Abstract
Brain trauma is associated with long-term decrements in synaptic plasticity and cognitive function, which likely reside on the acute effects of the injury on protein structure and function. Based on the action of proteasome on protein synthesis and degradation we have examined the effects of brain injury on proteasome level/activity and the potential of exercise to interact with the effects of the injury. Exercise has a healing ability but its action on proteasome function is not understood. Male Sprague-Dawley adult rats (n=19) were performed mild brain fluid percussion injury (FPI) prior to exercise. Animals were assigned to four groups: sedentary (Sed) or exercise (Exc) with sham surgery (Sham) or FPI: Sham/Sed, Sham/Exc, FPI/Sed, FPI/Exc. Animals were sacrificed after 14 days of treatment. FPI elevated levels of carbonyl (160.1±9.6% SEM, p<0.01) and reduced synapsin I levels (58.3±4.3% SEM, p<0.01) in the ipsilateral side of caudal cerebral cortex (FPI/Sed compared to Sham/Sed controls), and it appears that increased levels of carbonyls were associated with increased chymotripsin like activity. These results seem to indicate that proteasome function may be associated with levels of oxidative stress, and that these events may contribute to the action of exercise on synaptic plasticity. Interestingly, exercise attenuated changes in carbonyls, proteasome activity, and synapsin I following FPI, which may indicate an action of exercise on the molecular substrates that control protein turnover following brain trauma. Levels of the regulatory transcription factor of proteasome, Zif 268 were reduced by exercise in Sham and FPI animals and changed in proportion with proteasome activity/content. The overall results indicate that the action of exercise interfaces with that of brain injury on molecular systems involved with protein fate and function, which may be significant for synaptic plasticity.
Keywords: Fluid percussion injury, Proteasome, Synaptic plasticity, Oxidative stress, Exercise
1. Introduction
Brain trauma causes a long-term reduction in cognitive capacity (Rees et al., 2007; Royo et al. 2007), and these limitations likely derive from the events stemming from the period of the injury such as deteriorations in the molecular substrates that support synaptic plasticity and function. Most if not all forms of synaptic plasticity involve protein synthesis and limited protein degradation (James et al., 2005), which are under the spectrum of the action of proteasome complex. The proteasome system is responsible for the degradation of altered cellular proteins, including those modified by reactive oxygen species. Oxidized proteins are tagged for degradation either by the change of hydrophobicity or by ubiquitination, such that residual amino acids can be recycled for protein synthesis (Yao et al., 2008). Fluid percussion brain injury (FPI) reportedly results in oxidative modification of proteins (Wu et al., 2006); however, the role of proteasome complex in the pathobiology of the damage has been poorly investigated. The transcription factor Zif 268 is an early gene that may serve as a liaison between protein syntheses and degradation by modifying gene expression of proteasome subunits (Farmer et al., 2004). It appears that Zif 268 can alter the expression of proteasome subunits, with resulting effects on protein degradation and synapse formation (James et al., 2005).
Protein degradation has been suggested to contribute to synaptic plasticity (Opii et al., 2007) but the mechanisms involved are not well understood. Synapsin I is a vesicle-associated phosphoprotein engaged in transmitter release (Baekelandt et al., 1994) that secures synaptic vesicles to the actin cytoskeleton (Greengard et al., 1993). The supporting function of synapsin I on vesicle formation depends on proper protein synthesis and degradation, a process likely affected by brain trauma. In turn, regular exercise has been shown to promote changes in the activational stage of synapsin I that may reflect its action on the maintenance of a synaptic vesicle reserve pool. Exercise has also been shown to decrease the accumulation of oxidative protein modification in a variety of tissues including brain (Radak et al., 2008). In addition, it is becoming well established that exercise can benefit the traumatically injured brain, probably by reducing the deleterious effects of oxidative damage on synaptic plasticity and cognitive function (Gomez-Pinilla, 2008; Griesbach et al., 2008). Therefore, the objective of the current investigation is to determine the effects of brain trauma on the proteasome complex, and the possibility that voluntary exercise can counteract the effects of trauma, expecting to shed light on the involvement of protein turnover on the pathobiology of traumatic brain injury.
