Abstract
Monocyte recruitment and their differentiation into macrophages are both early events in native and accelerated atherosclerosis that follows angioplasty. We have investigated the putative functional role of the epidermal growth factor receptor (EGFR) present on rabbit monocytes/macrophages. The impact of periadventitial delivery of an EGFR-specific, blocking monoclonal antibody (ICR62, which inhibits EGF-binding to its receptor) was investigated in a rabbit model of accelerated atherosclerosis induced by a combination of carotid injury and 4 weeks of a 2% cholesterol-diet. Two weeks after the initiation of the diet, a balloon-catheter angioplasty of the left common carotid artery was performed and a collar placed around the injured carotid artery immediately, for the delivery of ICR62 antibody, isotype-matched antibody or saline control. Monocyte/macrophage accumulation, cell proliferation and neointimal thickening were determined 2 weeks after the delivery of the antibodies. The function of the EGFR on rabbit monocytes was also investigated in vitro, using chemotaxis assays. Treatment with ICR62 was associated with a significant reduction in macrophage accumulation and neointimal thickening and a 76% reduction in neointimal area of the vessel wall compared with controls. In vitro ICR62 inhibited macrophage and smooth muscle cell migration towards EGFR ligands including EGF and HB-EGF. These findings suggest that EGFR ligation may be important in the development of early atherosclerotic lesions following balloon-catheter angioplasty, and periadventitial delivery may provide a feasible approach for administration of the inhibitors of EGFR-binding such as ICR62.
Keywords: angioplasty, atherosclerosis, EGFR, macrophages, neointima formation
The accumulation of macrophages and vascular smooth muscle cells are early events in native and accelerated atherosclerosis associated with angioplasty, stenting and venous bypass graft failure (Ross 1993; Davies & Hagen 1995; Hansson 2005). Several growth factors, chemokines and cytokines elaborated by cells involved in atherogenesis may interact synergistically to trigger a network of biological signals that culminate in monocyte recruitment and differentiation and smooth muscle cell chemotaxis and proliferation, leading to neo-intimal thickening (Yarden & Sliwkowski 2001; Charo & Taubman 2004; Lamb et al. 2004). Whilst several studies have reported that the epidermal growth factor receptor (EGFR) and its family of ligands are present on human macrophages associated with melanoma and other carcinomas (Scholes et al. 2001; Normanno et al. 2006), few studies have identified their presence in atherosclerotic plaques (Miyagawa et al. 1995; Tamura et al. 2001; Dreux et al. 2006). EGFR has been demonstrated on intimal smooth muscle cells within human atherosclerotic plaque, cultured rat aortic smooth muscle cells and in the injured vessel wall (Tomita et al. 1986; Trieu et al. 2000; Tamura et al. 2001). Anti-EGFR blocking antibodies administered systemically in rodent models of restenosis inhibited neointimal hyperplasia caused predominantly by the accumulation of vascular smooth muscle cells (Trieu et al. 2000; Chan et al. 2003). Interestingly, our group has previously demonstrated the presence of EGFR on rabbit blood monocytes, and macrophages within the experimental atherosclerotic lesions, and we reported that EGFR in vitro mediates chemotactic and proliferative responses in monocytes/macrophages (Lamb et al. 2004). Despite the expression of EGFR on macrophages and on SMCs, no studies so far have focused on the functional significance of this receptor on monocytes/macrophages in a rabbit model of early accelerated atherogenesis (carotid injury and atherogenic diet).
The human EGF receptor (ErbB1, HER-1) is a 170-kDa trans-membrane glycoprotein with kinase activity (Modjtahedi et al. 1993). Three other members of the EGFR gene family have been identified; ErbB2, ErbB3 and ErbB4 (Dreux et al. 2006). Functional EGFRs consist of homo- and hetero-dimers that transduce tyrosine auto- and trans-phosphorylation, and activation of downstream signalling. EGFR is activated by binding to a number of peptide growth and differentiating factors, including epidermal growth factor (EGF), heparin-binding EGF (HB-EGF), transforming growth factor-α (TGF-α), amphiregulin (AR) and epiregulin (EPR), which are released from platelets, SMCs, endothelial cells and macrophages (Dreux et al. 2006). Several of these ligands have been identified on monocytes and in macrophage rich-areas of human aortic and coronary atherosclerotic lesions (Mograbi et al. 1997; Reape et al. 1997; Tamura et al. 2001; Panutsopulos et al. 2005). EGF and HB-EGF have been shown to stimulate macrophages and smooth muscle cells proliferation and migration in vitro (Higashiyama et al. 1993; Lamb et al. 2004). Moreover, non-EGFR ligands present in atherosclerotic lesions such as oxidized LDL and their oxidized derivatives (Suc et al. 1998) and thrombin (Kalmes et al. 2000) can transactivate EGFR, via G-protein-coupled receptors.
In this study, we have used a well established rabbit model of accelerated atherosclerosis, to investigate the effectiveness of a relatively short-term (2 week) local treatment of an EGFR blocking antibody to modulate monocyte/macrophage accumulation and neointimal thickening. Accelerated atherosclerosis was induced by a combination of balloon-injury to common carotid artery and a high cholesterol diet. The interesting feature of this model was that the angioplasty was performed when atherosclerotic lesions were established which consisted of abundant neointimal macrophages and macrophage-derived foam-cells. In this model, we studied the potential role of the monocyte/macrophage EGFR in an early accelerated atherogenesis. We also examined the effects of the anti-EGFR blocking antibody on the EGF and HB-EGF-stimulated macrophage and aortic SMC function in vitro.
Materials and methods
The monoclonal antibody ICR62 (rat monoclonal antibody that recognize human and rabbit macrophage EGFR) was produced, purified and characterized by Modjtahedi et al. (1993). All reagents including Histopaque were purchased from Sigma-Aldrich (Dorset, UK) unless indicated.
