparable to the spleen in tumor-bearing mice models, regardless of the existence of a metastatic tumor in the liver. The accumulation may be contributed by local hematopoiesis in the liver that was driven by inflammatory signals. This observation may have a particular bearing to islet transplantation, as the liver is the current site of choice for clinical islet transplantation, and it has been shown as a site where tDCs and other elements may reside to render it conducive to tolerance induction (23,143).
Evidence for a role of MDSCs in transplant tolerance is being gathered in different animal models of transplantation. Zhang et al (144) reported that in immunoglobulin-like transcript-2(ILT-2) transgenic mice, MDSCs expanded and their suppressive activity were enhanced. The enhanced MDSC suppression prolonged the skin allograft survival in this model. With a rat model of kidney transplant tolerance induced by anti-CD28 antibodies, Dugast et al (28) identified rat MDSCs with phenotypes and in vitro suppressive activity equivalent to their mouse counterparts. The cells expressed iNOS and accumulated in the tolerized kidney allograft. Inhibition of iNOS broke the established tolerance and led to graft rejection (28). In these transplantation studies (28,144), activated MDSCs were found in peripheral blood as well as at the transplant site, indicating a potential involvement of the cells in both systemic and local immune tolerance, perhaps in response to stages of transplant immunity and progression of tolerance induction.
CTLA4-B7 molecular interaction in a “crosstalk” between adaptive immunoregulators and innate immunosuppressors
A critical role for CTLA4 in immune tolerance
How exactly Treg cells exert their function in vivo remains a question, but clues come from characteristic molecules expressed by these cells. Among the suggestive markers is CTLA4, a member of CD28/B7 co-stimulatory family, which is expressed by Treg cells constitutively (94,102,114). Mice lacking CTLA4 exhibit devastating lymphoproliferative disorders, multi-organ inflammatory damage and lethality at early ages, unequivocally establishing CTLA4 as an inhibitory receptor for T lymphocytes (120). Very recently, Sakaguchi and colleagues reported that Treg-specific CTLA4-deficient mice exhibited systemic lymphoproliferation, definitively establishing a role of CTLA4 in Treg cells (136). However, CTLA4 expression by both Treg cells and Teff cells are apparently required for its full inhibitory function (35,89).
Molecular Models for CTLA4-based immunoregulation
Robust experimental evidence indicates that CTLA4 is not involved in central tolerance in the thymus (17,133). On the other hand, CTLA4 has been demonstrated to play a critical role in peripheral regulation of T lymphocytes through a variety of cellular and molecular mechanisms. First, CTLA4 is rapidly upregulated on Teff cells upon antigen stimulation through the specific T cell receptor (TCR) and counters CD28-mediated positive co-stimulatory signals. This direct modulation of Teff cell activation is achieved through two mechanisms that are not mutually exclusive. One mechanism is to competitively sequester B7 ligands from CD28, since CTLA4 has a much higher affinity for B7 than CD28. Engaging CTLA4 on Teff cells may also deliver an inhibitory signaling through the cytoplasmic tail of CTLA4. CTLA4 likely functions through both pathways because: the lymphoproliferation and inflammation destruction in mice can be curtailed by transgenic expression of either truncated CTLA4 without cytoplasmic domains (73) or mutated CTLA4 incapable of B7 binding (24); protein transduction with CTLA4 cytoplasmic domain inhibited experimental allergic asthma (25); and CTLA4-blockade accelerated rejection of allografts in CD28-deficient animals (67). Second, constitutive expression of CTLA4 by Treg cells suggests an important role of CTLA4 in Treg cell function. After much controversy in this crucial aspect of immune regulation, an essential role for CTLA4 in Treg cells was definitively characterized recently (136). Third, CTLA4, expressed by Treg or activated Teff cells, interacts with B7 molecules on APCs to induce expression of indoleamine 2,3-dioxygenase (IDO) in APCs and render the APCs tolerogenic (30).
Cellular “motility” model for CTLA4-mediated immunoregulation
Apparently, none of the afore-discussed molecular models can explain the full function of CTLA4. First, over expression of CTLA4 extracellular domain or signaling motif can only partially rescue the CTLA4-deficiency in animals. Second, despite the intricate overlap of function between CTLA4 and Treg cells, it has been demonstrated that CTLA4 and Treg cells have also distinct roles in immune tolerance (29,54). Third, IDO-deficient mice are apparently healthy, although IDO2 compensation is possible (77).
During the last few years, with the advanced technologies of imaging, it has been discovered that lymphocyte motility is a key element of immune responses. T cells are highly motile in steady-state physiology, but upon encountering with APC presenting specific antigens, T cells stop and engage with the APCs which leads to T-cell proliferation and differentiation (41). Thus, regulation of the T cell motility would serve as a “global” cellular control. Schneider et al (104) found that CTLA4 might reverse the antigen-induced T cell “arrest” and promoted T-cell motility, by ex vivo imaging of explanted lymph nodes maintained in oxygenated medium. The physiological relevance of the observations needs to be validated by noninvasive intravital imaging. However, the motility regulation model of CTLA4 function may offer a plausible explanation for a role of CTLA4 in regulating immune cell interaction in lymphoid and nonlymphoid target tissues. It also suggests T-cell motility control as a new venue of CTLA4-based immunotherapy for immune-mediated diseases.
The quantitative variations of CTLA4 expression are associated with making or breaking of immune tolerance in humans
Although the CTLA4o/o animal model served in many studies to address key questions in immunoregulation, the relevance of this model to human diseases is unclear. There is no report of complete loss of CTLA4 in humans. Genetic studies have linked the CTLA4 locus to specific human autoimmune diseases, including T1DM (119), rather than a lymphoproliferative disorder characteristic of the CTLA4o/o mice. No variation in the regions coding for CTLA4 mature proteins has been implicated, but several polymorphisms in the promoter or 3'-untranslated region (UTR) have been identified to affect promoter efficacy for CTLA4 expression (2,5,66,132) and/or the ratio of alternatively spliced forms of CTLA4 mRNA (127). In animal models, some studies found that autoimmunity -susceptible NOD mice expressed a lower level of full-length CTLA4 mRNA (72), whereas another study linked NOD mice to a modest decrease in expression of a CTLA4 splice variant (127). What is striking from these studies is that even a modest reduction of CTLA4 expression predisposes loss of immune tolerance.
In clinical transplantation, polymorphisms of the CTLA4 locus were linked with graft-versus-host diseases and acceptance of allotransplants (4,46,90,137), although conclusive evidence from a larger size of samples is still lacking. Increased frequencies of transplant rejection were associated with a polymorphism at the promoter region of the gene (−318C) that dictates only a modest reduction of CTLA4 expression (46,66,132,137), suggesting that a subtle quantitative variation of CTLA4 may reset the threshold for transplant tolerance or rejection.
To assess the impact of a modest reduction in CTLA4 expression on immunoregulation, a transgenic mouse model with CTLA4 RNAi has been established (22). In this model, the expression of CTLA4 is reduced 2- to 3-fold in both Treg cells and activated T cells. Unlike CTLA4o/o mice, which exhibit indiscriminate multi-organ inflammatory infiltrations, CTLA4 RNAi knockdown (KD) animals exhibited MHC-dependent and site-specific autoimmune damage of the pancreatic islet in the absence of global perturbation of the immune system (22), indicating that, indeed, a modest reduction of CTLA4 signals can have a profound but specific impact on immune tolerance.
The quantitative signals from CTLA4 might serve as a molecular “bridge” to facilitate “crosstalk” between innate and adaptive immunoregulators in inflammatory settings
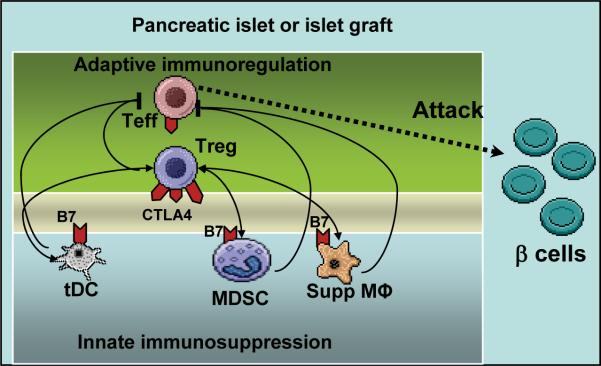
It remains to be studied how a modest variation of CTLA4 levels profoundly impacts immune tolerance induction. Presumably, evolution has shaped immune tolerance mechanisms into a system that integrates both the innate and adaptive branches. CTLA4 is constitutively expressed by Treg cells at high levels. It is further upregulated in the damage- controlling phase of a T1DM model in pancreatic target tissue (21). The B7 ligands of CTLA4, particularly B7.1 (CD80), can be expressed by cells in the innate immune system including tDCs (80) and suppressive macrophages (62). MDSCs also express B7.1 (81,110,141). The MDSC-mediated suppression of antitumor T cell responses depended on CTLA4-B7 interaction (141). Crystallography structural analysis of CTLA4-B7 interaction revealed that CTLA4 homodimers and alternating B7.1 homodimers can form a “zipper”-like structure (111). This interaction may serve to directly bridge T cells and tolerogenic innate immune cells expressing B7.1. On the other hand, since CTLA4 and B7 can transmit intracellular signals, CTLA4-B7 based interaction between T cells and the innate suppressors can trigger a circuitry of gene expression regulation among the regulatory cells. Taking together, the evidence suggests an integrative model of innate and adaptive immune regulation. Quantitative CTLA4 signals may regulate interactions between Treg cells and innate suppressor cells, by impacting, not mutually exclusive, the signal strength of potential regulatory “loops” for innate and adaptive immune molecules, or the strength of an intercellular CTLA4-B7 “zipper” between the adaptive and innate regulatory cells. The CTLA4-B7-mediated integration of innate and adaptive immunosuppressors can facilitate an effective control of the destructive activity of pathogenic T cell responses (Figure 1).
