Summary
Oxidative stress is a major challenge for all cells living in an oxygen-based world. Among reactive oxygen species, H2O2, is a well known toxic molecule and, nowadays, considered a specific component of several signalling pathways. In order to gain insight into the roles played by H2O2 in plant cells, it is necessary to have a reliable, specific and non-invasive methodology for its in vivo detection. Hence, the genetically-encoded H2O2 sensor HyPer was expressed in plant cells in different subcellular compartments such as cytoplasm and peroxisomes. Moreover, with the use of the new GFP-based Cameleon Ca2+ indicator, D3cpv-KVK-SKL, targeted to peroxisomes, we demonstrated that the induction of cytoplasmic Ca2+ increase is followed by Ca2+ rise in the peroxisomal lumen. The analyses of HyPer fluorescence ratios were performed in leaf peroxisomes of tobacco and pre- and post-bolting Arabidopsis plants. These analyses allowed us to demonstrate that an intraperoxisomal Ca2+ rise in vivo stimulates catalase activity, increasing peroxisomal H2O2 scavenging efficiency.
Keywords: plant peroxisomes, Cameleon, calcium, HyPer, hydrogen peroxide
Introduction
In their life cycle, plant cells synthetize different reactive oxygen species (ROS) whose production shows dramatic increases during senescence and under biotic and abiotic stress (Zentgraf, 2007). Regardless of how and where ROS are generated, an increase in intracellular oxidants has two important effects: damage to various cell components and activation of specific signalling pathways. This latter findings have led to the new concept of ROS as “oxidative signalling molecules” and not only as “oxidative stress by-products” (Foyer et al., 2009).
Within this scenario, H2O2 plays a key role. In contrast to other ROS, it has a relatively long half-life and can be produced in all cell compartments. Moreover, since it is highly diffusible, it can easily pass membranes. The endogenous H2O2 content of plant cells is usually much higher than that found in animals and bacteria; plant cells happily survive with H2O2 levels that would kill animal cells. This tolerance is linked to the presence in plant cells of highly efficient antioxidant systems.
Ascorbate peroxidase (APX) is probably the most important enzyme scavenging H2O2 produced in the chloroplasts; this enzymatic activity is also present in cytoplasm, peroxisomes and mitochondria (Jimenez et al., 1998a; Jimenez et al., 1998b; Asada and Takahashi, 1987, Shigeoka et al., 2002 and reference therein, Narendra et al., 2006, Locato et al., 2009). The other major H2O2-metabolizing enzymes, catalases (CATs), are located in the peroxisome lumen and are responsible for detoxification of high concentrations of H2O2 (Nyathi and Baker, 2006). Whereas APX has a high affinity for H2O2 and is able to detoxify low concentrations of H2O2, catalases have a higher Vmax, but a lower affinity for H2O2. Catalases also appear to play a key role in maintaining the redox balance in cells exposed to oxidative stresses (Willekens et al., 1997; Apel and Hirt, 2004; Queval et al., 2007).
In plants catalases belong to a small family of genes identified in several species (e.g. pea, maize and Arabidopsis) with different isoforms expressed at different levels (Wadsworth and Scandalios, 1989; McClung, 1997; Corpas et al., 1999). In Arabidopsis, there are three different catalase genes (CAT1, CAT2 and CAT3), but six enzymatically distinguishable isoforms described in different organs of the adult plant (McClung, 1997). The three Arabidopsis catalases are regulated both at the transcriptional and posttranscriptional level, and CAT2 and CAT3 show circadian regulation too (McClung, 1997; Orendi et al., 2001; Zimmermann et al., 2006; Queval et al., 2007; Du et al., 2008). Moreover, the levels of CAT3 vary substantially during the plant life span and, in particular, a significant increase both of transcription and of enzymatic activity is observed in leaves of senescent Arabidopsis plants (Zimmermann et al., 2006; Du et al., 2008). CAT3 is particularly interesting because is predicted to be regulated, at least in vitro, by Ca2+ and Calmodulin (CaM) (Yang and Poovaiah, 2002). The same authors demonstrated that Arabidopsis CAT3 can bind CaM in a Ca2+-dependent way and that the peptide corresponding to the predicted CAT3 CaM binding region is able to competitively inhibits in vitro the Ca2+-dependent stimulation of tobacco catalase activity (Yang and Poovaiah, 2002). Whether and how this Ca2+ regulation of CAT3 is relevant in intact cells remain to be established. In particular, it is still unknown whether (and when) the peroxisomal Ca2+ concentration ([Ca2+]p) can increase in intact cells and whether CAT3 within peroxisome can be indeed activated by these Ca2+ increases (if they occur).
In this study, we have developed an in vivo approach to investigate the problem of H2O2 metabolism by plant cells and its modulation by Ca2+ signalling. To this end, we have developed and characterized two genetically encoded probes targeted to the peroxisomal lumen (and to the cytoplasm) to monitor directly and quantitatively both H2O2 and Ca2+ concentration (HyPer and D3cpv, respectively) in intact living cells (Belousov et al., 2006, Palmer et al., 2006). We have also generated Arabidopsis transgenic plants stably expressing such probes. We here demonstrate, through in vivo imaging analyses, that H2O2 content into the leaf peroxisomes is modulated during the life cycle of Arabidopsis, in particular the Ca2+-dependent H2O2 catabolism is greatly accelerated when pre- and post-bolting phases are compared. In addition, we have investigated intraperoxisomal Ca2+ dynamics in plant cells subjected to stimuli that increase cytoplasmic Ca2+ levels and we demonstrate that [Ca2+]p rapidly equilibrates with that in the cytosol. Last, but not least, we demonstrate that the peroxisomal Ca2+ increases potently stimulates the scavenging of H2O2 and provide compelling evidence supporting that this is, at least in part, mediated by Ca2+ activation of peroxisomal CAT3 activity.
Results
Cytoplasmic HyPer can specifically sense H2O2 in plant cell cytoplasm
A genetically-encoded YFP-based H2O2 sensor, named HyPer (Belousov et al., 2006), has been recently described and it has been shown that HyPer in bacteria and animal cells is highly sensitive to H2O2 and not to other ROS. In order to investigate H2O2 metabolism in plant cells we thus generated stable transgenic Arabidopsis lines constitutively expressing HyPer within the cytoplasm (Figure 1a-d and S1a-c). HyPer cDNA was cloned downstream of the double CaMV35S promoter and was thus expected to be expressed in all cells. Figures 1(a-c) show confocal images of a representative transgenic Arabidopsis plant leaf expressing cytoplasmic HyPer (cHyPer) that, as expected, presents a diffuse cell fluorescence, including a clear signal in the nucleus. Stomata guard cells also strongly expressed the probe (Figure 1d).
Figure 1.
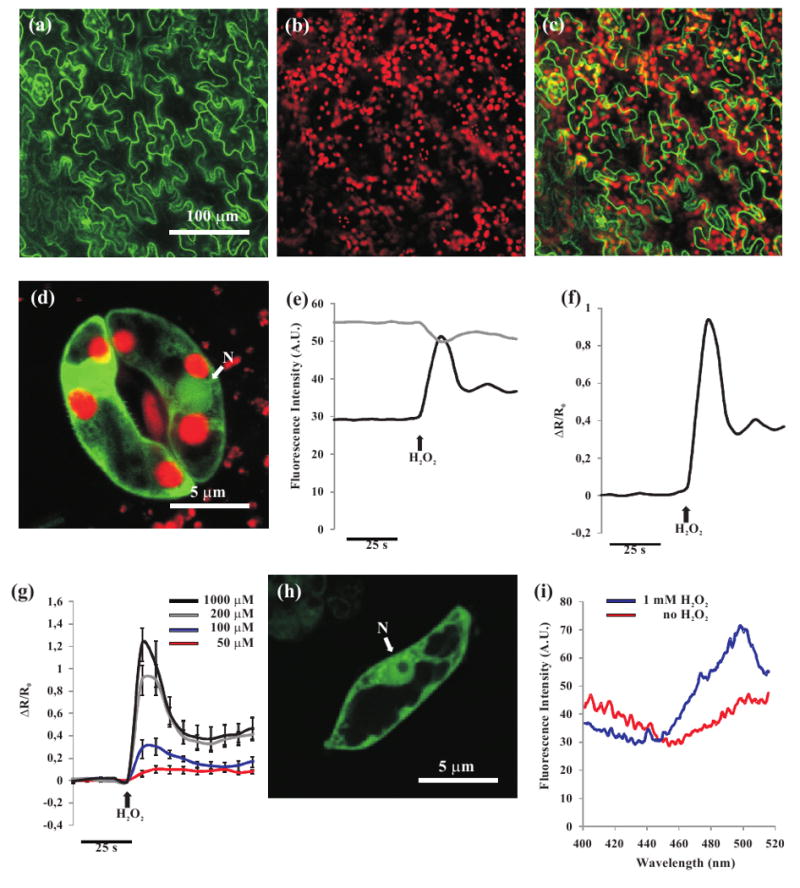
HyPer targeted to the cytoplasm of plant cells is able to sense exogenously applied H2O2.
