Abstract
Regeneration of skeletal muscle following injury is dependent on numerous factors including age, the inflammatory response, revascularization, gene expression of myogenic and growth factors and the activation and proliferation of endogenous progenitor cells. It is our hypothesis that oxidative stress preceding a contusion injury to muscle modulates the inflammatory response to inhibit muscle regeneration and enhance fibrotic scar formation. Male F344/BN rats were assigned to one of four groups. Group 1: uinjured control; Group 2: ischaemic occlusion of femoral vessels for 2 h followed by reperfusion (I-R); Group 3: contusion injury of the tibialis anterior (TA); Group 4: I-R, then contusion injury. The acute inflammatory response (8 h, 3 days) was determined by expression of the chemokine CINC-1, TGF-β1, IFN-γ and markers of neutrophil (myeloperoxidase) and macrophage (CD68) activity and recruitment. Acute oxidative stress caused by I-R and/or contusion, was determined by measuring GP91phox and lipid peroxidation. Muscle recovery (21 days) was assessed by examining the fibrosis after I-R and contusion injuries to the TA with Sirius Red staining and quantification of collagen I expression. Consistent with our hypothesis, I-R preceding contusion increased all markers of the acute inflammatory response and oxidative stress after injury and elevated the expression of collagen. We conclude that ischaemia-induced oxidative stress exacerbated the inflammatory response and enhanced fibrotic scar tissue formation after injury. This response may be attributable to increased levels of TGF-β1 and diminished expression of IFN-γ in the ischaemic contused muscle.
Keywords: chemokines, inflammation, myeloperoxidase, scar formation, TGF-β
Damage to skeletal muscle can be a common occurrence after workplace injuries, sports injuries, motor vehicle accidents or surgery. Under ideal conditions, skeletal muscle is capable of extensive muscle regeneration after several types of injury including strains, contusions and lacerations (Crisco et al. 1994; Menetrey et al. 1999; Malm et al. 2004). Regeneration of skeletal muscle or conversely, the formation of a fibrotic scar following injury, is dependent on numerous factors including type and severity of injury, age, oxidative stress, revascularization of the tissue and gene expression of several myogenic and growth factors (Charge & Rudnicki 2004). The relative activation and proliferation of two cell types: endogenous muscle progenitor cells and fibroblasts may be the determining variable in deciding the ultimate fate of the injured skeletal muscle (Conboy et al. 2003; Charge & Rudnicki 2004; Ehrhardt & Morgan 2005). Muscle progenitor cells (MPC, satellite cells) become myoblasts and repair muscle by fusing with injured, but surviving myofibres or by de novo formation of new myotubes that regenerate lost myofibres. After injury, fibroblasts proliferate in the damaged muscle and begin to produce a collagen-rich extracellular matrix to restore the muscle’s connective tissue framework (Lehto et al. 1986; Menetrey et al. 1999; Li & Huard 2002). Activated fibroblasts also release chemoattractants which recruit additional fibroblasts and inflammatory cells to the injured tissue (Kovacs & DiPietro 1994; Toumi & Best 2003). Excess proliferation and activation of fibroblasts can lead to extensive fibrosis and formation of dense scar tissue, which can obstruct the muscle regenerative process and result in incomplete recovery (Lehto et al. 1986; Kasemkijwattana et al. 1998; Menetrey et al. 1999).
It is the hypothesis of this project that after muscle injury, factors affecting the inflammatory response may also regulate fibrotic scar formation. To test this hypothesis, ischaemia–reperfusion-induced oxidative stress will be used to modulate the conditions affecting the inflammatory response. Ischaemia–reperfusion (I-R) was chosen as a stressor because I-R is known to generate reactive oxygen and nitrogen species (RONS) and to activate inflammatory cells (Pattwell et al. 2003; Huda et al. 2004; Zimiani et al. 2005). Oxidative stress also increases the expression of proinflammatory mediators such as cytokines, chemokines and adhesion molecules. In response, first neutrophils and then macrophages infiltrate the tissue which leads to further production of RONS, cytokines, chemokines and cytotoxic proteases by these leukocytes and exacerbation of the inflammatory cascade (Taoka et al. 1995; Prisk & Huard, 2003; Aoi et al. 2004). In addition, activated macrophages produce transforming growth factor (TGF)-β1 that induces fibrosis after injury or disease in a variety of tissues (Lagord et al. 2002; Flanders 2004; Kuusniemi et al. 2005; Nieto & Cederbaum 2005; Izumi et al. 2006). Since oxidative stress occurs in aging tissues (Lass et al. 1998; Wickens 2001) or during intense anaerobic exercise (Ulloa et al. 1999; Cuevas et al. 2005), regulation of inflammation in the presence of oxidative stress has implications for recovery from muscle injuries in the elderly and in athletes.
In the experiments described in this paper, I-R was used to produce an oxidative environment to determine its subsequent effects on the inflammatory response and histological recovery of muscle following injury. It is predicted that I-R and subsequently increased oxidative free radicals, will enhance TGF-β1-mediated scar formation after injury. It is hypothesized that: (i) 2 h of ischaemia followed by 2 h of reperfusion is sufficient to cause an increase in RONS and oxidative stress in skeletal muscle and exacerbate the inflammatory response to contusion injury; and (ii) I-R occurring prior to a contusion injury will result in increased collagen levels and fibrotic scar tissue formation in the skeletal muscle.
Materials and methods
Animals
Specific pathogen-free Fischer 344/Brown Norway (F344/BN) F1 hybrid male rats, aged 2 months, were obtained (Harlan, Indianapolis, IN, USA). The animals were housed two per microisolator cage and maintained in a temperature- and light-controlled room. All animal procedures were performed according to the Canadian Council on Animal Care (CCAC) Guide to the Care and Use of Experimental Animals (Olfert et al. 1993). The animals were assigned to one of the following four groups: (a) uninjured controls; (b) I-R; (c) contusion injury of the tibialis anterior (TA); and (d) I-R followed by contusion injury of the TA. For each group, the injuries were bilateral and individual muscles were used for either histology or biochemistry. All animals were anesthetized by inhalation of isoflurane gas (4%). Animals were given 0.1 ml daily injections of Enrofloxacin (Baytril, 50 mg/ml; Bayer, Pittsburgh, PA, USA) subcutaneously for 3 days to prevent infection after surgery and individually housed in clean microisolator cages.
I-R and contusion injury
Ischaemia of the lower limb musculature, including the TA, was induced by occluding the femoral artery using a small microvascular clamp (B2-V; S&T, Fine Science Tools, Vancouver, BC, Canada) for 2 h followed by reperfusion. If the animal was to receive a contusion injury in addition to I-R, it was performed after 2 h of reperfusion. An incision was made in the skin overlying the anterior compartment of the lower limb and the TA muscle was separated from the extensor digitorum longus using a fine pair of scissors. A Yasargil Phynox aneurysm clip (FE 623 K; Center Valley, PA, USA) with a closing force of 200 g was then placed around the TA muscle for 20 s to cause a compression contusion injury. This contusion injury was adapted from a similar method used commonly on the spinal cord (Weaver et al. 2001). Animals assigned to the uninjured control group were anesthetized and received a 1.0 cm incision in the skin overlying the TA muscle. All incisions in the skin were closed with silk suture.
Muscle harvesting and preparation
The animals were euthanized at 8 h (n=8 per group) and 3 days (n=8 per group) postinjury to examine oxidative stress and acute inflammation. At 21 days postinjury, rats (n=8 per group) were euthanized to examine chronic fibrosis in the muscle. The rats were euthanized with an intraperitoneal injection of 25% urethane in saline (3.0 ml) and the entire TA muscle was immediately harvested from each limb and frozen in liquid nitrogen-cooled isopentane. If a muscle was to be sectioned for histological purposes, it was frozen in a 2:1 mixture of optimal cutting temperature (OCT) compound (Tissue-Tek 4583; Sakura Finetek Inc., Torrence, CA, USA) and 20% sucrose in Phosphate Buffered Saline (PBS). The muscle samples were kept at −80 °C until further analysis.
