Abstract
Soluble gp130 (sgp130) has been shown to suppress the inflammatory response of autoimmune pathologies; however, its effects on virus infection are not known. Here, we report that intraperitoneal treatment of mice with sgp130-Fc fusion protein at the time of oral reovirus serotype 3 infection resulted in altered morphopathological changes that were evident by less shortening of intestinal villi length and crypt depth after infection. That the effect mediated by sgp130 treatment was due to an increase in intestinal crypt cell proliferation was demonstrated by an increase in the number of crypt mitotic figures. This was further confirmed by increased immunoreactivity to the Cdc47 proliferation-associated antigen in crypts of sgp130-treated virus-infected mice compared to infected non-treated mice. These findings suggest that sgp130 may have a beneficial effect during intestinal virus infection by disrupting interleukin-6 trans-signalling, thereby reducing the local inflammatory response.
Keywords: crypt, cytokine, enteric, intestine, signalling, villus
There are two modalities by which interleukin-6 (IL-6) can induce an inflammatory response. Because expression of membrane-bound IL-6 receptor (IL-6Rα) displays an expression pattern that is mostly (though not exclusively) restricted to leukocytes (Shirota et al. 1990; Bethin et al. 2000; Jones & Rose-John 2002), direct binding of IL-6 to the cognate IL-6Rα results in leukocyte activation. However, IL-6 also can function in a soluble manner by binding to soluble IL-6R. In both situations, subsequent binding to homodimeric membrane gp130 (CD130/IL-6Rβ) is required for cell signalling. In as much as the gp130 molecule is ubiquitously expressed across many types of tissue cells (Saito et al. 1992), binding of soluble IL-6/IL-6R to membrane gp130 can activate cells that do not express IL-6Rα─ a process referred to as trans-signalling (Jones & Rose-John 2002; Jones et al. 2005). Additionally, circulating sgp130 can act as a natural antagonist of IL-6/IL-6R trans-signalling (Kallen 2002). In vivo treatment with sgp130 has been shown to have beneficial therapeutic effects in systems of experimental rheumatoid arthritis (Nowell et al. 2003), and for chronic intestinal inflammation in IL-10-/- mice or mice with trinitrobenzene sulfonic acid-induced intestinal inflammation (Atreya et al. 2000). In the latter, treatment of IL-10-/- mice with sgp130-Fc fusion protein curtailed animal wasting and significantly reduced pathology in the colon in a manner similar to mice treated with anti-IL-6R antibody, suggesting either that direct blockade of IL-6 binding, or disruption of IL-6 trans-signalling, is effective for reducing disease symptomatology.
In the present study, we sought to determine the extent to which sgp130 might alter the effects of acute reovirus serotype 3 Dearing (T3D reovirus) infection in mice. That virus strain was selected since it causes minimal pathology in normal mice but results in a disseminated infection with extensive pathology in severe-combined immunodeficient mice (George et al. 1990), thus indicating that host factors are critical for curtailing the infectious process. Hence, based studies demonstrating a suppression of intestinal inflammation in sgp130 treated mice (Atreya et al. 2000), it was reasoned that exposure of mice to sgp130 at the time of T3D reovirus infection would lead to less intestinal pathology. As reported here, treatment of mice with sgp130 at the time of infection resulted in higher levels of viral RNA with less villus and crypt morphopathological changes after infection.
Materials and methods
Mice
Adult female C57BL/6 mice, 6–8 weeks of age, were purchased from Harlan Laboratories (Indianapolis, IN, USA). Animals were used in accordance with institutional animal welfare guidelines.
Virus, animal infection, and sgp130 treatment
T3D reovirus was purchased from American Type Culture Collection (Manassa, VA, USA). Virus was grown in L929 cells and virus titre was quantified as previously described (Montufar-Solis et al. 2006). Mice were infected with 107.5 pfu by oral gavage under isoflurane (Abbot Laboratories; North Chicago, IL, USA) anaesthesia. Recombinant mouse sgp130/Fc chimera and recombinant human IL-6 receptor, which has activity in murine systems (Saito et al. 1991) were purchased from R&D Systems (Minneapolis, MN, USA). For sgp130 treatment, mice were given a single i.p. injection of 250 ng of sgp130 at the time of infection. That dose is consistent with other studies using sgp130 (Hurst et al. 2001; McLoughlin et al. 2005).