(a-d) Confocal images of stable transgenic Arabidopsis plants transformed with cHyPer.
(a) HyPer fluorescence (green) in Arabidopsis epidermal leaf cells.
(b) Chlorophyll fluorescence of the same leaf shown in (a).
(c) Overlay image of (a) and (b).
(d) Overlay image of HyPer (green) and chlorophyll (red) fluorescences in stomata guard cells. Nucleus (N).
(e,f) Microscope imaging analysis performed in transgenic Arabidopsis guard cell expressing the HyPer sensor in the cytoplasm.
(e) Traces corresponding to single emission wavelengths used for the ratio calculation shown in (f). Upon addition of H2O2 (100 μM), a clear opposite response was observed in the two independent channels: the 420 nm excitation led to a fluorescence decrease (grey trace) whereas a sharp fluorescence increase was observed with 480 nm excitation (black trace).
(f) cHyPer H2O2-dependent fluorescence ratio increase expressed as ΔR/R0.
(g) cHyPer fluorescence ratio increase (ΔR/R0) in Arabidopsis guard cells subjected to different concentrations of exogenous H2O2.
(h) Confocal image of a representative suspension cultured cHyPer Arabidopsis cell. Nucleus (N).
(i) A cHyPer Arabidopsis suspension cell culture was transferred into a spectrophotometric cuvette and scanned with a Perkin-Elmer lambda spectrophotometer (Perkin-Elmer, USA). For the scan of oxidized HyPer the H2O2 was directly added into the cuvette. A clear change in the excitation spectrum of cHyPer before (red trace) and after (blue trace) H2O2 (1 mM) was observed. Emission was measured at 530 nm.
To test whether HyPer maintains its unique properties also in plant cells in situ, guard cells in an intact epidermis of Arabidopsis expressing cHyPer were challenged with H2O2 added to the bath (Figure 1e and 1f). As already reported both in vitro and in animal cells expressing HyPer addition of H2O2 caused a sharp increase in the fluorescence emitted (530 nm), when the probe was excited at 480 nm and a decrease when excited at 420 nm (Figure 1e). No appreciable delay was ever observed between H2O2 addition and the fluorescence changes. The fluorescence ratio change, here presented as ΔR/R0, was proportional to the amount of exogenously applied H2O2 with a clear dose-dependence (Figure 1g). Thus, not only HyPer is sensitive to H2O2 also in plant cells in situ, but this result clearly demonstrates that the plasma membrane of guard cells is highly permeable to H2O2, possibly favoured by aquaporin channels (Bienert et al., 2006, 2007). The spectral characteristics of HyPer in live Arabidopsis cells were further characterized in suspension cell culture lines obtained from Arabidopsis plants expressing cHyPer (Figure 1h). The HyPer excitation spectra obtained in presence or absence of exogenously added H2O2 showed essentially the same properties reported by Belousov and colleagues (2006) in vitro, i.e. the two excitation peaks at 420 and 500 nm and an isosbestic point at 450 nm (Figure 1i). The possibility of using HyPer in a ratiometric way is particularly advantageous for analyses of highly mobile organelles such as peroxisomes (see below).
Generation of Arabidopsis transgenic plants expressing peroxisome-targeted HyPer probe
Peroxisome metabolism of H2O2 is of major importance in plant cells: not only peroxisome photorespiration has been demonstrated to produce massive amounts of H2O2 (50 times more than mitochondria) (Foyer and Noctor, 2003), but these organelles are the major site of H2O2 scavenging, due to the high concentration in their lumen of catalases (Baker and Graham, 2002; Nyathi and Baker, 2006).
Although H2O2 should be capable of permeating all natural membranes, the specific permeability of the peroxisomal membrane to H2O2 has not been determined yet. In order to investigate the kinetics of H2O2 catabolism within peroxisome we thus generated another HyPer construct, including at its C-terminal the KSRM peptide, a well-known specific peroxisomal targeting sequence (Dammann et al., 2003; Reumann et al., 2004). Figures 2(a-d) and S1(d-f) show respectively confocal images of a representative transgenic Arabidopsis plant leaf and root expressing HyPer-KSRM: in this case, the fluorescence signal is restricted to typical small vesicular structures corresponding to peroxisomes. Peroxisomes were clearly detectable also in stomata guard cells (Figure 2d).
Figure 2.
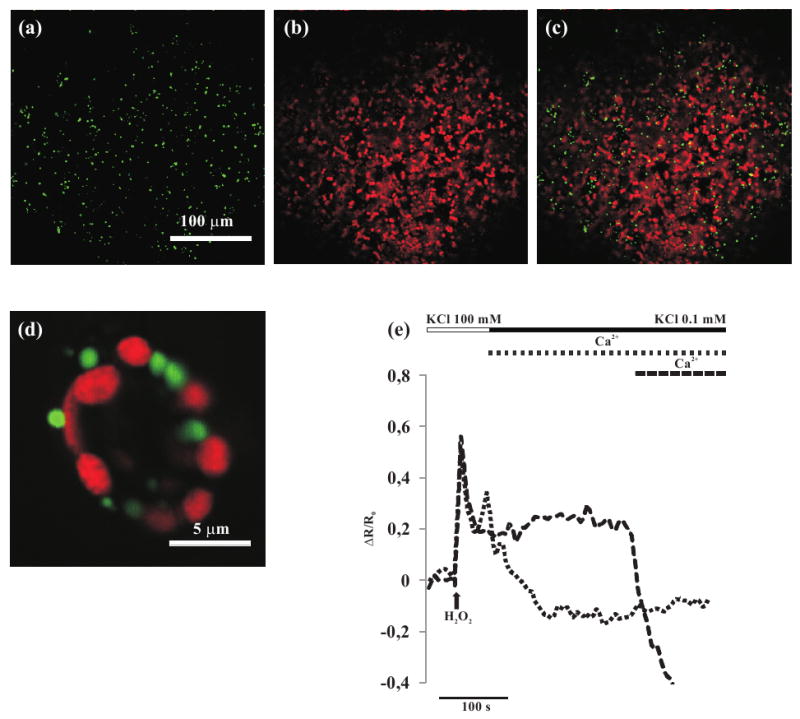
Ca2+-dependent peroxisomal H2O2 scavenging in Arabidopsis guard cells expressing the HyPer-KSRM probe.
(a-d) Confocal images of stable transgenic Arabidopsis plants transformed with HyPer-KSRM.
(a) HyPer fluorescence (green) in Arabidopsis leaf epidermal cells.
(b) Chlorophyll fluorescence of the same leaf shown in (a).
(c) Overlay image of (a) and (b).
(d) Overlay image of HyPer (green) and chlorophyll (red) fluorescences in stomata guard cells.
(e) Arabidopsis guard cells were bathed in high K+ concentration buffer (depolarization buffer, white bar), and H2O2 (100 μM) was added (arrow). The cells were then perfused with a buffer with low K+ (hyperpolarization buffer, black bar) and Ca2+ was added at the same time of hyperpolarization (dotted trace) or after (dashed trace). A steep decrease in the HyPer fluorescence ratio was clearly and only observed when Ca2+ was allowed to enter.
Ca2+-dependent H2O2 scavenging in Arabidopsis peroxisomes
As can be seen in Figure 2(e), in guard cells expressing HyPer-KSRM, addition of H2O2 to the bath, resulted, as in the case of cells expressing cHyPer (Figure 1f), in a sharp increase of the fluorescence emission ratio again, with no lag phase, indicating that also the peroxisomal membrane is freely permeable to H2O2. As noticed before for the cHyPer signal, the ratio peak was followed by a slight decrease and then by a sustained, slowly decaying phase (Figure 2e).
The next series of experiments were carried out to investigate whether conditions can be found in which H2O2 metabolism within the cytoplasm and/or peroxisomes could be altered. Given that a number of indirect evidence suggests that Ca2+ can affect H2O2 metabolism in plants (Apel and Hirt, 2004 and references therein), we subjected the cells to protocols that can induce cell Ca2+ changes (Pei et al., 2000; Allen et al., 2001; Yang et al., 2008). The experiments presented in Figure 2(e) were carried out in a standard depolarizing medium (high K+ concentration, see Experimental procedures), without added Ca2+. When cells were hyperpolarized (low K+ concentration) after adding H2O2 (100 μM), (Figure 2e, dashed trace) no significant variation in H2O2 level was observed. However, when Ca2+ was added during the hyperpolarization protocol, a stimulus that activates the hyperpolarization-activated calcium channels, leading to a [Ca2+]c increase (Pei et al., 2000; Allen et al., 2001; Yang et al., 2008) a fast acceleration of the fluorescence decay was observed. Similarly, when Ca2+ was present in the bath solution from the beginning of the hyperpolarization, HyPer fluorescence ratio was immediately followed by a fast decrease of the signal (Figure 2e, dotted trace) to, and often below, the initial level. Addition of Ca2+ was able to efficiently trigger the HyPer fluorescence ratio drop even when a higher dose of H2O2 (1 mM) was used (not shown).