Tissue homogenization
The TA muscle was crushed to a powder with a liquid nitrogen-cooled mortar and pestle and sonicated in 10 volumes of MPO buffer [25 mM potassium phosphate buffer (pH 6), 0.25% hexadecyltrimethyl ammonium bromide, 10 μg/ml aprotinin, 0.5 mM dithiothreitol (DTT), Complete EDTA-free protease inhibitor cocktail tablet (Roche, Mississauga, ON, Canada)] and centrifuged at 10,000 g for 20 min at 4 °C (Nakauchi et al. 1996; Bao et al. 2004;). The supernatant (cytosolic protein fraction) was collected and the pellet was resuspended on ice with a sonicator in 200 μl of membrane lysis buffer (pH 7.6) [100 mM potassium chloride (KCl), 10 mM sodium phosphate (Na2HPO4), 1 mM ethylenediamine tetraacetic acid (EDTA), 1% Triton-X 100, 0.1% sodium dodecyl sulphate (SDS), 0.5% sodium deoxycholate, 10 μg/ml aprotinin, 0.5 mM dithiothreitol (DTT), complete EDTA-free protease inhibitor cocktail] and centrifuged at 20,000 g for 30 min at 4 °C. The supernatant (membranous protein fraction) was collected and the pellet was discarded. Cytosolic and membranous protein fractions were assayed for total protein concentration using the BCA Protein Assay (Pierce, Rockford, IL, USA) and stored at −80 °C until needed.
Western blot analysis
For Western blot analyses of GP91phox, CD68, and TGF-β1, 40 μg of total protein from TA muscle homogenates was separated by SDS polyacrylamide gel electrophoresis (SDS-PAGE) followed by transfer to polyvinylidene fluoride (PVDF) membranes. Standard Western blotting techniques were used using Tris-Buffered Saline with 0.1% Tween-20 (TBS-T) for wash steps and 5% non-fat milk in TBS-T for blocking. Primary antibodies used were specific to GP91phox (1:500; 611414, BD Biosciences, Mississauga, ON, Canada), CD68 (1:500; MCA 341R, Serotec via Cedarlane Laboratories, Hornby, ON, Canada) and TGF-β1 (1:1000; LC-1-30.1 Dr. Kathy Flanders Laboratory, NCI/NIH, Bethesda, MD, USA). Measurement of TGF-β1 by Western blot with this antibody will determine total TGF-β1 that includes both latent and active forms of the protein. After incubation in anti-mouse IgG-, or anti-rabbit IgG-conjugated horseradish peroxidase (HRP) (1:15,000; Jackson Immunoresearch Laboratories, West Grove, PA, USA). The protein was detected using an ECL chemiluminescence kit (Amersham, Piscataway, NJ, USA), exposed to film and then quantified as a percentage of controls using an Alpha Imager 2200 and software (Alpha Innotech, San Leandro, CA, USA). Membranes were then stripped and re-probed with anti-glyceraldehyde phosphate dehydrogenase (GAPDH) (1:10,000; AB9485; Abcam, Cambridge, MA, USA) as a loading control.
Lipid peroxidation (TBARS) assay
The membranous protein fraction of the TA muscle homogenates were used to measure lipid peroxidation with a colorimetric assay. Lipid peroxidation was quantitated using a thiobarbituric acid reactive substances (TBARS) assay with malondialdehyde (MDA) standards (Bao et al. 2004; Dawn-Linsley et al. 2005). Samples and standards (25 μl) were mixed with 25 μl of 8.1% SDS, 200 μl of 20% acetic acid (pH 3.5), 200 μl of 0.8% thiobarbituric acid (TBA) in 50% acetic acid (pH 3.5), 50 μl of distilled water, and then heated for 1 h at 95 °C. Following heating, 250 μl of a 15:1 mixture of n-butanol and pyridine were added to the mixture and samples were vortexed and centrifuged for 10 min at 4000 rpm. Two hundred microlitres of the organic layer was pipetted in duplicates on 96 well microplates with MDA standards and the absorbance was measured at 532 nm using a Synergy HT universal plate reader with KC4 software (Bio-Tek Instruments, Winooski, VT, USA).
Myeloperoxidase assay
The cytosolic protein fraction of the TA muscle homogenates were used to measure myeloperoxidase (MPO) activity with a colorimetric assay (Taoka et al. 1997). Samples and standards (20 μl) were pipetted in duplicates onto 96-well plates and 200 μl of substrate solution (0.167 mg/ml o-dianisidine hydrochloride, 0.24 mM hydrogen peroxide (H2O2), 10 mM potassium phosphate buffer) was added to initiate the reaction. The change in absorbance was measured over 5 min at 450 nm using a plate reader (described above).
Enzyme-linked immunosorbant assays
IFN-γ and CINC-1 levels were measured in the cytosolic protein fraction of TA muscle homogenates using enzyme-linked immunosorbant assay (ELISA) kits from R&D Systems (IFN-γ DY585; CINC-1 DY515) (Minneapolis, MN, USA). Both ELISA kits used horse radish peroxidase (HRP)-conjugated antibodies with a 1:1 mixture of 0.01% hydrogen peroxide (H2O2) and tetramethylbenzidine (TMB) as the substrate. Plates were read at 450 nm with correction at 540 nm on a plate reader (described above).
Glutathione and glutathione disulfide assay
The ratio of glutathione (GSH) and glutathione disulfide (GSSG) is a widely-used indicator of oxidative stress and the cellular redox environment in tissues (Schafer & Buettner 2001; Shi & Liu 2006). The GSH and GSSG were measured with a glutathione assay kit (Cayman Chemical, Cat. #703002) from approximately 100 mg of muscle tissue homogenized in the buffer provided. The GSH:GSSG ratio was calculated according to the manufacturer’s instructions.
Immunohistochemistry
The TA muscles were embedded in OCT/sucrose, frozen in liquid nitrogen and sectioned (40 μm) on a cryostat. Thaw-mounted sections were fixed for 10 min in −20 °C acetone, washed in PBS and then placed in 4% formaldehyde for 2 min. The sections were incubated with 5% normal goat serum (NGS) in TBS with 0.1% Triton-X 100 (TBS-X) for 1 h and probed overnight at room temperature with anti-collagen I (AB6038; Abcam, Cambridge, MA, USA). Protein expression was revealed after incubation with Alexa-Fluor 568 conjugated secondary antibody (1:300; A11004, Molecular Probes/Invitrogen, Burlington, ON, Canada). Images were captured on a Zeiss Axioplan II microscope equipped with Axiocam HRC camera and software. The intensity and integrated area of immunoreactive staining was quantified on inverted grayscale images using Image Pro Discovery software (MediaCybernetics, Silver Spring, MD, USA). Approximately 10 images per muscle were quantified and then averaged.
Histology
Cryostat sections of TA were fixed in Bouin’s Fixative and processed for Hematoxylin and Eosin (H&E) and Sirius Red staining to examine morphology and connective tissue, respectively, in the uninjured and injured muscles (Kiernan 1999).
Statistical analysis
An analysis of variance (anova) was used to determine whether significant differences occurred at the P<0.05 level using SPSS statistical software. Student–Neuman–Keuls post hoc comparisons were used to assess significant differences relative to uninjured controls and among experimental groups.