2. Results
2.1. Effects of brain trauma
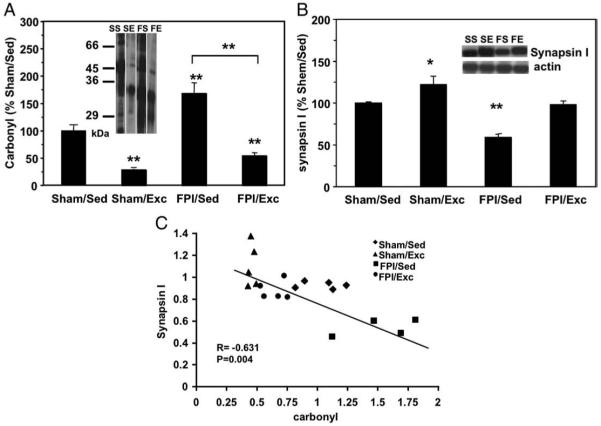
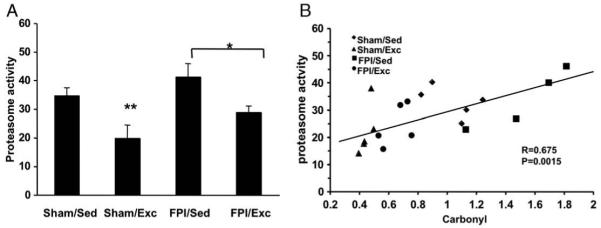
Fluid percussion injury (FPI) was used to evaluate the effects of brain trauma on oxidative stress, proteasome parameters, and correlates of synaptic plasticity. Our data show that the carbonyl concentration increased significantly in the injured side of the caudal cerebral cortex compared to sham control rats (160.1±9.6%, p<0.01) (Fig. 1A). The other significant effect of FPI was a reduction in the levels of synapsin I, a vesicle associated protein important for synaptic transmission (58.3 ±4.3%, p<0.01, FPI/Sed compared to Sham/Sed controls) (Fig. 1B). Indeed, a strong negative correlation was found between the level of carbonyl groups and the contents of synapsin I (r=−0.675, p<0.01) (Fig. 1C). FPI also showed a tendency to elevate levels of proteasome activity (Fig. 2A).
Fig. 1.
The effects of FPI and exercise on levels of carbonyls and synapsin I. Panel at A shows densitometric data of the level of carbonyl groups in amino acid residues. The levels of carbonyls were increased significantly in FPI/Sed animals. Panel at B shows that FPI decreased levels of synapsin I (FPI-Sed), and that exercised counteracted the effects of FPI (FPI/exc). Exercise also elevated levels of synapsin I in sham animals (Sham/Ex). Values are mean±SEM percentage of control group. *p<0.05, **p<0.01. Representative bands of Western blots are shown. Panel at C reveals a significant negative correlation between the level of oxidative protein damage (carbonyl groups), and synapsin I protein concentration. Values were calculated from the densitometric data obtained by Western blot analysis. (N=19, p<0.05, r=−0.63).
Fig. 2.
Effects of FPI and exercise on proteasome activity. Panel at A shows that exercise (FPI/Exc) reduced the FPI-induced elevation (FPI/Sed) of the chymotripsin like activity of proteasome. Exercise also reduced chymotripsin like activity in sham animals (Sham/Exc). Scatter plot at B reveals a positive correlation between the activity of proteasome and the level of carbonyl groups (N=19, p<0.05, r=0.68). Values are mean±SEM percentage of control group. *p<0.05, **p<0.01.
2.2. Effects of exercise
Voluntary exercise decreased the accumulation of carbonyl groups in both sham (31.5±2.2% SEM, p<0.01, Shma/Exc compared to Sham/Sed controls) and FPI (56.75±4.3% SEM, p<0.01, FPI/Exc compared to Sham/Sed controls), suggesting that exercise can promote a condition of increased resistance or increased repair of oxidative protein damage (Fig. 1A). Moreover, voluntary exercise decreased the chymotripsin-like activity of proteasome in the FPI (FPI/Sed: 115±17.1% SEM; FPI/Exc: 74.4±10.4% SEM, p<0.05, compared to Sham/Sed controls) and sham rats (Sham/Exc: 55.8±5.5% SEM, p<0.01, compared to Sham/Sed controls) (Fig. 2A). We have found a linear relationship between carbonyl content and proteasome activity (r=0.675, p<0.01) (Fig. 2B), which could mean that proteasome action is associated with the level of oxidative protein damage. In addition, the protein content of the main subunit of proteasome, 20S alpha decreased in exercise groups (Sham/Exc: 55±5.2% SEM, FPI/Exc: 60±17.3% SEM, p<0.01) compared to control groups (100±6.1% SEM) (Fig. 3A).