The experiments presented in Figure 2(e) were then repeated in guard cells expressing cHyPer. Also in this case the hyperpolarizing protocol, when carried out in the absence of Ca2+, resulted in no significant alteration of the H2O2 levels, while a rapid decrease in cHyPer fluorescence ratio occurred when Ca2+ was included in the hyperpolarizing medium (not shown).
The possibility that the observed behaviour of HyPer fluorescence ratio in the presence of Ca2+ was due to effects other than an accelerated H2O2 metabolism should be taken into account. A major potential artifact may arise from the sensitivity of HyPer to pH. Indeed, in the original HyPer work, Belousov and colleagues (2006) reported that pH can affect HyPer similarly to other GFP-based sensors such as Pericam (Nagai et al., 2001). Indeed, when Arabidopsis guard cells expressing the HyPer-KSRM were challenged with ammonium chloride (NH4Cl) or sodium acetate (CH3COONa), to induce alkalinization or acidification of both cytoplasm and organelle pH, major changes of the ΔR/R0 ratio were observed. In particular, the addition of NH4Cl (1 mM) results in an increase of ΔR/R0, ratio while CH3COONa (5 mM) caused a sharp ΔR/R0 ratio decrease (Figure S2a). To exclude major cellular pH changes upon Ca2+ entry, we applied the hyperpolarization protocol to guard cells of transgenic Arabidopsis plants expressing the cytosolic pH sensor Pt-GFP (Schulte et al., 2006). No appreciable fluorescence changes were observed when Ca2+ entered the cell (Figure S2b). The pH probe, on the contrary, responded as expected to the pH changes induced by NH4Cl or CH3COONa, that is, clear fluorescence increases and decreases, respectively. Taken together these experiments not only demonstrate that Ca2+ does not have a short-time influence on cytoplasmic or peroxisomal pH, given the rapid equilibration of peroxisome pH with that of the cytosol (Drago et al., 2008), but also that the sharp drop of HyPer fluorescence ratio, as induced by hyperpolarization in the presence of Ca2+, is causally linked to a rapid catabolism of H2O2 with a reduction of its level in both the cytoplasm and peroxisomes.
Intraperoxisomal Ca2+ dynamics analyses in Arabidopsis transgenic plants expressing the peroxisome-targeted D3cpv Cameleon probe
Given the high permeability of the peroxisome membrane to H2O2, our data do not allow to distinguish whether the Ca2+-activated degradation of H2O2 occurs in the cytoplasm, within peroxisomes or both. However, while there is no evidence in favour of the existence of cytoplasmic, Ca2+-activated, H2O2 scavenging enzymes, a potential Ca2+ target in peroxisomes has been described: the Arabidopsis catalase isoform CAT3 (Yang and Poovaiah, 2002). CAT3 is predicted to be activated by Ca2+-CaM in vitro (Yang and Poovaiah, 2002) and it is highly expressed in plant peroxisomes. In the previous experiments we showed that a protocol known to induce cytoplasmic Ca2+ increase in plant cells affects the H2O2 scavenging efficiency, but whether indeed Ca2+ increases occur within plant peroxisome has never been demonstrated. Hence, in order to follow the Ca2+ dynamics in the peroxisomal lumen of plant cells we took advantage of the recent generation of the peroxisomal targeted Ca2+ sensor D3cpv-KVK-SKL (Drago et al., 2008) (Figure 3 top panel). Cameleons and the Dcpv family of indicators derived from them (Palmer et al., 2006) are genetically encoded indicators in which two GFP variants, CFP and YFP (or circularly permuted variants of YFP) are linked together by CaM and a CaM-binding peptide, M13. Upon Ca2+ binding, the conformational change of CaM and its binding to M13 results in reduced distance between CFP and YFP and increase in Fluorescence Resonance Energy Transfer, FRET. FRET, and thus the [Ca2+] increases, can be conveniently measured by the increase in the ratio between the emission intensity of YFP and CFP upon CFP excitation (Rudolf et al., 2003). In the D family indicators, while the basic structure remains unchanged, both CaM and M13 have been mutated to abolish, or strongly reduce, the interference from endogenous CaM. We directly subcloned the DNA coding for the mammalian peroxisomal Ca2+ probe D3cpv-KVK-SKL in a plant expression vector under the control of the CaMV35S promoter and stable transgenic Arabidopsis plants were generated. Figures 3(a-c) show the confocal analysis of a leaf from one of the transgenic Arabidopsis lines selected. The Cameleon fluorescent signal is clearly recognizable in the peroxisomes of different cell types, such as epidermal, mesophyll and trichomes (Figure 3a-c) and no D3cpv-KVK-SKL mistargeting to other cellular structures was observed. D3cpv-KVK-SKL was expressed and properly localized also in Arabidopsis guard cells (Figures 3d-f). We also tested transient expression of D3cpv-KVK-SKL in tobacco cells as obtained by agroinfiltration. Figure 3(g-i) shows the fluorescent images of epidermal cells from tobacco leaves, upon transient co-transformation by agroinfiltration with D3cpv-KVK-SKL and the Red Fluorescent Protein (RFP) targeted to peroxisomes (RFP-KSRM; PTS1 signal peptide) (Dammann et al., 2003). The new probe (Figure 3g) is localized in discrete structures, with high motility (not shown), also labelled by RFP-KSRM (Figure 3h). The overlay image (Figure 3i) clearly shows a good merge (yellow) of the two signals in these punctated structures. The selective D3cpv-KVK-SKL peroxisome localization is supported also by the recognition of the crystalline inclusion, a typical characteristics of plant peroxisomes (Huang, 1983), in the fluorescent organelles (Figure 3j,l).
Figure 3.
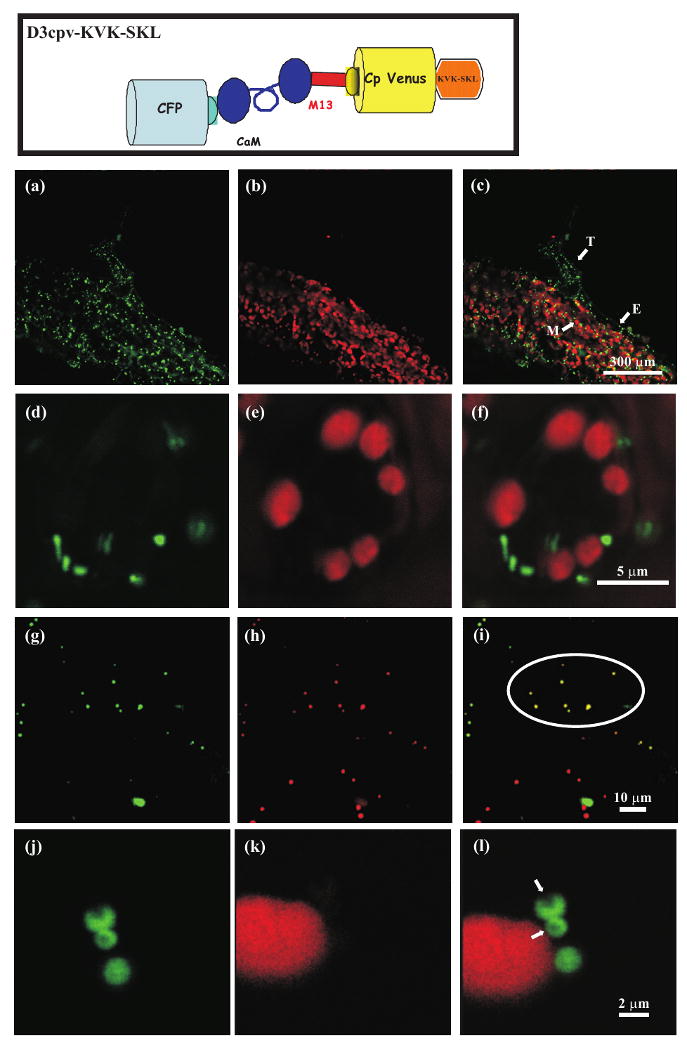
Subcellular distribution of D3cpv-KVK-SKL in stable transgenic Arabidopsis plants and in transiently transformed tobacco epidermal cells. Top panel. Schematic structure of the D3cpv-KVK-SKL Cameleon probe.
(a-f) Confocal images of stable transgenic Arabidopsis plants transformed with D3cpv-KVK-SKL.
(a) Cameleon YFP fluorescence in Arabidopsis leaf epidermal cells.
(b) Chlorophyll fluorescence of the same leaf shown in (a).
(c) Overlay image of (A) and (B). Mesophyll (M), epidermis (E), trichome (T).
(d) Cameleon YFP fluorescence in stomata guard cells.
(e) Chlorophyll fluorescence of the same stomata guard cell shown in (d).
(f) Overlay image of (d) and (e).