Figure 1.
Bridging adaptive and innate immune regulators by CTLA4 and B7 molecules at inflammatory sites. Illustrated are potential interplays between immune regulatory cells and pathogenic T cells, at inflammatory sites exemplified by pancreatic islets either undergoing autoimmune attack at the onset of type 1 diabetes mellitus, or becoming the target of graft-directed immunity following islet transplantation. CTLA4-B7 interaction could function as a molecular “zipper” between Treg cells and innate suppressors, or impact a signaling “loop” of gene expression that regulates the “crosstalk” between the innate and adaptive immunoregulators. CTLA4 is constitutively expressed by Treg cells at high levels but also expressed on Teff cells upon activation. The schematic drawing is oversimplified, omitting well-characterized elements which are however beyond the scope of the review. Treg: regulatory T cells; Teff: effector T cells; tDC: tolerogenic dendritic cell; MDSC: myeloid-derived suppressor cell. Supp MФ: suppressive macrophage. Two-way arrows indicate a potential “crosstalk” between the innate and adaptive suppressor cells. An integrated immune regulatory network “brakes” pathogenic activity of Teff cells.
Challenges of immunoregulatory cell therapies
In the past decade, Treg cells have attracted persistent and extensive efforts of most immunologists. Yet, an efficacy of Treg cell therapies in clinical trials still awaits to be documented, reflecting the daunting tasks to bring this promising therapy to benefit patients. The logistic and scientific challenges facing clinical tests of Treg cell therapies are well explained in a recent review by Riley et al (97). Among all difficulties and uncertainties, the requirement of antigen-specificity of Treg cells may pose the most compounding factor.
In murine models of T1DM, β-cell-specific Treg cells are far more effective in controlling autoimmune damage than non-specific polyclonal cells (115,117). In a mouse model of fulminant autoimmune diabetes caused by genetic mutation of the Foxp3 gene, only the β-cell-specific Treg cells, but not the non-specific cells, could successfully reconstitute the regulatory control of diabetes development (21). Proof-of-principle preclinical studies have been conducted to enrich and amplify β-cell-specific Treg cells (74,116), but successful expansion of human β-cell-specific Treg cells has yet to be documented. If it indeed takes “a billion” (97) antigen-specific Treg cells to elicit a protective efficacy in T1DM trials, it might take an extended period of time and effort to realize a substantial impact by this therapy in clinics.
In transplantation, the superiority of alloantigen-specific T cells has also been well-documented (44,56,125). On the other hand, antigen-specificity may not be an absolute requirement (123). The efficacy of non-specific Treg cells in a transplantation setting may be due to the potential of the transferred Treg cells in suppressing the homeostatic proliferation of pathogenic alloreactive Teff cells. Although lymphodepleting agents have been used successfully to suppress acute rejection of allografts and promote grafts from alloimmune damage, lymphopenia created by immunodepleting agents is followed with homeostatic proliferation of the residual Teff cells and heightened memory T cell formation. In animal models, lymphopenia-driven homeostatic proliferation has been shown to present a formidable obstacle for immune tolerance induction (139). In human patients, the transient lymphopenia caused by immunosuppression in the “Edmonton Protocol” was attributed to homeostatic expansion of autoreactive memory T cells that was believed to play a critical role in rejecting islet grafts in patients (79). Treg cells can efficiently modulate the pace and amplitude of lymphopenia-driven homeostatic proliferation in a manner that is apparently not limited by antigen-specificity (3,6). Thus, a therapy with nonspecific Treg cells may still benefit graft survival by modulating homeostatic proliferation and activation of Teff cells in transplant recipients. The peri-transplantation lymphopenic conditioning should also facilitate engraftment of the adoptive Treg cells, providing that the Treg cell therapies are administered in conjunction with a regimen that is not deleterious to the transferred cells or at a time window when lymphodepleting agents are cleared from circulation and homeostatic recovery of the host T cells has yet to begin.
As for MDSCs, rigorous efforts are devoted by many groups to examine their potential for clinical translations. The cells may be generated in vitro by culture of bone marrow cells with a cocktail of cytokines. However, MDSC therapies will probably be more challenging to materialize in clinical trials than Treg cell therapies. Aside from the relative early stage of knowledge in MDSC biology compared to that of Treg cells, myeloid cells are notoriously `fragile” to in vitro processing. In addition, a large number of the cells or more frequent dosing will likely be needed because unlike lymphocytes, myeloid cells are believed to have a short life span and have no capability of homeostatic expansion upon transfer to a new host. The potential of the cells to further differentiate to mature myeloid cells, which may mediate immune destruction, makes it a challenge to study their suppressive role in vivo, and poses a potential risk in its clinical translation.
A local solution—immune tolerance induction at the transplant site
Regulated unfolding of T1DM highlighting the potency of local immunoregulation
T1DM exhibits a protracted course of development. In humans, autoantibodies against islet antigens, evidence of autoreactivity against β-cells, can precede diagnosis of clinical diabetes by years. In NOD mice, insulitis begins to develop in the animal at about 4 weeks after birth, but inflammatory pathology can be kept under control and diabetes is not evident until ~12 week of age. To track pathogenic T cells during diabetes development, several lines of T cell receptor (TCR) transgenic models have been developed, among which is the BDC2.5 TCR transgenic line. This line expresses the TCR of a diabetogenic CD4+, Th1-like, T cell clone (51,58) that recognizes an unknown islet antigen presented by the NOD MHC class II molecule, Ag7, but the islet antigen itself is shared by all different strains of mice tested (52). In BDC2.5/NOD mice, massive insulitis begins sharply around 2 weeks of age in all mice, immediately after the activation of the islet-specific T cells in the pancreatic draining lymph nodes (PLN) (58). The development of overt diabetes, however, is not accelerated but suppressed in BDC2.5/NOD mice; most animals never develop full-blown diabetes even with rampant inflammation present in the pancreatic islets (45). The diabetogenic T cells in these animals, despite their invasion in high numbers into the pancreas, are kept in check and a sufficient number of β-cells remain to keep the animals free of diabetes.
Studies with a genetic model of Treg cell deficit (21) established an identity of regulatory cells in the BDC2.5 models. Foxp3 deficient BDC2.5 mice are devoid of CD4+CD25+ Treg cells, and develop diabetes with 100% penetrance at a “juvenile” age (by 20 days). Treating these animals with even a small number of islet-specific CD4+CD25+ T cells substantially inhibits diabetes development, whereas non-specific CD4+CD25+ T cells are not effective. In this genetic model of Treg cell function, Treg cells did not inhibit the initial activation, proliferation and Th1-subset differentiation in the PLN, nor did they delay the onset of T cell infiltration of pancreatic islets. It appeared that Treg cells enforced protection of pancreatic islets by suppressing aggression of pathogenic cells in the pancreas, halting progression from insulitis to overt diabetes (21).
Action of Treg cells local at the transplantation sites and tumor tissues
There is strong evidence that Treg cells act in the transplant target tissue (121). For example, re-graft experiments demonstrated that Treg cells from tolerated skin grafts transferred dominant tolerance into new recipients (47). In kidney transplantation in nonhuman primates, presence of Treg cells in the inflammatory infiltrates of allografts correlated with graft acceptance (122). Conversely, preventing intragraft accumulation of Treg cells correlated with rejection of cardiac allografts precipitated by toll-like-receptor stimulation (19). A recent study by Shapiro's group found that the intra-graft immunopathological balance established in tolerized islet grafts enabled the grafts to resist allorejection when re-transplanted into a new host (83). Treg cells are also present in lymphocytes infiltrating tumors, where they were thought to dampen the immune destruction of tumor cells at the microenvironment of tumor tissues (145).
A “local” approach to achieve immune tolerance with a bioengineered platform
The potency of Treg cells in local protection of pancreatic islets (21) illustrates a concept of local immune tolerance in the target tissue of immune damage, and suggests venues of local immunomodulation at the immune action site. Immunotherapeutic agents are often administered in a way that results in systemic distribution in the body, but immunomodulation does not have to be systemic. One routine example in medicine is the topical application of anti-inflammatory agents to treat skin diseases. Local immunosuppression can efficiently deliver the agents with a much lower dose and effectively circumvent systemic side-effects of the drugs. Indeed, promising leads of local immunosuppression have been reported in some experimental transplantation settings (49).
