Abstract
Rationale
Although mesenchymal stem cell (MSC) transplantation has been shown to promote cardiac repair in acute myocardial injury in vivo, its overall restorative capacity appears to be restricted mainly due to poor cell viability and low engraftment in the ischemic myocardium. Specific chemokines are upregulated in the infarcted myocardium. However the expression levels of the corresponding chemokine receptors (e.g. CCR1, CXCR2) in MSCs are very low. We hypothesized that this discordance may account for the poor MSC engraftment and survival.
Objective
To determine whether overexpression of CCR1 or CXCR2 chemokine receptors in MSCs augments their cell survival, migration and engraftment after injection in the infarcted myocardium.
Methods and Results
Overexpression of CCR1, but not CXCR2, dramatically increased chemokine-induced murine MSC migration and protected MSC from apoptosis in vitro. Moreover, when MSCs were injected intramyocardially one hour after coronary artery ligation, CCR1-MSCs accumulated in the infarcted myocardium at significantly higher levels than control-MSCs or CXCR2-MSCs three days post-myocardial infarction (MI). CCR1-MSC injected hearts exhibited a significant reduction in infarct size, reduced cardiomyocytes apoptosis and increased capillary density in injured myocardium three days post-MI. Furthermore, intramyocardial injection of CCR1-MSCs prevented cardiac remodeling and restored cardiac function 4 weeks post-MI.
Conclusions
Our results demonstrate the in vitro and in vivo salutary effects of genetic modification of stem cells. Specifically, overexpression of chemokine receptor enhances the migration, survival and engraftment of MSCs, and may provide a new therapeutic strategy for the injured myocardium.
Keywords: mesenchymal stem cells, engraftment, chemokine receptor, myocardial repair
Introduction
Over the past few years adult stromal mesenchymal stem cells have been extensively investigated for their potential in developing cell based therapies for the treatment of cardiac injury and/or other regenerative diseases1, 2. Numerous studies have shown that transplantation of those cells in post-infarct mice decreased mortality, reduced infarct size and improved cardiac function2. More recently, preliminary data from a clinical study of MSCs in 69 post-infarct patients also demonstrated improved left ventricular function3. Despite the progress, many barriers for translating the promise of stem cells therapy into practice still exist. Homing, engraftment and survival of the transplanted cells in the ischemic area still pose major problems, with most of the cells being lost within hours of the transplantation. Moreover, the mechanisms determining these processes are still not well understood.
Chemokine induction is one of the prominent features in the post-ischemic heart associated with neutrophil infiltration and potential angiogenic effects4. Importantly various chemokine/chemokine receptor axes are essential and potent regulators of chemotactic activities for a wide range of cell types such as monocytes and stem cells. We and others have demonstrated that many chemokines, including chemokine (c-c motif) ligand 7 (CCL7), chemokine (c-x-c motif) ligand 1 (CXCL1), chemokine (c-x-c motif) ligand 2 (CXCL2) and others were significantly upregulated in the heart following myocardial infarction and might be implicated in regulating engraftment and homing of MSCs to infarcted myocardium5–7. Although functionally active receptors for those chemokines have been identified in MSCs, their respective expression levels are relatively low8, 9.
In this study we have hypothesized that genetic modifications of the MSCs to enhance levels of specific chemokine receptors can improve the engraftment and survival of the cells to injured myocardium. Specifically, we asked whether MSCs overexpressing chemokine (c-c motif) receptor 1 (CCR1) which is the receptor for CCL7, or chemokine (c-x-c motif) receptor 2 (CXCR2) which is the receptor for both CXCL1 and CXCL2, exhibited improved engraftment and myocardial salvage after acute myocardial infarction.
Materials and Methods
An expanded Materials and Methods section is available in the online data supplement at http://circres.ahajournals.org and includes detail information regarding the following: RT-PCR and real-time RT-PCR of chemokine and chemokine receptors, isolation and culture of MSC, retroviral constructs design, retroviral packaging and producer cell lines, transduction of MSCs, Western blotting, transmigration assay, ELISA for VEGF, HUVEC culture and tube formation assay on matrix gels, BrdU incorporation assay, caspase 3/7 assay and TUNEL assay, siRNA transfection, intramyocardial MSC delivery, tracking of the GFP+ cells injected in mice, area at risk and infarct size determination, capillary and arteriolar density assessment, assessment of the collagen deposition and fibrosis, histochemical and immunofluorescent examination, echocardiographic study, cytokine arrays, and statistical analysis.
Results
Chemokine gene expression was up-regulated in the infarcted tissue
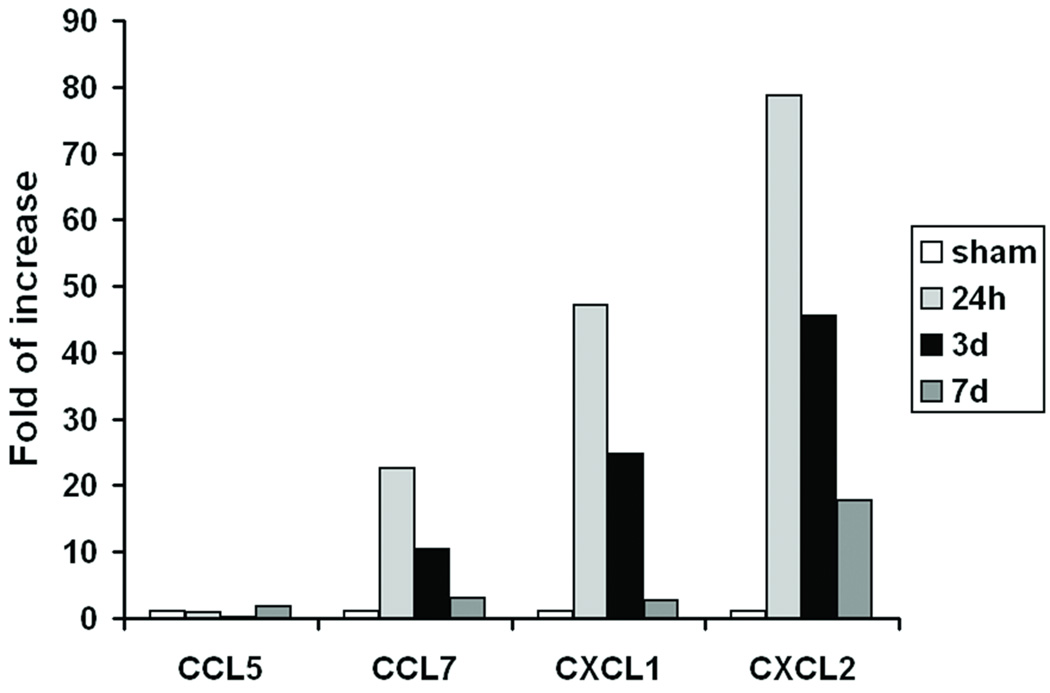
We have shown previously that specific chemokines are upregulated in the injured murine myocardium6, 10. To determine the role of the local chemokine milieu in MSC transplantation after myocardial infarction, we first confirmed the expression levels of a set of known chemokines in the border zone of the injured area by RT-PCR and real time RT PCR (Figure 1A, B). The results confirmed that mRNA levels of CXCL1, CXCL2 (both ligands for CXCR2), and CCL7 (a ligand for CCR1) were markedly upregulated after MI. The highest levels of expression were observed at 24h, although increased levels could be still detected at 7 days post-MI. The mRNA levels of CCL5, another ligand for CCR1, were not upregulated significantly. To investigate a possible role for a chemokine/chemokine receptor axis in MSC fate, we then evaluated the gene expression of the corresponding chemokine receptors, namely CCR1, CXCR2 and CXCR4, in MSCs. Since chemokine receptors are robustly expressed in different bone marrow (BM) leukocyte subtypes11, we isolated mRNA from whole bone marrow cells (wBMCs) as positive control. As expected CCR1, CXCR2 and CXCR4 were all expressed in wBMCs. CXCR4 was detected in MSCs, albeit at reduced levels compared to the wBMCs. Interestingly, very low expression of the CCR1 and CXCR2 genes was found in the MSCs under the conditions tested (Figure 1C).