Quantitative real-time PCR
At days 2, 4 and 6 post-infection, mice were euthanized; tissues were obtained and homogenized by disruption for 15 s using an Omni Tissue Master 240 (Omni International; Marietta, GA, USA). Total RNA was extracted using an RNeasy kit (Qiagen; Valencia, CA, USA). RNA concentrations were estimated spectrophotometrically at A260. T3D reovirus real-time PCR was done using a TaqMan system using the following primers and probe: forward primer: 5′-TGATTTCCATTACTTCTGCTGCTT-3′, reverse primer: 5-′TCCTGTTCACGATTCCATCAGAT-3′, probe: 5′/56-FAM/CGCCGTGCTTGACCACCCACTT/3BHQ1/-3′. Comparisons of viral RNA levels were done using the 2-ΔΔCt method of Livak and Schmittgen (Livak & Schmittgen 2001).
Quantitative histomorphometric analyses
Representative haematoxylin and eosin stained intestinal sections of control and experimental animals were examined for mucosal injury and inflammation. A total of 29 slides from reovirus T3D-infected mice that were not treated with sgp130 or were treated with sgp130-treated were used; five slides from non-infected mice were used. Twenty-five well-oriented villi and crypts were randomly selected and measured using a Nikon Eclipse E200 microscope with image analysis software (Image Pro plus 5.0) to determine the villi length and crypt depths. Crypt cell proliferation rate in control and experimental animals was evaluated by counting the mitotic figures and by immunohistochemical analysis using an antibody (Biocare Medical; Concord, CA, USA) to the Cdc47 cell proliferation-associated antigen. The total number of mitotic figures and Cdc47+ cells were counted in 30 randomly selected crypts under high power (×40) by an investigator blinded to the experimental groups. Intestinal crypt cell proliferation rates of experimental and control animals were expressed as number of mitotic figures and Cdc47+ cells per crypt.
Statistical analyses
Statistical comparisons of mean values were done using a two-sided t-test using Microsoft Excel software; P-values of <0.05 were considered to be statistically significant.
Results and discussion
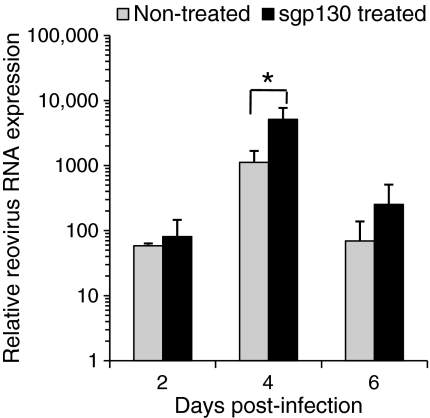
The results of virus-specific real-time PCR analysis are shown in Figure 1 for the three time points tested. Viral RNA levels were not significantly different in infected mice treated with sgp130 compared to non-treated infected mice, with the exception of day 4 post-infection when there was a statistically-significant difference in mice treated with sgp130 compared to non-treated mice (Figure 1).
Figure 1.
T3D reovirus gene expression at days 2, 4, and 6 post-infection in small intestinal tissues from non-treated and sgp130-treated infected mice. Data are mean values ± SEM; statistically-significant difference (*P< 0.05). No viral RNA was detected in non-infected mice (data not shown).
Two approaches were used to evaluate the effects of sgp130 treatment on intestinal pathology in T3D reovirus-infected mice. First, histopathological analysis of hematoxylin and eosin-stained tissues from the jejunum was done to identify pathological changes that occurred between treated and non-treated mice. Although T3D reovirus produces minimal pathology in mice, the possibility was considered that changes may occur following sgp130 treatment due to an altered host response to infection. However, histopathological analysis of jejunal mucosae of infected mice did not reveal any remarkable intestinal mucosal injury, oedema, necrosis, or inflammation (e.g. leucocytic infiltrate), at the three time points tested (data not shown).