Fig. 3.
The effects of FPI and exercise on contents of proteasome subunit and Zif 268. Panel at A shows that voluntary exercise decreased the 20S alfa subunit of the proteasome complex in Sham (Sham/Exc) and injured animals (FPI/Exc). Panel at B shows that exercise reduced the content of Zif 268 in a similar fashion to that observed for 20S alfa subunit. Values are mean±SEM percentage of control group. Panel at C shows a positive correlation between Zif 268 and proteasome activity (r=0.71, p<0.01), suggesting an association between Zif 268 and proteasome function. **p<0.01).
The protein content of Zif 268 decreased in response to exercise in sham (Sham/Exc: 80±7% SEM, p<0.01) and FPI (FPI/Exc: 78±6% SEM, p<0.01) rats compared to Sham/Sed controls (Fig. 3B), following a similar pattern to that shown by the effects of exercise on proteasome activity (Fig. 2A) and content (Fig. 3A). Indeed, a positive correlation (r=0.708, p=0.001) was found between Zif 268 and proteasome activity (Fig. 3C), which may suggest an association between proteasome activity and Zif 268, in agreement with the possibility that Zif 268 can serve as a regulating factor of proteasome complex.
3. Discussion
The present results indicate that experimental brain trauma elevates the levels of protein oxidation in the injured cerebral cortex. Correlation analysis showed that protein oxidation was positively associated with proteasome activity, while inversely related to levels of the synaptic protein synapsin I. An interesting observation was that voluntary exercise reduced levels of proteasome (Fig. 3A), proteasome activity (Fig. 2A), and the protein content of Zif 268 (Fig. 3B) in the animals that had received FPI as well as those that received sham surgery. The overall results seem to suggest that exercise can affect synaptic plasticity in the traumatically injured brain by acting on molecular systems that influence protein turnover (Fig. 4).
Fig. 4.
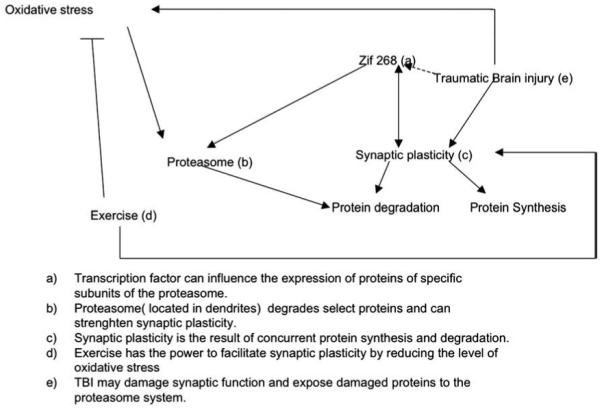
Diagrammatic representation for the potential effects of brain trauma and exercise on proteasome function and synaptic plasticity.
3.1. FPI effects on proteasome
Our results showed that brain trauma elevated levels of oxidative stress, which could be detrimental for brain function (Radak et al., 2001). It is also interesting that the positive correlation between protein oxidation and proteasome activity, as well as proteasome activity and Zif 268 indicate that protein oxidation and proteasome function could be interrelated events. FPI is known to induce oxidative stress (Wu et al., 2006), necrosis, apoptosis, and inflammation (Chen et al., 2008). The clearance of damaged proteins is indispensable in order to provide the necessary conditions for cellular repair and plasticity. Proteasome complex is a major housekeeping system, and its activity can be induced by oxidative stress. Indeed, induction of proteasome has been reported after different traumatic stressors, such as hyperoxia, radiation or oxidative damage (Chambellan et al., 2006; Radak et al., 2000). In the present study, FPI elevated protein oxidation and there was a linear correlation between the extent of carbonyl modification and the activity of proteasome. The fact that the effects of FPI on proteasome activity did not reach statistic significance suggests that FPI may affect proteasome via elevations in protein oxidation, and that higher levels of protein oxidation would have been necessary to elevate protein contents and activity. It is known that the oxidized proteins are tagged for degradation (Yao et al., 2008) and that activity of proteasome complex can be induced by oxidative protein damage in cell culture (Sitte et al., 1998).