(g-i) Confocal images of tobacco agroinfiltrated epidermal cells co-transformed with D3cpv-KVK-SKL and the peroxisomal marker RFP-KSRM.
(g) Cameleon YFP fluorescence.
(h) RFP fluorescence.
(i) Overlay image of (g) and (h).
(j-l) High magnification confocal images of tobacco agroinfiltrated epidermal cells transformed with D3cpv-KVK-SKL showing the presence of the crystalline (arrows) inclusion in labelled peroxisomes.
(j) Cameleon YFP fluorescence.
(k) Chlorophyll fluorescence of the same cell shown in (j).
(l) Overlay image of (j) and (k).
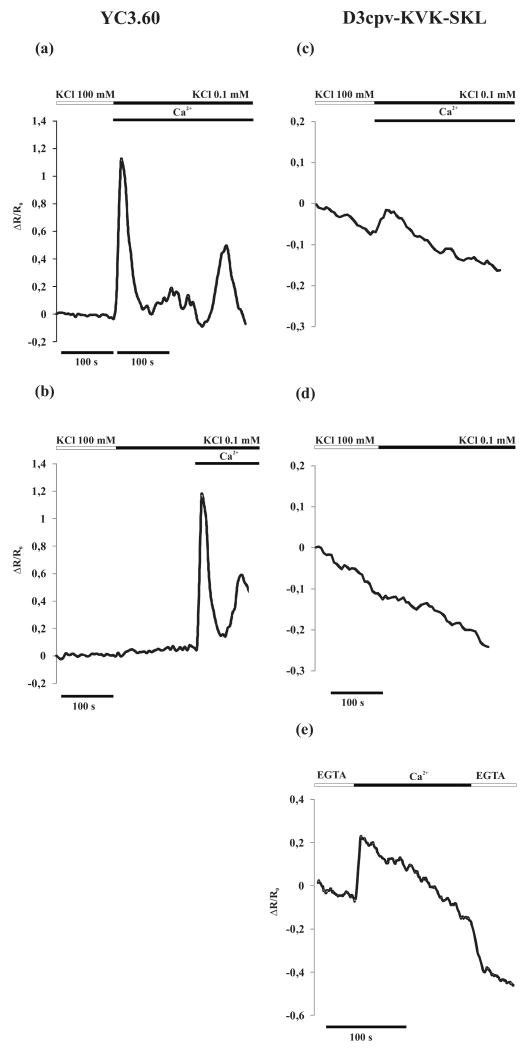
We first checked under our conditions that the hyperpolarization protocol employed in the experiments described in Figure 2(e) indeed results in a [Ca2+]c increase, using again guard cells of Arabidopsis plants stably expressing the cytoplasmic Cameleon Ca2+ indicator YC3.60 (Yang et al., 2008). As expected (Figures 4a,b), cell hyperpolarization caused a sharp increase in the fluorescence emitted at 540 nm (YFP) and a decrease of the signal at 480 nm (CFP) and thus an increase in the 540/480 nm fluorescence emission ratio (Figure 4a) here presented as ΔR/R0, which is proportional to the [Ca2+]c. When the hyperpolarization was performed in absence of external Ca2+, no appreciable [Ca2+]c changes were observed (Figure 4b), but the subsequent addition of CaCl2 (10 mM) induced a large [Ca2+]c increase. These results confirm previous data (Allen et al., 1999; Pei et al., 2000; Allen et al., 2001; Young et al., 2006; Yang et al., 2008) and demonstrate that [Ca2+]c increase due to hyperpolarization is mainly due to Ca2+ entry from the apoplast.
Figure 4.
[Ca2+]c and [Ca2+]p monitoring in Arabidopsis guard cells subjected to change in the bath solution form high K+ concentration (depolarization buffer) to low K+ concentration (hyperpolarization buffer).
(a-b) [Ca2+]c dynamics monitored in Arabidopsis guard cells expressing Cameleon YC3.60: Arabidopsis guard cells were bathed in depolarization buffer (white bar) and perfused with the hyperpolarization buffer (black bar) and Ca2+ was added at the same time of hyperpolarization (a) or after (b). The plasma membrane hyperpolarization causes a [Ca2+]c increase only in presence of external Ca2+.
(c-e) [Ca2+]p dynamics monitored in Arabidopsis guard cells expressing Cameleon D3cpv-KVK-SKL.
(c) The same protocol used in (a) was able to induce a [Ca2+]p increase.
(d) The [Ca2+]p increase was absent when plasma membrane hyperpolarization was performed in absence of external Ca2+.
(e) Guard cells expressing D3cpv-KVK-SKL were permeabilized with digitonin (1 mM) in an intracellular-like medium supplemented with EGTA (0.5 mM) and then perfused with saturating [Ca2+] in order to establish the in vivo dynamic range of the D3cpv-KVK-SKL probe.
Figure 4(c) shows a typical kinetic pattern of the D3cpv-KVK-SKL fluorescence ratio in guard cells of Arabidopsis plants exposed to the hyperpolarization protocol. Notably, upon hyperpolarization the [Ca2+]p increased, but the maximum ΔR/R0 increase (0.061 ± 0.027) (n=11) was reached more slowly within peroxisomes compared to the cytoplasm, similar to what already reported for peroxisomes in mammalian cells (Drago et al., 2008). When hyperpolarization was applied without Ca2+ in the medium, no [Ca2+]p variations were observed (Figure 4d). The ΔR/R0 in peroxisomes is rather small, as also observed in mammalian peroxisomes (Drago et al., 2008), and this is due, at least in part, to the reduced maximal Ca2+-induced ΔR/R0 change of the probe within the organelles (0.303 ± 0.0342) (n=11), as demonstrated by experiments performed in permeabilized guard cells (Figure 4e, see Experimental procedures). Similar results were obtained both in the cytoplasm and in peroxisomes of tobacco epidermal cells transiently expressing the Ca2+ indicators (not shown). The above results confirm and extend to plant cells the recent findings on peroxisome Ca2+ handling in animal cells by Drago et al. (2008) i.e. that [Ca2+]p essentially mimics the behaviour of [Ca2+]c.
Quantification of CAT and APX activities in leaves of Arabidopsis plants at different developmental stages
CAT3 has been predicted to be activated by Ca2+-CaM in vitro (Yang and Poovaiah, 2002) and it is highly expressed in plant peroxisomes. CAT3 is also known as SENESCENCE 2 since CAT3 activity increases with plant age -more dramatically during the time of bolting and at the onset of the senescence program- and when plants are exposed to stress (Orendi et al., 2001; Du et al., 2008).
The prediction thus is that Ca2+-dependent H2O2 catabolism should increase with plant age (Figure 5a), i.e. as a function of increased levels of CAT3. Figure 5(b) shows that the level of CAT3 transcript in the leaves of HyPer-KSRM expressing plants increased with age, confirming previous results (Zimmermann et al., 2006; Du et al., 2008), whereas the transcript level of another catalase, CAT2, was essentially unchanged. To verify whether the CAT3 transcript level correlates with an increase in CAT3 activity, an activity staining of a native PAGE was performed by using crude protein extracts from leaves of 4- and 6-week-old plants. In 6-week-old plants, the activity of CAT3 was more pronounced than in 4-week-old plants (upper band in Figure 5c), whereas a slight decrease in CAT2 activity was observed (lower band in Figure 5c).
Figure 5.
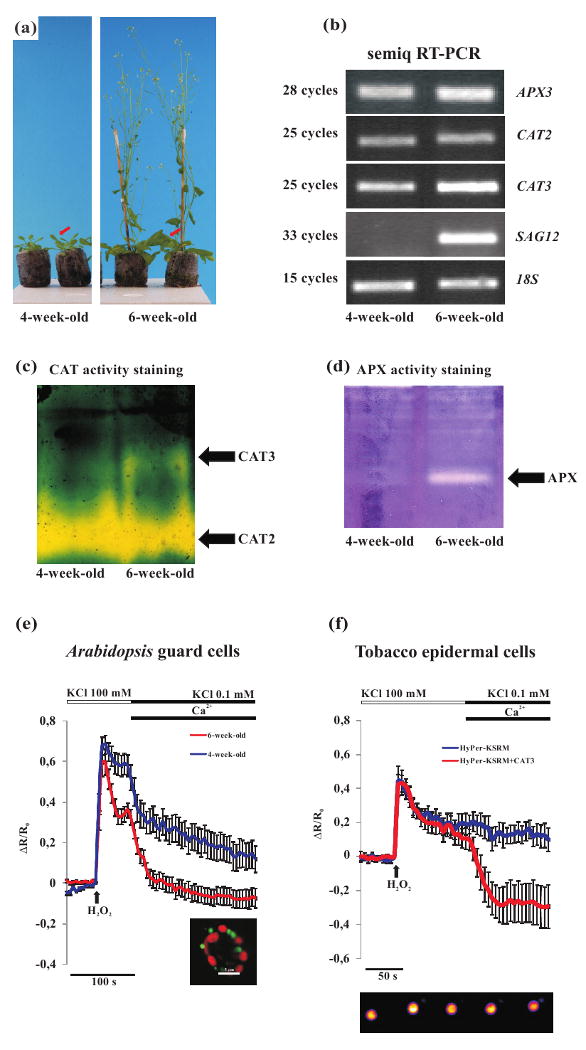
Ca2+-dependent peroxisomal H2O2 scavenging efficiency correlates with changes of CAT3 expression.