However, local delivery and retention of immunomodulatory agents are difficult to achieve in most transplantation settings, including islet transplantation. Currently clinical islet transplantation is performed by infusion of purified donor islets into the liver through the portal vein. There are increasing concerns that the liver might represent a less than optimal environment for islets. Factors associated with liver physiology and metabolism may have contributed to the progressive loss of islet transplants. The physiological role of the liver as a portal to the systemic circulation also makes it difficult to limit systemic distribution for agents applied to the liver. To tackle the limitations of intraportal infusion of islets in current clinical islet transplantation, alternative anatomical sites are explored for islet transplantation (128). Neosites are also created by bioengineering approaches. A biohybrid (BHD) device has shown promise in animal models to create an alternative, easy-to-access site of islet transplantation (91). The novel transplantation site engineered with the BHD device was shown to sustain a long-term survival and function of islet transplants (91). Since the site can support the complex physiology of β cells, it is reasonable to expect that such a site will also be compatible with the survival and function of Treg cells and innate suppressors. This platform may also be substantially improved in combination with tissue engineering approaches such as scaffolding, nanoparticle coating and pegylation (34,86,134). The success of such a bioengineering platform would create a defined niche for local therapies with immunoregulatory cells.
Local therapies could circumvent the risk of general immune suppression associated with systemic infusion of a large number of Treg cells (97). Importantly, it may substantially reduce the number of cells required for a therapeutic effect. In addition, it might by-pass or mitigate the requirement of antigen-specificities of Treg cells. One reason that antigen-specific Treg cells work far more efficiently may be due to effective recruitment of Treg cells to the local site of inflammatory damage. This premise would suppose that local delivery of activated Treg cells can suppress immune damage regardless of antigen-specificity, since one of the trade-mark activities of Treg cells in vitro is suppressing immune responses non-specifically. Furthermore, a cocktail of suppressive cytokine milieu at the local inflammatory site, contributed by local regulatory and other cells, may even convert Teff cells to antigen-specific Treg cells (130), inducing a long-lasting local immune tolerance.
Immunomodulation to enhance endogenous regulatory mechanisms
See no evil
Immunoregulatory cell therapy presents an appealing venue in future medicine. However, immunomodulation to elicit the potentials of patients' endogenous tolerogenic elements may also be critical for effective induction of immune tolerance. Immunomodulatory agents generally are viewed as necessary “evils” in transplantations. Recent evidence, however, suggest that there may be “good” (tolerogenic potential) in the “evil”, in that some immunosuppressive agents appear conducive to tolerance induction, whereas others may be counterproductive to immune tolerance, as indicated by their effect on CTLA4 and Treg cells. For example, low-dose anti-CD3 have been found to expand Treg cells (18). Such an effect is consistent with their therapeutic effect. In newly-diabetic NOD mice, low dose anti-CD3 treatment induced remission of the disease. The finding provoked clinical trials, using two humanized non-mitogenic anti-CD3 antibodies, which showed that anti-CD3 treatment restrained the progression of autoimmune attacks against β cells and partially preserved the residual β cell mass in recent-onset diabetic patients. Enhanced immunoregulation was found to be the main mechanism of restored tolerance to β cells (18). Anti-thymocyte globulins (ATG), a prototype of lymphodepleting agents, may also have the potentials to expand or induce Treg cells, but this effect remains debated and more concrete evidence needs to be provided (14,33,99). Evidence for the tolerogenic potentials of B-cell depletion therapies has been documented in clinical trials and experimental studies with animal models (53,69). The exact mechanism of Treg cell expansion and induction in vivo by immunomodulation remains to be elucidated. It might even implicate cells in the nonhematopoietic lineage. Indeed, bone marrow mesenchymal stem cells have been shown to suppress immune cells in various settings (63). Their immunomodulatory role could be partially mediated by Treg cell induction (106).
Some conventional immunosuppressive agents, including calcineurin inhibitors and cyclophosphamide, were shown to be deleterious to Treg cells (15,105). On the other hand, rapamycin, a routinely used Immunosuppressive drug in transplantations, was found to expand Treg cells (7), making it an appealing choice of agent in combination with Treg cell therapies, although the direct cytotoxicity of rapamycin to transplants, for example, to β cells in islet transplantation, is a substantial concern (57,85) that needs to be addressed with appropriate dosage and duration of its use. As for CTLA4 induction, cyclosporine was reported to be inhibitory (32), but dexamethasone enhanced CTLA4 expression on activated T cells (140).
The best example of CTLA4-based immunotherapeutic agent is perhaps CTLA4Ig fusion proteins. The disappointing outcome from trials with CTLA4Ig treatment in islet transplantation in non-human primate models dampened the initial enthusiasm for the agent (12). However, clinical interest in CTLA4Ig and its derivatives has been reignited by its recent success in treating chronic inflammatory diseases or reversing late-stage inflammatory pathology against allotransplants (1,12,129). The effect of CTLA4Ig on late stage inflammation is surprising since the agent was developed to inhibit the early step of T-cell activation through blocking B7/CD28 co-stimulation. Untowardly, despite the long-time research of CTLA4Ig, it is unclear how this agent controls inflammatory damage. It also remains uncertain whether it can impede or promote Treg cells (12,109). Evidence strongly suggests that CTLA4Ig may induce IDO expression in innate immune cells including DCs. IDO induction in DCs may deliver a tolerogenic signal to T cells (76).
Coordinated patterns of gene expression in adaptive and innate regulatory circuits as biomarkers for tolerance progression
One challenge for immune tolerance induction in clinical transplantation is how to measure tolerance progression in patients, so the right patients can be weaned off from immunosuppressive drugs at the right times with the right tempo. A variety of approaches have been used to test molecular and cellular parameters associated with responses to grafts (27,84,126), but reliable assays have yet to be presented for clinical utility. Encouragingly, key molecules of immunoregulation may be useful as biomarkers for immune tolerance induction. For example, FOXP3 mRNA levels in urine predicted the reversibility of acute renal graft rejection (82), and high levels of FOXP3 mRNA existed in tolerized kidney grafts (60). Likewise, clinical evidence suggests that enhanced CTLA4 expression may predicate immune tolerance in humans. A natural example is presented from the studies of HIV immunity. Kaufmann et al found that CTLA4 upregulation by CD4+ T cells in HIV-infected patients correlates with persistence of the virus; conversely, lower CTLA4 expression by T cells correlates with effective viral elimination (59). This example, together with immune damages associated with subtle CTLA4 reductions dictated by genetic polymorphism (46,66,132,137), suggests that quantitative variations of CTLA4 may serve as indicators of immune tolerance progression in humans.
Another challenge in the clinical settings is that peripheral blood is currently the practical access to gauge the function of immune cells. Analyses of blood samples may not always reflect disease activity in a specific organ. However, a status of transplant immunity may be revealed in circulating immune cells with a specific pattern of gene regulation. For example, elevation of mRNA levels for cytotoxic lymphocyte genes predicted islet graft rejection in human patients (50).
Given the well-documented roles of CTLA4 and Foxp3 in immunoregulation, their expression is expected to serve as indicators for tolerance induction. Potential relationships may exist between CTLA4 and Foxp3 levels and expression of genes associated with innate suppressors, such as arginase I, iNOS and IDO. A dynamic relationship between the two branches may suggest effective control of pathogenic T cell responses, manifested in suppressed expression of cytotoxic genes such as IFNγ, granzyme b and perforin. With the premise that immune tolerance induction requires integrated functioning by both innate and adaptive regulatory cells, coordinated patterns of gene expressions in adaptive and innate regulatory circuits may be prognostic of immune tolerance progression.
Conclusion
Advances in transplant immunology and clinical management in the last decade have substantially improved short-term transplant survival. However, long-term graft survival remains an elusive goal in most cases. Immune tolerance induction remains a “holy grail” for control of autoimmunity and alloimmunity in the absence of life-long immunosuppression. The struggle is perhaps best epitomized in pancreatic islet transplantation. For clinical and technical reasons, it has served as a model for transplantation immunology, except that survival of islet grafts in patients with T1DM faces the unusual double challenge of alloimmunity and recurrence of autoimmunity. Technological breakthroughs achieved with decades of efforts (64,95) enabled the achievement of reproducible success in clinical islet transplantation, with a multicenter clinical trial demonstrating that it is possible to obtain insulin independence within the first year post islet transplantation, with most recipients who demonstrated continued islet function for more than 2 years after transplantation (108). Nonetheless, despite the use of a substantial combinatory immunosuppressive strategy, the function of islet grafts decreased in most patients within 3 to 5 years, indicating that the specific immunosuppressive strategy impaired the ability of insulin producing cells to replicate and replenish themselves in the long term, or was ineffective to control the chronic immune response, or a combination of the two. Although the enthusiasm for the field appears dampened by these challenges, islet transplantation has been proven to be a clinically viable remedy for severe cases of T1DM that cannot be controlled by intensive insulin therapy without increasing the risk for severe hypoglycemic episodes (96).
