Abstract
Rhinovirus (RV) infections trigger asthma exacerbations. Genome-wide expression analysis of RV1A-infected primary bronchial epithelial cells from normal and asthmatic donors was performed to determine whether asthma is associated with a unique pattern of RV-induced gene expression. Virus replication rates were similar in cells from normal and asthmatic donors. Overall, RV downregulated 975 and upregulated 69 genes. Comparisons of transcriptional profiles generated from microarrays and confirmed by quantitative reverse transcription PCR and cluster analysis showed some up- and downregulated genes in asthma cells involved in immune responses (IL1B, IL1F9, IL24, IFI44) and airway remodeling (LOXL2, MMP10, FN1). Notably, most of the asthma-related differences in RV-infected cells were also present in the cells before infection. These findings suggest that differences in RV-induced gene expression profiles of cells from normal and mild asthmatic subjects could affect the acute inflammatory response to RV and subsequent airway repair and remodeling.
INTRODUCTION
Human rhinoviruses (RVs) cause the common cold and are frequently detected in asthma exacerbations. RV typically induces neutrophilic inflammation in the upper airways of both asthmatic and non-asthmatic patients; however, in asthmatics these infections can lead to more severe lower respiratory symptoms, as well as reductions in lung function. Interestingly, the severity of asthma symptoms may not be related to viral load, prolonged viral shedding, or to differences in proinflammatory cytokines in the upper airway secretions.1,2 Understanding of the mechanisms provoking RV-induced airway inflammation in asthma, as well as mechanisms linking RV infection to asthma exacerbations, may offer significant opportunities for improved disease management.
RV infection of airway epithelial cells induces the production of a wide range of mediators involved in inflammatory and immune processes.3,4 Transcriptional profiling of differentiated cultures of human primary bronchial epithelial (PBE) cells from two normal subjects has shown that RV infection induces a number of genes in the interferon (IFN)-β-dependent pathway.5 Furthermore, cultured epithelial cells from the airways of subjects with asthma have been found to have deficient innate immune responses to RV16 infection, characterized by increased viral replication, impaired early induction of apoptosis and reduced type I and type III IFN production.6,7 Collectively, these studies suggest that RV-induced airway disease could be due to asthma-related changes in gene expression in airway epithelium. The aim of this study is to compare RV-induced genome-wide gene expression profiles of cultured airway epithelial cells obtained from subjects with and without asthma to identify genes that may play a role in virus-induced asthma exacerbations.
RESULTS
RV1A infection of PBE cells and viral RNA quantification
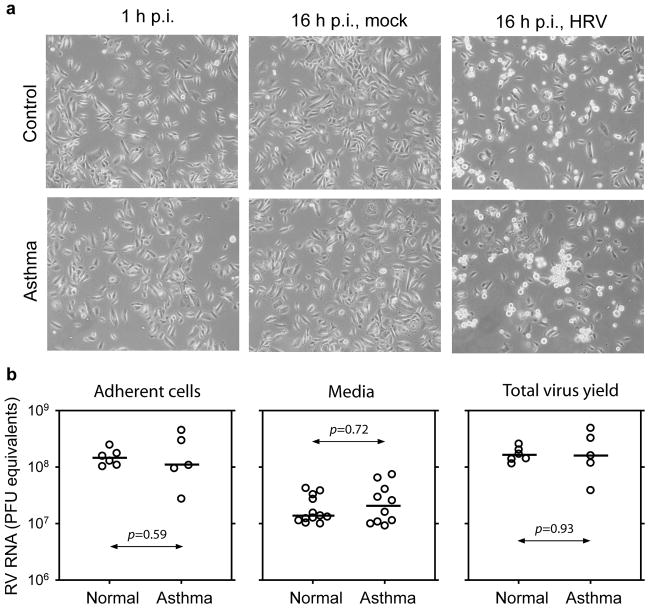
Primary bronchial epithelial cells (samples 7–18) from six normal subjects and six subjects with asthma (Table 1) were inoculated with RV1A (MOI (multiplicity of infection) of 10 PFU (plaque-forming units) per cell); for one subject with asthma (sample 11), the RNA yield after infection was insufficient for further analysis. Total RNA isolated from adherent cells collected 16 h post infection (p.i.) was analyzed using microarrays. Viral RNA was measured by quantitative reverse transcription (qRT)-PCR in growth media from the PBE cell cultures. We then repeated the process of infecting cells from these donors so that the results of the microarray experiments could be retested in separate experiments and using a different technology (qRT-PCR) to measure changes in host gene expression and viral RNA. Virus-induced cytopathic effect, as determined by light microscopy (Figure 1a), was similar in cells from normal and asthmatic subjects. In addition, there were no group-specific differences in the amount of viral RNA released into the media (including floating cells), in adherent cells, or in the total amount of RNA per well (sum of RNA in media and floating and adherent cells) (Figure 1b, P>0.05).
Table 1.
