Abstract
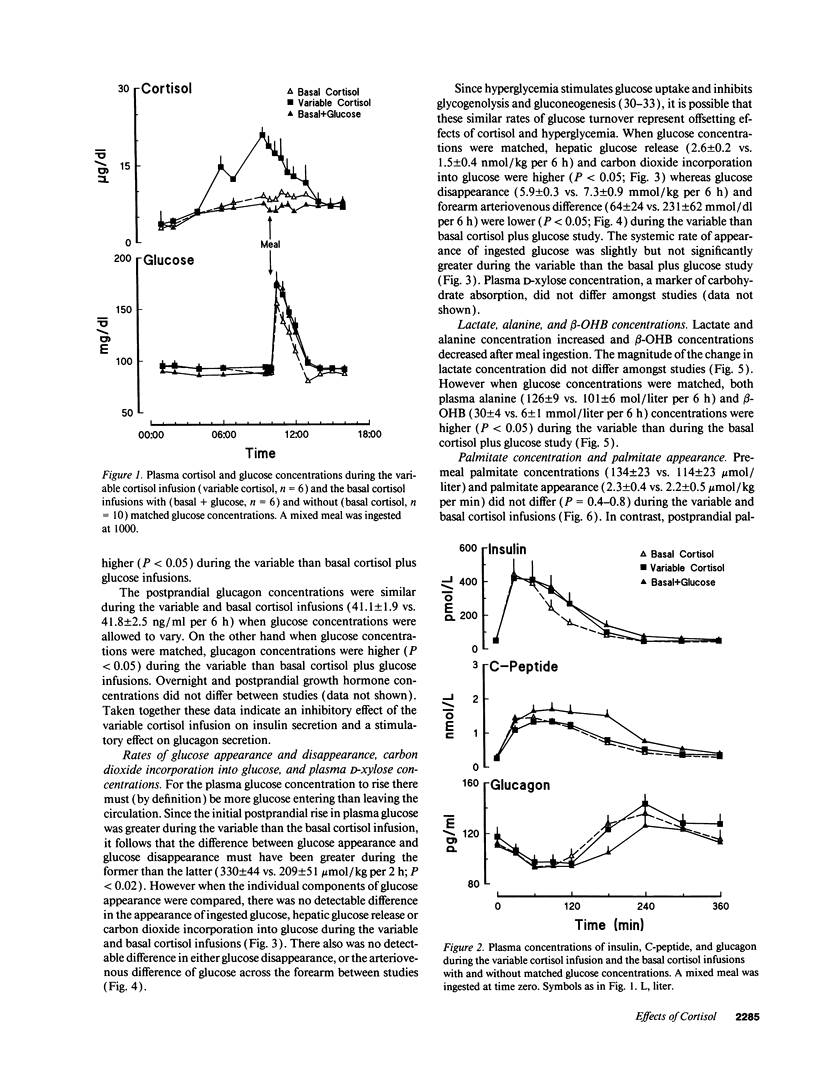
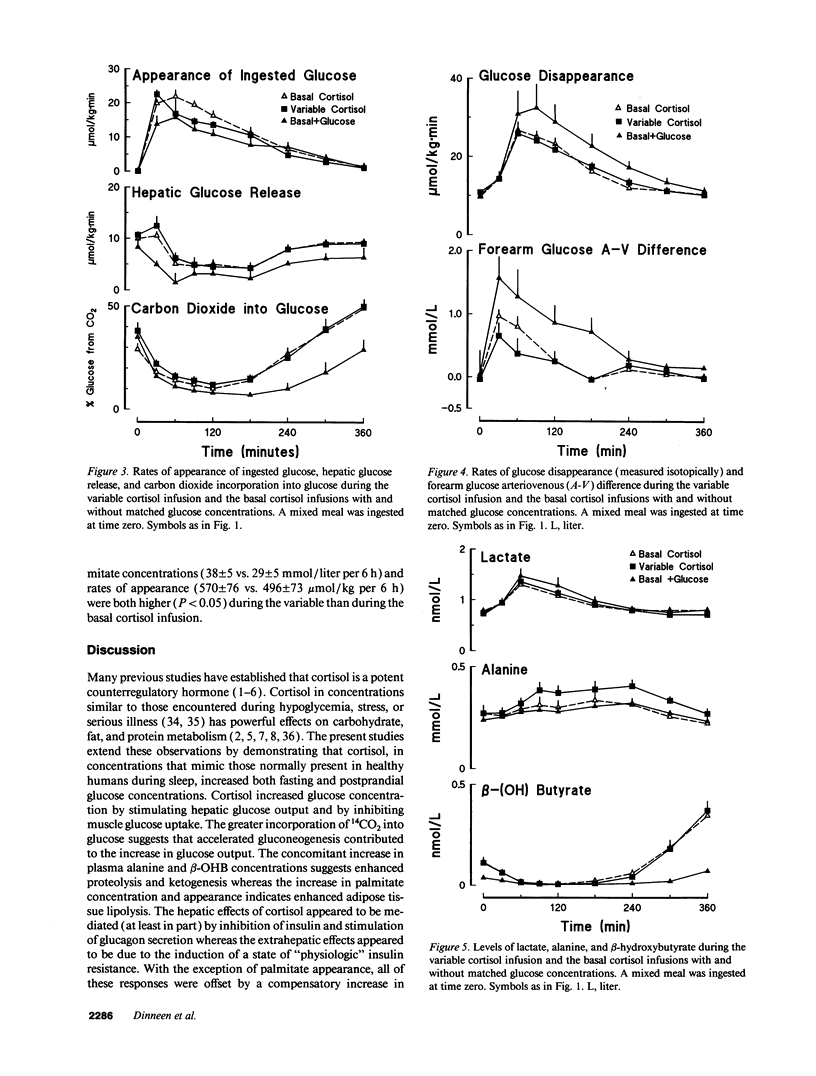
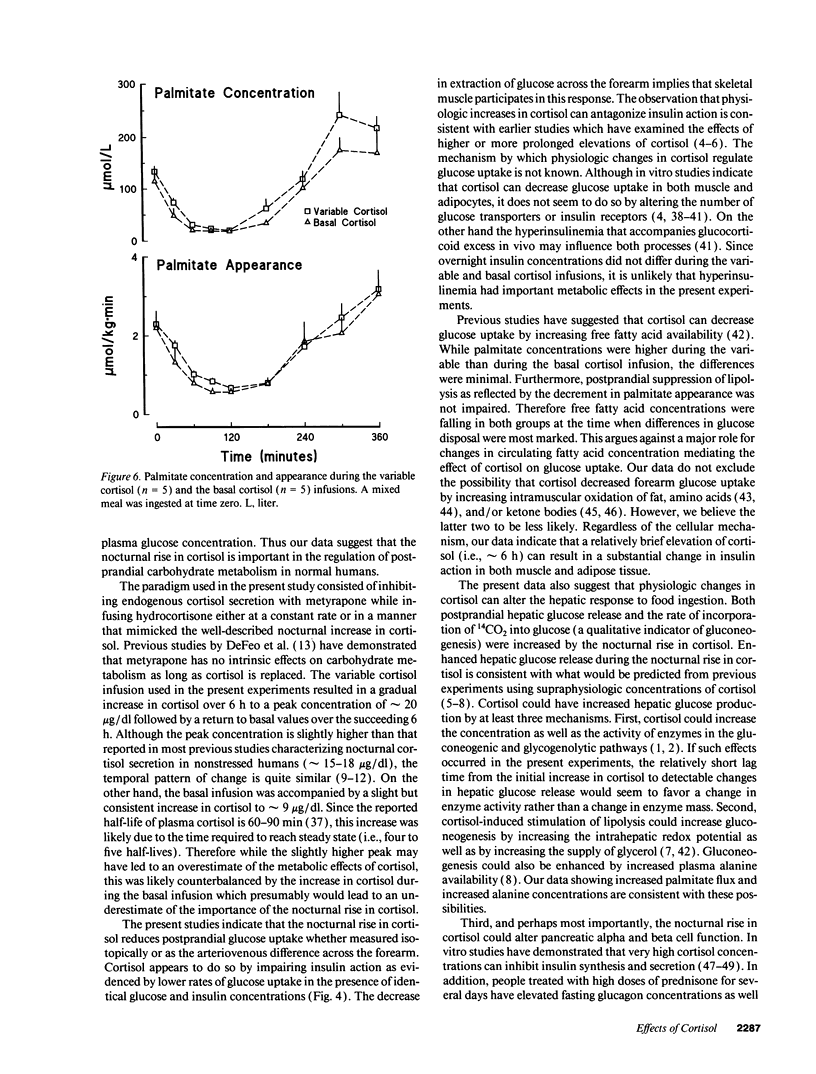
Glucocorticoid concentrations vary throughout the day. To determine whether an increase in cortisol similar to that present during sleep is of physiologic significance in humans, we studied the disposition of a mixed meal when the nocturnal rise in cortisol was mimicked or prevented using metyrapone plus either a variable or constant hydrocortisone infusion. When glucose concentrations were matched with a glucose infusion, hepatic glucose release (2.6 +/- 0.2 vs. 1.5 +/- 0.4 nmol/kg per 6 h) was higher (P < 0.05) while glucose disappearance (5.9 +/- 0.3 vs. 7.3 +/- 0.9 mmol/kg per 6 h) and forearm arteriovenous glucose difference (64 +/- 24 vs. 231 +/- 62 mmol/dl per 6 h) were lower (P < 0.05) during the variable than basal infusion. The greater hepatic response during the variable cortisol infusion was mediated (at least in part) by inhibition of insulin and stimulation of glucagon secretion as reflected by lower (P < 0.05) C-peptide (0.29 +/- 0.01 vs. 0.38 +/- 0.04 mmol/liter per 6 h) and higher (P < 0.05) glucagon (42.7 +/- 2.0 vs. 39.3 +/- 1.8 ng/ml per 6 h) concentrations. In contrast, the decreased rates of glucose uptake appeared to result from a state of "physiologic" insulin resistance. The variable cortisol infusion also increased (P < 0.05) postprandial palmitate appearance as well as palmitate, beta-hydroxybutyrate, and alanine concentrations, suggesting stimulation of lipolysis, ketogenesis, and proteolysis. We conclude that the circadian variation in cortisol concentration is of physiologic significance in normal humans.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adkins B. A., Myers S. R., Hendrick G. K., Stevenson R. W., Williams P. E., Cherrington A. D. Importance of the route of intravenous glucose delivery to hepatic glucose balance in the conscious dog. J Clin Invest. 1987 Feb;79(2):557–565. doi: 10.1172/JCI112847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell P. M., Firth R. G., Rizza R. A. Assessment of insulin action in insulin-dependent diabetes mellitus using [6(14)C]glucose, [3(3)H]glucose, and [2(3)H]glucose. Differences in the apparent pattern of insulin resistance depending on the isotope used. J Clin Invest. 1986 Dec;78(6):1479–1486. doi: 10.1172/JCI112739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman R. N., Bucolo R. J. Interaction of insulin and glucose in the control of hepatic glucose balance. Am J Physiol. 1974 Dec;227(6):1314–1322. doi: 10.1152/ajplegacy.1974.227.6.1314. [DOI] [PubMed] [Google Scholar]