Animals, induction of atherosclerosis and experimental protocol
All experiments were performed under a Home Office licence that had been approved by the Ethics Committee of the University of Surrey, Guildford, UK. Twenty-nine male New Zealand White rabbits (2.8–3.6 kg; B&K Universal Ltd, Hull, UK) were used in this study and of which 26 were fed a 2% cholesterol-enriched diet (Special Diet Service, Essex, UK) for 2 weeks before balloon-catheter angioplasty and collaring and than for further 2 weeks of experiments.
Rabbits were anaesthetized with Hypnorm (2% fluanisone/fentanyl citrate 0.3 ml/kg, i.m., Janssen Pharmaceutica, Leeds, UK) and inhalation anaesthesia (isoflurane), followed by diazepam (2 mg/kg i.v., Phoenix Pharmaceuticals Ltd, Kent, UK); Baytril (0.2 ml/kg s.c.) and then Vetragesic (0.03 mg/kg s.c.) during surgery.
Plasma total cholesterol concentration was determined by Accutrend meter with test strips (Roche, East Sussex, UK) at weekly intervals after the initiation of the cholesterol-diet. The cholesterol content of the diet was adjusted to create individually tailored diets to enable the plasma cholesterol level of each animal to be maintained between 20 and 30 mmol/l.
Six animals (one saline, five isotype matched) had completely occluded injured carotid arteries by extensive neointima and thrombus formation at the time of culling, and were therefore excluded from subsequent analysis.
Balloon injury, collaring and antibody delivery
Two weeks after the initiation of the cholesterol-rich diet, both carotid arteries were surgically exposed and dissected free from the surrounding tissues. The left common carotid artery was clamped and a direct arteriotomy performed. A deflated embolectomy catheter (2F; Mantis Surgical Ltd, Newbury, UK) was inserted via this opening into the lumen and advanced 5 cm cephalically, inflated with 0.02 ml saline, and then withdrawn to the site of arteriotomy before inflation. After repeating this sequence twice more, the catheter was removed, the small incision sutured and the arterial blood flow re-established. A sham procedure was conducted on the contralateral carotid artery in terms of its exposure and the degree of manipulation and was used as a self control.
Following the balloon-catheter angioplasty a biologically inert, silastic collar (Ark Therapeutics Ltd, Kuopio, Finland) was placed around the middle part of an injured carotid artery to delivery antibodies periadventitially as described (Khurana et al. 2004). Immediately after the balloon injury, 200 μl of EGFR blocking antibody ICR62 (200 nM n= 8), isotype control (n= 8) or vehicle alone (n= 8) was instilled within the space between collar and artery using a pipette. The wound was sutured and the animal allowed to regain consciousness. One week later a second dose of the antibodies or saline was introduced into the collar.
Tissue harvesting and processing
Two weeks post-angioplasty, the animals were killed and segments of carotid arteries were excised, fixed and paraffin embedded as described previously (Khurana et al. 2004). Serial cross-sections (5 μm) were cut at four levels, 50 μm apart. Sections from each level were stained with haematoxylin and eosin for morphometry and immunohistochemical analysis.
Immunohistochemistry
The following antibodies were used: macrophage specific mouse RAM 11 (1:100; Dako, Cambridgeshire, UK); VSMC-specific α-actin HHF-35 (1:200; Dako) and proliferating cell nuclear antigen specific anti-PCNA clone PC-10 (1:150; AbD Serotec, Oxford, UK). Negative controls for immunostaining included incubation with class- and species-matched antibodies or omitting the primary antibody. Deparaffinized sections were boiled for 10 min in citric acid (10 mM) for antigen retrieval after which immunostaining was performed as described by the mouse or rat Elite ABC kit and visualized with DAB substrate (Vector Laboratories, Peterborough, UK).
For identification of proliferating macrophages, immunostaining with RAM 11 was combined with PCNA staining. First staining of the serial sections with RAM 11 antibody was performed and visualized with DAB as described above. Sections were than blocked and the entire staining procedure was repeated with PCNA antibody and than visualized with Vector VIP.
Morphometry and image analysis
RAM 11, HHF-35 and PCNA positive cells were identified and quantitated using an image analysis system (QWin) interfaced with a Leica DLMB microscope equipped with a high resolution digital camera (JCV CCD). Images of cross-sections at ×2.5 or at ×4 magnification for neointimal thickness were acquired and analysed using Image J NIH programme. The intima:media ratios were averaged and expressed as mean ± SEM. Total RAM 11 positive macrophages were counted in the intima and were expressed as macrophage density (cells/mm2). Intima was defined as the region between the luminal endothelium and the internal elastic lamina (IEL). The media was defined as the area between the internal and the external elastic laminas (EEL). To study vascular remodelling, total vessel area (i.e. area encompassed by the EEL) plus the absolute lumen area (A) was calculated (A = C2/4π; where C is the perimeter length), to normalize for any changes that may occur during fixation procedure.
To identify intimal PCNA positive cells that were co-located with RAM 11 positivity, each ×40 field of view (total of 8 fields/section) was counted directly for PCNA(+)-macrophages and the total number of RAM 11 +ve cells. The percentage of macrophages proliferating was expressed as number of PCNA(+)-RAM 11 +ve cells/total number of RAM 11 +ve cells × 100%.
Mononuclear isolation from blood
Mononuclear cells were isolated from whole blood of a normocholesterolaemic rabbit by density centrifugation using Histopaque-1083r g/ml as described (Lamb et al. 2004). Mononuclear cells were incubated in Dulbecco’s Modified Eagles Medium (DMEM) containing 10% foetal bovine serum (FBS), 50 IU penicillin/ml and 50 μg streptomycin/ml (Gibco, Paisley, UK) to allow monocytes to adhere as macrophages for 48 h. Non-adherent cells were washed off.