Characteristics of subjects with asthma and controls
| Subjecta | Asthma | Gender | Age | PC20 mg/ml | FEV1 % predicted | Reversibility (%) | Allergen skin test |
|---|---|---|---|---|---|---|---|
| 1 | No | F | 20 | 20 | 110 | 3 | − |
| 2 | No | F | 21 | 20 | 113 | 1 | − |
| 3 | No | F | 20 | 20 | 83 | 4 | − |
| 4 | Yes | F | 19 | 0.3 | 69 | 36 | + |
| 5 | Yes | F | 22 | 1.0 | 103 | 16 | + |
| 6 | Yes | M | 21 | 2.5 | 91 | 12 | + |
| 7b | No | M | 24 | 20 | 86 | 7 | − |
| 8b | No | F | 19 | 20 | 99 | 3 | − |
| 9b | No | M | 21 | 20 | 99 | 5 | − |
| 10b | Yes | F | 22 | 1.0 | 73 | 12 | + |
| 11c | Yes | M | 25 | 2.2 | 80 | 16 | + |
| 12b | Yes | M | 25 | 0.3 | 82 | 4 | + |
| 13b | No | F | 41 | 20 | 91 | 5 | − |
| 14b | No | F | 24 | 20 | 106 | 6 | − |
| 15b | No | M | 37 | 20 | 110 | 1 | − |
| 16b | Yes | F | 32 | 0.5 | 63 | 23 | + |
| 17b | Yes | M | 35 | 4.6 | 90 | 7 | + |
| 18b | Yes | F | 28 | 2.5 | 72 | 17 | + |
F, female; FEV1, forced expiratory volume in one sec; M, male; PC20, provocative concentration of methacholine causing a 20% fall in FEV1;.
Cells from subjects 1–6 were analyzed in preliminary experiments using HG Focus GeneChips; cells from subjects 7–18 were tested using HG U133 Plus 2.0 GeneChips.
Samples were used for quantitative PCR validation and viral RNA quantification in independent experiments.
Sample was not analyzed by GeneChip because of the insufficient total RNA yield.
Figure 1.
RV1A infection induces similar cytopathic effect and viral RNA yield in cells obtained from donors with asthma and normal donors. (a) Representative microphotographs (magnification ×100) of primary bronchial epithelial (PBE) cells from one normal donor (no. 13) and one patient with asthma (no. 18) taken just after virus attachment period (left panel) and 16 hours post infection (p.i.) with medium alone (middle panel) or medium containing 10 plaque-forming units (PFU) per cell RV1A (right panel). (b) Quantification of viral RNA in adherent cells and growth media (including floating cells) collected 16 h p.i. of PBE cell cultures. Media samples both after microarray and quantitative PCR validation experiments were summarized. Viral RNA for each graph was calculated from each well of a six-well plate. Horizontal bars indicate medians. HRV, human RV.
Gene expression changes in response to infection
To determine the transcriptional response of normal and asthmatic PBE cells to RV1A infection, we started by comparing genome-wide gene expression profiles in infected cells and mock-infected controls in each group. We identified a total of 1,317 probe sets corresponding to 1,044 known human genes with at least a twofold change in expression in RV-infected PBE cells vs. mock-infected control cells, in both groups. Combined lists of the 40 most highly up- and downregulated transcripts found in normal and asthmatic cells are shown in Table 2, and complete lists of genes are provided in Supplementary Tables 1 and 2 online.
Table 2.
RV-induced changes in gene expression: combined asthma and normal groups
| Gene symbol | Gene name | Biological function | Normal FDa | Asthma FDa |
|---|---|---|---|---|
| Upregulated genes | ||||
| CXCL3 | chemokine (C-X-C motif) ligand 3 | chemokine | 12.2 / 36.7 | 12.6 / 5.6 |
| CXCL2 | chemokine (C-X-C motif) ligand 2 | chemokine | 6.7 / 9.4 | 7.3 / 6.6 |
| IL8 | interleukin 8 | chemokine | 5.7 / 20.7 | 5.7 / 4.4 |
| CSF3 | colony stimulating factor 3 (granulocyte) | cytokine | 5.4 | 7.2 |
| IL1R2 | interleukin 1 receptor, type II | cytokine receptor | 5.1 | 7.5 |
| CXCL1 | chemokine (C-X-C motif) ligand 1 | chemokine | 4.7 / 6.2 | 4.1 / 3.9 |
| EGR1 | early growth response 1 | transcription factor | 4.6 | 4.4 |
| SERPINB2 | serpin peptidase inhibitor, clade B (ovalbumin), member 2 | endopeptidase inhibitor | 4.4 | 5.3 |