- Bougnères P. F., Karl I. E., Hillman L. S., Bier D. M. Lipid transport in the human newborn. Palmitate and glycerol turnover and the contribution of glycerol to neonatal hepatic glucose output. J Clin Invest. 1982 Aug;70(2):262–270. doi: 10.1172/JCI110613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen A. J., Reeves R. L. Diurnal variation in glucose tolerance. Arch Intern Med. 1967 Mar;119(3):261–264. [PubMed] [Google Scholar]
- Bradley E. M., Waterhouse C. Effect of estrogen administration on extravascular cortisol. J Clin Endocrinol Metab. 1966 Jul;26(7):705–714. doi: 10.1210/jcem-26-7-705. [DOI] [PubMed] [Google Scholar]
- Bright G. M., Melton T. W., Rogol A. D., Clarke W. L. Failure of cortisol blockade to inhibit early morning increases in basal insulin requirements in fasting insulin-dependent diabetics. Diabetes. 1980 Aug;29(8):662–664. doi: 10.2337/diab.29.8.662. [DOI] [PubMed] [Google Scholar]
- Brosnan J. T. Pathways of carbon flux in gluconeogenesis. Fed Proc. 1982 Jan;41(1):91–95. [PubMed] [Google Scholar]
- Cahill G. F., Jr, Herrera M. G., Morgan A. P., Soeldner J. S., Steinke J., Levy P. L., Reichard G. A., Jr, Kipnis D. M. Hormone-fuel interrelationships during fasting. J Clin Invest. 1966 Nov;45(11):1751–1769. doi: 10.1172/JCI105481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell P. J., Bolli G. B., Cryer P. E., Gerich J. E. Sequence of events during development of the dawn phenomenon in insulin-dependent diabetes mellitus. Metabolism. 1985 Dec;34(12):1100–1104. doi: 10.1016/0026-0495(85)90153-2. [DOI] [PubMed] [Google Scholar]
- Caruso M., Divertie G. D., Jensen M. D., Miles J. M. Lack of effect of hyperglycemia on lipolysis in humans. Am J Physiol. 1990 Oct;259(4 Pt 1):E542–E547. doi: 10.1152/ajpendo.1990.259.4.E542. [DOI] [PubMed] [Google Scholar]
- Cherrington A. D., Williams P. E., Harris M. S. Relationship between the plasma glucose level and glucose uptake in the conscious dog. Metabolism. 1978 Jul;27(7):787–791. doi: 10.1016/0026-0495(78)90213-5. [DOI] [PubMed] [Google Scholar]
- Clore J. N., Helm S. T., Nestler J. E., Blackard W. G. Impaired modulation of hepatic glucose output overnight after a 72-h fast in normal man. J Clin Endocrinol Metab. 1990 Apr;70(4):865–868. doi: 10.1210/jcem-70-4-865. [DOI] [PubMed] [Google Scholar]