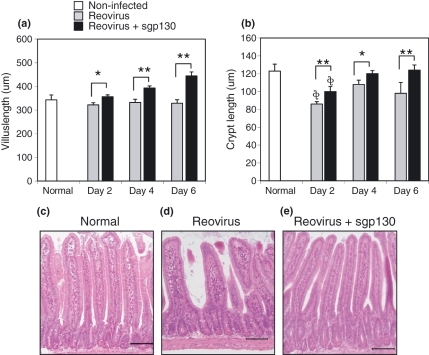
The second approach to determine pathological changes was to measure villi length and crypt depths of normal non-infected mice, reovirus infected mice, and reovirus-infected mice treated with sgp130. Differences in villi lengths and crypt depths between the control mice and the experimental groups would be indicative of altered cell proliferation and regeneration. As seen in Figure 2a,b, on days 2, 4 and 6 post-infection there was a statistically-significant increase in villi length and crypt depths in virus-infected sgp130-treated mice compared to infected non-treated animals. Representative tissue sections from reovirus-infected mice without (Figure 2d) and with (Figure 2e) sgp130 treatment are shown at the day 6 post-infection time point, as well as for a normal non-infected mouse (Figure 2c). Note the blunted characteristic of villi from infected non-treated mice (Figure 2d).
Figure 2.
(a) Villi length and (b) crypt depths at days 2, 4, and 6 post-infection in small intestinal tissues from non-treated and sgp130-treated infected mice. Panels C-E are representative tissue sections from reovirus-infected mice without (d) and with (e) sgp130 treatment at the day 6 post-infection time point, and (c) a tissue section of a normal non-infected mouse. Bars are 100 μm. Data are mean values ± SEM; treatment with sgp130 resulted in statistically-significant less (*P< 0.05; **P< 0.01) villus and crypt shortening at all time points. Statistically shorter (**P< 0.05) crypt length compared to non-infected mice.
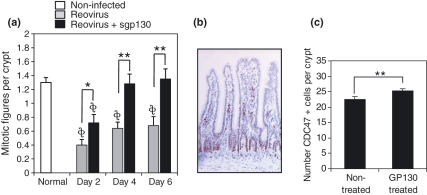
Those findings were confirmed by determining crypt cell proliferation rates. The number of mitotic figures per crypt were counted in haematoxylin and eosin stained tissue sections from non-treated and sgp130-treated virus infected mice. At days 2, 4 and 6 post-infection, intestinal crypts of sgp130-treated mice had a statistically-significant increase in mitotic figures compared to non-treated virus-infected mice (Figure 3a). This was further confirmed by counting the number of Cdc47+ cells in crypts; Cdc47 is a marker of DNA synthesis (Dalton & Whitbread 1995; Dery et al. 2003), including intestinal epithelia (Hiraiwa et al. 1997). Cdc47 staining is shown in Figure 3b. Analysis was done at day 4 post-infection - the time of maximum virus load. Consistent with the findings obtained from mitotic figure analysis, there were statistically-significant greater numbers of Cdc47+ cells in the crypts of virus-infected mice treated with sgp130 compared to virus-infected non-treated mice (Figure 3c). Taken together, these findings suggest that there is a generalized beneficial effect of sgp130 treatment in intestinal tissue recovery from reovirus infection.
Figure 3.
(a) Treatment of reovirus-infected mice with sgp130 resulted in statistically-significant (*P< 0.05; **P< 0.01) more cells undergoing mitosis at days 2, 4, and 6 post-infection compared to non-treated infected mice. Statistically fewer (*,**P< 0.01) crypt mitotic figures compared to non-infected mice. (b) Example of Cdc47 staining. (c) Crypts of reovirus-infected mice treated with sgp130 had statistically-significant (P < 0.01) more Cdc47+ cells compared to infected non-treated mice. Data are mean values ± SEM.