Results
Oxidative stress
To determine the level of oxidative stress in our muscle samples we measured the ratio of the reduced (GSH) to the oxidized (GSSG) forms of glutathione (Shi & Liu 2006), lipid peroxidation and quantified expression of the marker protein, GP91phox at 8 h and 3 days postinjury. GP91phox, together with P21phox, form an integral membrane cytochrome b558 which interacts with various cytosolic proteins (P67 phox, P47 phox, P40 phox, rac2) to produce RONS (Javesghani et al. 2002). The results are presented in Figures 1 and 2.
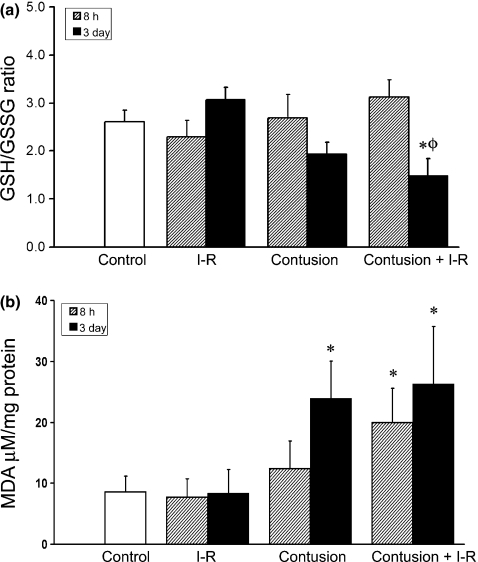
Figure 1.
(a) The ratio of reduced (GSH) to oxidized (GSSG) forms of glutathione in ischaemia–reperfused (I-R) and contused muscle is illustrated at 8 h and 3 days postinjury. A decrease in GSH:GSSG indicates elevated oxidative stress. (b) Lipid peroxidation in I-R and contused muscle. Lipid peroxidation was quantified using the TBARS assay to measure levels of malondialdehyde (MDA). * denotes a significant difference relative to uninjured control, P<0.05. ø denotes a significant difference from earlier time point within the same experimental group, P<0.05. ψ denotes a significant difference between contusion and IR-contusion at same time point, P<0.05. Error bars represent the SE of the mean (SEM, n=8 per group).
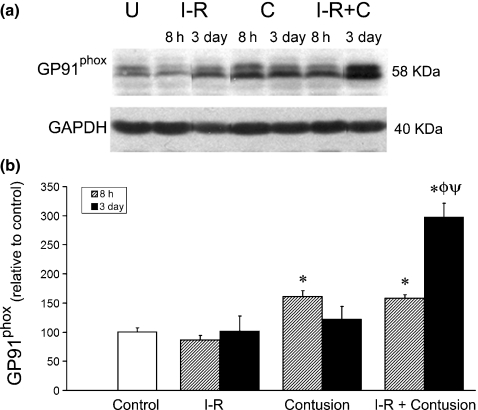
Figure 2.
(a) Representative samples at 3 days postinjury quantified by Western blot analysis for GP91phox. The protein glyceraldehyde phosphate dehydrogenase (GAPDH) was used as a loading control. Samples were all from the same gel, but cut with Adobe Photoshop for appropriate presentation. Photoshop editing was performed to all samples equally. (b) Relative GP91phox levels in control, ischaemia–reperfused and clip-injured muscle at 8 h (dimpled fill) and 3 days postinjury as determined by densitometry. * denotes a significant difference from control, P<0.05. ø denotes a significant difference earlier time point within the same experimental group, P<0.05. ψ denotes a significant difference between contusion and IR-contusion at same time point, P<0.05. Error bars represent the SE of the mean (SEM, n=8 per group).
The enzyme glutathione peroxidase uses GSH as a cofactor as it metabolizes hydrogen peroxide to oxygen and water. When levels of hydrogen peroxide are high, glutathione peroxidase activity depletes GSH and is unable to metabolize all the hydrogen peroxide. Excess hydrogen peroxide then can react with metal ions to become highly reactive hydroxyl radical. Therefore, a low GSH:GSSG ratio infers elevated levels of oxidative stress. Compared to uninjured control muscles the ratio of GSH:GSSG was unaltered 8 h or 3 days after I-R or muscle contusion injury. In contrast, when ischaemia preceded contusion, the GSG:GSSG ratio was significantly decreased 3 days after injury (Figure 1a).
Lipid peroxidation was measured using an assay for malondialdehyde (MDA) content. The MDA assay measured a significant increase in lipid peroxidation at 8 h only when the contusion injury was preceded by I-R (19.9 ± 5.6 μM/mg) compared to uninjured control muscle (7.9 ± 2.5 μM/mg). Three days after injury, the lipid peroxidation was further increased in the contused (23.8 ± 6.2 μM/mg) and I-R contused (26.2 ± 9.5 μM/mg) TA muscle homogenates compared to the homogenate of uninjured control TA (Figure 1b).
GP91phox levels were measured relative to uninjured control TA muscles at 8 h and 3 days postinjury. The GP91phox levels were elevated in TA muscle homogenates 8 h after contusion alone or after ischaemia and contusion combined relative to uninjured control. At 3 days post injury, the levels of GP91phox were similar among uninjured controls, ischaemia–reperfused (I-R) muscle and muscle after contusion. However, when I-R preceded the contusion there was a greater level of GP91phox in the TA muscle samples (Figure 2).
Inflammation
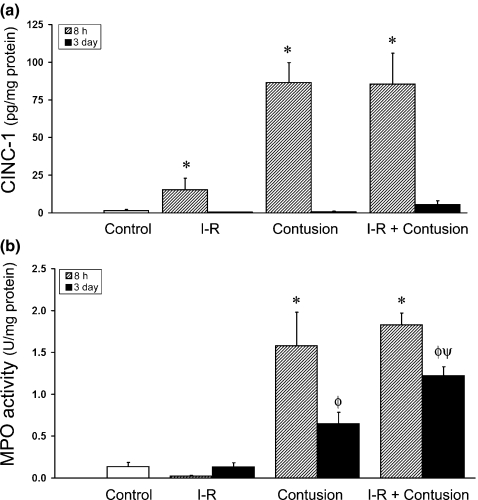
Neutrophil recruitment and oxidative activity was measured using assays for the concentration of cytokine-induced neutrophil chemoattractant-1 (CINC-1; rat homolog of Il-8) and for myeloperoxidase (MPO) activity at 8 h and 3 days following injury. The results of these independent assays are shown in Figure 3. At 8 h postinjury, there was a significant increase in CINC-1 concentration after I-R (15.2 ± 7.8 pg/mg), after contusion (86.5 ± 13.2 pg/mg muscle) and after I-R combined with contusion (85.4 ± 20.5 pg/mg) when compared to uninjured muscle (1.8 ± 0.8 pg/mg). By the third day after the injury, the level of CINC-1 had decreased in all groups that had received an insult (Figure 3a).
Figure 3.
(a) The concentration of CINC-1 in ischaemia–reperfused (I-R) and contused muscle at 8 h and 3 days postinjury. CINC-1 concentration was quantified by ELISA. (b) Myeloperoxidase (MPO) activity in I-R and contused muscle after injury. MPO activity was quantified by a kinetic enzymatic assay. * denotes a significant difference from control, P<0.05. ø denotes a significant difference from earlier time point within the same experimental group, P<0.05. ψ denotes a significant difference between contusion and IR-contusion at same time point, P<0.05. Error bars represent the SE of the mean (SEM, n=8 per group).