Aortic smooth muscle cell culture
Rabbit aortic SMCs were grown from aortic medial explants (n= 3) in DMEM containing 20% FBS as described (Lamb et al. 2005). Cellular identity was confirmed by α-actin staining.
Chemotaxis assay
Cell migration was measured using a modified Boyden micro-chemotaxis chamber (Neuroprobe, Gaitherburg, MD, USA), using a polycarbonate membrane filter (5 μm pores for monocytes and 8 μm pore pre-coated with 1% gelatine for SMCs), which separated each well into two compartments as described previously (Ferns et al. 1990). The lower compartment contained the putative chemokines (EGF or HB-EGF, positive control MCP-1 and PDGF-BB). The upper compartment contained either mononuclear (50 μl ∼ 3–5 ×106 cells/ml) or SMCs (1.3 × 106 cells/ml) together with increasing concentrations of the ICR62 antibody (10, 50 and 200 nM), isotype control antibody (200 nM) or medium alone. The total number of migrated cells were counted by light microscopy in 10 high-powered fields per well.
Statistical analysis
Results are expressed as means ± SEM. The integrated cholesterol concentrations were calculated as the area under the cholesterol concentration vs. time curve. Differences in plasma cholesterol levels and morphometeric differences between the means of controls and treated groups were evaluated by the unpaired Student’s t-test, and differences between different antibody concentrations were analysed by anova (spss). P≤ 0.05 was considered significant.
Results
The high cholesterol diet significantly raised plasma cholesterol levels
The plasma total cholesterol concentration in all rabbits (n= 26) increased at the end of the first week and was maintained at approximately 20 mmol/l through out the experiment [mean 20.9 ± 1.0 mmol/l (integrated total area of 52.2 ± 2.6 mmol/l for 1–4 week)]. There was no significant difference in the mean integrated plasma cholesterol concentrations between the three experimental groups over 4 weeks.
ICR62 inhibits neointimal lesion formation and intimal thickening
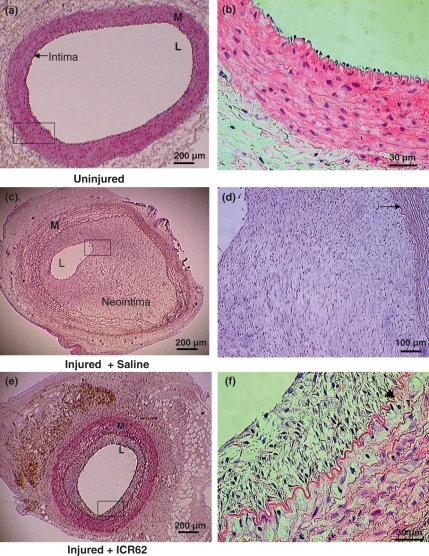
Neointima formation was histologically evident in all the balloon-catheter injured carotid arteries, with largely intact internal and external elastic laminae and media (Figure 1). The sham-operated contra-lateral carotid arteries showed normal histology despite cholesterol feeding (Figure 1a) with intact endothelial cell monolayer directly on internal elastic lamina (Figure 1b). The saline-treated arteries had an extensive neointima with abundant foam cells concentrated primarily in the deeper intima adjacent to the IEL and closely packed smooth muscle cells orientated longitudinally, growing into lumina and almost completely occluding with intimal proliferation (Figure 1c,d), whereas in ICR62-treated animals (Figure 1e), the lumen was larger and the neointima formation was markedly smaller with concentric intimal thickening consisting of smooth muscle cells, macrophages and foam cells between the media and intima (Figure 1f).
Figure 1.
Neointima formation in representative H & E stained cross sections of balloon-catheter injured and uninjured carotid arteries of cholesterol-fed rabbits 2 weeks post delivery of antibody or saline. The uninjured contralateral carotid artery show a normal morphology (a, b), whereas injured saline-treated (c, d) and ICR62 antibody treated (e, f) showed various degree of neointimal formation. The vessel lumen of saline-treated (c) was very much smaller than the ICR62-treated artery (e). Arrows indicate IEL. L, lumen; M, media.
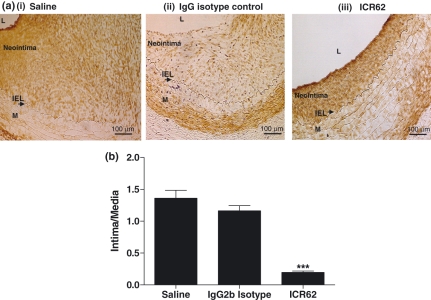
The effect of treatment with the ICR62 antibody on neointima formation was examined (Figure 2). Immunostaining with α-actin showed that the ICR62 antibody caused a marked reduction in VSMCs accumulation in the neointima (Figure 2aiii). Quantification of the intima: media ratio in Figure 2b showed that the mean ratio (0.20 ± 0.02) was significantly reduced by 83% and 85% in comparison with the isotype match antibody or saline-treated group respectively (1.16 ± 0.08 or 1.36 ± 0.13 respectively) (P< 0.0001). The marked reduction in neo-intimal thickening was associated with a 76% decrease in intimal area [(0.34 ± 0.05) vs. the saline controls (1.42 ± 0.22 mm2), P< 0.0004]. Consistent with these results, ICR62 antibody also significantly increased the luminal area compared with the saline or the isotype control (0.061 ± 0.01 mm2vs. 0.011 ± 0.003 or 0.014 ± 0.003 mm2 respectively). There was no significant difference in external elastic lamina area between the ICR62 and saline (2.09 ± 0.3 mm2vs. 2.42 ± 0.3 mm2), and also no differences in neointima formation between the isotype control and saline treated arteries.
Figure 2.