The level of protein degradation could significantly affect the rate of protein turnover, which is curricular during the regeneration after cellular damage. Increasing evidence indicates that reversible proteasome inhibitors can be therapeutic agents even neuroprotective during ischemic brain injury (Williams et al., 2003; Zhang et al., 2001). Our data suggest that physical activity might act as a physiological regulator of proteasome to facilitate the recovery process and to maintain synaptic plasticity. In these terms, it is interesting that the application of exercise reduced levels of proteasome content or activity in animals that had received FPI. These data seem to suggest that exercise can reduce levels of protein turnover following TBI. More studies are required to determine the specific protein targets for the effects of the proteasome systems under study.
3.2. Exercise may benefit the injured brain by normalizing proteasome parameters
It is interesting that exercise was able to reverse the elevation of protein carbonyl and the reduction of synapsin I associated with the effects of FPI on the brain, and we suggest that these changes could affect synaptic plasticity. In addition, exercise reduced levels of proteasome parameters such as proteasome activity and Zif268. It is interesting that exercise reduced proteasome parameters in both intact and injured animals, which may be indicative of the housekeeping function of proteasome and the capacity of exercise to maintain plasticity under homeostatic and injured conditions.
3.3. Implications for synaptic plasticity and function
Results showed that proteasome activity changed proportionally to carbonyl levels while levels of synapsin I were reduced according to carbonyl levels. Besides the important house-keeping function of proteasome, they play an important regulatory role by targeting degradation of transcription factors important for synaptic plasticity (Hegde and Upadhya, 2007). Although, it has been shown a regulatory action of proteasome on postsynaptic density (Ehlers, 2003), it is not clear the mechanisms used by the proteasome complex to determine protein specificity in the exclusion of damaged proteins. Products of degradation are transported to class I major histocompatibility complex, which supplies peptides to synapse and which has been shown to strongly alter hippocampal synaptic plasticity in the form of LTP (Huh et al., 2001).
Our correlation analysis showed a strong association between proteasome activity and Zif 268 levels. Along this line of thought, it has been suggested that Zif 268 has an important regulatory role in tuning the function of proteasome by altering the expression of several subunits of the complex to selectively target degradation of specific short-lived proteins. A recent microarray study has revealed that four proteasome subunits and the activity responsible element of the complex are dependent on the Zif 268 gene (James et al., 2005). Early evidence indicated that other members of the synapsin family are regulated by Zif 268 (Petersohn et al., 1995), which could affect synaptic plasticity. The involvement of Zif 268 in LTP is well demonstrated as mice with genetic deletion of Zif 268 show a deficit in memory performance (Jones et al., 2001). Although further studies are required, the overall evidence seems to indicate that the proteasome complex could function to regulate synaptic plasticity under normal conditions and following insults. The lack of significant changes in proteasome related variables in the FPI rats does not exclude an effect of TBI on proteasome. For example, we used a low intensity FPI that may not been sufficient to trigger changes at the studied timepoint.
It has been shown that accumulation of carbonyl groups in amino acid residues of brain proteins results in loss of cognitive function (Carney et al., 1991; Radak et al., 2001). Taken all together, it appears that voluntary exercise following FPI fosters recovery by decreasing the level of oxidative protein damage and by modulation of proteasome action with the possible regulatory involvement of Zif 268. The effect of exercise on proteasome function could translate into mechanisms that support synaptic plasticity, i.e., restore levels of synapsin I. We hypothesize that proteasome could emerge as a potential target for therapeutic agents to reduce some of the consequences of traumatic brain injury.
4. Experimental procedures
A total of 19 male Sprague-Dawley adult rats (250–300 g) were utilized in these experiments. Rats underwent lateral fluid percussion injury (FPI; n=9) or sham surgery (n=10) and were housed with or without access to a running wheel from post injury day 0 to 14. All animals were continually monitored and cared for by an IACUC-approved veterinary care staff upon arrival at UCLA. During the experiments, all rats were single housed in opaque plastic bins, which were lined with bedding material. Each animal was checked for weight loss, loss of stereospecific behavior, and any changes in coat color. All procedures were approved by the UCLA Chancellor’s Animal Research Committee.
4.1. Voluntary running wheel exercise
Rats were individually caged with or without access to a running wheel (Exc) from post injury day 0 to 14 [Sham/Exc (n=5) or FPI/Exc (n=5)]. This post injury period was selected given that it has previously been associated with behavioral deficits. Exercising animals were placed in standard cages equipped with running wheels (diameter = 31.8 cm, width=10 cm; Nalge Nunc International) that rotated against a resistance of 100 g. Wheel revolutions were recorded using an appropriate software (VitalViewer Data Acquisition System; Mini Mitter, Sunriver, OR). Sedentary animals (Sed) were left undisturbed in their home cages [Sham/sed (n=5) or FPI/-sed (n=4)]. All rats had ad lib access to food and water and were maintained on a 12/12-h light-dark cycle. The mean number of revolutions was calculated for each night, given that this was the most active period.