- DEBODO R. C., STEELE R., ALTSZULER N., DUNN A., BISHOP J. S. ON THE HORMONAL REGULATION OF CARBOHYDRATE METABOLISM; STUDIES WITH C14 GLUCOSE. Recent Prog Horm Res. 1963;19:445–488. [PubMed] [Google Scholar]
- De Feo P., Perriello G., Ventura M. M., Brunetti P., Santeusanio F., Gerich J. E., Bolli G. B. The pancreatic-adrenocortical-pituitary clamp technique for study of counterregulation in humans. Am J Physiol. 1987 Apr;252(4 Pt 1):E565–E570. doi: 10.1152/ajpendo.1987.252.4.E565. [DOI] [PubMed] [Google Scholar]
- De Pirro R., Green A., Kao M. Y., Olefsky J. M. Effects of prednisolone and dexamethasone in vivo and in vitro: studies of insulin binding, deoxyglucose uptake and glucose oxidation in rat adipocytes. Diabetologia. 1981 Aug;21(2):149–153. doi: 10.1007/BF00251283. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Ferrannini E., Hendler R., Felig P., Wahren J. Regulation of splanchnic and peripheral glucose uptake by insulin and hyperglycemia in man. Diabetes. 1983 Jan;32(1):35–45. doi: 10.2337/diab.32.1.35. [DOI] [PubMed] [Google Scholar]
- Dinneen S., Gerich J., Rizza R. Carbohydrate metabolism in non-insulin-dependent diabetes mellitus. N Engl J Med. 1992 Sep 3;327(10):707–713. doi: 10.1056/NEJM199209033271007. [DOI] [PubMed] [Google Scholar]
- Divertie G. D., Jensen M. D., Miles J. M. Stimulation of lipolysis in humans by physiological hypercortisolemia. Diabetes. 1991 Oct;40(10):1228–1232. doi: 10.2337/diab.40.10.1228. [DOI] [PubMed] [Google Scholar]
- Eberts T. J., Sample R. H., Glick M. R., Ellis G. H. A simplified, colorimetric micromethod for xylose in serum or urine, with phloroglucinol. Clin Chem. 1979 Aug;25(8):1440–1443. [PubMed] [Google Scholar]
- Ekstrand A., Saloranta C., Ahonen J., Grönhagen-Riska C., Groop L. C. Reversal of steroid-induced insulin resistance by a nicotinic-acid derivative in man. Metabolism. 1992 Jul;41(7):692–697. doi: 10.1016/0026-0495(92)90306-u. [DOI] [PubMed] [Google Scholar]
- Fantus I. G., Ryan J., Hizuka N., Gorden P. The effect of glucocorticoids on the insulin receptor: an in vivo and in vitro study. J Clin Endocrinol Metab. 1981 May;52(5):953–960. doi: 10.1210/jcem-52-5-953. [DOI] [PubMed] [Google Scholar]