Periadventitial delivery of ICR62 inhibits neointimal formation in injured carotid arteries of hypercholesterolaemic rabbits. VSMCs identified by immunostaining with anti-α-actin (top panel ai–iii). Arrows indicate IEL. L, lumen; M, media. Quantification of absolute intima/media ratios (b) of the ICR62 (n= 8), isotype matched control antibody (n= 3) and saline-treated (n= 7) were as described in Methods. ICR62 antibody significantly reduced intimal thickening compared with the saline or isotype controls: ***P< 0.0001. Bars represent mean ± SEM.
ICR62 decreases macrophages accumulation
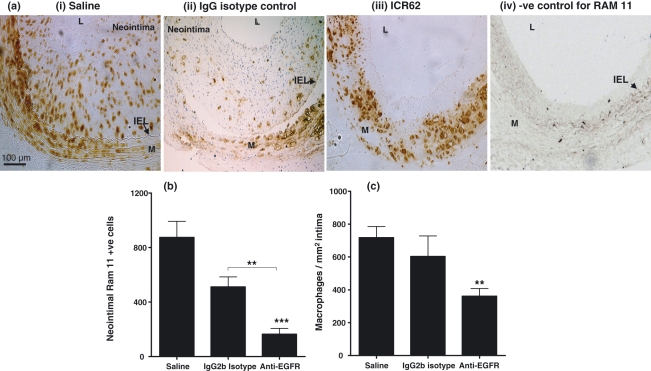
Beside a decrease in inimal thickness, ICR62 antibody also reduced the neointimal macrophage accumulation (Figure 3). In the saline-treated carotid arteries there were numerous macrophages and foam cells throughout the neointima and adjacent media (Figure 3ai), whereas in the ICR62 treated carotids the macrophages were more sparsely distributed in the neointima (Figure 3aiii). In fact ICR62 antibody caused a striking inhibition of macrophage accumulation in the neointima of injured carotid arteries. The number of neointimal RAM 11 positive cells (Figure 3b) and their density (expressed as macrophage/mm2 intima; Figure 3c) decreased by 81% (164.4 ± 42.9 vs. 874.9 ± 118; P< 0.0001) and 50% (360.8 ± 47 vs. 716.9 ± 69; P< 0.002), respectively, in comparison to controls. In comparison to the isotype matched antibody, the differences in neointimal macrophages cell number and their density were less than 10% of arteries from the saline treated animals. In isotype matched antibody-treated carotids (Figure 3aii) the number of RAM 11 positive cells appeared to be reduce compared to the saline-treated controls (Figure 3ai); but this reduction was not statistically significant (874.9 ± 118 vs. 511.0 ± 174; P< 0.083).
Figure 3.
Macrophage accumulation and their colocalization with EGFR in injured carotid arteries. The total macrophage content of the saline-treated (n= 6), IgG2b Isotype control (n= 3) and ICR62 antibody (n= 7) injured arteries was determined by immunostaining with RAM 11 (top panel a). In the saline-injured carotid artery (ai) numerous tissue macrophages and foam cells are apparent within the neointima and adjacent media, where as in the ICR62 fewer numbers are noticed and the neointima thickness is less (aiii). In IgG2b isotype-treated carotid artery (aii) the number of RAM 11 +ve cells appeared to be reduced compared to the saline carotid (ai). (aiv) a negative control (isotype matched control antibody for RAM 11). Arrows indicate IEL. Macrophages numbers are expressed as neointimal RAM 11 positive cells in injured carotid artery sections from six rabbits per group, except for IgG2b isotype controls (n= 3) (b). Macrophage density is expressed as neointimal RAM 11 positive cells per mm2 (total neointimal area) (c). ICR62 antibody significantly reduced neointimal macrophage content compared with the saline and IgG2b isotype matched control. **P< 0.002; ***P< 0.0001.
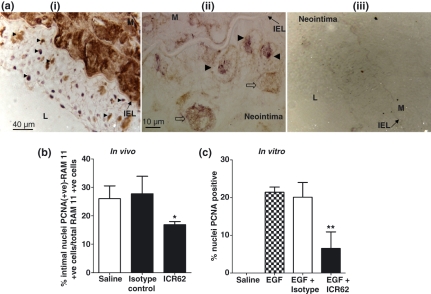
To elucidate the potential mechanisms responsible for the decreased macrophage accumulation, we determined the effect of ICR62 antibody on neointimal macrophage proliferation by performing double immunostaining with RAM 11 and PCNA antibodies (Figure 4a). The number of neointimal PCNA-positive nuclei that co-localized with RAM 11 positive cells corresponding to proliferating macrophages were significantly lower in ICR62-treated carotid arteries compared to the saline or the isotype controls (P< 0.05) (Figure 4b). PCNA +ve cells were predominantly expressed immediately beneath the endothelium, whereas most of the PCNA(+)-RAM 11 cells were in close proximity to the IEL. No significance of difference was observed between saline and the isotype controls.
Figure 4.
Macrophage proliferative activity as assessed by double immunostaining of rabbit carotid artery sections and adherent macrophages in vitro. Panel a shows a representative colocalization of PCNA-positive stained nuclei (purple staining) with RAM 11 positive cells (brown cytoplasmic staining). Arrowheads in the carotid neointimal lesion indicate proliferating macrophages (ai & aii; double-positive) and the open-arrows points to non-proliferating single-positive cells (only cytoplasmic staining without nuclear involvement) at a higher magnification (aii). (aiii) a negative control for RAM 11 and PCNA antibodies. Arrows indicate IEL. M, Media. (b) Quantification of neointimal macrophage proliferation expressed as percentage means of PCNA(+)-RAM 11 +ve cells (double-positive)/total number of RAM 11 +ve cells (single positive) in sections from the saline- (n= 4), IgG2b isotype (n= 3) and ICR62 antibody (n= 6) treated carotid arteries. (c) Inhibition of EGF-induced PCNA expression by ICR62 antibody in adherent macrophages in vitro. Bars represent the mean ± SEM of four independent experiments performed in duplicates. Significance of difference compared to the saline or isotype-matched antibody; *P< 0.05; **P< 0.005.