Regardless of the recent set-backs in islet transplantation, clinical experience with this approach and other areas of transplantation have indeed generated promising leads, as significant improvement in long term results reported in recent clinical trials, with a small fraction of transplant recipients who was able to maintain long-term graft function even after discontinuation of immunosuppressive treatment (“operational tolerance”) (8,9,31,78,126). Therefore, even though evolution may have shaped robust and multiple layers of mechanisms in the immune system to reject “foreign” antigenic substances, which prevent the selective acceptance of harmless allografts, the induction of transplant tolerance in humans is indeed an objective within reach. However, “re-educating” the immune system to accept a “foreign” transplant may require understanding and successful manipulation of a number of selected immune tolerance pathways, with integration and synergy of innate and adaptive immunoregulatory functions that could require cellular and molecular manipulation of the microenvironment at the transplant site.
Acknowledgement
This work is partly supported by the Diabetes Research Institute Foundation, Juvenile Diabetes Research Foundation International, Stanley J. Glaser Foundation, Bankhead-Coley Cancer Res.earch Program, and the National Institute of Diabetes and Digestive and Kidney Diseases (award number DP3DK085696). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health.
Footnotes
Disclosures The authors have no conflict of interests.
References
- 1.Adams AB, Shirasugi N, Durham MM, Strobert E, Anderson D, Rees P, Cowan S, Xu H, Blinder Y, Cheung M, Hollenbaugh D, Kenyon NS, Pearson TC, Larsen CP. Calcineurin inhibitor-free CD28 blockade-based protocol protects allogeneic islets in nonhuman primates. Diabetes. 2002;51(2):265–270. doi: 10.2337/diabetes.51.2.265. [DOI] [PubMed] [Google Scholar]
- 2.Anjos SM, Tessier MC, Polychronakos C. Association of the cytotoxic T lymphocyte-associated antigen 4 gene with type 1 diabetes: evidence for independent effects of two polymorphisms on the same haplotype block. J. Clin. Endocrinol. Metab. 2004;89(12):6257–6265. doi: 10.1210/jc.2004-0881. [DOI] [PubMed] [Google Scholar]
- 3.Annacker O, Pimenta-Araujo R, Burlen-Defranoux O, Barbosa TC, Cumano A, Bandeira A. CD25+ CD4+ T cells regulate the expansion of peripheral CD4 T cells through the production of IL-10. J. Immunol. 2001;166(5):3008–3018. doi: 10.4049/jimmunol.166.5.3008. [DOI] [PubMed] [Google Scholar]
- 4.Azarian M, Busson M, Lepage V, Charron D, Toubert A, Loiseau P, de Latour RP, Rocha V, Socie G. Donor CTLA-4 +49 A/G*GG genotype is associated with chronic GVHD after HLA-identical haematopoietic stem-Cell Transplantations. Blood. 2007;110(13):4623–4624. doi: 10.1182/blood-2007-08-106385. [DOI] [PubMed] [Google Scholar]
- 5.Baniasadi V, Narain N, Goswami R, Das SN. Promoter region −318 C/ T and −1661 A/G CTLA-4 single nucleotide polymorphisms and type 1 diabetes in North Indians. Tissue Antigens. 2006;67(5):383–389. doi: 10.1111/j.1399-0039.2006.00595.x. [DOI] [PubMed] [Google Scholar]
- 6.Barthlott T, Kassiotis G, Stockinger B. T cell regulation as a side effect of homeostasis and competition. J. Exp. Med. 2003;197(4):451–460. doi: 10.1084/jem.20021387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105(12):4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 8.Bellin MD, Kandaswamy R, Parkey J, Zhang HJ, Liu B, Ihm SH, Ansite JD, Witson J, Bansal-Pakala P, Balamurugan AN, Papas K, Sutherland DE, Moran A, Hering BJ. Prolonged insulin independence after islet allotransplants in recipients with type 1 diabetes. Am. J. Transplant. 2008;8(11):2463–2470. doi: 10.1111/j.1600-6143.2008.02404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berney T, Ferrari-Lacraz S, Buhler L, Oberholzer J, Marangon N, Philippe J, Villard J, Morel P. Long-term insulin-independence after allogeneic islet transplantation for type 1 diabetes: over the 10-year mark. Am. J. Transplant. 2009;9(2):419–423. doi: 10.1111/j.1600-6143.2008.02481.x. [DOI] [PubMed] [Google Scholar]
- 10.Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature. 1953;172(4379):603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 11.Bjornvold M, Amundsen SS, Stene LC, Joner G, Dahl-Jorgensen K, Njolstad PR, Ek J, Ascher H, Gudjonsdottir AH, Lie BA, Skinningsrud B, Akselsen HE, Ronningen KS, Sollid LM, Undlien DE. FOXP3 polymorphisms in type 1 diabetes and coeliac disease. J. Autoimmun. 2006;27(2):140–144. doi: 10.1016/j.jaut.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 12.Bluestone JA, St Clair EW, Turka LA. CTLA4Ig: bridging the basic immunology with clinical application. Immunity. 2006;24(3):233–238. doi: 10.1016/j.immuni.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Bretscher PA, Cohn M. Minimal model for the mechanism of antibody induction and paralysis by antigen. Nature. 1968;220(5166):444–448. doi: 10.1038/220444a0. [DOI] [PubMed] [Google Scholar]
- 14.Broady R, Yu J, Levings MK. ATG-induced expression of FOXP3 in human CD4+ T cells in vitro is associated with T cell activation and not the induction of FOXP3+ T regulatory cells. Blood. 2009 doi: 10.1182/blood-2009-04-214437. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 15.Brode S, Raine T, Zaccone P, Cooke A. Cyclophosphamide-induced type-1 diabetes in the NOD mouse is associated with a reduction of CD4+CD25+Foxp3+ regulatory T cells. J. Immunol. 2006;177(10):6603–6612. doi: 10.4049/jimmunol.177.10.6603. [DOI] [PubMed] [Google Scholar]
- 16.Brusko T, Wasserfall C, McGrail K, Schatz R, Viener HL, Schatz D, Haller M, Rockell J, Gottlieb P, Clare-Salzler M, Atkinson M. No alterations in the frequency of FOXP3+ regulatory T-cells in type 1 diabetes. Diabetes. 2007;56(3):604–612. doi: 10.2337/db06-1248. [DOI] [PubMed] [Google Scholar]
- 17.Chambers CA, Cado D, Truong T, Allison JP. Thymocyte development is normal in CTLA-4-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 1997;94(17):9296–9301. doi: 10.1073/pnas.94.17.9296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chatenoud L, Bluestone JA. CD3-specific antibodies: a portal to the treatment of autoimmunity. Nat. Rev. Immunol. 2007;7(8):622–632. doi: 10.1038/nri2134. [DOI] [PubMed] [Google Scholar]
- 19.Chen L, Wang T, Zhou P, Ma L, Yin D, Shen J, Molinero L, Nozaki T, Phillips T, Uematsu S, Akira S, Wang CR, Fairchild RL, Alegre ML, Chong A. TLR engagement prevents transplantation tolerance. Am. J. Transplant. 2006;6(10):2282–2291. doi: 10.1111/j.1600-6143.2006.01489.x. [DOI] [PubMed] [Google Scholar]
- 20.Chen Z, Benoist C, Mathis D. How defects in central tolerance impinge on a deficiency in regulatory T cells. Proc. Natl. Acad. Sci. U. S. A. 2005;102(41):14735–14740. doi: 10.1073/pnas.0507014102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Z, Herman AE, Matos M, Mathis D, Benoist C. Where CD4+CD25+ T reg cells impinge on autoimmune diabetes. J. Exp. Med. 2005;202(10):1387–1397. doi: 10.1084/jem.20051409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Z, Stockton J, Mathis D, Benoist C. Modeling CTLA4-linked autoimmunity with RNA interference in mice. Proc. Natl. Acad. Sci. U. S. A. 2006;103(44):16400–16405. doi: 10.1073/pnas.0607854103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiang YJ, Lu L, Fung JJ, Qian S. Liver-derived dendritic cells induce donor-specific hyporesponsiveness: use of sponge implant as a Cell Transplant model. Cell Transplant. 2001;10(3):343–350. doi: 10.3727/000000001783986729. [DOI] [PubMed] [Google Scholar]
- 24.Chikuma S, Abbas AK, Bluestone JA. B7-independent inhibition of T cells by CTLA-4. J. Immunol. 2005;175(1):177–181. doi: 10.4049/jimmunol.175.1.177. [DOI] [PubMed] [Google Scholar]