Figure 1.
Chemokine gene expression in infarcted myocardium and the expression of their corresponding receptors in MSCs and wBMCs. (A) RT-PCR and (B) real-time quantitative RT-PCR analysis of several chemokine mRNA levels in cardiac tissue (infarct adjacent border zone area) at 24h, 3d and 7d post-MI, as compared to sham mice. Fold changes are expressed as the ratio over the sham control. (C) RT-PCR detection of CCR1, CXCR2 and CXCR4 gene expression in MSCs and wBMCs.
CCR1 overexpression increased MSC chemotaxis in vitro
To test the potential of an effect of the receptors in chemokine-induced MSC migration, murine MSCs were retrovirally transfected with GFP, CCR1-GFP, or CXCR2-GFP constructs (Figure 2A), and sorted by flow cytometry for GFP expression. Cells with robust green fluorescence signal were separated and used in this study (Figure 2B). Western blot analysis performed in parallel, confirmed the overexpression (3–4 X) of the chemokine receptors on targeted cells (Figure 2C). The functionality of the engineered MSCs was then assessed using a migration transwell assay. In detail, control GFP-MSCs, CCR1-MSCs, or CXCR2-MSCs were stimulated with 1 µg/mL of chemokines known to correspond to the receptors overexpressed on these cells (CCL5 for receptor CCR1 and interleukin-8/IL-8 for receptor CXCR2). As shown in Figure 2D, overexpression of CCR1 significantly increased chemokine-induced MSC chemokinesis (~90% increase, P<0.05). In contrast, MSCs overexpressing CXCR2 did not demonstrate an increased response to IL-8-mediated chemotaxis.
Figure 2.
Generation of genetically modified IRES-AcGFP, CCR1-IRES-AcGFP, and CXCR2-IRES-AcGFP MSCs. (A) Simplified scheme of the retroviral vector constructs of CCR1-IRES-AcGFP, CXCR2-IRES-AcGFP, and IRES-AcGFP. 5’LPR: 5’ long terminal repeat; Ψ: the extended viral packaging signal; IRES: internal ribosome entry site; AcG: Aequorea coerulescens green fluorescent protein (GFP). (B) Retrovirus infected MSCs exhibited robust GFP signal. Phase contrast (top) and green channel (bottom) images. (C) Western blot analysis of chemokine receptor expression in retrovirally transduced MSCs (top) and densitometric evaluation of western blot data showing CCR1 and CXCR2 signal normalized to GAPDH (bottom). The data are shown as fold change over control GFP vector transfected MSCs. Mean ± SD of 3 experiments are shown; *P < 0.05. (D) Overexpression of CCR1, but not CXCR2, enhanced chemotaxis of murine MSCs in vitro. Transwell cell migration assays were performed and the number of migrated cells was counted. Data represent mean ± SD; n = 3; *P < 0.05 versus chemokine treated control cells; # P < 0.05 versus non treated chemokine receptor-overexpressing cells; N.S.: no significance.
CCR1 overexpression protects MSC from serum deprivation induced apoptosis in vitro
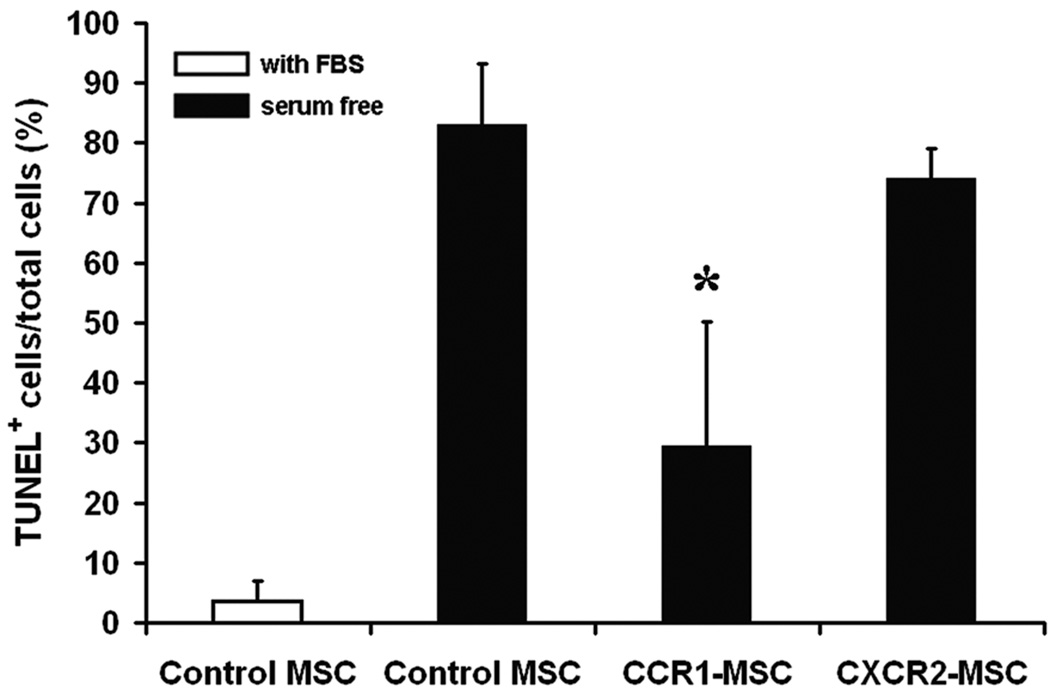
To further study the biology of the genetically modified cells, we first examined the rate of cell proliferation by performing 5-bromo-2′-deoxyuridine (BrdU) incorporation assays. As shown in Figure 3A, neither CCR1 nor CXCR2 overexpression affected the proliferation of MSCs compared with control MSCs. However, when serum deprivation was used as in vitro model to mimic the stress conditions found in the injured heart, the CCR1, but not the CXCR2, overexpressing MSCs did show enhanced levels of survival. Characteristically, after 12 hours of serum withdrawal, the activity of proapoptotic caspase 3/7 of control MSCs exceeded that of CCR1-MSCs by 1.5-fold. Overexpression of CXCR2 did not affect caspase 3/7 activity (Figure 3B). Follow up analysis by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining showed that the percentage of TUNEL positive cells was significantly reduced in CCR1-overexpressing MSCs as compared with control cells. CXCR2 overexpression did not render significant survival advantage to MSCs (Figure 3C, D).