To our knowledge, the present study is the first to demonstrate that T3D reovirus results in a shortening of villus and crypt lengths and reduced numbers of crypt mitotic figures, and that there is a beneficial effect of sgp130 during infection. Presumably, the action of sgp130 would be to block IL-6 trans-signalling to intestinal leukocytes and enterocytes that are a source of chemotactic mediators and receptors that contribute to the inflammatory process (Adams & Eksteen 2006; Rivera-Nieves et al. 2006). In a previous study from our laboratory we demonstrated, using a protocol for detecting 22 immune response analytes (chemokines and cytokines), that thirteen were produced at elevated levels by day 4 post-infection (Montufar-Solis & Klein 2005). Among those analytes were several that are known to drive the inflammatory response (IL-6, IL-12, IL-17, IFN-γ, GM-CSF and MCP-1). Binding of IL-6 produced during virus infection to sIL-6 receptor would activate lymphocytes and enterocytes via membrane bound gp130. Predictably, sgp130 would inhibit that process, thereby ameliorating the proinflammatory, and possibly tissue destructive, effects of IL-6. Additionally, this would suppress the activation of CCL2, CCL4, CCL5, CCL11, CCL17, and CXCL10 responses, all of which can be propagated by IL-6 or IL-6/IL-6R trans-signalling (McLoughlin et al. 2005). Although we did not detect an increase in mononuclear cell infiltration into intestinal regions during infection, this may reflect the fact that T3D reovirus is minimally virulent in mice. Additional studies using the reovirus serotype 1 Lang strain or rotavirus may shed light in that regard. Regardless, it is possible that sgp130 served to inhibit the immune response of resident intestinal mononuclear cells, thus providing a more suitable environment for tissue repair. Further evidence of this is supported by the fact that there was a slight increase in virus load at days 2 and 6 post-infection, and a statistically-significant increase at day 4 post-infection, in sgp130-treated mice.
Simultaneously, sgp130 may have contributed to the healing process by blocking trans-signalling, thus suppressing the release of monocyte/macrophage-derived deleterious products such as TNFα, IL-1β, and IL-12. The involvement of TNFα in intestinal cell destruction is seen in Crohn’s disease and other intestinal inflammatory conditions (Bradley 2008), possibly due to cytotoxic effects targeted to proliferating cells that are attempting to remedy local tissue damage (Palombella & Vilcek 1989). In that scenario, the effect of sgp130 would be to block the adverse effects of TNFα and other potent inflammatory cytokines. The balance in this, although perhaps not readily apparent in T3D reovirus infection, may be critical during enteric virus infections in which a strong immune response occurs.
Acknowledgments
This work was supported by NIH grants DK035566 and DE015355.
References
- Adams DH, Eksteen B. Aberrant homing of mucosal T cells and extra-intestinal manifestations of inflammatory bowel disease. Nat. Rev. Immunol. 2006;6:244–251. doi: 10.1038/nri1784. [DOI] [PubMed] [Google Scholar]
- Atreya R, Mudter J, Finotto S, et al. Blockade of interleukin 6 trans signalling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in Crohn disease and experimental colitis in vivo. Nat. Med. 2000;6:583–588. doi: 10.1038/75068. [DOI] [PubMed] [Google Scholar]
- Bethin KE, Vogt SK, Muglia LJ. Interleukin-6 is an essential, corticotropin-releasing hormone-independent stimulator of the adrenal axis during immune system activation. Proc. Natl Acad. Sci. USA. 2000;97:9317–9322. doi: 10.1073/pnas.97.16.9317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley JR. TNF-mediated inflammatory disease. J. Pathol. 2008;214:149–160. doi: 10.1002/path.2287. [DOI] [PubMed] [Google Scholar]