- 25.Choi JM, Ahn MH, Chae WJ, Jung YG, Park JC, Song HM, Kim YE, Shin JA, Park CS, Park JW, Park TK, Lee JH, Seo BF, Kim KD, Kim ES, Lee DH, Lee SK, Lee SK. Intranasal delivery of the cytoplasmic domain of CTLA-4 using a novel protein transduction domain prevents allergic inflammation. Nat. Med. 2006;12(5):574–579. doi: 10.1038/nm1385. [DOI] [PubMed] [Google Scholar]
- 26.D'Alise AM, Auyeung V, Feuerer M, Nishio J, Fontenot J, Benoist C, Mathis D. The defect in T-cell regulation in NOD mice is an effect on the T-cell effectors. Proc. Natl. Acad. Sci. U. S. A. 2008;105(50):19857–19862. doi: 10.1073/pnas.0810713105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Derks RA, Burlingham WJ. In vitro parameters of donor-antigen-specific tolerance. Curr. Opin. Immunol. 2005;17(5):560–564. doi: 10.1016/j.coi.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 28.Dugast AS, Haudebourg T, Coulon F, Heslan M, Haspot F, Poirier N, Vuillefroy de Silly R, Usal C, Smit H, Martinet B, Thebault P, Renaudin K, Vanhove B. Myeloid-derived suppressor cells accumulate in kidney allograft tolerance and specifically suppress effector T cell expansion. J. Immunol. 2008;180(12):7898–7906. doi: 10.4049/jimmunol.180.12.7898. [DOI] [PubMed] [Google Scholar]
- 29.Eggena MP, Walker LS, Nagabhushanam V, Barron L, Chodos A, Abbas AK. Cooperative roles of CTLA-4 and regulatory T cells in tolerance to an islet cell antigen. J. Exp. Med. 2004;199(12):1725–1730. doi: 10.1084/jem.20040124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, Belladonna ML, Fioretti MC, Alegre ML, Puccetti P. Modulation of tryptophan catabolism by regulatory T cells. Nat. Immunol. 2003;4(12):1206–1212. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 31.Faradji RN, Tharavanij T, Messinger S, Froud T, Pileggi A, Monroy K, Mineo D, Baidal DA, Cure P, Ponte G, Mendez AJ, Selvaggi G, Ricordi C, Alejandro R. Long-term insulin independence and improvement in insulin secretion after supplemental islet infusion under exenatide and etanercept. Transplantation. 2008;86(12):1658–1665. doi: 10.1097/TP.0b013e31818fe448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fecteau S, Basadonna GP, Freitas A, Ariyan C, Sayegh MH, Rothstein DM. CTLA-4 up-regulation plays a role in tolerance mediated by CD45. Nat. Immunol. 2001;2(1):58–63. doi: 10.1038/83175. [DOI] [PubMed] [Google Scholar]
- 33.Feng X, Kajigaya S, Solomou EE, Keyvanfar K, Xu X, Raghavachari N, Munson PJ, Herndon TM, Chen J, Young NS. Rabbit ATG but not horse ATG promotes expansion of functional CD4+CD25highFOXP3+ regulatory T cells in vitro. Blood. 2008;111(7):3675–3683. doi: 10.1182/blood-2008-01-130146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fort A, Fort N, Ricordi C, Stabler CL. Biohybrid devices and encapsulation technologies for engineering a bioartificial pancreas. Cell Transplant. 2008;17(9):997–1003. doi: 10.3727/096368908786991498. [DOI] [PubMed] [Google Scholar]
- 35.Friedline RH, Brown DS, Nguyen H, Kornfeld H, Lee J, Zhang Y, Appleby M, Der SD, Kang J, Chambers CA. CD4+ regulatory T cells require CTLA-4 for the maintenance of systemic tolerance. J. Exp. Med. 2009;206(2):421–434. doi: 10.1084/jem.20081811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fu F, Li Y, Qian S, Lu L, Chambers F, Starzl TE, Fung JJ, Thomson AW. Costimulatory molecule-deficient dendritic cell progenitors (MHC class II+, CD80dim, CD86-) prolong cardiac allograft survival in nonimmunosuppressed recipients. Transplantation. 1996;62(5):659–665. doi: 10.1097/00007890-199609150-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fu YX, Watson G, Jimenez JJ, Wang Y, Lopez DM. Expansion of immunoregulatory macrophages by granulocyte-macrophage colony-stimulating factor derived from a murine mammary tumor. Cancer Res. 1990;50(2):227–234. [PubMed] [Google Scholar]
- 38.Gabrilovich DI, Bronte V, Chen SH, Colombo MP, Ochoa A, Ostrand-Rosenberg S, Schreiber H. The terminology issue for myeloid-derived suppressor cells. Cancer Res. 2007;67(1):425–426. doi: 10.1158/0008-5472.CAN-06-3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009;9(3):162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garrovillo M, Ali A, Oluwole SF. Indirect allorecognition in acquired thymic tolerance: induction of donor-specific tolerance to rat cardiac allografts by allopeptide-pulsed host dendritic cells. Transplantation. 1999;68(12):1827–1834. doi: 10.1097/00007890-199912270-00001. [DOI] [PubMed] [Google Scholar]
- 41.Germain RN, Bajenoff M, Castellino F, Chieppa M, Egen JG, Huang AY, Ishii M, Koo LY, Qi H. Making friends in out-of-the-way places: how cells of the immune system get together and how they conduct their business as revealed by intravital imaging. Immunol. Rev. 2008;221:163–181. doi: 10.1111/j.1600-065X.2008.00591.x. [DOI] [PubMed] [Google Scholar]
- 42.Gilboa E. DC-based cancer vaccines. J. Clin. Invest. 2007;117(5):1195–1203. doi: 10.1172/JCI31205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gilboa E. The promise of cancer vaccines. Nat. Rev. Cancer. 2004;4(5):401–411. doi: 10.1038/nrc1359. [DOI] [PubMed] [Google Scholar]
- 44.Golshayan D, Jiang S, Tsang J, Garin MI, Mottet C, Lechler RI. In vitro-expanded donor alloantigen-specific CD4+CD25+ regulatory T cells promote experimental transplantation tolerance. Blood. 2007;109(2):827–835. doi: 10.1182/blood-2006-05-025460. [DOI] [PubMed] [Google Scholar]
- 45.Gonzalez A, Katz JD, Mattei MG, Kikutani H, Benoist C, Mathis D. Genetic control of diabetes progression. Immunity. 1997;7(6):873–883. doi: 10.1016/s1074-7613(00)80405-7. [DOI] [PubMed] [Google Scholar]
- 46.Gorgi Y, Sfar I, Abdallah TB, Abderrahim E, Ayed SJ, Aouadi H, Bardi R, Ayed K. Ctla-4 exon 1 (+49) and promoter (−318) gene polymorphisms in kidney transplantation. Transplant. Proc. 2006;38(7):2303–2305. doi: 10.1016/j.transproceed.2006.06.132. [DOI] [PubMed] [Google Scholar]
- 47.Graca L, Cobbold SP, Waldmann H. Identification of regulatory T cells in tolerated allografts. J. Exp. Med. 2002;195(12):1641–1646. doi: 10.1084/jem.20012097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389(6652):737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 49.Gruber SA. Locoregional immunosuppression of organ transplants. Immunol. Rev. 1992;129:5–30. doi: 10.1111/j.1600-065x.1992.tb01417.x. [DOI] [PubMed] [Google Scholar]
- 50.Han D, Xu X, Baidal D, Leith J, Ricordi C, Alejandro R, Kenyon NS. Assessment of cytotoxic lymphocyte gene expression in the peripheral blood of human islet allograft recipients: elevation precedes clinical evidence of rejection. Diabetes. 2004;53(9):2281–2290. doi: 10.2337/diabetes.53.9.2281. [DOI] [PubMed] [Google Scholar]
- 51.Haskins K, Portas M, Bergman B, Lafferty K, Bradley B. Pancreatic islet-specific T-cell clones from nonobese diabetic mice. Proc. Natl. Acad. Sci. U. S. A. 1989;86(20):8000–8004. doi: 10.1073/pnas.86.20.8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haskins K, Portas M, Bradley B, Wegmann D, Lafferty K. T-lymphocyte clone specific for pancreatic islet antigen. Diabetes. 1988;37(10):1444–1448. doi: 10.2337/diab.37.10.1444. [DOI] [PubMed] [Google Scholar]
- 53.Hu CY, Rodriguez-Pinto D, Du W, Ahuja A, Henegariu O, Wong FS, Shlomchik MJ, Wen L. Treatment with CD20-specific antibody prevents and reverses autoimmune diabetes in mice. J. Clin. Invest. 2007;117(12):3857–3867. doi: 10.1172/JCI32405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hwang KW, Sweatt WB, Mashayekhi M, Palucki DA, Sattar H, Chuang E, Alegre ML. Transgenic expression of CTLA-4 controls lymphoproliferation in IL-2-deficient mice. J. Immunol. 2004;173(9):5415–5424. doi: 10.4049/jimmunol.173.9.5415. [DOI] [PubMed] [Google Scholar]