When MPO activity was determined, similar results were observed. At 8 h postinjury, MPO levels were significantly greater after contusion (1.58 ± 0.40 U/mg) and when ischaemia preceded contusion (1.83 ± 0.14 U/mg) when compared to ischaemic (I-R) muscle (0.02 ± 0.01 U/mg) or uninjured control muscle homogenates (0.12 ± 0.05 U/mg). The level of MPO activity decreased in both the contused muscle and I-R contused muscle by the third day, but still remained greater than uninjured control muscle. However, 3 days postinjury, MPO levels remained elevated to a greater extent in ischaemic-contused muscles (1.22 ± 0.10 U/mg) than MPO levels after contusion alone (0.65 ± 0.13 U/mg) (Figure 3b). At this time point, oxidative activity of macrophages may be contributing to the activity of persisting neutrophils in the MPO assay.
Macrophage infiltration
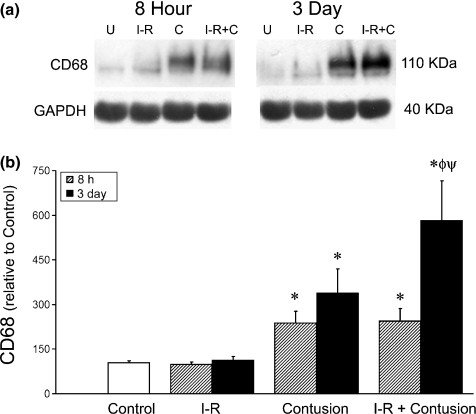
CD68 is a lysosomal protein found in macrophages. CD68 protein levels were used to assess the extent of macrophage infiltration into the TA muscle following injury. Western blot analysis of TA muscle homogenates at 8 h and 3 days postinjury are shown in Figure 4. At 8 h postinjury there was a significant increase in the levels of CD68 in contusion and I-R contusion groups when compared to uninjured controls. At 3 days, macrophage content had increased in the contused muscles, but this increase occurred to a greater extent when ischaemia (I-R) was combined with contusion injury. The infiltration of monocytes 3 days postinjury was also readily apparent in haematoxylin and eosin stained sections of muscle tissue (Figure 6). The dark purple-stained nuclei were more abundant after contusion compared to ischaemic (I-R) or uninjured muscles. The combination of ischaemia and contusion appeared to cause the most infiltration of monocytes into the skeletal muscle tissue (Figures 4 and 6).
Figure 4.
(a) Representative samples at 8 h and 3 days postinjury quantified by Western blot analysis for CD68. (b) Relative CD68 levels in ischaemia–reperfused and clip-injured muscle at 8 h and 3 days postinjury measured by densitometry. * denotes a significant difference from control, P<0.05. ø denotes a significant difference from control and earlier time point within the same experimental group, P<0.05. ψ denotes a significant difference between contusion and IR-contusion at same time point, P<0.05. Error bars represent the SE of the mean (SEM, n=8 per group).
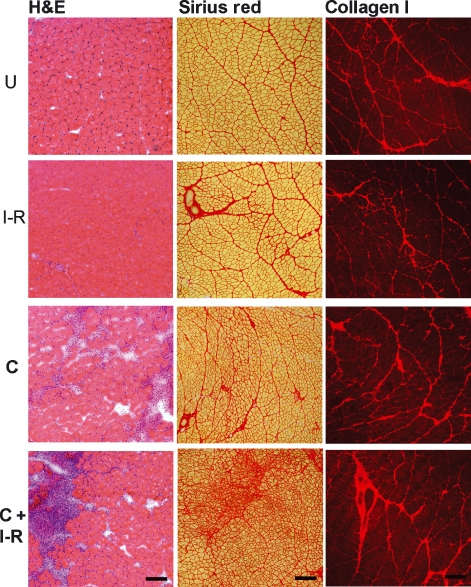
Figure 6.
Muscle morphology and collagen expression in uninjured and injured tibialis anterior muscle cross-sections. Panel (a) Hematoxylin and eosin staining of ischaemia–reperfused (I-R), contusion (C) and I-R and contusion combined (C+I-R) muscle cross-sections at 3 days postinjury compared with uninjured controls (U). Scale Bar = 100 μm. Panel (b) Sirius Red staining of ischaemia–reperfused (I-R), contusion (C) and I-R and contusion combined (C+I-R) muscle cross-sections at 3 days postinjury compared with uninjured controls (U). Scale Bar = 100 μm. Panel (c). Representative images illustrating immunoreactivity for collagen I expression in uninjured, ischaemia–reperfused (I-R), contused (C) and ischaemic-contused (C+I-R) muscles. Scale Bar = 200 μm.
Fibrotic response
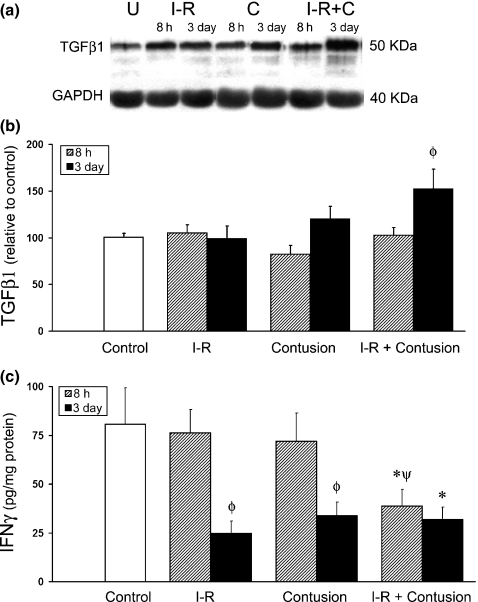
TGF-β1 is produced by macrophages during inflammation and is a known mediator of fibrosis (Flanders 2004; Nieto & Cederbaum 2005). As shown in Figure 5b, quantification of total TGF-β1 at 3 days postinjury by Western blot analysis revealed a significant increase in TGF-β1 when the contusion injury was preceded by I-R (152 ± 21%) compared to uninjured control muscle (100 ± 4%). In contrast, TGF-β1 levels were similar to uninjured control muscle 3 days after either I-R (99 ± 14%) or contusion injury (120 ± 13%). At 8 h postinjury, TGF-β1 levels in all groups were similar to that of uninjured control muscle (Figure 5b).
Figure 5.
Expression of TGF-β1 and IFN-γ in injured and control tibialis anterior (TA) muscle homogenates. (a) Representative samples at 8 h postinjury quantified by Western blot analysis for TGF-β1. Samples were all from the same gel, but cut with Adobe Photoshop for appropriate presentation. Photoshop editing was performed to all samples equally. (b) Relative TGF-β1 levels in ischaemia–reperfused (I-R) and contused muscle at 8 h and 3 days postinjury were determined by densitometry of Western blots and averages from each group are displayed in centre panel. (c) IFN-γ concentration (pg/mg total protein) was quantified by ELISA in TA muscles after I-R, contusion and I-R combined with contusion and compared to uninjured controls. * denotes a significant difference from control, P<0.05. ø denotes a significant difference from earlier time point within the same experimental group, P<0.05. ψ denotes a significant difference between contusion and IR-contusion at same time point, P<0.05. Error bars represent the SE of the mean (SEM, n=8 per group).
IFN-γ concentrations, as measured by ELISA, are shown in Figure 5c. A significant decrease in IFN-γ concentration was observed in ischaemic (I-R) contused muscle (39 ± 8 pg/mg) at 8 h postinjury when compared to uninjured control muscle (84 ± 18 pg/mg). In contrast, IFN-γ levels in the I-R muscle or contused muscle, were both similar to the uninjured control muscle. By 3 days postinjury, IFN-γ levels were significantly decreased in all injury groups relative to uninjured control muscle.
Connective tissue
Sirius Red staining was used to visualize muscle morphology and the distribution of connective tissue in the muscle 21 days after injury. With this stain, muscle tissue is stained pale yellow and collagen fibres are stained dark red (Figure 6). Cross-sections of uninjured and ischaemic (I-R) TA muscles revealed similar morphology, whereas contused TA muscles appeared to contain an increase in fibrotic tissue and a dissolution of muscle fibre morphology. When ischaemia was combined with contusion, there was a dramatic increase in collagen deposition and fibrotic connective tissue. There also appeared to be excessive tissue destruction and several damaged muscle fibres.