We determined the effect of ICR62 on the proliferative response of adherent macrophages to EGF (100 nM) for 4 h in vitro to confirm that ICR62 antibody bona fide inhibit their proliferation (Figure 4c). ICR62-treatment resulted in 69.6% decrease in macrophage proliferation compared to the EGF alone (6.5 ± 2.5%vs. 21.4 ± 0.8% nuclei PCNA +ve; n= 4, P< 0.005). In contrast, isotype control antibody had no influence in suppressing macrophage proliferation (20.1 ± 1.9%). In the absence of EGF, saline treated macrophages showed no proliferative activity.
ICR62 inhibits HB-EGF and EGF-induced monocyte and smooth muscle cells migration in vitro
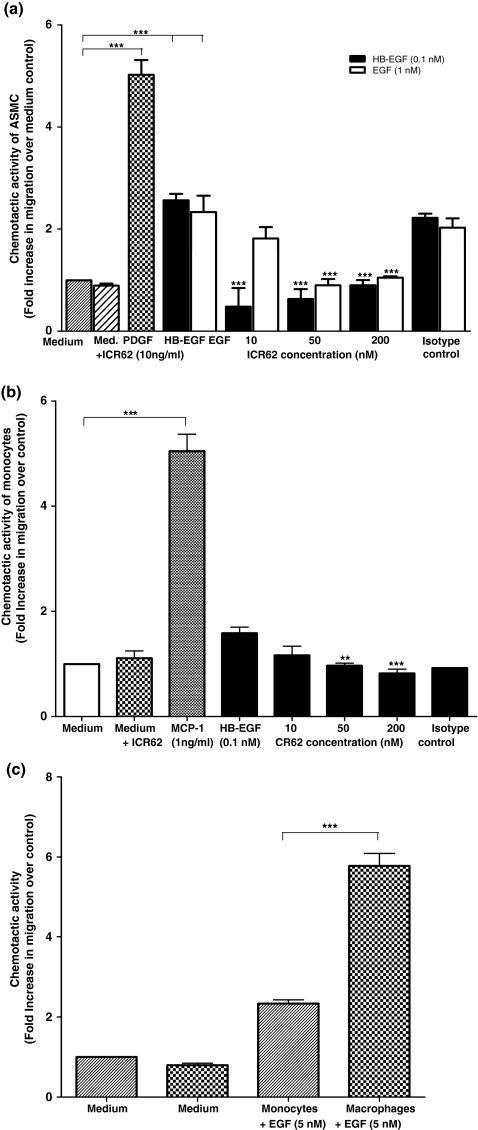
To explore the possible mechanisms of reduced macrophage accumulation and neointimal thickening in the ICR62 injured carotid arteries, we compared the chemotaxis of peripheral blood monocytes, monocyte derived macrophage and aortic SMCs from non-cholesterol fed rabbits in vitro. Checker-board analysis in our previous study revealed that the response of monocytes to EGF was a chemotactic as opposed to a chemokinetic response (Lamb et al. 2004). In the presence MCP-1 and PDGF-BB, used as a positive controls for monocytes/macrophages and SMCs respectively, the fold increase in cell response was 4.91 and 5.05, respectively, and as expected, these were greater than for unstimulated cells (Figure 5). The HB-EGF and EGF-induced migratory responses of both SMCs and monocytes were significantly inhibited by the presence of ICR62 antibody when compared to their relevant controls (Figure 5a,b; P< 0.0001; P< 0.001), except at lower ICR62 concentration (10 nM), which had no significant effect on monocyte migration. The response of monocytes to HB-EGF was markedly inhibited in a dose dependent manner by ICR62 (Figure 5b), whereas for the SMCs (Figure 5a) the chemotactic response was significantly inhibited by ICR62 (P< 0.0001). Both HB-EGF and EGF showed similar responses in SMCs. EGF-induced migration in the presence of increasing concentrations of ICR62 also decreased significantly in a dose dependent manner (Figure 5a; P< 0.0001). Furthermore, the chemotactic response of monocyte-derived macrophages towards EGF was significantly different (5.8 ± 0.3 vs. 2.3 ± 0.1 fold over control, n= 3; P< 0.0005) from that of peripheral blood monocytes (Figure 5c). These results show that EGFR mediates EGF and HB-EGF-induced monocyte/macrophage and SMC migration. Isotype matched antibody had no significant effect on EGF and HB-EGF-induced SMC migration.
Figure 5.
ICR62 antibody inhibits HB-EGF and EGF-induced (a) smooth muscle cells and (b) monocytes migration. A Chemotaxis assay was used to determine whether increasing concentrations of ICR62 (10, 50 and 200 nM) or the isotype control antibody (200 nM) inhibited HB-EGF and EGF-induced migration of peripheral blood monocytes and aortic SMCs. (c) Comparison of peripheral blood monocyte and monocyte-derived macrophage chemotactic response to EGF. The chemotactic response is expressed as mean fold induction over medium control ± SEM of three independent experiments performed in triplicate, except for the isotype control in monocyte migration, where n= 2. The chemotactic response of the cells induced by HB-EGF, EGF, MCP-1 and PDGF-BB was significantly higher as compared to the medium control; ***P< 0.0001. Significance of differences in the inhibition of HB-EGF and EGF-induced cell migration by ICR62 antibody compared to the relevant controls: ***P< 0.0001; **P< 0.001. Significance of difference of the chemotactic response of monocyte-derived macrophages compared to monocytes; ***P< 0.0005.