- 55.Ilkovitch D, Lopez DM. The liver is a site for tumor-induced myeloid-derived suppressor cell accumulation and immunosuppression. Cancer Res. 2009;69(13):5514–5521. doi: 10.1158/0008-5472.CAN-08-4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Joffre O, Santolaria T, Calise D, Al Saati T, Hudrisier D, Romagnoli P, van Meerwijk JP. Prevention of acute and chronic allograft rejection with CD4+CD25+Foxp3+ regulatory T lymphocytes. Nat. Med. 2008;14(1):88–92. doi: 10.1038/nm1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johnson JD, Ao Z, Ao P, Li H, Dai L, He Z, Tee M, Potter KJ, Klimek AM, Meloche RM, Thompson DM, Verchere CB, Warnock GL. Different effects of FK506, rapamycin, and mycophenolate mofetil on glucose-stimulated insulin release and apoptosis in human islets. Cell Transplant. 2009 doi: 10.3727/096368909X471198. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 58.Katz JD, Wang B, Haskins K, Benoist C, Mathis D. Following a diabetogenic T cell from genesis through pathogenesis. Cell. 1993;74(6):1089–1100. doi: 10.1016/0092-8674(93)90730-e. [DOI] [PubMed] [Google Scholar]
- 59.Kaufmann DE, Kavanagh DG, Pereyra F, Zaunders JJ, Mackey EW, Miura T, Palmer S, Brockman M, Rathod A, Piechocka-Trocha A, Baker B, Zhu B, Le Gall S, Waring MT, Ahern R, Moss K, Kelleher AD, Coffin JM, Freeman GJ, Rosenberg ES, Walker BD. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat. Immunol. 2007;8(11):1246–1254. doi: 10.1038/ni1515. [DOI] [PubMed] [Google Scholar]
- 60.Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, Shaffer J, Preffer FI, Ding R, Sharma V, Fishman JA, Dey B, Ko DS, Hertl M, Goes NB, Wong W, Williams WW, Jr., Colvin RB, Sykes M, Sachs DH. HLA-mismatched renal transplantation without maintenance immunosuppression. N. Engl. J. Med. 2008;358(4):353–361. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim J, Rasmussen J, Rudensky A. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat. Immunol. 2007;8(2):191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 62.Kryczek I, Zou L, Rodriguez P, Zhu G, Wei S, Mottram P, Brumlik M, Cheng P, Curiel T, Myers L, Lackner A, Alvarez X, Ochoa A, Chen L, Zou W. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J. Exp. Med. 2006;203(4):871–881. doi: 10.1084/jem.20050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kuo TK, Ho JH, Lee OK. Mesenchymal Stem Cell Therapy for Non-musculoskeletal Diseases: Emerging Applications. Cell Transplant. 2009 doi: 10.3727/096368909X471206. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 64.Lakey JR, Warnock GL, Shapiro AM, Korbutt GS, Ao Z, Kneteman NM, Rajotte RV. Intraductal collagenase delivery into the human pancreas using syringe loading or controlled perfusion. Cell Transplant. 1999;8(3):285–292. doi: 10.1177/096368979900800309. [DOI] [PubMed] [Google Scholar]
- 65.Lawson JM, Tremble J, Dayan C, Beyan H, Leslie RD, Peakman M, Tree TI. Increased resistance to CD4+CD25hi regulatory T cell-mediated suppression in patients with type 1 diabetes. Clin. Exp. Immunol. 2008;154(3):353–359. doi: 10.1111/j.1365-2249.2008.03810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ligers A, Teleshova N, Masterman T, Huang WX, Hillert J. CTLA-4 gene expression is influenced by promoter and exon 1 polymorphisms. Genes Immun. 2001;2(3):145–152. doi: 10.1038/sj.gene.6363752. [DOI] [PubMed] [Google Scholar]
- 67.Lin H, Rathmell JC, Gray GS, Thompson CB, Leiden JM, Alegre ML. Cytotoxic T lymphocyte antigen 4 (CTLA4) blockade accelerates the acute rejection of cardiac allografts in CD28-deficient mice: CTLA4 can function independently of CD28. J. Exp. Med. 1998;188(1):199–204. doi: 10.1084/jem.188.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Linsley PS, Clark EA, Ledbetter JA. T-cell antigen CD28 mediates adhesion with B cells by interacting with activation antigen B7/BB-1. Proc. Natl. Acad. Sci. U. S. A. 1990;87(13):5031–5035. doi: 10.1073/pnas.87.13.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu C, Noorchashm H, Sutter JA, Naji M, Prak EL, Boyer J, Green T, Rickels MR, Tomaszewski JE, Koeberlein B, Wang Z, Paessler ME, Velidedeoglu E, Rostami SY, Yu M, Barker CF, Naji A. B lymphocyte-directed immunotherapy promotes long-term islet allograft survival in nonhuman primates. Nat. Med. 2007;13(11):1295–1298. doi: 10.1038/nm1673. [DOI] [PubMed] [Google Scholar]
- 70.Long SA, Walker MR, Rieck M, James E, Kwok WW, Sanda S, Pihoker C, Greenbaum C, Nepom GT, Buckner JH. Functional islet-specific Treg can be generated from CD4+CD25− T cells of healthy and type 1 diabetic subjects. Eur. J. Immunol. 2009;39(2):612–620. doi: 10.1002/eji.200838819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lu L, Rudert WA, Qian S, McCaslin D, Fu F, Rao AS, Trucco M, Fung JJ, Starzl TE, Thomson AW. Growth of donor-derived dendritic cells from the bone marrow of murine liver allograft recipients in response to granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 1995;182(2):379–387. doi: 10.1084/jem.182.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lundholm M, Motta V, Lofgren-Burstrom A, Duarte N, Bergman ML, Mayans S, Holmberg D. Defective induction of CTLA-4 in the NOD mouse is controlled by the NOD allele of Idd3/IL-2 and a novel locus (Ctex) telomeric on chromosome 1. Diabetes. 2006;55(2):538–544. doi: 10.2337/diabetes.55.02.06.db05-1240. [DOI] [PubMed] [Google Scholar]
- 73.Masteller EL, Chuang E, Mullen AC, Reiner SL, Thompson CB. Structural analysis of CTLA-4 function in vivo. J. Immunol. 2000;164(10):5319–5327. doi: 10.4049/jimmunol.164.10.5319. [DOI] [PubMed] [Google Scholar]
- 74.Masteller EL, Warner MR, Tang Q, Tarbell KV, McDevitt H, Bluestone JA. Expansion of functional endogenous antigen-specific CD4+CD25+ regulatory T cells from nonobese diabetic mice. J. Immunol. 2005;175(5):3053–3059. doi: 10.4049/jimmunol.175.5.3053. [DOI] [PubMed] [Google Scholar]
- 75.Mathis D, Benoist C. Back to central tolerance. Immunity. 2004;20(5):509–516. doi: 10.1016/s1074-7613(04)00111-6. [DOI] [PubMed] [Google Scholar]
- 76.Mellor A. Indoleamine 2,3 dioxygenase and regulation of T cell immunity. Biochem. Biophys. Res. Commun. 2005;338(1):20–24. doi: 10.1016/j.bbrc.2005.08.232. [DOI] [PubMed] [Google Scholar]
- 77.Metz R, Duhadaway JB, Kamasani U, Laury-Kleintop L, Muller AJ, Prendergast GC. Novel tryptophan catabolic enzyme IDO2 is the preferred biochemical target of the antitumor indoleamine 2,3-dioxygenase inhibitory compound D-1-methyl-tryptophan. Cancer Res. 2007;67(15):7082–7087. doi: 10.1158/0008-5472.CAN-07-1872. [DOI] [PubMed] [Google Scholar]
- 78.Mineo D, Pileggi A, Alejandro R, Ricordi C. Point: steady progress and current challenges in clinical islet transplantation. Diabetes Care. 2009;32(8):1563–1569. doi: 10.2337/dc09-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Monti P, Scirpoli M, Maffi P, Ghidoli N, De Taddeo F, Bertuzzi F, Piemonti L, Falcone M, Secchi A, Bonifacio E. Islet transplantation in patients with autoimmune diabetes induces homeostatic cytokines that expand autoreactive memory T cells. J. Clin. Invest. 2008;118(5):1806–1814. doi: 10.1172/JCI35197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Morelli AE, Thomson AW. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat. Rev. Immunol. 2007;7(8):610–621. doi: 10.1038/nri2132. [DOI] [PubMed] [Google Scholar]
- 81.Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, De Baetselier P, Van Ginderachter JA. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111(8):4233–4244. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- 82.Muthukumar T, Dadhania D, Ding R, Snopkowski C, Naqvi R, Lee JB, Hartono C, Li B, Sharma VK, Seshan SV, Kapur S, Hancock WW, Schwartz JE, Suthanthiran M. Messenger RNA for FOXP3 in the urine of renal-allograft recipients. N. Engl. J. Med. 2005;353(22):2342–2351. doi: 10.1056/NEJMoa051907. [DOI] [PubMed] [Google Scholar]