4.2. Fluid percussion injury
Lateral fluid percussion injury has been done accordingly to the method described previously (Griesbach et al., 2004). In brief, rats were initially anesthetized with 4% isofluorane (in 100% O2) and were then maintained to 1.5–2% isofluorane (in 100% O2). The head was secured in a stereotactic frame, shaved and prepped with betadine and ethanol. Body temperature was monitored and maintained at 37 °C with a heating pad (Braintree Scientific; Braintree, MA). A midline sagittal incision was made. With the aid of a microscope (Wild; Heerburg, Switzerland), a 3-mm diameter craniotomy was made with a high-speed drill (Dremel; Racine, WI), 3-mm posterior to bregma and 6-mm lateral to the midline, on the left side. A plastic injury cap was placed over the craniotomy with silicone adhesive, cyanoacrylate, and dental cement. When the dental cement hardened, the cap was filled with 0.9% saline solution. Anesthesia was discontinued and the animal was removed from the stereotactic device. The injury cap was attached to the fluid percussion device. At the first sign of hind-limb withdrawal to a paw pinch, a mild fluid percussion pulse (1.5 atm) was administered. Apnea times were determined as the time from injury to the moment of spontaneous breathing. Time of unconsciousness was operationally defined as the time from the injury to the return of a hind-limb withdrawal reflex. Sham animals underwent an identical preparation with the exception of the FPI. Immediately upon responding to a paw pinch, anesthesia was restored, the injury cap removed, and the scalp was sutured. Neomycin was applied on the suture and the rat was placed in a heated recovery chamber for approximately 1 h before returning to its cage.
4.3. Proteasome activity assessment
The last day of the exercise period or equivalent in sedentary rats, animals were quickly sacrificed by decapitation and tissue from the posterior one third of the cerebral cortex (caudal cortex) affected by FPI was saved at −70 degrees for biochemical analyses. The chymotripsin like activity of proteasome was measured as described previously (Hayashi and Goto, 1998). In brief, caudal cortex was homogenized (100 mg) in a 10× volume lysis buffer containing 10 mM Tris, 0.25MSucrose, 1.5mMMgCl2, 1mMDTT, 10% Glycerol, 10 KCl, 5 mM ATP, complete solution should be at pH 7.4. The homogenates were centrifuged, the supernatants were collected and total protein concentration was determined by Micro BCA procedure (Pierce, Rockford, IL) using bovine serum albumin as a standard. The samples were diluted to same concentration (1 mg/ml). For the assay we mixed 5× reaction buffer (500 mM Tris-HCl pH 8.0, 5 mM DTT, 25 mM MgCl2), homogenization buffer, BSA, SDS, sample, and substrate (Suc-leu-Leu-Val-Tyr-AMC) with the total volume of 200 μl to measure chymotripsin like activity of proteasome complex. The samples were incubated at 37 °C for 30 min, blocked the reaction with ice-cold methanol, and centrifuged at 10,000 g for 5 min. We took the supernatant, mixed with Tris-HCl buffer (pH 9.0) and we read the fluorescent intensity at Ex: 320 nm Em: 460 nm. The activity was calculated as described previously (Hayashi and Goto, 1998).
4.4. Protein levels assessment by Western blotting
Synapsin I, Zif 268, 20S alfa core protein of 26S proteasome complex were analyzed by Western blot as previously described (Griesbach et al., 2004). Membranes were incubated with the following primary antibodies: anti-synapsin I (1:2000; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), anti-total Zif 268 (1:2000; Cell Signaling Technology, Inc., Beverly, MA, USA), followed by anti-mouse IgG horseradish peroxidase conjugated secondary antibody, anti-20S alfa subunit of proteasome (1:2000; Cell Signaling Technology, Inc., Biotechnology), and anti-actin (1:2000; Santa Cruz Biotechnology) followed by anti-goat IgG horseradish peroxidase conjugate for synapsin I, and actin.