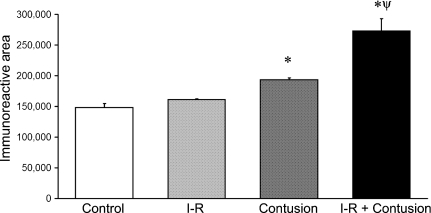
The results observed after immunohistochemistry for collagen I expression are also illustrated in Figure 6. The specific immunohistochemistry for type I collagen in the uninjured, ischaemic, contused and ischaemic-contused TA muscles was similar to the staining with Sirius Red. At 21 days postinjury, computer-aided image analysis of collagen I expression in muscle revealed a significant increase in collagen I expression. Collagen I expression was increased in contused TA muscles. This increase in collagen I expression was enhanced by ischaemia (Figure 7).
Figure 7.
Quantification of collagen I immunoreactivity in ischaemia–reperfused (I-R) and contused muscle at 21 days postinjury. Representative images are illustrated in Figure 9. * denotes a significant difference from control, P<0.05. ø denotes a significant difference from earlier time point within the same experimental group, P<0.05. ψ denotes a significant difference between contusion and IR-contusion at same time point, P<0.05. Error bars represent the SE of the mean (SEM, n=6 per group).
Discussion
Infiltration and activation of inflammatory cells are important factors in the response of muscle to injury and are modulated by TGF-β1 and the cycoloxygenase (COX)-2 pathway (Bondesen et al. 2004; Shen et al. 2008). Muscle regeneration has been shown to be diminished after depletion of macrophages by clodronate liposomes (Shen et al. 2008), by specific pharmacological inhibition of COX-2 and in COX-2 null mice (Bondesen et al. 2004; Shen et al. 2005). These results highlight the importance of sufficient inflammation-derived macrophage infiltration and TGF-β1-induced activation of COX-2 pathways in muscle growth and regeneration. However, if excessive, the phagocytic activities of neutrophils and macrophages after injury are not benign. The infiltration and activation of neutrophils are especially cytotoxic and have been linked to secondary tissue damage (Tonai et al. 2001; Pizza et al. 2005). The balance between beneficial inflammation that facilitates muscle regeneration and deleterious inflammation that inhibits muscle regeneration and promotes fibrotic scar formation, may depend on the local environment and presence of oxidative stress. Oxidative stress prior to or during inflammation, enhances the production of proinflammatory cytokines and cytotoxic free radicals, including RONS, by neutrophils and macrophages (Bartnik et al. 2000; Lu & Wahl 2005).
A model of transient I-R was used in this study to increase oxidative stress and to modulate the inflammatory response prior to contusion injury of the TA muscle. The I-R paradigm used has been shown previously to produce RONS and elevate oxidative stress without causing any significant damage to the muscle (Sayan et al. 2001; Hatoko et al. 2002; Dupouy et al. 2006). If the ischaemia is prolonged more than 2 h, significant morphological damage to the muscle has been shown to occur (Sayan et al. 2001; Hatoko et al. 2002; Dupouy et al. 2006). Interestingly, brief ischaemic preconditioning has been shown to minimize structural damage of skeletal muscle to subsequent prolonged ischaemia (Bushell et al. 2002) by a mechanism that may included reduced production of RONS (Vanden Hoek et al. 2000). The purpose of this study was to compare the inflammatory response and fibrotic scar formation in the TA muscle among control, I-R, contused and I-R contused muscles. The results obtained from this study suggest that I-R prior to contusion modulated the muscle’s regenerative response to trauma, such that inflammation and fibrosis were more robust. Sirius Red staining and the deposition of type I collagen, indicate that fibrotic scar formation has occurred to a greater extent after ischaemia and contusion, than after contusion injury alone (Figures 6 and 7).
Acutely after muscle injury, TGF-β1 is an important modulator of inflammation and macrophage activity (Shen et al. 2008). Chronic, elevated TGF-β1 expression is a potent mediator of fibrosis via receptor-mediated Smad signalling pathways (Dennler et al. 2002; Roberts et al. 2003). The increased content of total TGF-β1 in ischaemic contused muscle 3 days postinjury (Figure 5b) is likely attributable to macrophage activation and recruitment to the injury site (McTigue et al. 2000). Macrophages are a primary source of TGF-β1 and we observed that macrophage content was greatest in the I-R contused muscle 3 days postinjury (Figure 4). Overproduction of TGF-β1 is a major cause of fibrosis after injury or disease in various tissues and is increased after injury in regenerating muscle (Kovacs & DiPietro 1994; Logan et al. 1999; Lijnen et al. 2000; Foster et al. 2003; Flanders 2004; Venkatesan et al. 2004). Although there was a significant increase in TGF-β1 following injury to the I-R muscle, it was surprising to observe little change in TGF-β1 levels following injury alone. Differentiating between total and active forms of TGF-β1 present in the TA muscles of each experimental group may have provided additional, more relevant information for comparison.
TGF-β1 stimulates fibroblasts to increase synthesis of collagen, vimentin, proteoglycans, fibronectin and other extracellular matrix components which lead to an overall increase in fibrotic scar tissue (Bassols & Massague 1988; Ritzenthaler et al. 1993; Carey & Zehner 1995). In addition, TGF-β1 has also been shown to inhibit the regenerative process of skeletal muscle both in vitro and in vivo (Brennan et al. 1991; Liu et al. 2001; Li et al. 2004; Zhu et al. 2004). In cultures of C2C12 myoblasts, TGF-β1 was shown to increase proliferation, but diminish MyoD and myogenin levels and subsequently reduce terminal differentiation and myosin heavy chain expression (Schabort et al. 2009). In addition, TGF-β1 has been suggested to convert myoblasts and muscle-derived stem cells into a fibroblast lineage that eventually contributes to the development of fibrosis. Furthermore, inhibiting, or disrupting TGF-β1 signal transduction 1–2 weeks after injury, has been associated with reduced fibrosis and enhanced muscle regeneration (Foster et al. 2003; Sato et al. 2003). In the current study, increased collagen content in the TA muscle 21 days following combined ischaemia and contusion injury is associated with increased levels of total TGF-β1 (Figure 5b and 7).
It has been shown in previous studies that IFN-γ can act as an anti-fibrosis agent, reducing scar tissue to facilitate improved muscle functional recovery (Fukushima et al. 2001; Foster et al. 2003; Prisk & Huard 2003). In this study, IFN-γ levels were significantly diminished in I-R contusion TA muscles (Figure 8). The anti-fibrotic actions of IFN-γ reportedly occur within the TGF-β1 signalling pathway and increase the production of Smad 7 (Ulloa et al. 1999). Smad 7 inhibits the nuclear translocation of Smad 3, which is an important transcription factor associated with transcription with extracellular matrix- and fibrosis-related genes (Ulloa et al. 1999; Flanders 2004). In this study, a decrease in IFN-γ in injured muscle was correlated with TGF-β1-mediated fibrosis that occurs in the I-R contused TA muscle. Indeed, it has been reported that exogenous IFN-γ injected into injured muscle inhibits TGF-β-mediated fibrosis (Foster et al. 2003). These acute changes in the balance of TGF-β1 to IFN-γ levels, ultimately lead to increased type I collagen and vimentin in the recovering muscle which form a fibrotic scar capable of impeding regeneration.