(a) 4-and 6-week-old Arabidopsis HyPer-KSRM plant phenotypes. Red arrows indicate the leaf type used for imaging analyses and molecular analyses.
(b) Semi-quantitative RT-PCR analysis of APX3, CAT2 and CAT3 transcripts abundance in leaves harvested from Arabidopsis plants at different growth stages. The results show an increase in APX3 and CAT3 transcripts abundance in leaves of 6-week-old plants compared to 4-week-old plants. No significative changes of CAT2 transcript level were observed, on the other hand a clear SAG12 induction was observed.
(c) CAT zymogram of pooled leaves from 4- and 6-week-old Arabidopsis plants respectively. In leaves from 6-week-old plants a clear increase in CAT3 activity was observed, whereas a decrease of CAT2 occurred. The experiment was repeated six times showing always the same activity pattern.
(d) APX zymogram of pooled leaves from 4- and 6-week-old Arabidopsis plants respectively. A clear APX activity was detected only in leaves from 6-week-old Arabidopsis plants. The experiment was repeated three times showing always the same activity pattern.
(e) Leaves from 4-and 6-week-old plants were bathed in depolarization buffer and H2O2 (100 μM) was added at the indicated time point. The perfusion of the guard cells with the hyperpolarization solution in presence of external Ca2+ induced a different response in the leaf guard cells of the different plant ages. In the guard cells peroxisomes of 6-week-old plants the Ca2+-dependent HyPer fluorescence ratio drop was more consistent (red trace), compared with the responses observed in guard cells of 4- (blue trace) plants.
(f) Tobacco epidermal cells expressing HyPer-KSRM were subjected to H2O2 (1 mM) administration and afterwards hyperpolarization in presence of Ca2+ was applied. A steep and prolonged Ca2+-dependent H2O2 scavenging occurred only in cells of tobacco leaves co-transformed with the HyPer-KSRM and the 35S-CAT3. Bottom panel. An example of HyPer fluorescence emitted fluorescence (530 nm), with the 480 nm excitation wavelength, in a tobacco epidermal cell peroxisome subjected to H2O2 addition.
In plants, besides catalases, the major H2O2 scavenging system is represented by the APXs and, for this reason, the activity of such enzymes in leaves of 4- and 6-week-old plants was also investigated. APXs are extremely unstable enzymes, so only the cytoplasmic forms can be visualized in native PAGE using crude extract (Zimmermann et al., 2006). In particular, with our samples, the APX activity was observed in 6-week-old plants, while the activity was not measurable in 4-week-old plants (Figure 5d). The assay did not allow the discrimination of the APX3 activity, the Arabidopsis peroxisomal isoform (Narendra et al., 2006) from that of the other isoforms. The transcript level of APX3 in leaves of 4- and 6-week-old plants was thus analyzed. As for the CAT3, the level of APX3 transcript was higher in 6-week-old plants (Figure 5b). The establishment of the onset of the senescence program in 6-week-old plants was then confirmed by a high expression level of SAG12 a specific senescence marker gene (Noh and Amasino, 1999).
Variation in the level of catalases expression affects the in vivo Ca2+-dependent H2O2 scavenging in peroxisomes
Figure 5(e) shows that upon imposing a Ca2+ increase caused by hyperpolarization an increase in the rate of H2O2 scavenging was observed in guard cell peroxisomes of both leaf ages, but in leaves of 6-week-old plants the effect of Ca2+ entry was much stronger than in 4-week-old plants, indicating that Ca2+-activated peroxisomal H2O2 scavenging correlates with CAT3 and APX3 expression level. Interestingly, in 6-week-old plants the observed initial drop of HyPer fluorescence ratio before the plasma membrane hyperpolarization, that constitute the Ca2+-independent component of the H2O2 scavenging system, showed to be more pronounced than in 4-week-old plants. The increased rate of H2O2 catabolism induced by Ca2+ in cells from older plants is consistent with, but does not demonstrate that it is due to, the increased levels of CAT3, given that several other parameters may change during plant senescence, e.g. the increased APX activity. To demonstrate that this phenomenon is indeed due to CAT3, among other possible approaches (see Discussion), we choose to overexpress CAT3 by transient transformation. A highly efficient transient transformation, such as agroinfiltration, is unfortunately not possible in Arabidopsis (Zipfel et al., 2006), but can be easily performed in tobacco leaves (Batoko et al., 2000). Tobacco leaves were thus agroinfiltrated with HyPer-KSRM alone or with a 35S-CAT3 construct, and the capacity of epidermal cells (controls and CAT3 overexpressing) to metabolize H2O2 was compared. While in the absence of a Ca2+ rise the rate of H2O2 catabolism (measured by HyPer-KSRM) was indistinguishable in control and CAT3 overexpressing cells, a Ca2+-dependent fast H2O2 catabolism was observed in the cells overexpressing CAT3 (Figure 5f). It should be also noted that in control tobacco cells the rise in [Ca2+] resulted in no, or marginal, acceleration of H2O2 catabolism. This latter observation suggests that tobacco epidermal cells express lower levels of the endogenous catalase homologue of Arabidopsis CAT3.
Discussion
The development of genetically-encoded fluorescent probes represent a milestone for the understanding at the subcellular level of the dynamic changes of key cellular parameters such as second messenger levels, protein-protein interactions or metabolic activation. This approach has found thus far a relatively limited application in plant cells. One of the first aims of this study was to develop new tools for studying in vivo and with high temporal and spatial resolution the kinetics of H2O2 metabolism as well as organelle [Ca2+] in plant cells. To this end we have generated new compartiment targeted transgenic plants expressing on the one hand the genetically encoded HyPer probe (Belousov et al., 2006) targeted to the cytoplasm and peroxisome lumen, on the other a new GFP-based fluorescent Ca2+ indicator selectively located within peroxisomes. As to HyPer, the probe specificity (HyPer fluorescence is practically insensitive to other ROS (Belousov et al., 2006)) is of particular importance for the unambiguous detection of the dynamic changes of H2O2 levels, since under normal plant growth conditions or during both biotic and abiotic stress many different ROS can be synthesized (Mittler, 2002). Targeting HyPer to the peroxisomes appears of major interest because not only it is known that these organelles are the main scavenging site for H2O2 produced during photorespiration, but new evidence suggests that peroxisomes are also very important sites for production and sensing of different signalling molecules, including H2O2 and nitric oxide (NO) (Corpas et al., 2001; Nyathi and Baker, 2006; Palma et al., 2009).
A first conclusion obtained by using HyPer in the two compartments is the confirmation that cellular membranes (plasma and peroxisome) do not represents a significant physical barrier to the diffusion of H2O2 from outside to inside of the cell and from the cytosol to the peroxisomes. Indeed no appreciable lag phase has ever been observed between the addition of H2O2 to the bath and the rise in HyPer fluorescence ratio in either compartment. This result confirms and extends previous data concerning the high permeability of H2O2 through natural membranes (Bienert et al., 2006, 2007).
A second observation concerns the importance of Ca2+ for H2O2 scavenging efficiency: we demonstrate that it depends on Ca2+ increases within peroxisomes. It should be stressed that the first data concerning the mechanisms of Ca2+ handling in peroxisomes have been published only very recently in mammalian cells (Drago et al., 2008; Lasorsa et al., 2008), while nothing was known until now about Ca2+ homeostasis in these organelles in plants. Here we demonstrate that [Ca2+]p rises follow [Ca2+]c variations, as in mammalian cells. Most important, we found that the intracellular Ca2+ elevation accelerated dramatically H2O2 scavenging (Figure 2e). The question then arises as to whether this Ca2+-dependent activation of H2O2 scavenging depends on enzymes localized in the cytosol, in the peroxisomes or in both compartments. The only known enzyme potentially endowed with this characteristic is CAT3, located within peroxisomes. Given that membranes are highly permeable to H2O2 the catabolism of this molecule within the peroxisomes organelles would lead to a rapid drop of H2O2 level also in the cytosol. Different experimental evidence supports the conclusion that indeed peroxisomal CAT3 is responsible at least in part for the accelerated metabolism of H2O2 observed upon increase of cellular Ca2+ activated by hyperpolarization. Zimmermann et al. (2006), analyzed gene expression and enzyme activities of CATs during Arabidopsis leaf senescence and showed that the expression and activity of CAT3 increased with age. Not only we confirmed these findings, but, by means of HyPer imaging analyses in living cells, we showed a strict correlation between CAT3 expression levels and efficiency of the Ca2+-dependent H2O2 scavenging system. This was demonstrated both by showing a correlation of the two phenomena in aging Arabidopsis and by using transient transformation of CAT3 (by agroinfiltration) in tobacco leaves. This latter was preferred respect to the generation of stable transgenic plants, with overexpressed or downregulated CAT3, because they do not provide unambiguous results, given that in stable mutants adaptive phenomena may be generated (Willekens et al., 1997; Apel and Hirt, 2004). On the contrary, transient transformation minimizes adaptive phenomena.