4.5. Statistical analyses
We used an analysis of variance (ANOVA) with repeated measures, and Fischer-test for cross group comparisons. Results were expressed as the mean percent of control values for graphic clarity and represent the mean±standard error of the mean (SEM). A linear regression analysis was performed on individual samples to evaluate association between variables.
Acknowledgments
This study was supported by the National Institutes of Health award NS50465 and by UCLA Brain Injury Research Center. We thank Erika Koltai for helpful assistance with the statistic analysis.
REFERENCES
- Baekelandt V, Arckens L, Annaert W, Eysel UT, Orban GA, Vandesande F. Alterations in GAP-43 and synapsin immunoreactivity provide evidence for synaptic reorganization in adult cat dorsal lateral geniculate nucleus following retinal lesions. Eur. J. Neurosci. 1994;6:754–765. doi: 10.1111/j.1460-9568.1994.tb00987.x. [DOI] [PubMed] [Google Scholar]
- Carney JM, Starke-Reed PE, Oliver CN, Landum RW, Cheng MS, Wu JF, Floyd RA. Reversal of age-related increase in brain protein oxidation, decrease in enzyme activity, and loss in temporal and spatial memory by chronic administration of the spin-trapping compound N-tert-butyl-alpha-phenylnitrone. Proc. Natl. Acad. Sci. U. S. A. 1991;88:3633–3636. doi: 10.1073/pnas.88.9.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambellan A, Cruickshank PJ, McKenzie P, Cannady SB, Szabo K, Comhair SA, Erzurum SC. Gene expression profile of human airway epithelium induced by hyperoxia in vivo. Am. J. Respir. Cell Mol. Biol. 2006;35:424–435. doi: 10.1165/rcmb.2005-0251OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Shi J, Qi M, Yin H, Hang C. Glutamine decreases intestinal nuclear factor kappa B activity and pro-inflammatory cytokine expression after traumatic brain injury in rats. Inflamm. Res. 2008;57:57–64. doi: 10.1007/s00011-007-7101-7. [DOI] [PubMed] [Google Scholar]
- Ehlers MD. Activity level controls postsynaptic composition and signaling via the ubiquitin-proteasome system. Nat. Neurosci. 2003;6:231–242. doi: 10.1038/nn1013. [DOI] [PubMed] [Google Scholar]
- Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience. 2004;124:71–79. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F. The influences of diet and exercise on mental health through hormesis. Ageing Res. Rev. 2008;7:49–62. doi: 10.1016/j.arr.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard P, Valtorta F, Czernik AJ, Benfenati F. Synaptic vesicle phosphoproteins and regulation of synaptic function. Science. 1993;259:780–785. doi: 10.1126/science.8430330. [DOI] [PubMed] [Google Scholar]
- Griesbach GS, Hovda DA, Molteni R, Wu A, Gomez-Pinilla F. Voluntary exercise following traumatic brain injury: brain-derived neurotrophic factor upregulation and recovery of function. Neuroscience. 2004;125:129–139. doi: 10.1016/j.neuroscience.2004.01.030. [DOI] [PubMed] [Google Scholar]
- Griesbach GS, Hovda DA, Gomez-Pinilla F, Sutton RL. Voluntary exercise or amphetamine treatment, but not the combination, increases hippocampal brain-derived neurotrophic factor and synapsin I following cortical contusion injury in rats. Neuroscience. 2008;154:530–540. doi: 10.1016/j.neuroscience.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Goto S. Age-related changes in the 20S and 26S proteasome activities in the liver of male F344 rats. Mech. Ageing Dev. 1998;102:55–66. doi: 10.1016/s0047-6374(98)00011-6. [DOI] [PubMed] [Google Scholar]
- Hegde AN, Upadhya SC. The ubiquitin-proteasome pathway in health and disease of the nervous system. Trends Neurosci. 2007;30:587–595. doi: 10.1016/j.tins.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Huh GY, Glantz SB, Je S, Morrow JS, Kim JH. Calpain proteolysis of alpha II-spectrin in the normal adult human brain. Neurosci. Lett. 2001;316:41–44. doi: 10.1016/s0304-3940(01)02371-0. [DOI] [PubMed] [Google Scholar]