We hypothesized that 2 h of I-R would increase oxidative stress in the TA muscle and exacerbate the inflammatory response to contusion injury. A 2 h I-R paradigm was selected to be sufficient to elevate oxidative stress (Buttemeyer et al. 2002), but not to induce inflammation or tissue damage on its own (Hatoko et al. 2002). We found this to be only partly true as an overt increase in oxidative stress (GSH:GSSG ratio, GP91phox) was not observed after I-R alone (Figures 1 and 2). However, the I-R paradigm was effective as it elevated Cinc-1 (Figure 3a) and exacerbated inflammation in ischaemic-contused muscle (Figures 3 and 4). This effect was not evident acutely (8 h), but was evident 3 days after muscle contusion injury. This time course suggests that neutrophil activity may have created the environment, but it was recruitment and activation of macrophages that was required for elevated oxidative stress after injury. The CINC-1 and CD68 data all indicate that by 3 days, recruitment of neutrophils is negligible and that macrophages are the dominant population. At 3 days postinjury, the MPO assay is measuring phagocytic activity of neutrophils and macrophages. The elevated MPO observed in ischaemic-contused muscle is attributable to increased macrophage content and activity. The delay in observing the I-R effect on our markers of oxidative stress, while possibly attributable to macrophage activity, may also be due to the necessary time for the activation of signal transduction pathways by RONS and oxidative stress (Hensley et al. 2000) and the subsequent transactivation of nuclear factor NFκB (Meyer et al. 1993; Christman et al. 2000; Kefaloyianni et al. 2006). NFκB is a transcription factor that has been implicated in stimulating pro-inflammatory gene expression and modulating subsequent inflammatory response and muscle recovery from injury (Callejas et al. 2003; Cuevas et al. 2005; Hnia et al. 2008).
The main finding of this study is that I-R of skeletal muscle is capable of exacerbating inflammation and fibrosis after contusion injury. This is true despite a lack of a significant increase in levels of oxidative stress measured in the muscle after I-R. In this study, when ischaemic muscle was subjected to a contusion injury, oxidative stress and expression of neutrophil chemoattractant was enhanced and leukocyte infiltration increased resulting in a positive feedback loop that ultimately exacerbated the inflammatory response to the injury. Consequently, greater amounts of TGF-β1 are released from macrophages and the production of collagen, vimentin and other fibrotic proteins is stimulated in the recovering muscle. There are implications of these findings for sports-related injuries or performing surgery involving skeletal muscle and ischaemia. If ischaemic or hypoxic, conditions exist in the muscle prior to, or at the time of injury, then the risk of poor muscle regeneration and enhanced scar formation is significant.
Acknowledgments
This research was funded by the Natural Sciences and Engineering Research Council of Canada. A.G. was supported by a studentship from the Nova Scotia Health and Research Foundation. We wish to thank Emily Alexander, Paula Mackie and Melanie Wilcox for their excellent technical assistance.
References
- Aoi W, Naito Y, Takanami Y, et al. Oxidative stress and delayed-onset muscle damage after exercise. Free Radic. Biol. Med. 2004;37:480–487. doi: 10.1016/j.freeradbiomed.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Bao F, Chen Y, Dekaban GA, Weaver LC. Early anti-inflammatory treatment reduces lipid peroxidation and protein nitration after spinal cord injury in rats. J. Neurochem. 2004;88:1335–1344. doi: 10.1046/j.1471-4159.2003.02240.x. [DOI] [PubMed] [Google Scholar]
- Bartnik BL, Juurlink BH, Devon RM. Macrophages: their myelinotrophic or neurotoxic actions depend upon tissue oxidative stress. Mult. Scler. 2000;6:37–42. doi: 10.1177/135245850000600108. [DOI] [PubMed] [Google Scholar]
- Bassols A, Massague J. Transforming growth factor beta regulates the expression and structure of extracellular matrix chondroitin/dermatan sulfate proteoglycans. J. Biol. Chem. 1988;263:3039–3045. [PubMed] [Google Scholar]
- Bondesen BA, Mills ST, Kegley KM, Pavlath GK. The COX-2 pathway is essential during early stages of skeletal muscle regeneration. Am. J. Physiol. Cell. Physiol. 2004;287:C475–C483. doi: 10.1152/ajpcell.00088.2004. [DOI] [PubMed] [Google Scholar]
- Brennan TJ, Edmondson DG, Li L, Olson EN. Transforming growth factor beta represses the actions of myogenin through a mechanism independent of DNA binding. Proc. Natl. Acad. Sci. USA. 1991;88:3822–3826. doi: 10.1073/pnas.88.9.3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushell AJ, Klenerman L, Taylor S, et al. Ischaemic preconditioning of skeletal muscle. 1. Protection against the structural changes induced by ischaemia/reperfusion injury. J. Bone Joint Surg. Br. 2002;84:1184–1188. doi: 10.1302/0301-620x.84b8.9361. [DOI] [PubMed] [Google Scholar]
- Buttemeyer R, Philipp AW, Mall JW, Ge B, Scheller FW, Lisdat F. In vivo measurement of oxygen-derived free radicals during reperfusion injury. Microsurgery. 2002;22:108–113. doi: 10.1002/micr.21733. [DOI] [PubMed] [Google Scholar]
- Callejas NA, Fernandez-Martinez A, Castrillo A, Bosca L, Martin-Sanz P. Selective inhibitors of cyclooxygenase-2 delay the activation of nuclear factor kappa B and attenuate the expression of inflammatory genes in murine macrophages treated with lipopolysaccharide. Mol. Pharmacol. 2003;63:671–677. doi: 10.1124/mol.63.3.671. [DOI] [PubMed] [Google Scholar]
- Carey I, Zehner ZE. Regulation of chicken vimentin gene expression by serum, phorbol ester, and growth factors: identification of a novel fibroblast growth factor-inducible element. Cell Growth Differ. 1995;6:899–908. [PubMed] [Google Scholar]
- Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol. Rev. 2004;84:209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- Christman JW, Blackwell TS, Juurlink BH. Redox regulation of nuclear factor kappa B: therapeutic potential for attenuating inflammatory responses. Brain Pathol. 2000;10:153–162. doi: 10.1111/j.1750-3639.2000.tb00252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Smythe GM, Rando TA. Notch-mediated restoration of regenerative potential to aged muscle. Science. 2003;302:1575–1577. doi: 10.1126/science.1087573. [DOI] [PubMed] [Google Scholar]
- Crisco JJ, Jokl P, Heinen GT, Connell MD, Panjabi MM. A muscle contusion injury model. Biomechanics, physiology, and histology. Am. J. Sports Med. 1994;22:702–710. doi: 10.1177/036354659402200521. [DOI] [PubMed] [Google Scholar]
- Cuevas MJ, Almar M, Garcia-Glez JC, et al. Changes in oxidative stress markers and NF-kappaB activation induced by sprint exercise. Free Radic. Res. 2005;39:431–439. doi: 10.1080/10715760500072149. [DOI] [PubMed] [Google Scholar]