The work by Yang and Poovaiah (2002) had clearly shown that CAT3 in vitro can bind CaM in a Ca2+-dependent way but, due the lack of activity of the recombinant Arabidopsis CAT3, they were unable to demonstrate directly its stimulation by Ca2+. The present data demonstrate the Ca2+ activation of CAT3 in vivo and the dependence of H2O2 metabolism on cellular Ca2+ dynamics. Indeed, our results demonstrate that CAT3 can be responsible for at least part of Ca2+-dependent H2O2 scavenging, though they do not exclude the existence of other H2O2 scavenging systems, whose activity could be stimulated by Ca2+. Several antioxidant systems, besides catalases, exist in peroxisomes including superoxide dismutases (SODs), NADP-dehydrogenases, and the ascorbate-glutathione cycle and their activity can change during senescence (del Rio et al., 1998, 2002, 2006; Palma et al., 2009), but, to the best of our knowledge, none of them has ever been demonstrated to be Ca2+ dependent. In conclusion, in this study we have described the successful use in plant cells of genetically-encoded H2O2 and Ca2+ sensors localized within cell organelles. Moreover, we presented the analysis of H2O2 and Ca2+ dynamics within plant peroxisomes providing unambiguous in vivo evidence that [Ca2+]p elevations allows a more rapid catabolism of H2O2 by the increase of CAT3 within peroxisomes. Such efficient H2O2 elimination can be of major relevance in cells undergoing stimulation of H2O2 production (e.g., under stress conditions) or when the other scavenging enzymes decrease. Several physiological and pathological conditions exist under which plant cells have to cope with biotic and abiotic stimuli that induce both Ca2+ and H2O2 overproduction. We hence suggest that Ca2+ increase within peroxisomes, by causing the activation of CAT3 and possibly of other H2O2 scavenging enzymes and non-enzymatic systems, may represent a fine highly efficient cellular mechanism to strictly control H2O2 levels.
Experimental procedures
Plant material and growth condition
All the Arabidopsis thaliana plants used in this study were of the Columbia ecotype. Plants were grown on Jiffy Pot (http://www.jiffypot.com/) 16/8 h cycles of light (70 μE m-2 sec-1) at 22°C and 75% RH. The transgenic pGC1-YC3.60 Arabidopsis plants were generated and reported in a previous study (Yang et al., 2008). Seeds of Arabidopsis plants expressing Pt-GFP (Schulte et al., 2006) were obtained from the European Arabidopsis Stock Centre (NASC).
DNA constructs
The RFP-KSRM construct was digested from the pRTL2 vector by PstI digestion and ligated in the pGreen0029 binary vector (Hellens et al., 2000).
The D3-KVK-SKL construct was digested from the pcDNA3 vector with HindIII and EcoRI and ligated in the 35S-CaMV cassette vector (http://www.pgreen.ac.uk/JIT/JIT_fr.htm). The entire cassette was then partially digested with EcoRV and ligated in the pGreen0179 binary vector.
The HyPer cDNA was amplified by PCR by using the Phusion® DNA Polymerase (Finnzymes, Finland) from the purchased pHyPer-Cyto vector (http://www.evrogen.com/products/HyPer/HyPer.shtml, Evrogen, Russia). For both the cytoplasmic and peroxisomes localization of HyPer we used the same forward primer: 5′-CATGCCATGGAGATGGCAAGCCAGCA-3′ where a NcoI restriction site was introduced at the 5′ end. The 3′ reverse primer for the subcloning of the cytoplasmic localized HyPer was: 5′-TGGAAGATCTTTAAACCGCCTGTTTTAAAACT-3′, for the peroxisomes localization we inserted the KSRM peptide coding sequence upstream of the HyPer stop codon 5′-TGGAAGATCTCACATCCTGGATTTAACCGCCTGTTTTAAA-3″. In both cases we introduced a BglII restriction site. The amplicons were then digested and inserted in the pAVA554 vector downstream of the double 35S promoter and the translational enhancer sequence of TEV. The entire expression cassettes of both cHyPer and HyPer-KSRM were thereafter isolated from the pAVA554 modified vectors by digestion with KpnI/SacI and ligated in the pGreen 0179 binary vector.
The Arabidopsis CAT3 coding sequence was obtained by RT-PCR using as template cDNA from total leaf RNA extract. The primers were: 5′-CATGAAGCTTATGGATCCTTACAAGTATCGTCCTTCAA-3′ and CATGCTCGAGCTAGATGCTTGGCCTCACGTTCAGA. The PCR amplicon was digested with HindIII and XhoI restriction enzymes and ligated in the pKYLX7135S2 binary vector. All the constructs were sequenced to verify that no mistakes were introduced by PCR amplifications. All the binary vectors were then introduced in the Agrobacterium tumefaciens GV3101 strain.
RT-PCR of Arabidopsis leaves
Total RNA extraction from leaves of 4-, and 6-week-old HyPer-KSRM plants and the cDNA synthesis were performed as previously described (Costa et al., 2004). The PCR was repeated three times on pooled cDNA for each time point. Five different RNA extractions and cDNA synthesis were performed from leaves pools obtained from 15 different plants for each time point. The APX3, CAT2, CAT3 and SAG12 set of primers used for the RT-PCR reactions were respectively: 5′-CGGGAGAAAGGATTCAAATGTCTGC-3′(forward) and 5′-GAACTCCGGGTCTTCCAATAAGGTC-3′(reverse), 5′-TCCCGTCGAGGTATGACCAGGTT-3′ (forward) and 5′-CTTGCCAGCTTCTGTCCCAAAGACT-3′(reverse), 5′-CGCCTTGGACCGAATTATTTGCAG-3′ (forward) and 5′-TGAGACGTGGCTCCGATAGAATCTC-3′ (reverse), 5′-ACTGGAGGAAGAAAGGAGCTGT-3′(forward) and 5′-TGATCCGTTAGTAGATTCGCGT-3′(reverse). For the 18S transcript amplification the Ambion 18S Universal Primers were used (QuantumRNA 18S Internal Standards Kit; Ambion). The specificity of APX3, CAT2, CAT3 and SAG12 PCR products obtained were confirmed by direct PCR sequencing.
Transgenic plants
The Agrobacterium strains obtained were used for both tobacco agroinfiltration experiments or to generate transgenic Arabidopsis plants by floral-dip method. For each construct, different Arabidopsis independent transgenic lines were selected and for imaging experiments two independent lines were employed. None of the transgenic lines selected, with the different constructs, showed phenotypic differences or abnormalities in our standard growth conditions.
Suspension cell culture
Suspension cHyPer cell culture lines were generated starting from 6-day-old transgenic seedlings. Briefly, in order to induce callus formation, shoots of transgenic seedlings were placed on MS (Murashige and Skoog, 1962) solid medium (0.8% plant agar, Duchefa) supplemented with 3% sucrose, 4.44 μM 6-benzylaminopurine (6-BAP) and 9.04 μM 2,4-dichlorophenoxy-acetic acid (2,4-D); the pH was adjusted to 5.7. The obtained callus were maintained on the same solid medium with a subculture cycle of 3 weeks. After several subculture cycles, aliquots of callus were transferred to Erlenmeyer flasks in liquid medium with the same composition. The suspension cultures were subcultured in fresh medium every week and maintained in a climate growth chamber at 25 ± 1°C on an orbital shaker (80 rpm).
Confocal microscopy analyses
Confocal microscopy analyses were performed using a Nikon PCM2000 (Bio-Rad, Germany) laser scanning confocal imaging system. For Cameleon-dependent-YFP and HyPer detection, excitation was at 488 nm and emission between 530/560 nm. For the chlorophyll detection, excitation was at 488 nm and detection over 600 nm. For RFP detection, excitation was set at 548 nm and emission at 573 nm. Image analysis was done with the ImageJ bundle software (http://rsb.info.nih.gov/ij/).