Figure 3.
CCR1 overexpression enhanced MSC cells survival by protection from apoptosis. (A) BrdU incorporation assays were performed and the percentage of BrdU positive MSCs versus total MSCs was calculated. (B) Caspase 3/7 luminescence assay for MSCs after 12h serum withdrawal. RLU: relative light units. (C) Representative fluorescence images of TUNEL (red) DAPI (blue) and GFP (green) signal in MSCs after 12h serum deprivation. Co-localization (purple nuclei) of TUNEL and DAPI can be observed in some cells. GFP: green fluorescent protein. (D) Quantitative analysis of images represented in C. The percentage of TUNEL positive MSCs versus total MSCs is shown. Data represent mean ± SD; n = 3–4; *P < 0.05 versus control MSCs under serum deprivation.
We have previously demonstrated that MSCs secrete paracrine factors which potentially exert pro-angiogenic and cardioprotective effects in the injured myocardium12. To examine whether the vascular endothelial growth factor (VEGF) levels were affected in the CCR1 or CXCR2 overexpressing cells, we first performed ELISA to measure the VEGF levels in the culture medium of MSCs. After 12 hours of serum deprivation, the culture supernatants of CCR1-MSCs contained more VEGF compared with CXCR2-MSCs and control MSCs supernatants (Online Figure I, A). Moreover, when culture supernatants from CCR1-MSCs was added in human umbilical vein endothelial cells (HUVECs), significantly increased levels of tube formation was observed as compared to HUVECs treated with control MSCs culture supernatants (Online Figure I, B). However, addition of CXCR2-MSCs supernatants did not enhance tube formation in HUVECs. Interestingly, when the VEGF values in the culture supernatants of CCR1-MSCs, CXCR2-MSCs and control MSCs were normalized to the number of viable cells (TUNEL negative cells), the VEGF values were similar (Online Figure I, C), suggesting that the higher VEGF level in culture supernatant of CCR1-MSCs is due to the resistance of these cells to serum deprivation-induced apoptosis, rather than an increase in secretion per cell.
siRNA mediated down-regulation of endogenous ligands expression attenuated the protective effects of CCR1 overexpression against serum deprivation-induced apoptosis in MSCs
To characterize the role of endogenous CCR1 ligands such as CCL5 and CCL7 in protecting CCR1-MSCs from apoptosis, we down-modulated the expression of those genes in CCR1-MSCs using siRNA. After 72 hours transfection with either CCL5 or CCL7 siRNA, CCL5 mRNA content in CCR1-MSCs decreased 98% and CCL7 mRNA content decreased 46%, respectively, compared to negative control siRNA treated cells. siRNA treatment itself did not cause non-specific down-regulation of gene expression, as demonstrated by the β-actin control (Figure 4A, B). The effects of CCL5 and CCL7 gene silencing in CCR1-MSCs survival were then evaluated (Figure 4C). Following CCL7 siRNA transfection, 75 ± 21.1% of CCR1-MSCs underwent apoptosis vs. 38.3 ± 16.6% in the control siRNA transfected cells (~2X fold change). We also observed a trend for enhanced apoptosis in CCL5 siRNA transfected CCR1-MSCs. These data indicated that endogenous ligands of CCR1, especially CCL7, are critical for the high apoptosis resistance of CCR1-MSCs.
Figure 4.
Silencing of endogenous CCL7 ablated the protective effects of CCR1-overexpression. (A) Effect of siRNA on CCL5 and CCL7 mRNA expression in MSCs. RT-PCR for CCL5, CCL7 and β-actin at 72h after siRNA transfection. (B) Real-time RT-PCR for relative CCL5, CCL7 mRNA levels of MSCs after siRNA transfection. Changes are expressed as percentage of remaining mRNA versus control siRNA treated MSCs. (C) Quantitative analysis of apoptosis in percentage of TUNEL positive control MSCs or CCR1-MSCs transfected with siRNAs and then subjected to serum deprivation for 12h. Percentages of TUNEL positive control MSCs or CCR1-MSCs are shown and the data are expressed as mean ± SD. n = 3–4; *P < 0.05 compared with scrambled siRNA treated CCR1-MSCs; #P < 0.05 versus control MSCs; N.S.: no significance.