- Dalton S, Whitbread L. Cell cycle-regulated nuclear import and export of Cdc47, a protein essential for initiation of DNA replication in budding yeast. Proc. Natl Acad. Sci. USA. 1995;92:2514–2518. doi: 10.1073/pnas.92.7.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dery MC, Leblanc V, Shooner C, Asselin E. Regulation of Akt expression and phosphorylation by 17beta-estradiol in the rat uterus during estrous cycle. Reprod. Biol. Endocrinol. 2003;1:47. doi: 10.1186/1477-7827-1-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George A, Kost SI, Witzleben CL, Cebra JJ, Rubin DH. Reovirus-induced liver disease in severe combined immunodeficient (SCID) mice. A model for the study of viral infection, pathogenesis, and clearance. J. Exp. Med. 1990;171:929–934. doi: 10.1084/jem.171.3.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraiwa A, Fujita M, Nagasaka T, Adachi A, Ohashi M, Ishibashi M. Immunolocalization of hCDC47 protein in normal and neoplastic human tissues and its relation to growth. Int. J. Cancer. 1997;74:180–184. doi: 10.1002/(sici)1097-0215(19970422)74:2<180::aid-ijc7>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Hurst SM, Wilkinson TS, McLoughlin RM, et al. Il-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity. 2001;14:705–714. doi: 10.1016/s1074-7613(01)00151-0. [DOI] [PubMed] [Google Scholar]
- Jones SA, Rose-John S. The role of soluble receptors in cytokine biology: the agonistic properties of the sIL-6R/IL-6 complex. Biochim. Biophys. Acta. 2002;1592:251–263. doi: 10.1016/s0167-4889(02)00319-1. [DOI] [PubMed] [Google Scholar]
- Jones SA, Richards PJ, Scheller J, Rose-John S. IL-6 transsignaling: the in vivo consequences. J. Interferon Cytokine Res. 2005;25:241–253. doi: 10.1089/jir.2005.25.241. [DOI] [PubMed] [Google Scholar]
- Kallen KJ. The role of transsignalling via the agonistic soluble IL-6 receptor in human diseases. Biochim. Biophys. Acta. 2002;1592:323–343. doi: 10.1016/s0167-4889(02)00325-7. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-ΔΔCT) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- McLoughlin RM, Jenkins BJ, Grail D, et al. IL-6 trans-signalling via STAT3 directs T cell infiltration in acute inflammation. Proc. Natl Acad. Sci. USA. 2005;102:9589–9594. doi: 10.1073/pnas.0501794102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montufar-Solis D, Klein JR. Experimental intestinal reovirus infection of mice: what we know, what we need to know. Immunol. Res. 2005;33:257–265. doi: 10.1385/IR:33:3:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montufar-Solis D, Garza T, Teng BB, Klein JR. Upregulation of ICOS on CD43+ CD4+ murine small intestinal intraepithelial lymphocytes during acute reovirus infection. Biochem. Biophys. Res. Commun. 2006;342:782–790. doi: 10.1016/j.bbrc.2006.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowell MA, Richards PJ, Horiuchi S, et al. Soluble IL-6 receptor governs IL-6 activity in experimental arthritis: blockade of arthritis severity by soluble glycoprotein 130. J. Immunol. 2003;171:3202–3209. doi: 10.4049/jimmunol.171.6.3202. [DOI] [PubMed] [Google Scholar]
- Palombella VJ, Vilcek J. Mitogenic and cytotoxic actions of tumor necrosis factor in BALB/c 3T3 cells. Role of phospholipase activation. J. Biol. Chem. 1989;264:18128–18136. [PubMed] [Google Scholar]
- Rivera-Nieves J, Ho J, Bamias G, et al. Antibody blockade of CCL25/CCR9 ameliorates early but not late chronic murine ileitis. Gastroenterology. 2006;131:1518–1529. doi: 10.1053/j.gastro.2006.08.031. [DOI] [PubMed] [Google Scholar]
- Saito T, Yasukawa K, Suzuki H, et al. Preparation of soluble murine IL-6 receptor and anti-murine IL-6 receptor antibodies. J. Immunol. 1991;147:168–173. [PubMed] [Google Scholar]
- Saito M, Yoshida K, Hibi M, Taga T, Kishimoto T. Molecular cloning of a murine IL-6 receptor-associated signal transducer, gp130, and its regulated expression in vivo. J. Immunol. 1992;148:4066–4071. [PubMed] [Google Scholar]
- Shirota K, LeDuy L, Yuan SY, Jothy S. Interleukin-6 and its receptor are expressed in human intestinal epithelial cells. Virchows. Arch. B. Cell. Pathol. Incl. Mol. Pathol. 1990;58:303–308. doi: 10.1007/BF02890085. [DOI] [PubMed] [Google Scholar]