| TNFAIP3 | tumor necrosis factor, alpha-induced protein 3 | inhibitor of apoptosis | 3.9 / 4.9 | 3.9 / 2.0 |
| CCL20 | chemokine (C-C motif) ligand 20 | chemokine | 3.8 / 5.7 | 4.5 / 3.8 |
| IL13RA2 | interleukin 13 receptor, alpha 2 | cytokine receptor | 3.7 | 4.7 |
| CSF2 | colony stimulating factor 2 (granulocyte-macrophage) | cytokine | 3.6 / 4.1 | 3.2 |
| IL1F9 | interleukin 1 family, member 9 | cytokine | 3.5 | 6.0 |
| SPRR2B | small proline-rich protein 2B | structural molecule | 3.1 | 4.5 |
| ZC3H12A | zinc finger CCCH-type containing 12A | transcription regulator | 3.1 | 3.2 |
| IL6 | interleukin 6 | cytokine | 2.9 / 5.6 | 3.7 |
| NFKBIZ | Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, zeta | transcription regulator | 2.8 | 3.2 |
| 1558048_x_atb | cDNA clone IMAGE:4523513 | NA | 2.7 | 3.1 |
| S100A3 | S100 calcium binding protein A3 | calcium and zink binding | 2.7 | 2.6 / 2.0 |
| SOCS3 | suppressor of cytokine signaling 3 | JAK2 kinase inhibitor | 2.7 | 3.3 |
| Downregulated genes | ||||
| 225123_atb | FLJ33813 | NA | 9.1 | 8.4 |
| IVNS1ABP | influenza virus NS1A binding protein | response to virus | 8.6 | 8.3 |
| ITGB6 | integrin, beta 6 | receptor for fibronectin | 7.0 | 8.9 |
| DLG1 | discs, large homolog 1 (Drosophila) | cell-cycle regulation | 5.7 | 5.4 |
| TIA1 | TIA1 cytotoxic granule-associated RNA binding protein | inductor of apoptosis | 5.6 | 4.4 |
| FN1 | fibronectin 1 | ECM constituent | 5.6 | 4.1 |
| SLITRK6 | SLIT and NTRK-like family, member 6 | membrane protein | 5.4 | 4.2 |
| PCMTD1 | protein-L-isoaspartate (D-aspartate) O-methyltransferase domain containing 1 | protein modification | 5.3 | 5.2 |
| ACSL3 | acyl-CoA synthetase long-chain family member 3 | lipid biosynthesis | 5.2 | 4.3 |
| UHRF2 | ubiquitin-like, containing PHD and RING finger domains, 2 | cell-cycle regulation | 5.0 | 4.4 |
| CDKN2B | cyclin-dependent kinase inhibitor 2B (p15, inhibits CDK4) | cell-cycle regulation | 5.0 | 4.5 |
| PLOD2 | procollagen-lysine, 2-oxoglutarate 5-dioxygenase 2 | hydroxylation of lysyl residues in collagen | 5.0 | 4.7 |
| FLRT2 | fibronectin leucine rich transmembrane protein 2 | cell adhesion and/or receptor signaling | 4.9 | 5.2 |
| MAF | v-maf musculoaponeurotic fibrosarcoma oncogene homolog | transcription factor | 4.8 | 5.4 |
| GBP3 | guanylate binding protein 3 | GTP binding | 4.8 | 3.9 |
| SFRS12 | splicing factor, arginine/serine-rich 12 | splicing factor | 4.8 | 3.4 |
| KITLG | KIT ligand | ligand of the tyrosine- kinase receptor | 4.8 | 3.6 |
| SMC4 | structural maintenance of chromosomes 4 | chromosome organization | 4.8 | 2.7 |
| TTC37 | tetratricopeptide repeat domain 37 | binding | 4.8 | 3.1 |
| SLC39A10 | solute carrier family 39 (zinc transporter), member 10 | zink ion transport | 4.8 | 4.4 |
ECM, extracellular matrix; FD, fold difference (in expression); NA, not available; RV, rhinovirus.
Corresponding fold differences in expression (nominal P<0.05) generated by HG Focus microarray using cells from six additional donors (three normal and three asthmatic) are indicated after “slash”.
Affymetrix probe set ID is used when gene symbol is not available.
The majority of affected genes were down-regulated in infected cells compared to mock-infected control samples (Figure 2a). This finding is consistent with global host cell transcriptional shutoff due to RV-induced cleavages of multiple transcription factors and nuclear pore complex components.8,9 Virus infection decreased the expression of genes related to antiviral defense (influenza virus NS1A binding protein), apoptosis (TIA1 cytotoxic granule-associated RNA binding protein) and regulation of cell-cycle (discs, large homolog 1 (Drosophila); ubiquitin-like, containing PHD and RING finger domains; cyclin-dependent kinase inhibitor 2B) as well as multiple proteins participating in cell metabolism (Table 2).
Figure 2.