- Firth R. G., Bell P. M., Marsh H. M., Hansen I., Rizza R. A. Postprandial hyperglycemia in patients with noninsulin-dependent diabetes mellitus. Role of hepatic and extrahepatic tissues. J Clin Invest. 1986 May;77(5):1525–1532. doi: 10.1172/JCI112467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinsmann W. H., Hern E. P., Lynch A. Intrinsic regulation of glucose output by rat liver. Am J Physiol. 1969 Apr;216(4):698–703. doi: 10.1152/ajplegacy.1969.216.4.698. [DOI] [PubMed] [Google Scholar]
- Haber R. S., Weinstein S. P. Role of glucose transporters in glucocorticoid-induced insulin resistance. GLUT4 isoform in rat skeletal muscle is not decreased by dexamethasone. Diabetes. 1992 Jun;41(6):728–735. doi: 10.2337/diab.41.6.728. [DOI] [PubMed] [Google Scholar]
- Haymond M. W., Miles J. M. Branched chain amino acids as a major source of alanine nitrogen in man. Diabetes. 1982 Jan;31(1):86–89. doi: 10.2337/diab.31.1.86. [DOI] [PubMed] [Google Scholar]
- Heding L. G., Rasmussen S. M. Human C-peptide in normal and diabetic subjects. Diabetologia. 1975 Jun;11(3):201–206. doi: 10.1007/BF00422322. [DOI] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Issekutz B., Jr Studies on hepatic glucose cycles in normal and methylprednisolone-treated dogs. Metabolism. 1977 Feb;26(2):157–170. doi: 10.1016/0026-0495(77)90051-8. [DOI] [PubMed] [Google Scholar]
- Jacobs H. S., Nabarro J. D. Plasma 11-hydroxycorticosteroid and growth hormone levels in acute medical illnesses. Br Med J. 1969 Jun 7;2(5657):595–598. doi: 10.1136/bmj.2.5657.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen M. D., Heiling V., Miles J. M. Measurement of non-steady-state free fatty acid turnover. Am J Physiol. 1990 Jan;258(1 Pt 1):E103–E108. doi: 10.1152/ajpendo.1990.258.1.E103. [DOI] [PubMed] [Google Scholar]
- Jensen M. D., Rogers P. J., Ellman M. G., Miles J. M. Choice of infusion-sampling mode for tracer studies of free fatty acid metabolism. Am J Physiol. 1988 May;254(5 Pt 1):E562–E565. doi: 10.1152/ajpendo.1988.254.5.E562. [DOI] [PubMed] [Google Scholar]
- Kaihara S., Wagner H. N., Jr Measurement of intestinal fat absorption with carbon-14 labeled tracers. J Lab Clin Med. 1968 Mar;71(3):400–411. [PubMed] [Google Scholar]
- Kalhan S. C., Adam P. A. Inhibitory effect of prednisone on insulin secretion in man: model for duplication of blood glucose concentration. J Clin Endocrinol Metab. 1975 Sep;41(3):600–610. doi: 10.1210/jcem-41-3-600. [DOI] [PubMed] [Google Scholar]
- Karl I. E., Pagliara A. S., Kipnis D. M. A microfluorometric enzymatic assay for the determination of alanine and pyruvate in plasma and tissues. J Lab Clin Med. 1972 Sep;80(3):434–441. [PubMed] [Google Scholar]