- Dawn-Linsley M, Ekinci FJ, Ortiz D, Rogers E, Shea TB. Monitoring thiobarbituric acid-reactive substances (TBARs) as an assay for oxidative damage in neuronal cultures and central nervous system. J. Neurosci. Methods. 2005;141:219–222. doi: 10.1016/j.jneumeth.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Dennler S, Goumans MJ, ten Dijke P. Transforming growth factor beta signal transduction. J. Leukoc. Biol. 2002;71:731–740. [PubMed] [Google Scholar]
- Dupouy VM, Ferre PJ, Uro-Coste E, Lefebvre HP. Time course of COX-1 and COX-2 expression during ischemia–reperfusion in rat skeletal muscle. J. Appl. Physiol. 2006;100:233–239. doi: 10.1152/japplphysiol.00673.2004. [DOI] [PubMed] [Google Scholar]
- Ehrhardt J, Morgan J. Regenerative capacity of skeletal muscle. Curr. Opin. Neurol. 2005;18:548–553. doi: 10.1097/01.wco.0000177382.62156.82. [DOI] [PubMed] [Google Scholar]
- Flanders KC. Smad3 as a mediator of the fibrotic response. Int. J. Exp. Pathol. 2004;85:47–64. doi: 10.1111/j.0959-9673.2004.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster W, Li Y, Usas A, Somogyi G, Huard J. Gamma interferon as an antifibrosis agent in skeletal muscle. J. Orthop. Res. 2003;21:798–804. doi: 10.1016/S0736-0266(03)00059-7. [DOI] [PubMed] [Google Scholar]
- Fukushima K, Badlani N, Usas A, Riano F, Fu F, Huard J. The use of an antifibrosis agent to improve muscle recovery after laceration. Am. J. Sports Med. 2001;29:394–402. doi: 10.1177/03635465010290040201. [DOI] [PubMed] [Google Scholar]
- Hatoko M, Tanaka A, Kuwahara M, Yurugi S, Iioka H, Niitsuma K. Difference of molecular response to ischemia–reperfusion of rat skeletal muscle as a function of ischemic time: study of the expression of p53, p21(WAF-1), Bax protein, and apoptosis. Ann. Plast. Surg. 2002;48:68–74. doi: 10.1097/00000637-200201000-00010. [DOI] [PubMed] [Google Scholar]
- Hensley K, Robinson KA, Gabbita SP, Salsman S, Floyd RA. Reactive oxygen species, cell signaling, and cell injury. Free Radic. Biol. Med. 2000;28:1456–1462. doi: 10.1016/s0891-5849(00)00252-5. [DOI] [PubMed] [Google Scholar]
- Hnia K, Gayraud J, Hugon G, et al. L-arginine decreases inflammation and modulates the nuclear factor-kappaB/matrix metalloproteinase cascade in mdx muscle fibers. Am. J. Pathol. 2008;172:1509–1519. doi: 10.2353/ajpath.2008.071009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huda R, Solanki DR, Mathru M. Inflammatory and redox responses to ischaemia/reperfusion in human skeletal muscle. Clin. Sci. (Lond) 2004;107:497–503. doi: 10.1042/CS20040179. [DOI] [PubMed] [Google Scholar]
- Izumi N, Mizuguchi S, Inagaki Y, et al. BMP-7 opposes TGF-beta1-mediated collagen induction in mouse pulmonary myofibroblasts through Id2. Am. J. Physiol. Lung Cell Mol. Physiol. 2006;290:L120–L126. doi: 10.1152/ajplung.00171.2005. [DOI] [PubMed] [Google Scholar]
- Javesghani D, Magder SA, Barreiro E, Quinn MT, Hussain SN. Molecular characterization of a superoxide-generating NAD(P)H oxidase in the ventilatory muscles. Am. J. Respir. Crit. Care Med. 2002;165:412–418. doi: 10.1164/ajrccm.165.3.2103028. [DOI] [PubMed] [Google Scholar]
- Kasemkijwattana C, Menetrey J, Somogyl G, et al. Development of approaches to improve the healing following muscle contusion. Cell Transplant. 1998;7:585–598. doi: 10.1177/096368979800700609. [DOI] [PubMed] [Google Scholar]
- Kefaloyianni E, Gaitanaki C, Beis I. ERK1/2 and p38-MAPK signalling pathways, through MSK1, are involved in NF-kappaB transactivation during oxidative stress in skeletal myoblasts. Cell Signal. 2006;18:2238–2251. doi: 10.1016/j.cellsig.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Kiernan JA. Methods for connective tissue. In: Kiernan JA, editor. Histological and Histochemical Methods: Theory and Practice. Oxford: Butterworth-Heinemann; 1999. pp. 200–250. [Google Scholar]
- Kovacs EJ, DiPietro LA. Fibrogenic cytokines and connective tissue production. FASEB J. 1994;8:854–861. doi: 10.1096/fasebj.8.11.7520879. [DOI] [PubMed] [Google Scholar]
- Kuusniemi AM, Lapatto R, Holmberg C, Karikoski R, Rapola J, Jalanko H. Kidneys with heavy proteinuria show fibrosis, inflammation, and oxidative stress, but no tubular phenotypic change. Kidney Int. 2005;68:121–132. doi: 10.1111/j.1523-1755.2005.00386.x. [DOI] [PubMed] [Google Scholar]
- Lagord C, Berry M, Logan A. Expression of TGFbeta2 but not TGFbeta1 correlates with the deposition of scar tissue in the lesioned spinal cord. Mol. Cell Neurosci. 2002;20:69–92. doi: 10.1006/mcne.2002.1121. [DOI] [PubMed] [Google Scholar]
- Lass A, Sohal BH, Weindruch R, Forster MJ, Sohal RS. Caloric restriction prevents age-associated accrual of oxidative damage to mouse skeletal muscle mitochondria. Free Radic. Biol. Med. 1998;25:1089–1097. doi: 10.1016/s0891-5849(98)00144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehto M, Jarvinen M, Nelimarkka O. Scar formation after skeletal muscle injury. A histological and autoradiographical study in rats. Arch. Orthop. Trauma Surg. 1986;104:366–370. doi: 10.1007/BF00454432. [DOI] [PubMed] [Google Scholar]
- Li Y, Foster W, Deasy BM, et al. Transforming growth factor-beta1 induces the differentiation of myogenic cells into fibrotic cells in injured skeletal muscle: a key event in muscle fibrogenesis. Am. J. Pathol. 2004;164:1007–1019. doi: 10.1016/s0002-9440(10)63188-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Huard J. Differentiation of muscle-derived cells into myofibroblasts in injured skeletal muscle. Am. J. Pathol. 2002;161:895–907. doi: 10.1016/S0002-9440(10)64250-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lijnen PJ, Petrov VV, Fagard RH. Induction of cardiac fibrosis by transforming growth factor-beta(1) Mol. Genet. Metab. 2000;71:418–435. doi: 10.1006/mgme.2000.3032. [DOI] [PubMed] [Google Scholar]
- Liu D, Black BL, Derynck R. TGF-beta inhibits muscle differentiation through functional repression of myogenic transcription factors by Smad3. Genes Dev. 2001;15:2950–2966. doi: 10.1101/gad.925901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan A, Green J, Hunter A, Jackson R, Berry M. Inhibition of glial scarring in the injured rat brain by a recombinant human monoclonal antibody to transforming growth factor-beta2. Eur. J. Neurosci. 1999;11:2367–2374. doi: 10.1046/j.1460-9568.1999.00654.x. [DOI] [PubMed] [Google Scholar]
- Lu Y, Wahl LM. Oxidative stress augments the production of matrix metalloproteinase-1, cyclooxygenase-2, and prostaglandin E2 through enhancement of NF-kappa B activity in lipopolysaccharide-activated human primary monocytes. J. Immunol. 2005;175:5423–5429. doi: 10.4049/jimmunol.175.8.5423. [DOI] [PubMed] [Google Scholar]