Guard Cell Imaging
For guard cell imaging leaves of 6-week-old Arabidopsis plants, or otherwise indicated, were attached to microscope cover glasses using a Medical adhesive (Hollister Inc., Libertyville, IL). A paintbrush was used to gently press the leaf to the coverslip and upper cell layers were carefully removed using a razor blade. Cells expressing the fluorescent probes were analyzed using an inverted fluorescence microscope (Zeiss Axioplan) with immersion oil objectives (X100, N.A. 1.3 or X63, N.A. 1.40). Excitation light was produced by a monochromator (Polychrome II; TILL Photonics, Martinsried, Germany): 475 nm for Pt-GFP; 420 and 480 nm for HyPer. The two excitation wavelengths were rapidly alternated and the emitted light deflected by dichroic mirrors (HQ 520 LP for Pt-GFP and 455 DRPL for HyPer) was collected through emission filters (HQ 520 LP for Pt-GFP and 480 ELFP for HyPer). For the YC3.60 and D3cpv-KVK-SKL probes, the excitation light was 425 nm. The emitted light was collected through a beamsplitter (OES s.r.l., Padua, Italy) (emission filters HQ480/40M for cyan fluorescent protein and HQ 535/30M for yellow fluorescent protein) and a dichroic mirror (515 DCXR). Filters and dichroic mirrors were purchased from Omega Optical and Chroma. Images were acquired using a cooled CCD camera (Imago; TILL Photonics) attached to a 12-bit frame grabber. Synchronization of the monochromator and CCD camera was performed through a control unit run by TILLvisION v.4.0 (TILL Photonics); this software was also used for image analysis. For time course experiments, the fluorescence intensity was determined over regions of interests corresponding to an entire guard cell for Pt-GFP, cHyPer and YC3.60 or covering single or small groups of peroxisomes expressing HyPer-KSRM or D3cpv-KVK-SKL. Exposure time and frequency of image capture varied from 100 to 500 ms and from 1 to 0.2 Hz respectively. Cells were mounted into an open-top chamber and maintained in the depolarization (100 mM KCl, 0.5 mM EGTA and 10 mM Mes-Tris pH 6.15) or hyperpolarization (0.1 mM KCl, 0.5 mM EGTA and 10 mM Mes-Tris pH 6.15) buffers. For the Ca2+ addition the hyperpolarization buffer was prepared as follow: 0.1 mM KCl, 10 mM CaCl2, 0.5 mM EGTA and 10 mM Mes-Tris pH 6.15.
To perform the passive loading of Ca2+ in guard cell peroxisomes plasma membrane permeabilization was performed by treating cells for 2 min with 1 mM digitonin in an intracellular-like medium containing: 100 mM potassium-gluconate, 1 mM MgCl2, 10 mM Hepes, pH 7.5, at and 0.5 mM EGTA. Experiments with permeabilized cells were performed in the same medium; where indicated, the latter was supplemented with the same buffer containing 2 mM CaCl2.
Enzyme analyses
For the analyses of CATs activities, leaves pooled from different plants of 4- and 6-week-old Arabidopsis plants were homogenized in 50 mM Tris-HCl pH 7.4, 0.05% cysteine at 4°C. To determine the APXs activities the leaves were grounded in 50 mM Tris-HCl pH 7.4, 0.05% cysteine, and 1 mM ascorbic acid at 4 °C. After centrifugation at 13200 rpm for 20 min at 4° C, the protein content was quantified according to the method of Bradford and successively used for zymograms.
For the analysis of CAT and APX isozymes, the protein extracts containing respectively 20 μg and 75 μg of total protein extracts were loaded and separated in native polyacrylamide gels and gels were then assayed as reported previously by Zimmermann et al. (2006).
For the analysis of CAT activity six independent protein extractions from different plants for each condition were performed. For the APX activity three independent protein extractions from different plants of each condition were performed.
Statistical Analysis
All the data are representative of at least ten different experiments. Values are expressed as mean ± S.E.
Supplementary Material
Figure S1. Subcellular distribution of cHyPer (cytoplasmic) and HyPer-KSRM (peroxisomes) in stable transgenic Arabidopsis plants.
Figure S2. The Ca2+-dependent HyPer ratio drop is strictly dependent on H2O2 removal and not affected by pH changes.
Acknowledgments
We tank Paulo Magalhães for critical comments on the paper. We thank the European Arabidopsis Stock Centre (NASC) for providing PtGFP seeds. This work was supported by the “Ministero dell'Istruzione e della Ricerca, fondi PRIN” to F.L.S., grants from the Italian Ministry of University, the Veneto Region (Biotech 2) and the CARIPARO Foundation to T.P. and in part the National Institutes of Health to J.I.S. (GM060396-ES010337).
Abbreviations
- APX
Ascorbate peroxidase
- [Ca2+]c
cytosolic Ca2+ concentration
- [Ca2+]p
peroxisomal Ca2+ concentration
- CaM
Calmodulin
- CAT
Catalase
- FRET
Fluorescence Resonance Energy Transfer
- ROS
Reactive Oxygen Species
- YFP
Yellow fluorescent protein
- ΔR/R0
ratio at time “tn”-ratio at time “t0”/ratio at time “t0”
Footnotes
Supporting information: Additional Supporting Information may be found in the online version of this article.
References
- Allen GJ, Kwak JM, Chu SP, Llopis J, Tsien RY, Harper JF, Schroeder JI. Cameleon calcium indicator reports cytoplasmic calcium dynamics in Arabidopsis guard cells. Plant J. 1999;19:735–47. doi: 10.1046/j.1365-313x.1999.00574.x. [DOI] [PubMed] [Google Scholar]
- Allen GJ, Chu SP, Harrington CL, Schumacher K, Hoffmann T, Tang YY, Grill E, Schroeder JI. A defined range of guard cell calcium oscillation parameters encodes stomatal movements. Nature. 2001;411:1053–1057. doi: 10.1038/35082575. [DOI] [PubMed] [Google Scholar]
- Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- Asada K, Takahashi M. Production and scavenging of active oxygen in photosynthesis. In: Kyle DJ, Osborne CB, Arntzen CJ, editors. Photoinhibition. Amsterdam: Elsevier; 1987. pp. 227–87. [Google Scholar]
- Baker A, Graham IA. Plant Peroxisomes Biochemistry, Cell Biology and Biotechnological Applications. Kluwer Academic Publishers; Dordrecht, The Netherlands: 2002. [Google Scholar]
- Batoko H, Zheng HQ, Hawes C, Moore I. A Rab1 GTPase is required for transport between the endoplasmic reticulum and Golgi apparatus and for normal Golgi movement in plants. Plant Cell. 2000;12:2201–2218. doi: 10.1105/tpc.12.11.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belousov VV, Fradkov AF, Lukyanov KA, Staroverov DB, Shakhbazov KS, Terskikh AV, Lukyanov S. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nature Methods. 2006;3:281–286. doi: 10.1038/nmeth866. [DOI] [PubMed] [Google Scholar]
- Bienert GP, Schjoerring JK, Jahn TP. Membrane transport of hydrogen peroxide. Biochim Biophys Acta. 2006;1758:994–1003. doi: 10.1016/j.bbamem.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Bienert GP, Møller AL, Kristiansen KA, Schulz A, Møller IM, Schjoerring JK, Jahn TP. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J Biol Chem. 2007;282:1183–1192. doi: 10.1074/jbc.M603761200. [DOI] [PubMed] [Google Scholar]
- Corpas FJ, Palma JM, Sandalio LM, López-Huertas E, Romero-Puertas MC, Barroso JB, del Río LA. Purification of catalase from pea leaf peroxisomes: identification of five different isoforms. Free Radic Res. 1999;31:S235–S241. doi: 10.1080/10715769900301561. [DOI] [PubMed] [Google Scholar]
- Corpas FJ, Barroso JB, del Río LA. Peroxisomes as a source of reactive oxygen species and nitric oxide signal molecules in plant cells. Trends Plant Sci. 2001;6:145–50. doi: 10.1016/s1360-1385(01)01898-2. [DOI] [PubMed] [Google Scholar]
- Costa A, Carpaneto A, Varotto S, Formentin E, Marin O, Barizza E, Terzi M, Gambale F, Lo Schiavo F. Potassium and carrot embryogenesis: are K+ channels necessary for development? Plant Mol Biol. 2004;54:837–852. doi: 10.1007/s11103-004-0236-9. [DOI] [PubMed] [Google Scholar]
- Dammann C, Ichida A, Hong B, Romanowsky SM, Hrabak EM, Harmon AC, Pickard BG, Harper JF. Subcellular targeting of nine calcium-dependent protein kinase isoforms from Arabidopsis. Plant Physiol. 2003;132:1840–1848. doi: 10.1104/pp.103.020008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Río LA, Pastori GM, Palma JM, Sandalio LM, Sevilla F, Corpas FJ, Jiménez A, López-Huertas E, Hernández JA. The Activated Oxygen Role of Peroxisomes in Senescence. Plant Physiol. 1998;116:1195–1200. doi: 10.1104/pp.116.4.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Río LA, Corpas FJ, Sandalio LM, Palma JM, Gómez M, Barroso JB. Reactive oxygen species, antioxidant systems and nitric oxide in peroxisomes. J Exp Bot. 2002;53:1255–1272. [PubMed] [Google Scholar]