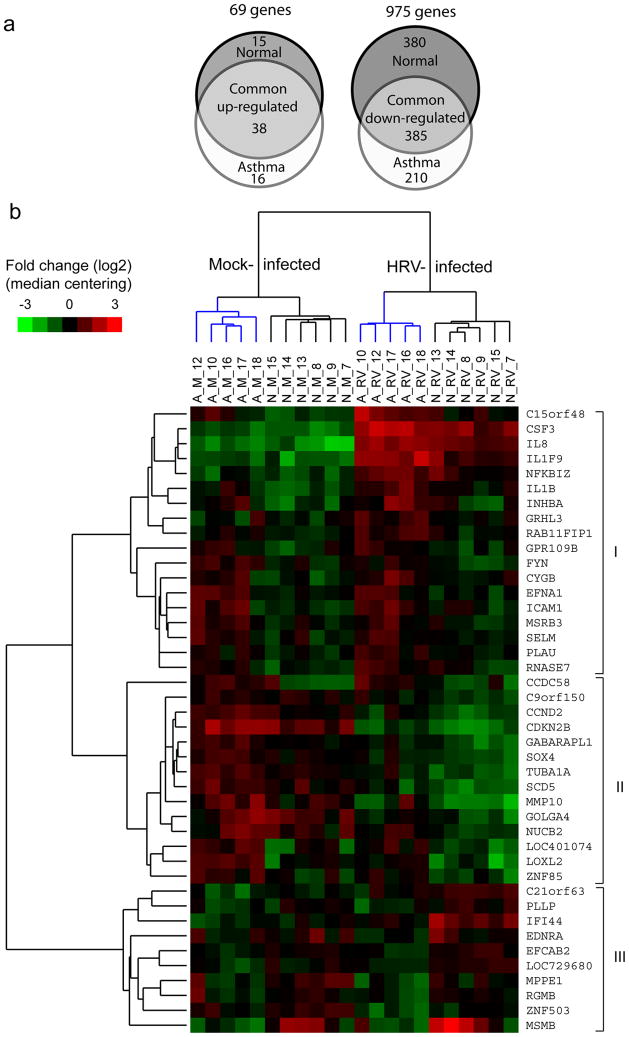
Patterns of gene expression in normal and asthmatic cells. (a) Area-proportional Venn diagrams showing up- and downregulated genes determined in asthma and normal group samples after rhinovirus (RV) infection. Changes in gene expression (greater than or equal to twofold, adjusted P<0.05) common to both groups are shown by overlapping areas. The diagrams were generated with an online tool available at http://www.venndiagram.tk/. (b) Clustering analysis of gene expression patterns. Genes (n=42) were selected based on differential expression in infected cells from asthma vs. normal group, and then expression intensity values of mock- and RV-infected samples were analyzed by hierarchical clustering of samples and genes. The gene expression patterns of asthma (A) and normal (N) samples clustered together for uninfected (M; mock), as well as RV-infected, cells. Clusters of asthma samples are shown in blue. Color bar represents fold changes (log2 scale) in expression for each gene compared with median. HRV, human RV.
Genes exhibiting an increase in expression (greater than or equal to twofold) included 53 found in normal samples and 54 in the asthma group that together comprise 69 unique genes (Supplementary Table 1 online). Among the induced genes were those encoding chemoattractants for granulocytes, macrophages and T lymphocytes (chemokine (C-X-C motif) ligand (CXCL) 1, 2 and 3; interleukin (IL) 8 and chemokine (C-C motif) ligand (CCL) 20), cytokines (colony stimulating factors (CSF) 2 and 3; IL1F9; IL6 and IL24), decoy cytokine receptors (IL1 receptor, type II (IL1R2); IL1 receptor antagonist (IL1RN); IL13 receptor, α 2) and transcription factors and regulators such as early growth response 1 (EGR1), FOS-like antigen 1 (FOSL1), nuclear factor-kappa B (NFκB) inhibitors Z and A (NFKBIZ and NFKBIA) and zinc finger CCCH-type containing 12A (Table 2). Two cytokine genes (IL1F9 and IL24) revealed more robust up-regulation after RV infection in the asthma group. We also observed an increase in expression of antiviral response genes (2′-5′-oligoadenylate synthetase-like (OASL), interferon-induced protein 44 (IFI44) and IL28A (IFN, λ 2)) in normal cells and of regulators of smooth muscle tone (adrenergic receptor, β 2 (ADRB2) and endothelin 1 (EDN1)) in both groups.
We then compared these gene expression changes with results from six additional PBE cell cultures (three normal donors and three donors with asthma, Table 1) that were similarly infected in preliminary studies and explored using the HG Focus chips (Affymetrix) with lower probe density (>8,700 probe sets). All of the probe sets from the smaller chip are also present in the higher-density HG U133 Plus 2.0 arrays to enable comparability. Although we observed some differences in magnitude of changes between two microarray data sets (Table 2), the overall core set of virus-induced genes was similar (Supplementary Table 3 online).
Asthma-specific gene expression profiles in PBE cells
We next compared RV-induced responses in the asthma vs. normal groups. In general, patterns of gene expression were quite similar in the two groups, with a few notable exceptions. Direct comparisons of transcriptional profiles after infection identified 9 genes with ≥ twofold up- or down-regulation in the asthma group compared to normal controls (Table 3). Genes with higher expression in the asthma group included those with functions related to inflammation (IL1F9), tumor suppressor activity (C15orf48) and airway repair and remodeling (inhibin, beta A (INHBA); lysyl oxidase-like 2 (LOXL2); matrix metallopeptidase 10 (MMP10)). In contrast, IFI44, an interferon response gene, and tumor suppressor gene microseminoprotein beta (MSMB) revealed lower expression in the asthma group. Six of these genes were also differentially expressed in mock-infected cells from asthma patients (Table 3).