- Knutson V. P. The acute and chronic effects of glucocorticoids on insulin receptor and insulin responsiveness. Transient fluctuations in intracellular receptor level parallel transient fluctuations in responsiveness. J Biol Chem. 1986 Aug 5;261(22):10306–10312. [PubMed] [Google Scholar]
- Maizels E. Z., Ruderman N. B., Goodman M. N., Lau D. Effect of acetoacetate on glucose metabolism in the soleus and extensor digitorum longus muscles of the rat. Biochem J. 1977 Mar 15;162(3):557–568. doi: 10.1042/bj1620557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco J., Calle C., Román D., Díaz-Fierros M., Villanueva M. L., Valverde I. Hyperglucagonism induced by glucocorticoid treatment in man. N Engl J Med. 1973 Jan 18;288(3):128–131. doi: 10.1056/NEJM197301182880305. [DOI] [PubMed] [Google Scholar]
- McMahon M., Gerich J., Rizza R. Effects of glucocorticoids on carbohydrate metabolism. Diabetes Metab Rev. 1988 Feb;4(1):17–30. doi: 10.1002/dmr.5610040105. [DOI] [PubMed] [Google Scholar]
- McMahon M., Marsh H. M., Rizza R. A. Effects of basal insulin supplementation on disposition of mixed meal in obese patients with NIDDM. Diabetes. 1989 Mar;38(3):291–303. doi: 10.2337/diab.38.3.291. [DOI] [PubMed] [Google Scholar]
- Miles J. M., Ellman M. G., McClean K. L., Jensen M. D. Validation of a new method for determination of free fatty acid turnover. Am J Physiol. 1987 Mar;252(3 Pt 1):E431–E438. doi: 10.1152/ajpendo.1987.252.3.E431. [DOI] [PubMed] [Google Scholar]
- Nielsen H. K., Brixen K., Kassem M., Charles P., Mosekilde L. Inhibition of the morning cortisol peak abolishes the expected morning decrease in serum osteocalcin in normal males: evidence of a controlling effect of serum cortisol on the circadian rhythm in serum osteocalcin. J Clin Endocrinol Metab. 1992 Jun;74(6):1410–1414. doi: 10.1210/jcem.74.6.1592888. [DOI] [PubMed] [Google Scholar]
- Nosadini R., Del Prato S., Tiengo A., Valerio A., Muggeo M., Opocher G., Mantero F., Duner E., Marescotti C., Mollo F. Insulin resistance in Cushing's syndrome. J Clin Endocrinol Metab. 1983 Sep;57(3):529–536. doi: 10.1210/jcem-57-3-529. [DOI] [PubMed] [Google Scholar]