Discussion
EGFR is expressed on rabbit peripheral blood monocytes, macrophages and rat smooth muscle cells and HB-EGF, BTC and EGF have been reported to be elaborated within atherosclerotic lesions (Tomita et al. 1986; Miyagawa et al. 1995; Tamura et al. 2001; Lamb et al. 2004; Panutsopulos et al. 2005; Dreux et al. 2006). The aim of this present study was to investigate the functional role of EGFR in accelerated atherosclerosis. We have, for the first time provided evidence from a rabbit model of atherosclerosis induced by a high cholesterol-diet and carotid injury and from in vitro studies that monocytes/macrophages EGFR have a potential role in the development of an early accelerated atherosclerosis and that HB-EGF and EGF are both potent chemotactic factors for rabbit macrophages and SMCs.
Evidence from in vitro Studies
Two ligands for the EGFR, specifically HB-EGF and EGF were used to investigate SMC and monocytes chemotaxis in vitro. We found that HB-EGF (0.1 nM) and EGF (1 nM) stimulated both the peripheral blood monocyte and SMC chemotaxis. Although both ligands bind to the EGFR, HB-EGF elicited a greater chemotaxis response for SMC than for monocytes. The differential effects of the ligands could be explained by their different affinities for the SMC EGFR. Higashsiyama et al. showed that HB-EGF requires the presence of cell surface heparan sulphate proteoglycans (HSPG) for this high affinity binding to occur on SMCs’ (Raab & Klagsbrun 1997) and these proteoglycans are present in excess amount only on macrophages and not on monocytes (Edwards et al. 1995). Interestingly, our results for monocyte-derived macrophages showed greater chemotactic response to EGF than monocytes, thereby, also suggesting that indeed cell surface proteoglycans may be essential for its optimal binding to EGFR and hence for its optimal ability to stimulate migration or that it may be simply related to the cell surface receptor density. The chemotactic response of peripheral monocytes to HB-EGF in the presence of increasing concentrations of ICR62 antibody was also significantly decreased in a dose dependent manner, suggesting that blockade of EGFR inhibited their migration. Similar reductions in SMC migration were also observed with the high concentrations of ICR62 (200 nM), which we than used in our in vivo studies. From these results we concluded that the effect of ICR62 antibody on HB-EGF and EGF-induced cell migration was due to the direct inhibition of ligand binding to the EGFR on monocytes, macrophages and SMCs and that this function was blocked by an anti-EGFR antibody. Previous studies have shown that this antibody binds specifically to the epitope ‘C’ region of the EGF receptor located within the ligand binding domain of the EGFR (Modjtahedi et al. 1993). Our results are consistent with our previous work that shown that EGFR blockade with ICR62 antibody caused an inhibition of the tumour growth (Modjtahedi et al. 1998a,b;). In addition we have also shown that ICR62 antibody act as EGFR antagonist and block the binding of EGFR ligands (EGF, TGF-α, HB-EGF and BTC) to the EGFR in a wide range of tumour cells in which it inhibits downstream cell signalling molecules (Modjtahedi et al. 1998a,b; Cunningham et al. 2006).
Miyagawa et al. have shown that HB-EGF production is increased in human aortic atherosclerotic plaques and that HB-EGF is expressed as membrane-bound integral precursor on macrophages and SMCs. Peripheral human monocytes express a family of proteases, that include ADAM-10 and -12 (Namba et al. 2001) and activated macrophages secrete several MMPs (MMP-1, -2 -3, -7 & -9) within the atherosclerotic plaque (George 1998; Yamashita et al. 2008). It has been demonstrated that during arterial injury these proteases and MMPs may become activated, thereby leading to the proteolytic cleavage and shedding of the EGFR ligands from monocytes and macrophages that may in turn activate the EGF receptor in an autocrine manner. MMPs inhibitors have been shown to block their release (Tokumaru et al. 2000). Boucher et al. (2007) have recently shown that injury triggers the release of adenine nucleotides (ATP/UTP/ADP), which stimulate P2Y purinoceptors to elicit the phosphorylation of EGFR tyrosine residues, ERK activation and subsequent cell migration by the proteases and HB-EGF. Therefore, a combination of processes may be responsible for activation of the EGFR following vessel wall injury. Suc et al. (1998) have reported that ox-LDL may induce tyrosine phosphorylation of the EGFR and activation of its signalling pathway, independent of any autocrine EGF effect. This activation triggered by ox-LDL, lipid perioxidation products and 4-HNE was associated with derivatization of EGFR-free reactive amino groups, which becomes the binding sites for a group of cytoplasmic signalling proteins including phosphatidylinositol 3-kinase (PI-3K). Several studies have confirmed that the EGFR signalling cascade results in the activation of PI-3K/Akt and ERK pathways, which is required for migration and/or proliferation in a variety of cell (Kim et al. 2007).
Evidence from in vivo studies
Our findings using double immunostaining confirms that EGFR is present on intimal monocytes/macrophages, which is consistent with previously published studies (Lamb et al. 2004). Our results clearly demonstrate that periadventitial delivery of EGFR blocking antibody ICR62 into injured carotid arteries of hypercholesterolaemic rabbits inhibits neointimal thickening, which to a large extent can be explained by the reduced accumulation of macrophages in the intima. This could be a consequence of the inhibition of monocyte migration from both the injured endothelium and from the adventitia via the internal elastic lamella and their subsequent proliferation. Various mitogenic factors, such as macrophage colony stimulating factor (M-CSF), products of ox-LDL and EGF have been shown to induce macrophage proliferation in vitro (Biwa et al. 2000; Lamb et al. 2004). We found numerous proliferating cells in the neointimas of saline and isotype matched antibody-treated carotid arteries compared to the ICR62-treated carotids. These results were corroborated by our in vitro adherent macrophage proliferation study, which showed that indeed, ICR62 antibody markedly inhibited EGF-stimulated macrophage proliferation. The most important finding is that the reduced neointimal thickening observed on ligation of the EGFR in the injured carotid arteries was associated with inhibition of inflammation (reduced macrophage infiltration). The fact that no such macrophage infiltration in the contralateral carotid artery was evident suggests that the extensive accumulation of neointimal macrophages in the saline-carotid arteries is an early and critical event and is dependent on intimal thickening induced by balloon-catheter injury in the presence of high blood cholesterol. Thus, it can be inferred that EGFR blockade with ICR62 antibody reduced macrophage accumulation in the carotid lesions and this effect may have been mediated by one or several of the following mechanisms: (i) partly through a direct or indirect inhibition of macrophage infiltration from both the subendothelium space and the adventitia into the neointima, (Yamashita et al. 2008); (ii) through a direct neointima-decreasing effect, whereby only a limited space may be available for the macrophages; (iii) indirectly by the inhibiting the activation of macrophages and reducing the release of potential growth factors within the intima; or (iv) via other mechanism(s). The first possibility is supported by our in vitro migration studies, discussed above in the text.