- James AB, Conway AM, Morris BJ. Genomic profiling of the neuronal target genes of the plasticity-related transcription factor — Zif268. J. Neurochem. 2005;95:796–810. doi: 10.1111/j.1471-4159.2005.03400.x. [DOI] [PubMed] [Google Scholar]
- Jones MW, Errington ML, French PJ, Fine A, Bliss TV, Garel S, Charnay P, Bozon B, Laroche S, Davis S. A requirement for the immediate early gene Zif268 in the expression of late LTP and long-term memories. Nat. Neurosci. 2001;4:289–296. doi: 10.1038/85138. [DOI] [PubMed] [Google Scholar]
- Opii WO, Nukala VN, Sultana R, Pandya JD, Day KM, Merchant ML, Klein JB, Sullivan PG, Butterfield DA. Proteomic identification of oxidized mitochondrial proteins following experimental traumatic brain injury. J. Neurotrauma. 2007;24:772–789. doi: 10.1089/neu.2006.0229. [DOI] [PubMed] [Google Scholar]
- Petersohn D, Schoch S, Brinkmann DR, Thiel G. The human synapsin II gene promoter. Possible role for the transcription factor zif268/egr-1, polyoma enhancer activator 3, and AP2. J. Biol. Chem. 1995;270:24361–24369. doi: 10.1074/jbc.270.41.24361. [DOI] [PubMed] [Google Scholar]
- Radak Z, Sasvari M, Nyakas C, Pucsok J, Nakamoto H, Goto S. Exercise preconditioning against hydrogen peroxide-induced oxidative damage in proteins of rat myocardium. Arch Biochem. Biophys. 2000;376:248–251. doi: 10.1006/abbi.2000.1719. [DOI] [PubMed] [Google Scholar]
- Radak Z, Kaneko T, Tahara S, Nakamoto H, Pucsok J, Sasvari M, Nyakas C, Goto S. Regular exercise improves cognitive function and decreases oxidative damage in rat brain. Neurochem. Int. 2001;38:17–23. doi: 10.1016/s0197-0186(00)00063-2. [DOI] [PubMed] [Google Scholar]
- Radak Z, Chung HY, Goto S. Systemic adaptation to oxidative challenge induced by regular exercise. Free Radic. Biol. Med. 2008;44:153–159. doi: 10.1016/j.freeradbiomed.2007.01.029. [DOI] [PubMed] [Google Scholar]
- Rees L, Marshall S, Hartridge C, Mackie D, Weiser M. Cognitive interventions post acquired brain injury. Brain Inj. 2007;21:161–200. doi: 10.1080/02699050701201813. [DOI] [PubMed] [Google Scholar]
- Royo NC, LeBold D, Magge SN, Chen I, Hauspurg A, Cohen AS, Watson DJ. Neurotrophin-mediated neuroprotection of hippocampal neurons following traumatic brain injury is not associated with acute recovery of hippocampal function. Neuroscience. 2007;148:359–370. doi: 10.1016/j.neuroscience.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitte N, Merker K, Grune T. Proteasome-dependent degradation of oxidized proteins in MRC-5 fibroblasts. FEBS Lett. 1998;440:399–402. doi: 10.1016/s0014-5793(98)01495-1. [DOI] [PubMed] [Google Scholar]
- Williams AJ, Hale SL, Moffett JR, Dave JR, Elliott PJ, Adams J, Tortella FC. Delayed treatment with MLN519 reduces infarction and associated neurologic deficit caused by focal ischemic brain injury in rats via antiinflammatory mechanisms involving nuclear factor-kappaB activation, gliosis, and leukocyte infiltration. J. Cereb. Blood Flow Metab. 2003;23:75–87. doi: 10.1097/01.WCB.0000039285.37737.C2. [DOI] [PubMed] [Google Scholar]
- Wu A, Ying Z, Gomez-Pinilla F. Dietary curcumin counteracts the outcome of traumatic brain injury on oxidative stress, synaptic plasticity, and cognition. Exp. Neurol. 2006;197:309–317. doi: 10.1016/j.expneurol.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Yao X, Liu J, McCabe JT. Alterations of cerebral cortex and hippocampal proteasome subunit expression and function in a traumatic brain injury rat model. J. Neurochem. 2008;104:353–363. doi: 10.1111/j.1471-4159.2007.04970.x. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhang ZG, Zhang RL, Lu M, Adams J, Elliott PJ, Chopp M. Postischemic (6-Hour) treatment with recombinant human tissue plasminogen activator and proteasome inhibitor PS-519 reduces infarction in a rat model of embolic focal cerebral ischemia. Stroke. 2001;32:2926–2931. doi: 10.1161/hs1201.100207. [DOI] [PubMed] [Google Scholar]