- Malm C, Sjodin TL, Sjoberg B, et al. Leukocytes, cytokines, growth factors and hormones in human skeletal muscle and blood after uphill or downhill running. J. Physiol. 2004;556:983–1000. doi: 10.1113/jphysiol.2003.056598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTigue DM, Popovich PG, Morgan TE, Stokes BT. Localization of transforming growth factor-beta1 and receptor mRNA after experimental spinal cord injury. Exp. Neurol. 2000;163:220–230. doi: 10.1006/exnr.2000.7372. [DOI] [PubMed] [Google Scholar]
- Menetrey J, Kasemkijwattana C, Fu FH, Moreland MS, Huard J. Suturing versus immobilization of a muscle laceration. A morphological and functional study in a mouse model. Am. J. Sports Med. 1999;27:222–229. doi: 10.1177/03635465990270021801. [DOI] [PubMed] [Google Scholar]
- Meyer M, Schreck R, Baeuerle PA. H2O2 and antioxidants have opposite effects on activation of NF-kappa B and AP-1 in intact cells: AP-1 as secondary antioxidant-responsive factor. EMBO J. 1993;12:2005–2015. doi: 10.1002/j.1460-2075.1993.tb05850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakauchi K, Ikata T, Katoh S, Hamada Y, Tsuchiya K, Fukuzawa K. Effects of lecithinized superoxide dismutase on rat spinal cord injury. J. Neurotrauma. 1996;13:573–582. doi: 10.1089/neu.1996.13.573. [DOI] [PubMed] [Google Scholar]
- Nieto N, Cederbaum AI. S-adenosylmethionine blocks collagen I production by preventing transforming growth factor-beta induction of the COL1A2 promoter. J. Biol. Chem. 2005;280:30963–30974. doi: 10.1074/jbc.M503569200. [DOI] [PubMed] [Google Scholar]
- Pattwell D, Ashton T, McArdle A, Griffiths RD, Jackson MJ. Ischemia and reperfusion of skeletal muscle lead to the appearance of a stable lipid free radical in the circulation. Am. J. Physiol. Heart Circ. Physiol. 2003;284:H2400–H2404. doi: 10.1152/ajpheart.00931.2002. [DOI] [PubMed] [Google Scholar]
- Pizza FX, Peterson JM, Baas JH, Koh TJ. Neutrophils contribute to muscle injury and impair its resolution after lengthening contractions in mice. J. Physiol. 2005;562:899–913. doi: 10.1113/jphysiol.2004.073965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prisk V, Huard J. Muscle injuries and repair: the role of prostaglandins and inflammation. Histol. Histopathol. 2003;18:1243–1256. doi: 10.14670/HH-18.1243. [DOI] [PubMed] [Google Scholar]
- Ritzenthaler JD, Goldstein RH, Fine A, Smith BD. Regulation of the alpha 1(I). collagen promoter via a transforming growth factor-beta activation element. J. Biol. Chem. 1993;268:13625–13631. [PubMed] [Google Scholar]
- Roberts AB, Russo A, Felici A, Flanders KC. Smad3: a key player in pathogenetic mechanisms dependent on TGF-beta. Ann. NY Acad. Sci. 2003;995:1–10. doi: 10.1111/j.1749-6632.2003.tb03205.x. [DOI] [PubMed] [Google Scholar]
- Sato K, Li Y, Foster W, et al. Improvement of muscle healing through enhancement of muscle regeneration and prevention of fibrosis. Muscle Nerve. 2003;28:365–372. doi: 10.1002/mus.10436. [DOI] [PubMed] [Google Scholar]
- Sayan H, Babul A, Ugurlu B. Effects of nitric oxide donor and inhibitor on prostaglandin E2-like activity, malondialdehyde and reduced glutathione levels after skeletal muscle ischemia–reperfusion. Prostaglandins Leukot. Essent. Fatty Acids. 2001;65:179–183. doi: 10.1054/plef.2001.0308. [DOI] [PubMed] [Google Scholar]
- Schabort EJ, van der MM, Loos B, Moore FP, Niesler CU. TGF-beta’s delay skeletal muscle progenitor cell differentiation in an isoform-independent manner. Exp. Cell Res. 2009;315:373–384. doi: 10.1016/j.yexcr.2008.10.037. [DOI] [PubMed] [Google Scholar]
- Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- Shen W, Li Y, Tang Y, Cummins J, Huard J. NS-398, a cyclooxygenase-2-specific inhibitor, delays skeletal muscle healing by decreasing regeneration and promoting fibrosis. Am. J. Pathol. 2005;167:1105–1117. doi: 10.1016/S0002-9440(10)61199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Li Y, Zhu J, Schwendener R, Huard J. Interaction between macrophages, TGF-beta1, and the COX-2 pathway during the inflammatory phase of skeletal muscle healing after injury. J. Cell Physiol. 2008;214:405–412. doi: 10.1002/jcp.21212. [DOI] [PubMed] [Google Scholar]
- Shi H, Liu KJ. Effects of glucose concentration on redox status in rat primary cortical neurons under hypoxia. Neurosci. Lett. 2006;410:57–61. doi: 10.1016/j.neulet.2006.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taoka Y, Naruo M, Koyanagi E, Urakado M, Inoue M. Superoxide radicals play important roles in the pathogenesis of spinal cord injury. Paraplegia. 1995;33:450–453. doi: 10.1038/sc.1995.98. [DOI] [PubMed] [Google Scholar]
- Taoka Y, Okajima K, Uchiba M, et al. Role of neutrophils in spinal cord injury in the rat. Neuroscience. 1997;79:1177–1182. doi: 10.1016/s0306-4522(97)00011-0. [DOI] [PubMed] [Google Scholar]
- Tonai T, Shiba K, Taketani Y, et al. A neutrophil elastase inhibitor (ONO-5046) reduces neurologic damage after spinal cord injury in rats. J. Neurochem. 2001;78:1064–1072. doi: 10.1046/j.1471-4159.2001.00488.x. [DOI] [PubMed] [Google Scholar]
- Toumi H, Best TM. The inflammatory response: friend or enemy for muscle injury? Br. J. Sports Med. 2003;37:284–286. doi: 10.1136/bjsm.37.4.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulloa L, Doody J, Massague J. Inhibition of transforming growth factor-beta/SMAD signaling by the interferon-gamma/STAT pathway. Nature. 1999;397:710–713. doi: 10.1038/17826. [DOI] [PubMed] [Google Scholar]
- Vanden Hoek T, Becker LB, Shao ZH, Li CQ, Schumacker PT. Preconditioning in cardiomyocytes protects by attenuating oxidant stress at reperfusion. Circ. Res. 2000;86:541–548. doi: 10.1161/01.res.86.5.541. [DOI] [PubMed] [Google Scholar]
- Venkatesan N, Pini L, Ludwig MS. Changes in Smad expression and subcellular localization in bleomycin-induced pulmonary fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 2004;287:L1342–L1347. doi: 10.1152/ajplung.00035.2004. [DOI] [PubMed] [Google Scholar]
- Weaver LC, Verghese P, Bruce JC, Fehlings MG, Krenz NR, Marsh DR. Autonomic dysreflexia and primary afferent sprouting after clip-compression injury of the rat spinal cord. J. Neurotrauma. 2001;18:1107–1119. doi: 10.1089/08977150152693782. [DOI] [PubMed] [Google Scholar]
- Wickens AP. Ageing and the free radical theory. Respir. Physiol. 2001;128:379–391. doi: 10.1016/s0034-5687(01)00313-9. [DOI] [PubMed] [Google Scholar]
- Zhu S, Goldschmidt-Clermont PJ, Dong C. Transforming growth factor-beta-induced inhibition of myogenesis is mediated through Smad pathway and is modulated by microtubule dynamic stability. Circ. Res. 2004;94:617–625. doi: 10.1161/01.RES.0000118599.25944.D5. [DOI] [PubMed] [Google Scholar]
- Zimiani K, Guarnier FA, Miranda HC, Watanabe MA, Cecchini R. Nitric oxide mediated oxidative stress injury in rat skeletal muscle subjected to ischemia/reperfusion as evaluated by chemiluminescence. Nitric Oxide. 2005;13:196–203. doi: 10.1016/j.niox.2005.07.002. [DOI] [PubMed] [Google Scholar]