Enhanced accumulation and survival of transplanted CCR1-overexpressing MSCs in injured murine myocardial tissue 3 days post-MI
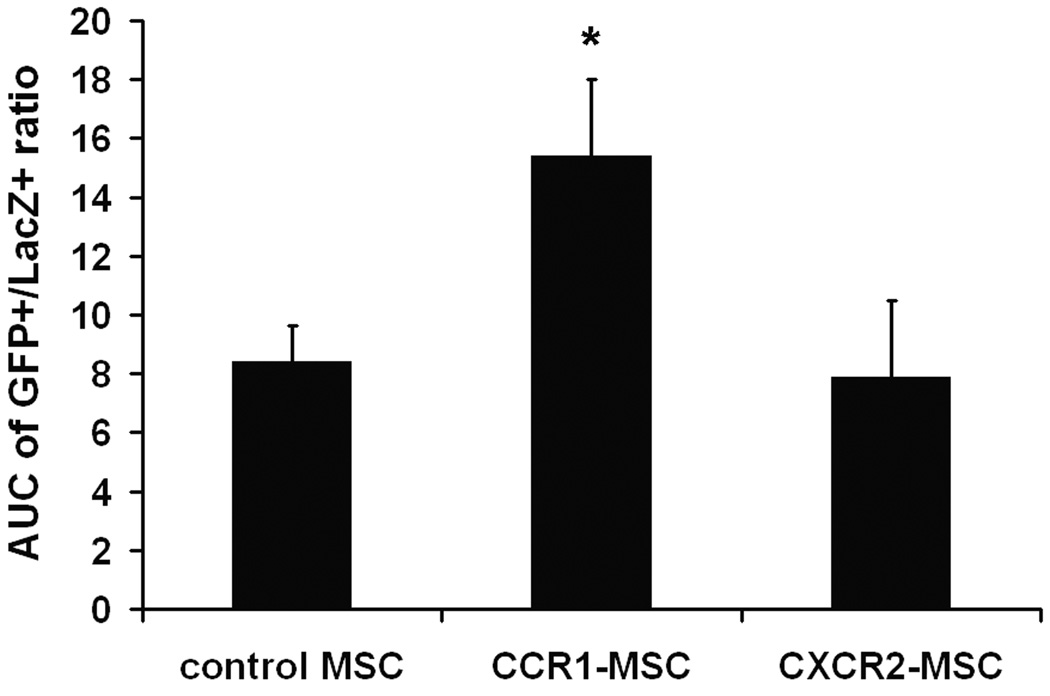
To examine the in vivo effects of chemokine receptor overexpression in MSCs, MI was produced in mice by permanent ligation of the LAD. Mice were randomly assigned to receive intramyocardial injection of a mixture of 0.75 × 105 LacZ+ MSCs added to 3 × 105 MSCs which were retrovirally transduced with GFP, CCR1-GFP, or CXCR2-GFP. The LacZ+ MSCs were injected to account for any technical variability with the cell delivery (see Material and Methods for details). Three days post-MI, GFP+ cells could be detected in the infarcted ventricular myocardium (Figure 5A). In sections from mouse hearts injected with the mixture of GFP-MSC and LacZ-MSC, the ratio of GFP+/LacZ+ ranged from 3:1 to 5:1 (Online Figure II), consistent with the 4:1 ratio of the injected cells (3 × 105 GFP-MSCs, 0.75 × 105 LacZ+ MSCs). In hearts injected with the mixture of CXCR2-GFP-MSCs and LacZ MSCs, the ratio of GFP/LacZ was greater in the first few sections but in the remainder of the sections, was very similar to that observed in the hearts injected with mixture of GFP-MSC and LacZ-MSC, suggesting the overexpression of CXCR2 had little or no effect of migration into the infarct zone (Online Figure II, bottom panel). In contrast, in mouse hearts injected with mixture of CCR1-MSC and LacZ-MSC (Online Figure II, top panel), the ratio of GFP/LacZ was higher in all sections compared to the ratio observed in hearts injected with mixture of GFP-MSC and LacZ-MSC, suggesting that expression of CCR1 enhanced MSC migration into the infarct zone. Calculation of the AUC for the three groups supported this observation (Figure 5B).
Figure 5.

Overexpression of CCR1, but not CXCR2, enhanced the migration ability of murine MSCs into infarcted myocardium. Mice received a mixture of 0.75 × 105 LacZ+ MSCs and 3 × 105 GFP+ MSCs (control GFP-MSC, CCR1-MSCs or CXCR2-MSCs) injected 1 mm above the ligation site 1 hour post infarct. Three days post-MI sections were stained using anti β-galactosidase antibody (red). Digital pictures for each section were taken. (A) Representative images of heart sections are presented. The control GFP, CCR1-, or CXCR2- MSCs are in green; LacZ-MSCs are in red; Nuclei are in blue (DAPI staining). (B) The green (GFP+) area and red (LacZ+) area were measured and the GFP+/LacZ+ area ratio was calculated for each section from the ligation site down to the apex. The GFP+/LacZ+ area ratio are plotted as a function of migration distance from the ligation site and the migration area was calculated (see Material and Methods). (C) Representative fluorescence images of TUNEL (red), DAPI (blue) and GFP (green) signals of border zone from control GFP MSC injected hearts (left) and CCR1-MSC injected hearts (right). (D) Quantitative analysis of images represented at C. The number of GFP+ cells (MSCs) per mm2 is shown. (E) Percentage of TUNEL and GFP double positive cells in sections of murine hearts represented at C. Data are expressed as mean ± SEM; n = 6–8 mice per group; *P < 0.05 versus control GFP MSCs injected mice.
When TUNEL staining was performed on these heart sections, a significantly greater number of viable GFP+ cells were observed in the myocardium of the CCR1-MSC as compared to control GFP MSC group (Figure 5 C, D). We also observed lower percentage of TUNEL and GFP double positive cells in CCR1-GFP MSC group as compared with CXCR2-MSC group and control-GFP MSC group, though it did not reach statistical significance (Figure 5E).
Intramyocardial injection of CCR1-overexpressing MSCs reduced the infarction size and increased cardiomyocytes survival and capillary density 3 days post-MI
To evaluate the functional significance of the overexpression of CCR1 or CXCR2 on MSCs, we measured the infarct area and AAR by Evans Blue dye perfusion and TTC staining in heart sections of treated mice three days post-MI. The AAR of infarction after coronary artery ligation was equivalent in all groups of animals (data not shown). As anticipated, injection of control MSCs caused a minor but significant decrease in infarct. Importantly, mice injected with MSCs overexpressing CCR1, but not CXCR2, exhibited a significantly reduced infarct/AAR ratio compared with mice injected with control MSCs (Figure 6A, B). Moreover, mouse hearts injected with CCR1-MSCs exhibited a significantly reduced initial collagen deposition compared to hearts injected with control MSCs or CXCR2-MSCs as evidenced by Mason’s trichrome staining (Online Figure III).