In addition to reduced neointimal thickening, the antibody may also promote constrictive remodelling of the injured arteries. Our results are in accord with those of Chan et al. (2003), in a rat initmal hyperplasia model, who showed that the vessel area encompassed by the EEL was not affected by EGFR blockade, suggesting that EGFR does not regulate vessel remodelling.
In conclusion, this study has provided the first direct evidence that the monocytes/macrophages EGFR may have an important role in the pathogenesis of atherosclerosis. Therapeutic strategies targeting EGFR may be an attractive and novel approach to the inhibition of macrophage accumulation underlying early events in atherosclerotic plaque formation including plaque rupture. These results also suggest that monoclonal antibodies against EGFR might be a novel therapeutic option in the treatment of post-angioplasty restenosis and vessel wall thickening after vascular manipulation.
Acknowledgments
This work was supported by British Heart Foundation. We would also like to thank Professor Susanna Hourani for proof reading of the manuscript.
References
- Biwa T, Sakai M, Shichiri M, Horiuchi S. Granulocyte/macrophage colony-stimulating factor plays an essential role in oxidized low density lipoprotein-induced macrophage proliferation. J. Atheroscler. Thromb. 2000;7:14–20. doi: 10.5551/jat1994.7.14. [DOI] [PubMed] [Google Scholar]
- Boucher I, Yang L, Mayo C, Klepeis V, Trinkaus-Randall V. Injury and nucleotides induce phosphorylation of epidermal growth factor receptor: MMP and HB-EGF dependent pathway. Exp. Eye Res. 2007;85:130–141. doi: 10.1016/j.exer.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AK, Kalmes A, Hawkins S, Daum G, Clowes AW. Blockade of the epidermal growth factor receptor decreases intimal hyperplasia in balloon-injured rat carotid artery. J. Vasc. Surg. 2003;37:644–649. doi: 10.1067/mva.2003.92. [DOI] [PubMed] [Google Scholar]
- Charo IF, Taubman MB. Chemokines in the pathogenesis of vascular disease. Circ. Res. 2004;95:858–866. doi: 10.1161/01.RES.0000146672.10582.17. [DOI] [PubMed] [Google Scholar]
- Cunningham MP, Thomas H, Fan Z, Modjtahedi H. Responses of human colorectal tumor cells to treatment with the anti-epidermal growth factor receptor monoclonal antibody ICR62 used alone and in combination with the EGFR tyrosine kinase inhibitor gefitinib. Cancer Res. 2006;66:7708–7715. doi: 10.1158/0008-5472.CAN-06-1000. [DOI] [PubMed] [Google Scholar]
- Davies MG, Hagen PO. Pathophysiology of vein graft failure: a review. Eur. J. Vasc. Endovasc. Surg. 1995;9:7–18. doi: 10.1016/s1078-5884(05)80218-7. [DOI] [PubMed] [Google Scholar]
- Dreux AC, Lamb DJ, Modjtahedi H, Ferns GA. The epidermal growth factor receptors and their family of ligands: their putative role in atherogenesis. Atherosclerosis. 2006;186:38–53. doi: 10.1016/j.atherosclerosis.2005.06.038. [DOI] [PubMed] [Google Scholar]
- Edwards IJ, Xu H, Obunike JC, Goldberg IJ, Wagner WD. Differentiated macrophages synthesize a heparan sulfate proteoglycan and an oversulfated chondroitin sulfate proteoglycan that bind lipoprotein lipase. Arterioscler. Thromb. Vasc. Biol. 1995;15:400–409. doi: 10.1161/01.atv.15.3.400. [DOI] [PubMed] [Google Scholar]
- Ferns GA, Sprugel KH, Seifert RA, et al. Relative platelet-derived growth factor receptor subunit expression determines cell migration to different dimeric forms of PDGF. Growth Factors. 1990;3:315–324. doi: 10.3109/08977199009003674. [DOI] [PubMed] [Google Scholar]
- George SJ. Tissue inhibitors of metalloproteinases and metalloproteinases in atherosclerosis. Curr. Opin. Lipidol. 1998;9:413–423. doi: 10.1097/00041433-199810000-00005. [DOI] [PubMed] [Google Scholar]
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- Higashiyama S, Abraham JA, Klagsbrun M. Heparin-binding EGF-like growth factor stimulation of smooth muscle cell migration: dependence on interactions with cell surface heparan sulfate. J. Cell Biol. 1993;122:933–940. doi: 10.1083/jcb.122.4.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmes A, Vesti BR, Daum G, Abraham JA, Clowes AW. Heparin blockade of thrombin-induced smooth muscle cell migration involves inhibition of epidermal growth factor (EGF) receptor transactivation by heparin-binding EGF-like growth factor. Circ. Res. 2000;87:92–98. doi: 10.1161/01.res.87.2.92. [DOI] [PubMed] [Google Scholar]
- Khurana R, Shafi S, Martin J, Zachary I. Vascular endothelial growth factor gene transfer inhibits neointimal macrophage accumulation in hypercholesterolemic rabbits. Arterioscler. Thromb. Vasc. Biol. 2004;24:1074–1080. doi: 10.1161/01.ATV.0000128127.57688.e0. [DOI] [PubMed] [Google Scholar]