- 83.Nanji SA, Hancock WW, Luo B, Schur CD, Pawlick RL, Zhu LF, Anderson CC, Shapiro AM. Costimulation blockade of both inducible costimulator and CD40 ligand induces dominant tolerance to islet allografts and prevents spontaneous autoimmune diabetes in the NOD mouse. Diabetes. 2006;55(1):27–33. [PubMed] [Google Scholar]
- 84.Newell KA, Larsen CP. Tolerance assays: measuring the unknown. Transplantation. 2006;81(11):1503–1509. doi: 10.1097/01.tp.0000222912.69532.1e. [DOI] [PubMed] [Google Scholar]
- 85.Nir T, Melton DA, Dor Y. Recovery from diabetes in mice by beta cell regeneration. J. Clin. Invest. 2007;117(9):2553–2561. doi: 10.1172/JCI32959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nolan K, Millet Y, Ricordi C, Stabler CL. Tissue engineering and biomaterials in regenerative medicine. Cell Transplant. 2008;17(3):241–243. doi: 10.3727/096368908784153931. [DOI] [PubMed] [Google Scholar]
- 87.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J. Immunol. 2009;182(8):4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ou D, Wang X, Metzger DL, Ao Z, Pozzilli P, James RF, Chen L, Warnock GL. Suppression of human T-cell responses to beta-cells by activation of B7-H4 pathway. Cell Transplant. 2006;15(5):399–410. [PubMed] [Google Scholar]
- 89.Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J. Exp. Med. 2009;206(8):1717–1725. doi: 10.1084/jem.20082492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Perez-Garcia A, De la Camara R, Roman-Gomez J, Jimenez-Velasco A, Encuentra M, Nieto JB, de la Rubia J, Urbano-Ispizua A, Brunet S, Iriondo A, Gonzalez M, Serrano D, Espigado I, Solano C, Ribera JM, Pujal JM, Hoyos M, Gallardo D. CTLA-4 polymorphisms and clinical outcome after allogeneic stem Cell Transplantation from HLA-identical sibling donors. Blood. 2007;110(1):461–467. doi: 10.1182/blood-2007-01-069781. [DOI] [PubMed] [Google Scholar]
- 91.Pileggi A, Molano RD, Ricordi C, Zahr E, Collins J, Valdes R, Inverardi L. Reversal of diabetes by pancreatic islet transplantation into a subcutaneous, neovascularized device. Transplantation. 2006;81(9):1318–1324. doi: 10.1097/01.tp.0000203858.41105.88. [DOI] [PubMed] [Google Scholar]
- 92.Powrie F, Carlino J, Leach MW, Mauze S, Coffman RL. A critical role for transforming growth factor-beta but not interleukin 4 in the suppression of T helper type 1-mediated colitis by CD45RB(low) CD4+ T cells. J. Exp. Med. 1996;183(6):2669–2674. doi: 10.1084/jem.183.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Putnam AL, Brusko TM, Lee MR, Liu W, Szot GL, Ghosh T, Atkinson MA, Bluestone JA. Expansion of human regulatory T-cells from patients with type 1 diabetes. Diabetes. 2009;58(3):652–662. doi: 10.2337/db08-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J. Exp. Med. 2000;192(2):295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ricordi C, Lacy PE, Finke EH, Olack BJ, Scharp DW. Automated method for isolation of human pancreatic islets. Diabetes. 1988;37(4):413–420. doi: 10.2337/diab.37.4.413. [DOI] [PubMed] [Google Scholar]
- 96.Ricordi C, Strom TB. Clinical islet transplantation: advances and immunological challenges. Nat. Rev. Immunol. 2004;4(4):259–268. doi: 10.1038/nri1332. [DOI] [PubMed] [Google Scholar]
- 97.Riley JL, June CH, Blazar BR. Human T regulatory cell therapy: take a billion or so and call me in the morning. Immunity. 2009;30(5):656–665. doi: 10.1016/j.immuni.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Roncarolo MG, Battaglia M. Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nat. Rev. Immunol. 2007;7(8):585–598. doi: 10.1038/nri2138. [DOI] [PubMed] [Google Scholar]
- 99.Ruzek MC, Waire JS, Hopkins D, Lacorcia G, Sullivan J, Roberts BL, Richards SM, Nahill SR, Williams JM, Scaria A, Dzuris J, Shankara S, Garman RD. Characterization of in vitro antimurine thymocyte globulin-induced regulatory T cells that inhibit graft-versus-host disease in vivo. Blood. 2008;111(3):1726–1734. doi: 10.1182/blood-2007-08-106526. [DOI] [PubMed] [Google Scholar]
- 100.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995;155(3):1151–1164. [PubMed] [Google Scholar]
- 101.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133(5):775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 102.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12(4):431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 103.Schneider A, Rieck M, Sanda S, Pihoker C, Greenbaum C, Buckner JH. The effector T cells of diabetic subjects are resistant to regulation via CD4+ FOXP3+ regulatory T cells. J. Immunol. 2008;181(10):7350–7355. doi: 10.4049/jimmunol.181.10.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schneider H, Downey J, Smith A, Zinselmeyer BH, Rush C, Brewer JM, Wei B, Hogg N, Garside P, Rudd CE. Reversal of the TCR stop signal by CTLA-4. Science. 2006;313(5795):1972–1975. doi: 10.1126/science.1131078. [DOI] [PubMed] [Google Scholar]
- 105.Segundo DS, Ruiz JC, Izquierdo M, Fernandez-Fresnedo G, Gomez-Alamillo C, Merino R, Benito MJ, Cacho E, Rodrigo E, Palomar R, Lopez-Hoyos M, Arias M. Calcineurin inhibitors, but not rapamycin, reduce percentages of CD4+CD25+FOXP3+ regulatory T cells in renal transplant recipients. Transplantation. 2006;82(4):550–557. doi: 10.1097/01.tp.0000229473.95202.50. [DOI] [PubMed] [Google Scholar]
- 106.Selmani Z, Naji A, Zidi I, Favier B, Gaiffe E, Obert L, Borg C, Saas P, Tiberghien P, Rouas-Freiss N, Carosella ED, Deschaseaux F. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 2008;26(1):212–222. doi: 10.1634/stemcells.2007-0554. [DOI] [PubMed] [Google Scholar]
- 107.Sgouroudis E, Piccirillo CA. Control of type 1 diabetes by CD4+Foxp3+ regulatory T cells: lessons from mouse models and implications for human disease. Diabetes Metab. Res. Rev. 2009;25(3):208–218. doi: 10.1002/dmrr.945. [DOI] [PubMed] [Google Scholar]
- 108.Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, Cagliero E, Alejandro R, Ryan EA, DiMercurio B, Morel P, Polonsky KS, Reems JA, Bretzel RG, Bertuzzi F, Froud T, Kandaswamy R, Sutherland DE, Eisenbarth G, Segal M, Preiksaitis J, Korbutt GS, Barton FB, Viviano L, Seyfert-Margolis V, Bluestone J, Lakey JR. International trial of the Edmonton protocol for islet transplantation. N. Engl. J. Med. 2006;355(13):1318–1330. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 109.Shevach EM. Immunology. Regulating suppression. Science. 2008;322(5899):202–203. doi: 10.1126/science.1164872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sinha P, Okoro C, Foell D, Freeze HH, Ostrand-Rosenberg S, Srikrishna G. Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells. J. Immunol. 2008;181(7):4666–4675. doi: 10.4049/jimmunol.181.7.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stamper CC, Zhang Y, Tobin JF, Erbe DV, Ikemizu S, Davis SJ, Stahl ML, Seehra J, Somers WS, Mosyak L. Crystal structure of the B7-1/CTLA-4 complex that inhibits human immune responses. Nature. 2001;410(6828):608–611. doi: 10.1038/35069118. [DOI] [PubMed] [Google Scholar]
- 112.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449(7161):419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 113.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J. Exp. Med. 1973;137(5):1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, Mak TW, Sakaguchi S. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J. Exp. Med. 2000;192(2):303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J, Masteller EL, McDevitt H, Bonyhadi M, Bluestone JA. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J. Exp. Med. 2004;199(11):1455–1465. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tarbell KV, Petit L, Zuo X, Toy P, Luo X, Mqadmi A, Yang H, Suthanthiran M, Mojsov S, Steinman RM. Dendritic cell-expanded, islet-specific CD4+ CD25+ CD62L+ regulatory T cells restore normoglycemia in diabetic NOD mice. J. Exp. Med. 2007;204(1):191–201. doi: 10.1084/jem.20061631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tarbell KV, Yamazaki S, Olson K, Toy P, Steinman RM. CD25+ CD4+ T cells, expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes. J. Exp. Med. 2004;199(11):1467–1477. doi: 10.1084/jem.20040180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Taylor PA, Noelle RJ, Blazar BR. CD4(+)CD25(+) immune regulatory cells are required for induction of tolerance to alloantigen via costimulatory blockade. J. Exp. Med. 2001;193(11):1311–1318. doi: 10.1084/jem.193.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Teft WA, Kirchhof MG, Madrenas J. A molecular perspective of CTLA-4 function. Annu. Rev. Immunol. 2006;24:65–97. doi: 10.1146/annurev.immunol.24.021605.090535. [DOI] [PubMed] [Google Scholar]