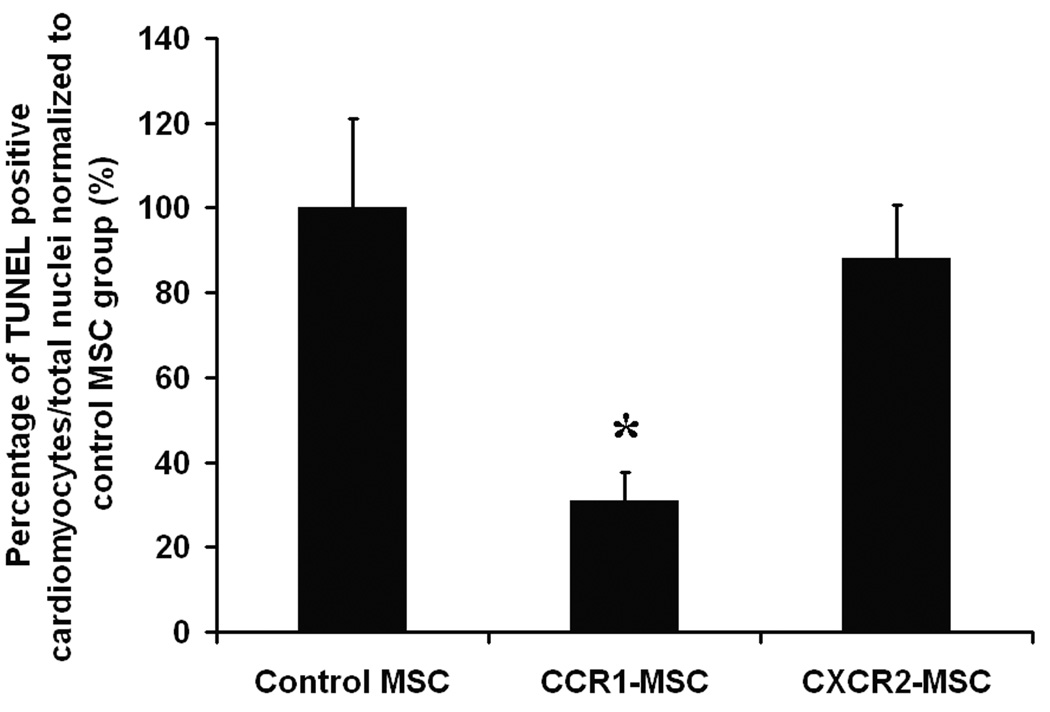
Figure 6.
(A, B) Intramyocardial injection of CCR1-MSCs, but not CXCR2-MSCs, reduced infarction size 3 d post-MI. Myocardial infarcts were assessed by Evans blue and TTC staining. (A) Representative heart sections are shown. Evans blue-perfused area, indicating area not at risk, blue; viable myocardium, red; infarcted myocardium, pale/white. (B) Percentage of injured area versus total area at risk from MI hearts from groups as indicated. (C) Quantification of the density of TUNEL positive cardiomyocytes. Percentage of TUNEL and α-sarcomeric actin double positive cells/total nuclei was calculated and the data presented are normalized to control MSCs group. Data represent mean ± SEM; n = 6–8 mice per group; *P <0.05 versus infarct/risk area of control MSC injected mice; #P <0.05 versus infarct/risk area of PBS injected mice. (D) Intramyocardial injection of CCR1-MSCs, but not CXCR2-MSCs enhanced capillary density in the infarcted myocardium 3d post-MI. The capillaries were stained by lectin in infarct adjacent border zone. The number of capillaries/mm2 was counted. Data represent mean ± SEM; n = 6–8 mice per group; *P < 0.05 versus control MSC injected mice.
A reduction in cardiomyocyte cell death was also evident when we examined for apoptosis. TUNEL and α-sarcomeric actin double positive cells were counted and the percentage of TUNEL-positive cardiomyocytes/total nuclei was calculated and normalized to control MSCs group. The data demonstrated that the percentage of apoptotic cardiomyocytes was lower in CCR1-MSC group as compared with CXCR2-MSC group and control-MSC group (Figure 6C). No changes were observed in cardiac myocyte regeneration as no significant effects in proliferation, fusion or differentiation were detected by GFP, α-sarcomeric actin and /or Ki67 costaining (data not shown).
Since we have shown that MSCs secrete angiogenic factors and promote angiogenesis in vitro, we measured capillary density in the infarction adjacent border zone (Online Figure IV, A). The capillary density increased significantly in CCR1-MSC transplanted hearts as compared to control cell transplanted hearts whereas, in CXCR2-MSC injected hearts, the capillary density was not increased significantly. Modest increase of capillary density was observed in the control GFP-MSC injected group compared with PBS injected group (Figure 6D). To address whether the injected MSCs differentiated to endothelial cells, we performed GFP and lectin co-staining and did not detect any double positive cells (data not shown).
Interestingly, when percentage of infarction/AAR ratio and number of capillaries/mm2 were analyzed together, there was a significant negative correlation between infarct size and capillary density (Online Figure IV, B; r = −0.90).
Intramyocardial injection of CCR1-overexpressing MSCs prevented cardiac remodeling, restored cardiac function 4 weeks post-MI
To investigate the long term effects of the CCR1-MSC transplantation, we measured collagen deposition and fibrosis by Masson’s trichrome staining in heart sections four weeks post-MI. Mouse hearts injected with MSCs overexpressing CCR1 exhibited a significantly reduced fibrotic volume compared to hearts injected with PBS and control MSCs (Figure 7A, B).
Figure 7.
Intramyocardial injection of CCR1-MSCs prevented cardiac remodeling and restored cardiac function 4 weeks post-MI. (A) Representative Masson’s trichrome staining for heart sections 4 weeks post-MI. (B) Quantification of the fibrotic volume. Fibrotic volume in each heart was expressed as a percentage by calculating [sum of infarct volume from all sections/sum of LV volumes from all sections]*100. Data are presented as mean ± SEM, n = 4–9. (C) Capillaries were stained by lectin in infarct adjacent border zone. The number of capillaries/mm2 was counted. Data represent mean ± SEM; n = 3–10 mice per group; *P <0.05 versus PBS group; #P <0.05 versus GFP-MSCs group.
We also measured capillary density in the infarction adjacent border zone. The capillary density increased significantly in CCR1-overexpressing MSC injected hearts as compared to PBS and GFP-MSCs injected hearts, while there was no significant difference between CCR1-MSCs group and Sham group (Figure 7C). Although we were able to detect TUNEL and α-sarcomeric actin double positive cells 4 weeks post MI (Online Figure V, A), the apoptotic cardiomyocyte number was very low and there was no difference among the MI groups. Additionally, double positive Ki67 and α-sarcomeric actin cells could be detected 4 weeks post MI (Online Figure V, B) but at very low number and again there was no difference among the MI groups. Although few GFP positive cells were detectable, we were not able to detect GFP and α-sarcomeric actin or GFP and lectin double positive cells.