- Philippe J., Giordano E., Gjinovci A., Meda P. Cyclic adenosine monophosphate prevents the glucocorticoid-mediated inhibition of insulin gene expression in rodent islet cells. J Clin Invest. 1992 Dec;90(6):2228–2233. doi: 10.1172/JCI116108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierluissi J., Navas F. O., Ashcroft S. J. Effect of adrenal steroids on insulin release from cultured rat islets of Langerhans. Diabetologia. 1986 Feb;29(2):119–121. doi: 10.1007/BF00456122. [DOI] [PubMed] [Google Scholar]
- Pisters P. W., Restifo N. P., Cersosimo E., Brennan M. F. The effects of euglycemic hyperinsulinemia and amino acid infusion on regional and whole body glucose disposal in man. Metabolism. 1991 Jan;40(1):59–65. doi: 10.1016/0026-0495(91)90193-z. [DOI] [PubMed] [Google Scholar]
- Plumpton F. S., Besser G. M. The adrenocortical response to surgery and insulin-induced hypoglycaemia in corticosteroid-treated and normal subjects. Br J Surg. 1969 Mar;56(3):216–219. doi: 10.1002/bjs.1800560315. [DOI] [PubMed] [Google Scholar]
- RIDDICK F. A., Jr, REISLER D. M., KIPNIS D. M. The sugar transport system in striated muscle. Effect of growth hormone, hydrocortisone and alloxan diabetes. Diabetes. 1962 May-Jun;11:171–178. [PubMed] [Google Scholar]
- Rizza R. A., Mandarino L. J., Gerich J. E. Cortisol-induced insulin resistance in man: impaired suppression of glucose production and stimulation of glucose utilization due to a postreceptor detect of insulin action. J Clin Endocrinol Metab. 1982 Jan;54(1):131–138. doi: 10.1210/jcem-54-1-131. [DOI] [PubMed] [Google Scholar]
- Ruderman N. B., Herrera M. G. Glucose regulation of hepatic gluconeogenesis. Am J Physiol. 1968 Jun;214(6):1346–1351. doi: 10.1152/ajplegacy.1968.214.6.1346. [DOI] [PubMed] [Google Scholar]
- STEELE R., WALL J. S., DE BODO R. C., ALTSZULER N. Measurement of size and turnover rate of body glucose pool by the isotope dilution method. Am J Physiol. 1956 Sep;187(1):15–24. doi: 10.1152/ajplegacy.1956.187.1.15. [DOI] [PubMed] [Google Scholar]
- Service F. J., Hall L. D., Westland R. E., O'Brien P. C., Go V. L., Haymond M. W., Rizza R. A. Effects of size, time of day and sequence of meal ingestion on carbohydrate tolerance in normal subjects. Diabetologia. 1983 Oct;25(4):316–321. doi: 10.1007/BF00253193. [DOI] [PubMed] [Google Scholar]
- Shaw J. H., Wolfe R. R. Influence of beta-hydroxybutyrate infusion on glucose and free fatty acid metabolism in dogs. Am J Physiol. 1984 Dec;247(6 Pt 1):E756–E764. doi: 10.1152/ajpendo.1984.247.6.E756. [DOI] [PubMed] [Google Scholar]