- 120.Thompson CB, Allison JP. The emerging role of CTLA-4 as an immune attenuator. Immunity. 1997;7(4):445–450. doi: 10.1016/s1074-7613(00)80366-0. [DOI] [PubMed] [Google Scholar]
- 121.Thomson AW, Fairchild RL. The last 5 years of basic science investigation in transplant immunology. Am. J. Transplant. 2006;6(8):1768–1773. doi: 10.1111/j.1600-6143.2006.01424.x. [DOI] [PubMed] [Google Scholar]
- 122.Torrealba JR, Katayama M, Fechner JH, Jr., Jankowska-Gan E, Kusaka S, Xu Q, Schultz JM, Oberley TD, Hu H, Hamawy MM, Jonker M, Wubben J, Doxiadis G, Bontrop R, Burlingham WJ, Knechtle SJ. Metastable tolerance to rhesus monkey renal transplants is correlated with allograft TGF-beta 1+CD4+ T regulatory cell infiltrates. J. Immunol. 2004;172(9):5753–5764. doi: 10.4049/jimmunol.172.9.5753. [DOI] [PubMed] [Google Scholar]
- 123.Trenado A, Charlotte F, Fisson S, Yagello M, Klatzmann D, Salomon BL, Cohen JL. Recipient-type specific CD4+CD25+ regulatory T cells favor immune reconstitution and control graft-versus-host disease while maintaining graft-versus-leukemia. J. Clin. Invest. 2003;112(11):1688–1696. doi: 10.1172/JCI17702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Truong W, Hancock WW, Anderson CC, Merani S, Shapiro AM. Coinhibitory T-cell signaling in islet allograft rejection and tolerance. Cell Transplant. 2006;15(2):105–119. doi: 10.3727/000000006783982160. [DOI] [PubMed] [Google Scholar]
- 125.Tsang JY, Tanriver Y, Jiang S, Xue SA, Ratnasothy K, Chen D, Stauss HJ, Bucy RP, Lombardi G, Lechler R. Conferring indirect allospecificity on CD4+CD25+ Tregs by TCR gene transfer favors transplantation tolerance in mice. J. Clin. Invest. 2008;118(11):3619–3628. doi: 10.1172/JCI33185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Turka LA, Lechler RI. Towards the identification of biomarkers of transplantation tolerance. Nat. Rev. Immunol. 2009;9(7):521–526. doi: 10.1038/nri2568. [DOI] [PubMed] [Google Scholar]
- 127.Ueda H, Howson JM, Esposito L, Heward J, Snook H, Chamberlain G, Rainbow DB, Hunter KM, Smith AN, Di Genova G, Herr MH, Dahlman I, Payne F, Smyth D, Lowe C, Twells RC, Howlett S, Healy B, Nutland S, Rance HE, Everett V, Smink LJ, Lam AC, Cordell HJ, Walker NM, Bordin C, Hulme J, Motzo C, Cucca F, Hess JF, Metzker ML, Rogers J, Gregory S, Allahabadia A, Nithiyananthan R, Tuomilehto-Wolf E, Tuomilehto J, Bingley P, Gillespie KM, Undlien DE, Ronningen KS, Guja C, Ionescu-Tirgoviste C, Savage DA, Maxwell AP, Carson DJ, Patterson CC, Franklyn JA, Clayton DG, Peterson LB, Wicker LS, Todd JA, Gough SC. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003;423(6939):506–511. doi: 10.1038/nature01621. [DOI] [PubMed] [Google Scholar]
- 128.van der Windt DJ, Echeverri GJ, Ijzermans JN, Cooper DK. The choice of anatomical site for islet transplantation. Cell Transplant. 2008;17(9):1005–1014. doi: 10.3727/096368908786991515. [DOI] [PubMed] [Google Scholar]
- 129.Vincenti F, Larsen C, Durrbach A, Wekerle T, Nashan B, Blancho G, Lang P, Grinyo J, Halloran PF, Solez K, Hagerty D, Levy E, Zhou W, Natarajan K, Charpentier B. Costimulation blockade with belatacept in renal transplantation. N. Engl. J. Med. 2005;353(8):770–781. doi: 10.1056/NEJMoa050085. [DOI] [PubMed] [Google Scholar]
- 130.Waldmann H, Adams E, Fairchild P, Cobbold S. Infectious tolerance and the long-term acceptance of transplanted tissue. Immunol. Rev. 2006;212:301–313. doi: 10.1111/j.0105-2896.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- 131.Waldmann H, Cobbold S. Exploiting tolerance processes in transplantation. Science. 2004;305(5681):209–212. doi: 10.1126/science.1099538. [DOI] [PubMed] [Google Scholar]
- 132.Wang XB, Zhao X, Giscombe R, Lefvert AK. A CTLA-4 gene polymorphism at position -318 in the promoter region affects the expression of protein. Genes Immun. 2002;3(4):233–234. doi: 10.1038/sj.gene.6363869. [DOI] [PubMed] [Google Scholar]
- 133.Waterhouse P, Bachmann MF, Penninger JM, Ohashi PS, Mak TW. Normal thymic selection, normal viability and decreased lymphoproliferation in T cell receptor-transgenic CTLA-4-deficient mice. Eur. J. Immunol. 1997;27(8):1887–1892. doi: 10.1002/eji.1830270811. [DOI] [PubMed] [Google Scholar]
- 134.Wee YM, Lim DG, Kim YH, Kim JH, Kim SC, Yu E, Park MO, Choi MY, Park YH, Jang HJ, Cho EY, Cho MH, Han DJ. Cell surface modification by activated polyethylene glycol prevents allosensitization after islet transplantation. Cell Transplant. 2008;17(10-11):1257–1269. doi: 10.3727/096368908787236657. [DOI] [PubMed] [Google Scholar]
- 135.Wildin RS, Smyk-Pearson S, Filipovich AH. Clinical and molecular features of the immunodysregulation, polyendocrinopathy, enteropathy, X linked (IPEX) syndrome. J Med Genet. 2002;39(8):537–545. doi: 10.1136/jmg.39.8.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322(5899):271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 137.Wisniewski A, Kusztal M, Magott-Procelewska M, Klinger M, Jasek M, Luszczek W, Nowak I, Kosmaczewska A, Ciszak L, Frydecka I, Gorski A, Kusnierczyk P. Possible association of cytotoxic T-lymphocyte antigen 4 gene promoter single nucleotide polymorphism with acute rejection of allogeneic kidney transplant. Transplant. Proc. 2006;38(1):56–58. doi: 10.1016/j.transproceed.2005.11.094. [DOI] [PubMed] [Google Scholar]
- 138.Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat. Rev. Immunol. 2003;3(3):199–210. doi: 10.1038/nri1027. [DOI] [PubMed] [Google Scholar]
- 139.Wu Z, Bensinger SJ, Zhang J, Chen C, Yuan X, Huang X, Markmann JF, Kassaee A, Rosengard BR, Hancock WW, Sayegh MH, Turka LA. Homeostatic proliferation is a barrier to transplantation tolerance. Nat. Med. 2004;10(1):87–92. doi: 10.1038/nm965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Xia M, Gasser J, Feige U. Dexamethasone enhances CTLA-4 expression during T cell activation. Cell. Mol. Life Sci. 1999;55(12):1649–1656. doi: 10.1007/s000180050403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yang R, Cai Z, Zhang Y, Yutzy W. H. t., Roby KF, Roden RB. CD80 in immune suppression by mouse ovarian carcinoma-associated Gr-1+CD11b+ myeloid cells. Cancer Res. 2006;66(13):6807–6815. doi: 10.1158/0008-5472.CAN-05-3755. [DOI] [PubMed] [Google Scholar]
- 142.Yi KH, Chen L. Fine tuning the immune response through B7-H3 and B7-H4. Immunol. Rev. 2009;229(1):145–151. doi: 10.1111/j.1600-065X.2009.00768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhang H, Ohmi K, Hirasawa A, Enosawa S, Hara Y, Tamura A, Tsujimoto G. Immunological tolerance-related genes in a spontaneous tolerant model of rat liver transplantation explored by suppression subtractive hybridization. Cell Transplant. 2008;17(1-2):195–201. doi: 10.3727/000000008783906955. [DOI] [PubMed] [Google Scholar]
- 144.Zhang W, Liang S, Wu J, Horuzsko A. Human inhibitory receptor immunoglobulin-like transcript 2 amplifies CD11b+Gr1+ myeloid-derived suppressor cells that promote long-term survival of allografts. Transplantation. 2008;86(8):1125–1134. doi: 10.1097/TP.0b013e318186fccd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat. Rev. Immunol. 2006;6(4):295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]