More importantly, dramatic functional changes could be detected in the CCR1-MSC treated mice as evidenced by echocardiography studies (Table 1). After 4 weeks MI, both LVEDD and LVESD were significantly reduced in the CCR1-MSC group compared to the PBS group indicating an attenuation of LV dilation. LVm was also significantly smaller in the CCR1-MSC treated group. Finally, similar changes were observed for the FS, which was significantly improved in the CCR1-MSCs group compared PBS control group (~12 % increase). The CCR1-MSC treated group also showed improved levels compared to the GFP-MSC in all parameters measured.
Table 1.
Serial echocardiographic measurement in conscious mice pre, 2 and 4 weeks post-MI.
| Parameter | Sham N=3 |
PBS N=4 |
GFP-MSC N=9 |
CCR1-MSC N=10 |
P Value CCR1 vs. PBS CCR1 vs. GFP |
||
|---|---|---|---|---|---|---|---|
|
LVEDD (mm) |
Pre | 2.76±0.11 | 2.91±0.08 | 3.04±0.05 | 2.96±0.05 | ||
| 2W | 2.91±0.08 | 4.17±0.22 | 4.03±0.25 | 3.66±0.22 | |||
| 4W | 3.02±0.02 | 4.27±0.22 | 4.21±0.33 | 3.63±0.14 | 0.030 | 0.069 | |
|
LVESD (mm) |
Pre | 1.03±0.10 | 1.03±0.05 | 1.14±0.02 | 1.11±0.04 | ||
| 2W | 1.12±0.01 | 2.79±0.42 | 2.86±0.35 | 2.34±0.37 | |||
| 4W | 1.23±0.12 | 3.09±0.31 | 2.94±0.42 | 2.17±0.22 | 0.027 | 0.066 | |
| LVm (mg) | Pre | 77.29±7.65 | 94.08±3.99 | 98.17±3.94 | 89.32±3.18 | ||
| 2W | 82.80±1.03 | 137.95±15.90 | 127.07±11.97 | 92.11±3.94 | |||
| 4W | 90.77±9.82 | 133.33±12.49 | 137.92±13.13 | 101.87±10.86 | 0.047 | 0.025 | |
| FS (%) | Pre | 63±2 | 65±1 | 62±1 | 63±1 | ||
| 2W | 62±1 | 34±7 | 31±5 | 39±7 | |||
| 4W | 59±4 | 28±5 | 33±5 | 42±4 | 0.029 | 0.086 | |
All values are expressed as mean ± SEM. LVEDD, left ventricular end diastolic dimension; LVESD, left ventricular end systolic dimension; LVm, left venticular mass; FS, fractional shortening. t-Test: Two-Sample Assuming Unequal Variances: 4 weeks post-MI CCR1-MSC group vs. PBS group and CCR1-MSC group vs. GFP-MSC group.
Discussion
Although stem cell transplantation shows great promise as a tool for cardiac reparative and regenerative therapy, effective engraftment and survival of the transplanted stem cells within the ischemic myocardium remain as major limitations. Incorporation of stem cells into cardiac tissue is regulated by multiple processes including cell recruitment, migration and adhesion. In addition, survival in the ischemic microenvironment is challenging for the transplanted cells due to the lack of oxygen and nutrients. A complete understanding of the mechanisms that enhance MSC migration and survival in injured tissue therefore is imperative for improving their repair capacity and therapeutic application. Yet, the predominant primary signaling pathways that orchestrate local engraftment of MSCs, remain to be fully elucidated.
The ischemic myocardium expresses an array of biological mediators, in particular chemokines and cytokines. Chemokine/chemokine receptor axes are essential and potent regulators of chemotactic activities for a wide range of cell types. In particular, the stromal cell-derived fractor-1 (SDF-1)/CXCR4 axis has been shown to play an important role in guiding hematopoietic stem cells (HSCs) into ischemic myocardium5, 13. However, neutralized antibody blockage of SDF-1/CXCR4 does not influence MSC homing into ischemic myocardium6, which might be explained by the relatively lower level of CXCR4 expression in MSCs as compared to HSCs5, 13. As a supportive evidence, overexpression of CXCR4 enhanced chemotaxis of MSCs to the myocardium14.
In the current study, we hypothesized that additional chemokine/receptor pairs, namely, CCL7/CCR1 and CXCL1, 2/CXCR2, might be involved in MSC migration to, and engraftment in injured myocardium. Our experiments confirm that specific chemokines are upregulated transiently in the murine ischemic myocardium, including CCL7, CXCL1 and CXCL2. However the expression levels of the corresponding receptors of these chemokines, namely, CCR1 and CXCR2, were low in MSCs. To investigate whether enhancement of the above chemotactic axis would affect the biological properties of stem cells, we overexpressed the CCR1 and CXCR2 receptors in MSCs by gene transfer.
Our data show that CCR1, but not CXCR2, overexpressing MSCs exhibit higher chemotactic activity. Furthermore, CCR1-MSCs, but not CXCR2-MSCs, show increased resistance against serum deprivation-induced apoptosis in vitro. Thus, overexpressing CCR1 has an effect on increasing cell viability in addition to migration. By using siRNA-mediated gene silencing, we also demonstrated that MSC expression of CCL5 and CCL7 (both CCR1 ligands) is important in protecting MSCs from serum withdrawal-induced apoptosis. Research into the targeting of CCR1 may lead to therapies in inflammation and immunosupression. However its potential positive role in stem cell biology or in cardiovascular disease has not been extensively evaluated. Interestingly, an interaction between CCR1/CCL5 has been shown recently to be involved in affecting intimal smooth muscle-like cells proliferation and migration15. In addition it has been reported that CCL5 which interacts with CCR1, can induce ERK-1, ERK-2, FAK, and STAT phosphorylation in MSCs8. The activation of these signaling pathways might be responsible for the enhanced cell survival observed in CCR1-MSCs. The exact mechanism of CCR1 prosurvival effect is worthy of future investigations.