- Shaw J. H., Wolfe R. R. The integrated effect of adrenergic blockade on glucose, fatty acid, and glycerol kinetics: responses in the basal state and during hormonal control with somatostatin-hormonal infusion. J Surg Res. 1987 Mar;42(3):257–272. doi: 10.1016/0022-4804(87)90142-9. [DOI] [PubMed] [Google Scholar]
- Simmons P. S., Miles J. M., Gerich J. E., Haymond M. W. Increased proteolysis. An effect of increases in plasma cortisol within the physiologic range. J Clin Invest. 1984 Feb;73(2):412–420. doi: 10.1172/JCI111227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skor D. A., White N. H., Thomas L., Santiago J. V. Influence of growth hormone on overnight insulin requirements in insulin-dependent diabetes. Diabetes. 1985 Feb;34(2):135–139. doi: 10.2337/diab.34.2.135. [DOI] [PubMed] [Google Scholar]
- Skor D. A., White N. H., Thomas L., Shah S. D., Cryer P. E., Santiago J. V. Examination of the role of the pituitary-adrenocortical axis, counterregulatory hormones, and insulin clearance in variable nocturnal insulin requirements in insulin-dependent diabetes. Diabetes. 1983 May;32(5):403–407. doi: 10.2337/diab.32.5.403. [DOI] [PubMed] [Google Scholar]
- Tessari P., Inchiostro S., Biolo G., Duner E., Nosadini R., Tiengo A., Crepaldi G. Hyperaminoacidaemia reduces insulin-mediated glucose disposal in healthy man. Diabetologia. 1985 Nov;28(11):870–872. doi: 10.1007/BF00291080. [DOI] [PubMed] [Google Scholar]
- Van Cauter E., Blackman J. D., Roland D., Spire J. P., Refetoff S., Polonsky K. S. Modulation of glucose regulation and insulin secretion by circadian rhythmicity and sleep. J Clin Invest. 1991 Sep;88(3):934–942. doi: 10.1172/JCI115396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cauter E., Shapiro E. T., Tillil H., Polonsky K. S. Circadian modulation of glucose and insulin responses to meals: relationship to cortisol rhythm. Am J Physiol. 1992 Apr;262(4 Pt 1):E467–E475. doi: 10.1152/ajpendo.1992.262.4.E467. [DOI] [PubMed] [Google Scholar]
- Weitzman E. D., Fukushima D., Nogeire C., Roffwarg H., Gallagher T. F., Hellman L. Twenty-four hour pattern of the episodic secretion of cortisol in normal subjects. J Clin Endocrinol Metab. 1971 Jul;33(1):14–22. doi: 10.1210/jcem-33-1-14. [DOI] [PubMed] [Google Scholar]
- Wolfe R. R., Peters E. J., Klein S., Holland O. B., Rosenblatt J., Gary H., Jr Effect of short-term fasting on lipolytic responsiveness in normal and obese human subjects. Am J Physiol. 1987 Feb;252(2 Pt 1):E189–E196. doi: 10.1152/ajpendo.1987.252.2.E189. [DOI] [PubMed] [Google Scholar]