Table 3.
Differentially expressed genes: asthma vs. normal groups
| Gene symbol | Gene name | Biological function | FDa | P-valueb |
|---|---|---|---|---|
| Differentially expressed after RV infection | ||||
| LOXL2 | lysyl oxidase-like 2 | cross-linking of collagen and elastin | 2.2 | 0.010 |
| MMP10 | matrix metallopeptidase 10 (stromelysin 2) | metalloproteinase | 2.2 | 0.040 |
| C15orf48 | chromosome 15 open reading frame 48 | tumor suppressor | 2.2 | 0.008 |
| LOC401074 | hypothetical LOC401074 | NA | 2.1 | 0.004 |
| CCDC58 | coiled-coil domain containing 58 | NA | 2.1 | 0.010 |
| INHBA | inhibin, beta A | growth/differentiation factor | 2.1 | 0.045 |
| IL1F9 | interleukin 1 family, member 9 | cytokine | 2.0 | 0.004 |
| MSMB | microseminoprotein, beta- | tumor suppressor | −3.6 | 0.031 |
| IFI44 | interferon-induced protein 44 | response to virus, cell-cycle arrest | −2.1 | 0.002 |
| Differentially expressed in mock-infected cells | ||||
| ADAM19 | ADAM metallopeptidase domain 19 | metalloproteinase, cell-matrix interactions | 2.8 | 0.023 |
| SPARC | secreted protein, acidic, cysteine-rich (osteonectin) | synthesis of ECM, changes in cell shape | 2.6 | 0.034 |
| MAP1Bc | microtubule-associated protein 1B | microtubule assembly | 2.6 | 0.014 |
| LOC401074 | NA | NA | 2.3 | 0.014 |
| 227140_atd | NA | NA | 2.3 | 0.038 |
| FN1 | fibronectin 1 | cell adhesion and migration | 2.3 | 0.050 |
| MAN1A1 | mannosidase, alpha, class 1A, member 1 | glycosyl hydrolase | 2.3 | 0.002 |
| AKAP12 | A kinase (PRKA) anchor protein 12 | scaffold protein, binds to protein kinase A | 2.3 | 0.040 |
| CCDC58 | coiled-coil domain containing 58 | NA | 2.2 | 0.014 |
| LOXL2 | lysyl oxidase-like 2 | cross-linking of collagen and elastin | 2.2 | 0.006 |
| CXCL5 | chemokine (C-X-C motif) ligand 5 | neutrophil activation | 2.2 | 0.046 |
| SCG5 | secretogranin V (7B2 protein) | calcium binding | 2.1 | 0.039 |
| TMSB15 | thymosin beta 15a | metastasis of human prostate and breast cancer | 2.1 | 0.015 |
| COL4A1c | collagen, type IV, alpha 1 | ECM structural protein | 2.1 | 0.022 |
| IGF2BP3 | insulin-like growth factor 2 mRNA binding protein 3 | RNA synthesis and metabolism | 2.0 | 0.035 |
| SLC46A3 | solute carrier family 46, member 3 | integral to membrane | 2.0 | 0.031 |
| C15orf48 | chromosome 15 open reading frame 48 | tumor suppressor | 2.0 | 0.019 |
| SERPINE1c | serpin peptidase inhibitor, clade E | plasminogen activator inhibitor | 2.0 | 0.035 |
| INHBA | inhibin, beta A | growth/differentiation factor | 1.9 | 0.028 |
| PPL | periplakin | structural protein, desmosomes | −2.0 | 0.025 |
| MSMBc | microseminoprotein, beta- | tumor suppressor | −2.6 | 0.014 |
ECM, extracellular matrix; FD, fold difference (in expression); NA, not available; RV, rhinovirus.
Negative numbers indicate down-regulation in asthmatic cells compared with normal cells.
Nominal P-values from unpaired t-test comparison of asthma vs. normal samples (either infected or mock-infected).
These genes had more than one probe set that revealed similar expression changes; corresponding fold changes and P-values were averaged.
Gene symbol is not available for the probe set.
Using a less stringent 1.5-fold criterion for group-specific differences in gene expression, a total of 42 genes were identified, including 32 with higher expression and 10 with lower expression in infected cells from the asthma vs. normal groups. (Supplementary Table 4 online). These genes separated the samples into four branches based on hierarchical clustering of gene expression patterns with or without viral infection (Figure 2b). Clusters of genes distinguished by this approach included those with higher expression in asthmatic cells after infection (Cluster I), those with higher expression in both mock-infected and RV-infected asthma samples (Cluster II), and those genes with increased expression in mock- or RV-infected cells from normal donors (Cluster III). Interestingly, one gene from this cluster (MSMB) revealed higher expression both at baseline (mock infection) and after RV infection in the samples from 3 normal female subjects.