Our previous data has shown that MSCs secrete paracrine factors that can mediate cell survival, repair processes, as well as a variety of angiogenic factors such as VEGF, basic fibroblast growth factor, and hepatocyte growth factor, many of which are upregulated by hypoxia16, 17. In support of this notion, other groups have demonstrated that intramyocardial translantation of MSCs induces myocardial angiogenesis and subsequently lead to increased cardiomyocyte survival18–20. In this report, we further investigated the production of VEGF by CCR1-overexpressing MSCs. Under normal culture conditions, no difference was detected in the levels of VEGF secretion between CCR1-MSCs and control MSCs. After serum deprivation, VEGF secretion was increased dramatically in CCR1-MSCs compared to control MSCs. However, the increased VEGF secretion in CCR1-MSCs is due to the resistance of these cells to serum deprivation-induced apoptosis, rather than an increase in secretion per cell.
The enhanced biological properties of CCR1-MSCs observed in vitro were also present upon injection of those cells into the murine myocardium post-MI. A greater number of CCR1-MSCs accumulated within myocardium tissue and those cells showed increased migratory capacity towards the area of injury compared to the control MSC or CXCR2-MSC groups.
More importantly the infarct size of the CCR1-MSC injected group was significantly reduced compared to the control MSC group. Additionally, a reduced number of apoptotic cardiomyocytes were observed in CCR1-MSC injected hearts indicating protective effects for the injured myocardium. Compared to the control MSC injected hearts, the CCR1-MSC injected hearts showed increased capillary density in the peri-infarct area, which was not observed in the CXCR2-MSC injected group. We also demonstrated a strong negative correlation between infarct size and capillary density in hearts, suggesting that improved blood perfusion of the myocardium might at least partially contribute to these protective effects. Furthermore, these beneficial effects were maintained beyond the acute phase of infarction (3 days time point). Intramyocardial injection of CCR1-overexpressing MSCs further prevented cardiac remodeling and restored cardiac function 4 weeks post-MI.
These observations are consistent with our previous observations that the release of biologically active mediators is significant in mediating the beneficial effects of stem cell therapy via a paracrine mechanism12, 16, 17. A possible interpretation of the data is that the relative preservation of capillary density simply reflects preservation of border zone viability through a direct effect on existing cardiac cells rather than cell regeneration. As a result, increased capillary density reflects reduced scar formation rather than regeneration. Indeed, we were unable to detect either the transdifferentiation of the injected CCR1-MSCs into cardiomyocytes or endothelial cells or the proliferation of the cardiomyocytes. An alternative hypothesis would be that MSCs injected into ischemic hearts, via paracrine effects, may attract and activate circulating and/or resident cardiac stem cells (CSCs). Higher levels of VEGF secreted due to the better survival of CCR1-MSCs in the ischemic environment may account at least partially for such effects. Indeed, VEGF has been shown to result in mobilization of bone marrow-derived endothelial progenitor cells21 as well as possibly contributing in the spontaneous differentiation of stem cells towards the cardiac myocyte fate22. It is also worthy to note that granulocyte colony-stimulating factor (G-CSF) production in day 3 post-MI hearts injected with CCR1-MSCs was significant higher compared to hearts injected with control MSCs, although other cytokine production did not change (Online Figure VI). It has been reported that G-CSF increases the mobilization of stem cells from BM into peripheral circulation resulting in myocardial protection after MI 23, 24. Whether the injection of CCR1-MSCs attracts and activates CSCs as well as MSCs requires further in depth investigation.
In summary, our efforts to genetically manipulate MSCs ex vivo by enhancing the expression of CCR1 show that overexpression of CCR1 enhances MSC survival, migration, and engraftment in ischemic myocardium. Meanwhile, CCR1-MSC injection reduced cell apoptosis and infarction size, increased capillary density, prevented cardiac remodeling, and restored cardiac function (Online Figure VII). This approach has advanced our understanding of chemokine/receptor axis and stem cell biology, and may offer a new strategy for improved stem cell therapy for injured myocardium.
Novelty and Significance
What Is Known?
Chemokine/chemokine receptor axes are essential and potent regulators of chemotactic activities for a wide range of cell types, including stem cells.
Chemokines CCL7, CXCL1 and CXCL2 are upregulated in the heart following myocardial infarction.
The expression levels of the corresponding receptors CCR1 and CXCR2 are low in MSCs.
What New Information Does This Article Contribute?
Overexpression of CCR1, but not CXCR2, increased MSC migration and viability in vitro and in vivo.
Intramyocardial injection of CCR1-MSCs protected myocardium injury, prevented cardiac remodeling, and restored cardiac function.
Despite the great promise of MSC therapy for acute myocardial injury, the effective engraftment of the transplanted stem cells into the ischemic myocardium still poses a challenge for improving their therapeutic application. Ischemic myocardium expresses high level of chemokines, including CCL7, CXCL1 and CXCL2. However the expression levels of the corresponding receptors CCR1 and CXCR2 are low in MSCs. We overexpressed the CCR1 and CXCR2 receptors in MSCs by gene transfer and demonstrated that overexpressing CCR1, but not CXCR2, increases MSC migration and viability in vitro. When we injected CCR1-MSCs into murine myocardium post-MI, a greater number of CCR1-MSCs accumulated in the myocardial tissue and migrated towards the area of injury. Injection of CCR1-MSCs produced protective effects for the injured myocardium 3 days post-MI and prevented cardiac remodeling and impairment of cardiac function 4 weeks post-MI. Genetic manipulation of MSCs ex vivo by enhancing the expression of CCR1 advances our understanding of the chemokine/receptor axis and of stem cell biology, and may offer a new strategy for improving stem cell therapy for injured myocardium.
Supplementary Material
Acknowledgements
We thank Drs. Lan Mao and Howard Rockman for assistance with echocardiographic measurements and data analysis.
Sources of Funding
This study was supported by the National Institutes of Health Grant R01-HL081744, R01-HL073219, R01-HL072010, and R01-HL035610 (to V.J.D.) from the National Heart, Lung, and Blood Institute, National Institute of Health. The study was also supported by grants from the Edna and Fred L. Mandel, Jr. Foundation (to V.J.D. and M.M.) and the Fondation Leducq (to V.J.D.).