- del Río LA, Sandalio LM, Corpas FJ, Palma JM, Barroso Juan B. Reactive Oxygen Species and Reactive Nitrogen Species in Peroxisomes. Production, Scavenging, and Role in Cell Signaling. Plant Physiol. 2006;141:330–335. doi: 10.1104/pp.106.078204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drago I, Giacomello M, Pizzo P, Pozzan T. Calcium dynamics in the peroxisomal lumen of living cells. J Biol Chem. 2008;283:14384–14390. doi: 10.1074/jbc.M800600200. [DOI] [PubMed] [Google Scholar]
- Du YY, Wang PC, Chen J, Song CP. Comprehensive functional analysis of the catalase gene family in Arabidopsis thaliana. J Integr Plant Biol. 2008;50:1318–1326. doi: 10.1111/j.1744-7909.2008.00741.x. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Noctor G. Redox sensing and signalling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiol Plant. 2003;119:355–364. [Google Scholar]
- Foyer CH, Noctor G, Buchanan B, Dietz KJ, Pfannschmidt T. Redox Regulation in Photosynthetic Organisms: Signaling, Acclimation, and Practical Implications. Antioxid Redox Signal. 2009;11:861–905. doi: 10.1089/ars.2008.2177. [DOI] [PubMed] [Google Scholar]
- Hellens RP, Edwards EA, Leyland NR, Bean S, Mullineaux PM. pGreen: a versatile and flexible binary Ti vector for Agrobacterium-mediated plant transformation. Plant Mol Biol. 2000;42:819–832. doi: 10.1023/a:1006496308160. [DOI] [PubMed] [Google Scholar]
- Huang AHC, Trelease RN, Moore TS. Plant Peroxisomes. Academic Press; New York: 1983. [Google Scholar]
- Jimenez A, Hernandez JA, Pastori G, del Rio LA, Sevilla F. Role of the ascorbate-glutathione cycle of mitochondria and peroxisomes in the senescence of pea leaves. Plant Physiol. 1998a;118:1327–1335. doi: 10.1104/pp.118.4.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez A, Hernández JA, Ros Barceló A, Sandalio LM, del Río LA, Sevilla F. Mitochondrial and peroxisomal ascorbate peroxidase of pea leaves. Physiol Plant. 1998b;104:687–692. [Google Scholar]
- Lasorsa FM, Pinton P, Palmieri L, Scarcia P, Rottensteiner H, Rizzuto R, Palmieri F. Peroxisomes as novel players in cell calcium homeostasis. J Biol Chem. 2008;283:15300–15308. doi: 10.1074/jbc.M800648200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locato V, de Pinto MC, De Gara L. Different involvement of the mitochondrial, plastidial and cytosolic ascorbate-glutathione redox enzymes in heat shock responses. Physiol Plant. 2009;135:296–306. doi: 10.1111/j.1399-3054.2008.01195.x. [DOI] [PubMed] [Google Scholar]
- McClung CR. Regulation of catalases in Arabidopsis. Free Radic Biol Med. 1997;23:489–496. doi: 10.1016/s0891-5849(97)00109-3. [DOI] [PubMed] [Google Scholar]
- Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–410. doi: 10.1016/s1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. [Google Scholar]
- Nagai T, Sawano A, Park ES, Miyawaki A. Circularly permuted green fluorescent proteins engineered to sense Ca2+ Proc Natl Acad Sci USA. 2001;98:3197–3202. doi: 10.1073/pnas.051636098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra S, Venkataramani S, Shen G, Wang J, Pasapula V, Lin Y, Kornyeyev D, Holaday AS, Zhang H. The Arabidopsis ascorbate peroxidase 3 is a peroxisomal membrane-bound antioxidant enzyme and is dispensable for Arabidopsis growth and development. J Exp Bot. 2006;57:3033–3042. doi: 10.1093/jxb/erl060. [DOI] [PubMed] [Google Scholar]
- Noh YS, Amasino RM. Identification of a promoter region responsible for the senescence specific expression of SAG12. Plant Mol Biol. 1999;41:181–194. doi: 10.1023/a:1006342412688. [DOI] [PubMed] [Google Scholar]
- Nyathi Y, Baker A. Plant peroxisomes as a source of signalling molecules. Biochim Biophys Acta. 2006;1763:1478–1495. doi: 10.1016/j.bbamcr.2006.08.031. [DOI] [PubMed] [Google Scholar]
- Orendi G, Zimmermann P, Baar C, Zentgraf U. Loss of stress-induced expression of catalase3 during leaf senescence in Arabidopsis thaliana is restricted to oxidative stress. Plant Sci. 2001;161:301–314. doi: 10.1016/s0168-9452(01)00409-5. [DOI] [PubMed] [Google Scholar]
- Palma JM, Corpas FJ, Del Río LA. Proteome of plant peroxisomes: new perspectives on the role of these organelles in cell biology. Proteomics. 2009;9:2301–2312. doi: 10.1002/pmic.200700732. [DOI] [PubMed] [Google Scholar]
- Palmer AE, Giacomello M, Kortemme T, Hires SA, Lev-Ram V, Baker D, Tsien RY. Ca2+ indicators based on computationally redesigned calmodulin-peptide pairs. Chem Biol. 2006;13:521–530. doi: 10.1016/j.chembiol.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Pei ZM, Murata Y, Benning G, Thomine S, Klüsener B, Allen GJ, Grill E, Schroeder JI. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature. 2000;406:731–734. doi: 10.1038/35021067. [DOI] [PubMed] [Google Scholar]
- Queval G, Issakidis-Bourguet E, Hoeberichts FA, Vandorpe M, Gakière B, Vanacker H, Miginiac-Maslow M, Van Breusegem F, Noctor G. Conditional oxidative stress responses in the Arabidopsis photorespiratory mutant cat2 demonstrate that redox state is a key modulator of daylength-dependent gene expression, and define photoperiod as a crucial factor in the regulation of H2O2-induced cell death. Plant J. 2007;52:640–657. doi: 10.1111/j.1365-313X.2007.03263.x. [DOI] [PubMed] [Google Scholar]
- Reumann S, Ma C, Lemke S, Babujee L. AraPerox. A database of putative Arabidopsis proteins from plant peroxisomes. Plant Physiol. 2004;136:2587–608. doi: 10.1104/pp.104.043695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolf R, Mongillo M, Rizzuto R, Pozzan T. Looking forward to seeing calcium. Nat Rev Mol Cell Biol. 2003;4:579–586. doi: 10.1038/nrm1153. [DOI] [PubMed] [Google Scholar]
- Schulte A, Lorenzen I, Böttcher M, Plieth C. A novel fluorescent pH probe for expression in plants. Plant Methods. 2006;6:2–7. doi: 10.1186/1746-4811-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeoka S, Ishikawa T, Tamoi M, Miyagawa Y, Takeda T, Yabuta Y, Yoshimura K. Regulation and function of ascorbate peroxidase isoenzymes. J Exp Bot. 2002;53:1305–1319. [PubMed] [Google Scholar]
- Wadsworth GJ, Scandalios JG. Differential expression of the maize catalase genes during kernel development: the role of steady-state mRNA levels. Dev Genet. 1989;10:304–310. doi: 10.1002/dvg.1020100405. [DOI] [PubMed] [Google Scholar]
- Willekens H, Chamnongpol S, Davey M, Schraudner M, Langebartels C, Van Montagu M, Inzé D, Van Camp W. Catalase is a sink for H2O2 and is indispensable for stress defence in C3 plants. EMBO J. 1997;15:4806–4816. doi: 10.1093/emboj/16.16.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Poovaiah BW. Hydrogen peroxide homeostasis: activation of plant catalase by calcium/calmodulin. Proc Natl Acad Sci USA. 2002;99:4097–4102. doi: 10.1073/pnas.052564899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Costa A, Leonhardt N, Siegel RS, Schroeder JI. Isolation of a strong Arabidopsis guard cell promoter and its potential as a research tool. Plant Methods. 2008;19:4–6. doi: 10.1186/1746-4811-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JJ, Mehta S, Israelsson M, Godoski J, Grill E, Schroeder JI. CO2 signaling in guard cells: calcium sensitivity response modulation, a Ca(2+)-independent phase, and CO2 insensitivity of the gca2 mutant. Proc Natl Acad Sci USA. 2006;103:7506–7511. doi: 10.1073/pnas.0602225103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zentgraf U. Senescence Processes in Plants. Annual Plant Reviews Wiley-Blackwell; Oxford: 2007. Oxidative stress and leaf senescence; pp. 69–86. [Google Scholar]
- Zimmermann P, Heinlein C, Orendi G, Zentgraf U. Senescence-specific regulation of catalases in Arabidopsis thaliana (L.) Heynh. Plant Cell Environ. 2006;29:1049–1060. doi: 10.1111/j.1365-3040.2005.01459.x. [DOI] [PubMed] [Google Scholar]
- Zipfel C, Kunze G, Chinchilla D, Caniard A, Jones JD, Boller T, Felix G. Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell. 2006;125:749–760. doi: 10.1016/j.cell.2006.03.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Subcellular distribution of cHyPer (cytoplasmic) and HyPer-KSRM (peroxisomes) in stable transgenic Arabidopsis plants.
Figure S2. The Ca2+-dependent HyPer ratio drop is strictly dependent on H2O2 removal and not affected by pH changes.