Alternative hierarchical clustering of samples using gene expression ratios (fold differences) revealed groupings of up- and down-regulated genes, but did not reveal differences related to asthma (Supplementary Figure 1 online). These findings indicate that most of the asthma-related differences in HRV-induced patterns of gene expression were also present without infection.
Functional analysis of genes affected by RV infection
Functional analysis based on the Gene Ontology classifications (Database for Annotation, Visualization and Integrated Discovery (DAVID)) demonstrated that most genes induced by RV infection of both asthma and normal samples were related to inflammatory responses. Interestingly, there were nine RV-induced genes (CCL5, prostaglandin-endoperoxide synthase 2 (PTGS2), superoxide dismutase 2, CSF2, tumor necrosis factor (TNF), IL1RN, EDN1, ADRB2 and suppressor of cytokine signaling 1 (SOCS1)) that have been associated with asthma in genetic studies (Genetic Association Database (http://geneticassociationdb.hih.gov/)). RV infection inhibited many important biological processes in the host cell including post-translational protein modification, ubiquitin cycle, intracellular transport and mRNA processing (Table 4). Of the 42 genes that were differentially expressed in the asthma vs. normal samples after RV infection, many were classified in the “defense response” and “cell-cell signaling” functional categories.
Table 4.
Functional groupings of genes that are down-regulated after RV infection or differentially expressed in asthma
| DAVID functional group term | Gene numbera | Genesb(%) | P-valuec |
|---|---|---|---|
| Down-regulated genes (975 genes) | |||
| Biological process (GO database) | |||
| post-translational protein modification | 120 | 14% | 3×10−8 |
| biopolymer metabolic process | 310 | 35% | 5×10−7 |
| intracellular transport | 65 | 7% | 2×10−6 |
| ubiquitin cycle | 50 | 6% | 1×10−5 |
| protein transport | 58 | 7% | 9×10−5 |
| mRNA processing | 29 | 3% | 1×10−4 |
| Differentially expressed in asthma compared with normal (42 genes) | |||
| Biological process (GO database) | |||
| defense response | 6 | 14% | 0.008 |
| cell-cell signaling | 7 | 16% | 0.003 |
| response to external stimulus | 7 | 16% | 0.002 |
| cell surface receptor linked signal transduction | 10 | 23% | 0.004 |
| immune response | 7 | 16% | 0.003 |
DAVID, Database for Annotation, Visualization and Integrated Discovery; GO; Gene Ontology; RV, rhinovirus.
Genes involved in the term.
Percentage of involved genes from total gene list.
Modified Fisher exact P-value.
Analysis of the same data set with gene set enrichment analysis (GSEA, Broad Institute, Cambridge, MA) revealed that RV infection of both groups up-regulated genes classified in NFκB, TNF and double-stranded RNA (poly I:C) pathways. Down-regulated genes were related to metabolic pathways for pyruvate, propanoate and steroid biosynthesis, and the Krebs-TCA cycle. Group-specific differences in gene expression patterns after RV infection were found in “local acute inflammatory response” and “genes up-regulated by NFκB” categories. Overall, both functional classification approaches revealed similar findings.
PCR validation of microarray results
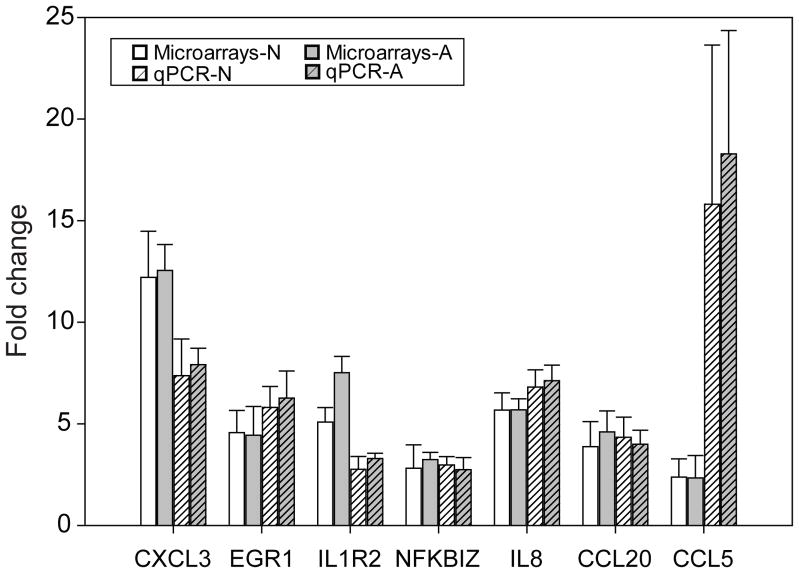
To test the validity of the microarray data, additional samples of cells from normal and asthmatic volunteers were grown, and host cell mRNA was analyzed by quantitative PCR. These additional experiments confirmed virus induction of 7 common up-regulated genes both in normal and asthmatic samples (Figure 3).
Figure 3.