Non-standard Abbreviations and Acronyms
- AAR
area at risk
- alpha-MEM
alpha minimal essential medium
- AUC
area under the curve
- BM
bone marrow
- BrdU
5-bromo-2′-deoxyuridine
- CCL5
chemokine (c-c motif) ligand 5
- CCL7
chemokine (c-c motif) ligand 7
- CCR1
chemokine (c-c motif) receptor 1
- CSCs
cardiac stem cells
- CXCL1
chemokine (c-x-c motif) ligand 1
- CXCL2
chemokine (c-x-c motif) ligand 2
- CXCR2
chemokine (c-x-c motif) receptor 2
- CXCR4
chemokine (c-x-c motif) receptor 4
- DMEM
Dulbecco Modified Eagle medium
- FACS
fluorescence-activated cell sorting
- FS
fractional shortening
- G-CSF
granulocyte colony-stimulating factor
- GFP
green fluorescent protein
- HPF
high power field
- HSCs
hematopoietic stem cells
- HUVECs
human umbilical vein endothelial cells
- IL
interleukin
- IRES
internal ribosome entry site
- LacZ
β-galactosidase
- LAD
left anterior descending
- LV
left ventricular
- LVEDD
left ventricular end diastolic dimension
- LVESD
left ventricular end systolic dimension
- LVm
left ventricular mass
- MI
myocardial infarction
- MSCs
mesenchymal stem cells
- RLU
relative light unit
- SDF-1
stromal cell-derived fractor-1
- siRNA
small interfering RNA
- TTC
triphenyltetrazolium chloride
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
- VEGF
vascular endothelial growth factor
- wBMCs
whole bone marrow cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Subject codes: [4] Acute myocardial infarction, [27] Other Treatment
Disclosures
None.
References
- 1.Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, Dzau VJ. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 2.Psaltis PJ, Zannettino AC, Worthley SG, Gronthos S. Concise review: mesenchymal stromal cells: potential for cardiovascular repair. Stem Cells. 2008;26:2201–2210. doi: 10.1634/stemcells.2008-0428. [DOI] [PubMed] [Google Scholar]
- 3.Chen SL, Fang WW, Ye F, Liu YH, Qian J, Shan SJ, Zhang JJ, Chunhua RZ, Liao LM, Lin S, Sun JP. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol. 2004;94:92–95. doi: 10.1016/j.amjcard.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 4.Frangogiannis NG. The immune system and cardiac repair. Pharmacol Res. 2008;58:88–111. doi: 10.1016/j.phrs.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abbott JD, Huang Y, Liu D, Hickey R, Krause DS, Giordano FJ. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110:3300–3305. doi: 10.1161/01.CIR.0000147780.30124.CF. [DOI] [PubMed] [Google Scholar]
- 6.Ip JE, Wu Y, Huang J, Zhang L, Pratt RE, Dzau VJ. Mesenchymal stem cells use integrin beta1 not CXC chemokine receptor 4 for myocardial migration and engraftment. Mol Biol Cell. 2007;18:2873–2882. doi: 10.1091/mbc.E07-02-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schenk S, Mal N, Finan A, Zhang M, Kiedrowski M, Popovic Z, McCarthy PM, Penn MS. Monocyte chemotactic protein-3 is a myocardial mesenchymal stem cell homing factor. Stem Cells. 2007;25:245–251. doi: 10.1634/stemcells.2006-0293. [DOI] [PubMed] [Google Scholar]
- 8.Honczarenko M, Le Y, Swierkowski M, Ghiran I, Glodek AM, Silberstein LE. Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells. 2006;24:1030–1041. doi: 10.1634/stemcells.2005-0319. [DOI] [PubMed] [Google Scholar]
- 9.Ponte AL, Marais E, Gallay N, Langonne A, Delorme B, Herault O, Charbord P, Domenech J. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25:1737–1745. doi: 10.1634/stemcells.2007-0054. [DOI] [PubMed] [Google Scholar]
- 10.Wu Y, Ip JE, Huang J, Zhang L, Matsushita K, Liew CC, Pratt RE, Dzau VJ. Essential role of ICAM-1/CD18 in mediating EPC recruitment, angiogenesis, and repair to the infarcted myocardium. Circ Res. 2006;99:315–322. doi: 10.1161/01.RES.0000235986.35957.a3. [DOI] [PubMed] [Google Scholar]
- 11.Jin T, Xu X, Hereld D. Chemotaxis, chemokine receptors and human disease. Cytokine. 2008;44:1–8. doi: 10.1016/j.cyto.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 13.Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, Rovner A, Ellis SG, Thomas JD, DiCorleto PE, Topol EJ, Penn MS. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362:697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 14.Cheng Z, Ou L, Zhou X, Li F, Jia X, Zhang Y, Liu X, Li Y, Ward CA, Melo LG, Kong D. Targeted migration of mesenchymal stem cells modified with CXCR4 gene to infarcted myocardium improves cardiac performance. Mol Ther. 2008;16:571–579. doi: 10.1038/sj.mt.6300374. [DOI] [PubMed] [Google Scholar]
- 15.Shimizu K, Minami M, Shubiki R, Lopez-Ilasaca M, MacFarlane L, Asami Y, Li Y, Mitchell RN, Libby P. CC chemokine receptor-1 activates intimal smooth muscle-like cells in graft arterial disease. Circulation. 2009;120:1800–1813. doi: 10.1161/CIRCULATIONAHA.109.859595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mirotsou M, Zhang Z, Deb A, Zhang L, Gnecchi M, Noiseux N, Mu H, Pachori A, Dzau V. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci U S A. 2007;104:1643–1648. doi: 10.1073/pnas.0610024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95:9–20. doi: 10.1161/01.RES.0000135902.99383.6f. [DOI] [PubMed] [Google Scholar]
- 19.Silva GV, Litovsky S, Assad JA, Sousa AL, Martin BJ, Vela D, Coulter SC, Lin J, Ober J, Vaughn WK, Branco RV, Oliveira EM, He R, Geng YJ, Willerson JT, Perin EC. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005;111:150–156. doi: 10.1161/01.CIR.0000151812.86142.45. [DOI] [PubMed] [Google Scholar]
- 20.Tang YL, Zhao Q, Zhang YC, Cheng L, Liu M, Shi J, Yang YZ, Pan C, Ge J, Phillips MI. Autologous mesenchymal stem cell transplantation induce VEGF and neovascularization in ischemic myocardium. Regul Pept. 2004;117:3–10. doi: 10.1016/j.regpep.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, Inai Y, Silver M, Isner JM. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. Embo J. 1999;18:3964–3972. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song YH, Gehmert S, Sadat S, Pinkernell K, Bai X, Matthias N, Alt E. VEGF is critical for spontaneous differentiation of stem cells into cardiomyocytes. Biochem Biophys Res Commun. 2007;354:999–1003. doi: 10.1016/j.bbrc.2007.01.095. [DOI] [PubMed] [Google Scholar]
- 23.Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci U S A. 2001;98:10344–10349. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, Ponomaryov T, Taichman RS, Arenzana-Seisdedos F, Fujii N, Sandbank J, Zipori D, Lapidot T. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3:687–694. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.