- Kim SE, Lee WJ, Choi KY. The PI3 kinase-Akt pathway mediates Wnt3a-induced proliferation. Cell. Signal. 2007;19:511–518. doi: 10.1016/j.cellsig.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Lamb DJ, Modjtahedi H, Plant NJ, Ferns GA. EGF mediates monocyte chemotaxis and macrophage proliferation and EGF receptor is expressed in atherosclerotic plaques. Atherosclerosis. 2004;176:21–26. doi: 10.1016/j.atherosclerosis.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Lamb DJ, Tickner ML, Hourani SM, Ferns GA. Dietary copper supplements modulate aortic superoxide dismutase, nitric oxide and atherosclerosis. Int. J. Exp. Pathol. 2005;86:247–255. doi: 10.1111/j.0959-9673.2005.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagawa J, Higashiyama S, Kawata S, et al. Localization of heparin-binding EGF-like growth factor in the smooth muscle cells and macrophages of human atherosclerotic plaques. J. Clin. Invest. 1995;95:404–411. doi: 10.1172/JCI117669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modjtahedi H, Styles JM, Dean CJ. The human EGF receptor as a target for cancer therapy: six new rat mAbs against the receptor on the breast carcinoma MDA-MB 468. Br. J. Cancer. 1993;67:247–253. doi: 10.1038/bjc.1993.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modjtahedi H, Affleck K, Stubberfield C, Dean C. EGFR blockade by tyrosine kinase inhibitor or monoclonal antibody inhibits growth, directs terminal differentiation and induces apoptosis in the human squamous cell carcinoma HN5. Int. J. Oncol. 1998a;13:335–342. doi: 10.3892/ijo.13.2.335. [DOI] [PubMed] [Google Scholar]
- Modjtahedi H, Komurasaki T, Toyoda H, Dean C. Anti-EGFR monoclonal antibodies which act as EGF, TGF alpha, HB-EGF and BTC antagonists block the binding of epiregulin to EGFR-expressing tumours. Int. J. Cancer. 1998b;75:310–316. doi: 10.1002/(sici)1097-0215(19980119)75:2<310::aid-ijc22>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Mograbi B, Rochet N, Imbert V, et al. Human monocytes express amphiregulin and heregulin growth factors upon activation. Eur. Cytokine Netw. 1997;8:73–81. [PubMed] [Google Scholar]
- Namba K, Nishio M, Mori K, et al. Involvement of ADAM9 in multinucleated giant cell formation of blood monocytes. Cell. Immunol. 2001;213:104–113. doi: 10.1006/cimm.2001.1873. [DOI] [PubMed] [Google Scholar]
- Normanno N, De Luca A, Bianco C, et al. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene. 2006;366:2–16. doi: 10.1016/j.gene.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Panutsopulos D, Arvanitis DL, Tsatsanis C, Papalambros E, Sigala F, Spandidos DA. Expression of heregulin in human coronary atherosclerotic lesions. J. Vasc. Res. 2005;42:463–474. doi: 10.1159/000088100. [DOI] [PubMed] [Google Scholar]
- Raab G, Klagsbrun M. Heparin-binding EGF-like growth factor. Biochim. Biophys. Acta. 1997;1333:F179–F199. doi: 10.1016/s0304-419x(97)00024-3. [DOI] [PubMed] [Google Scholar]
- Reape TJ, Wilson VJ, Kanczler JM, Ward JP, Burnand KG, Thomas CR. Detection and cellular localization of heparin-binding epidermal growth factor-like growth factor mRNA and protein in human atherosclerotic tissue. J. Mol. Cell. Cardiol. 1997;29:1639–1648. doi: 10.1006/jmcc.1997.0399. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Scholes AG, Hagan S, Hiscott P, Damato BE, Grierson I. Overexpression of epidermal growth factor receptor restricted to macrophages in uveal melanoma. Arch. Ophthalmol. 2001;119:373–377. doi: 10.1001/archopht.119.3.373. [DOI] [PubMed] [Google Scholar]
- Suc I, Meilhac O, Lajoie-Mazenc I, et al. Activation of EGF receptor by oxidized LDL. FASEB J. 1998;12:665–671. doi: 10.1096/fasebj.12.9.665. [DOI] [PubMed] [Google Scholar]
- Tamura R, Miyagawa J, Nishida M, et al. Immunohistochemical localization of Betacellulin, a member of epidermal growth factor family, in atherosclerotic plaques of human aorta. Atherosclerosis. 2001;155:413–423. doi: 10.1016/s0021-9150(00)00576-1. [DOI] [PubMed] [Google Scholar]
- Tokumaru S, Higashiyama S, Endo T, et al. Ectodomain shedding of epidermal growth factor receptor ligands is required for keratinocyte migration in cutaneous wound healing. J. Cell Biol. 2000;151:209–220. doi: 10.1083/jcb.151.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita M, Hirata Y, Uchihashi M, Fujita T. Characterization of epidermal growth factor receptors in cultured vascular smooth muscle cells of rat aorta. Endocrinol. Jpn. 1986;33:177–184. doi: 10.1507/endocrj1954.33.177. [DOI] [PubMed] [Google Scholar]
- Trieu VN, Narla RK, Myers DE, Uckun FM. EGF-genistein inhibits neointimal hyperplasia after vascular injury in an experimental restenosis model. J. Cardiovasc. Pharmacol. 2000;35:595–605. doi: 10.1097/00005344-200004000-00013. [DOI] [PubMed] [Google Scholar]
- Yamashita A, Shoji K, Tsuruda T, et al. Medial and adventitial macrophages are associated with expansive atherosclerotic remodeling in rabbit femoral artery. Histol. Histopathol. 2008;23:127–136. doi: 10.14670/HH-23.127. [DOI] [PubMed] [Google Scholar]
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]