Genes upregulated by rhinovirus (RV) infection: analysis by microarray vs. quantitative reverse transcription (qRT)-PCR. Seven target genes that were up-regulated in both RV-infected normal (N) and asthma (A) samples by microarray were analyzed in separate experiments using qRT-PCR. Expression levels in RV-infected cells were compared with those in mock-infected cells. Expression profiles were determined in six normal and five asthma samples.
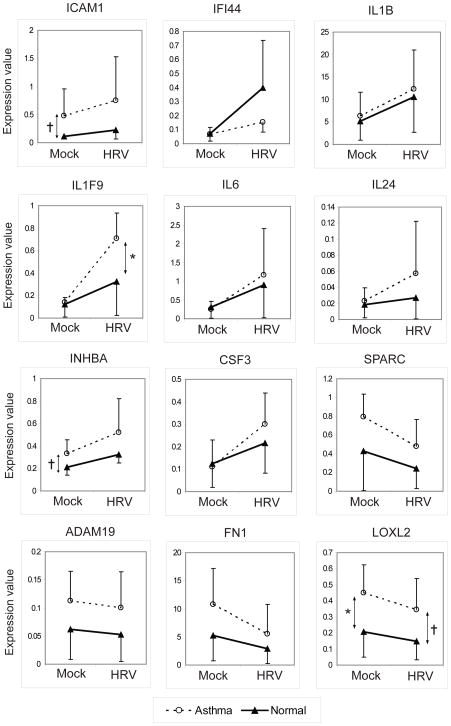
Of the mRNAs that appeared to be more highly expressed in asthma by microarray, similar patterns of expression were identified for several genes by qPCR (Figure 4). Notably, both INHBA and intercellular adhesion molecule 1 (ICAM1) tended to be expressed at higher levels in the uninfected asthma samples (P<0.1), and similar differences were present in infected samples. In contrast, LOXL2 was expressed at higher levels in the asthma samples (P<0.05), and was downregulated by HRV in both groups. Similar nonsignificant patterns were observed for fibronectin 1 (FN1); ADAM metallopeptidase domain 19 (ADAM19), and secreted protein, acidic, cysteine-rich (SPARC). IFI44 tended to be more up-regulated in normal samples after infection. Finally, IL1F9 had similar baseline expression in the two groups, but was more highly induced by RV infection in the asthma group (P<0.05), and similar nonsignificant patterns were noted for CSF3, IL6 and IL24.
Figure 4.
Quantitative PCR analysis of genes differentially expressed in asthma. Genes that were induced (n = 8) or inhibited (n = 4) by rhinovirus (RV) infection, and also differentially expressed in asthma samples by microarray were analyzed in separate experiments using quantitative reverse transcription PCR. Expression values are 2−ΔCt values determined by relative quantification method. Each line represents the mean and standard deviation. *P<0.05; †P<0.1. HRV, human RV.
The microarray analysis identified increased expression of IL28A but not IFNB1 mRNA after RV infection despite the availability of the corresponding probes in genechips. In the validation experiments using qPCR, both IFNB1 and IL28 mRNAs were up-regulated after infection of both normal (7.5-fold (P=0.01) and 6.3-fold (P=0.04), respectively) and asthmatic (8.2-fold (P<0.01) and 5.9-fold (P=0.03), respectively) cells. IL29 gene was also up-regulated after infection, but its very low expression levels were not sufficient for reliable comparisons. There were no significant group-specific differences in RV-induced IFN mRNA expression.
Virus infection induces expression of inflammatory cytokines in vitro
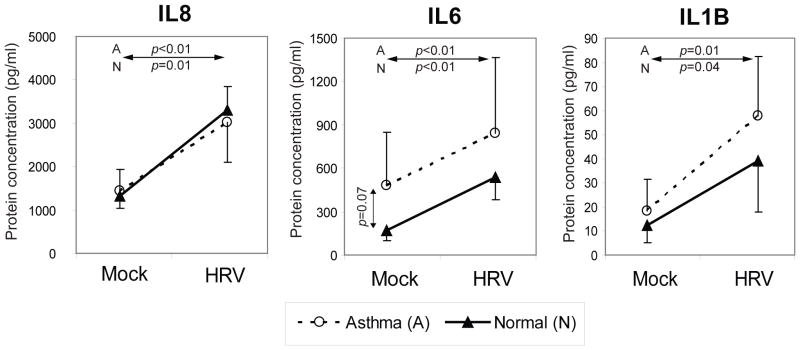
Reagents were available for a subset of differentially expressed genes to test for group-specific differences in RV-induced protein expression. We quantified protein expression of three secreted pro-inflammatory cytokines IL1B, IL6 and IL8 in cell culture media of RV-infected and control samples. Virus infection increased protein levels of all three cytokines both in normal and asthmatic samples (P<0.05) 16 hrs p.i. (Figure 5). IL6 tended to be up-regulated in asthma samples with or without infection, but there were no significant differences between groups.
Figure 5.
Expression of three secreted pro-inflammatory cytokines in cell culture supernatants. Three tested cytokines were significantly induced at 16 h after rhinovirus (RV) infection, both in normal and asthmatic primary bronchial epithelial (PBE) cells (P<0.05); differences between asthma and normal groups were not significant (P>0.05). Each line represents the mean and standard deviation. HRV, human RV.
DISCUSSION
Genome-wide transcriptional analysis was employed to determine whether asthma is associated with a unique pattern of epithelial cell gene expression after RV infection. RV1A induced characteristic CPE and efficiently replicated in PBE cell monolayers producing similar amounts of viral RNA in cells from donors with vs. without asthma. The transcriptional response to RV infection, characterized by robust up-regulation of pro-inflammatory pathways, and down-regulation of cell metabolic processes, was also similar in normal and asthmatic cells. However, both paired (infected vs. mock) and unpaired (asthma vs. normal) comparisons revealed sets of differentially expressed genes related to inflammatory mechanisms and epithelial repair that clearly separated the asthma and normal groups by hierarchical clustering. Notably, most genes that were differentially expressed in the asthma group after RV infection were also differentially expressed in uninfected cells.
Different technical approaches can be used to study responses to viral infection in vitro. We chose to use the undifferentiated cell culture model that has the advantage of allowing analysis of cells that are fairly uniform in their susceptibility to infection. Air-liquid interface cultures of the well-differentiated cells are more resistant to RV infection with a relatively small proportion of infected cells (~5%).5,10,11 Therefore, the transcriptional response to RV infection is measured primarily in uninfected cells. In our system, the much higher rate of cellular infection may account for the fact that RV infection inhibits global host transcription and induces relatively few antiviral genes.12,13
Recent studies using cultured PBE monolayers demonstrated increased (≥10-fold) RV replication in cell monolayers obtained from subjects with atopic asthma while cells from normal volunteers were more resistant to infection.6,7 In contrast to these reports, but in a good agreement with the recent findings in differentiated PBE cell cultures14, we found very similar amounts of viral RNA by qRT-PCR both in supernatants, adherent cells and in total virus yields in cells from normal and asthmatic subjects. Mechanisms that have been proposed to explain enhanced RV replication in asthmatic cells in previous studies are deficient production of type I and III IFN and impaired early induction of apoptosis. In our microarray analysis of RV1A infection, we detected a 2.1-fold induction of the type III IFN mRNA (IL28A) after RV infection in normal cells, and somewhat less (1.6-fold, P=0.115) in cells from the asthma group. Although IFNB1 expression was not detected by microarrays, we used more sensitive qPCR methodology to confirm induction of both IL28 and IFNB1 mRNAs. However, there were no significant differences in IFN expression related to asthma.
It is possible that the different findings were due to differences in either experimental technique, virus strain or subject selection. The subjects in our studies all had mild persistent atopic asthma, and we are currently conducting studies with cells obtained from donors with more severe disease. Interestingly, IFNB1 mRNA induction after RV16 infection was not detected in another study using similar Affymetrix arrays5 indicating a possible problem with sensitivity of this probe set.
RV, like several other picornaviruses, induces gene expression shutoff in host cells via activities of two viral proteinases, 2Apro and 3Cpro that cleave multiple translation and transcription factors and nuclear pore complex proteins.15 Accordingly, more than 90% of differentially expressed genes in our study were reduced in expression, and many of these genes are involved in cell metabolism pathways.
The most highly up-regulated genes were enriched for inflammatory mechanisms, and many of the induced factors (e.g. CSF2, CSF3, IL6, IL8 and TNF) have been previously identified in experimental models and clinical infections3,4,16,17 and shown to play roles in airway inflammation.18–21 Additional inflammatory factors were also upregulated by infection, including cytokines (IL1F9 and IL24) and transcription factors that regulate inflammatory responses in airways (EGR1 and FOSL1).22–25 Notably, some of the up-regulated cytokines and their receptors (e.g. TNF, CCL5, CSF2 and IL1RN) and inflammatory factors (SOCS1, PTGS2, serpin peptidase inhibitor B2 (SERPINB2), and EDN1) have been linked to asthma in genetic and microarray studies and mouse models of asthma.26–34
The main goal of our study was to identify genes that were differentially-expressed with RV infection in the asthma vs. normal cell groups. Two categories of factors were identified by the analysis and hierarchical clustering: 1) different expression levels at baseline and after infection (most common), and 2) similar expression at baseline and different expression after infection. The first category included genes implicated in airway repair and remodeling (INHBA, MMP10, LOXL2, FN1, SPARC), and interestingly, ICAM1, which is used as a receptor by major group RV. These findings provide evidence that epithelial cells from individuals with asthma may be fundamentally different at baseline in the absence of infection. There were relatively few genes that were differentially expressed in asthmatic cells after infection but not at baseline (IL1F9, CSF3, IFI44, IL24). Notably, IL1F9 is up-regulated in PBE cells after microbial exposure,22 and IL24 is the key cytokine to trigger the up-regulation of class I IFNs.35 Additional clinical studies are required to determine whether these cytokines contribute to the increased morbidity of RV infections in patients with asthma.
One of the limitations of our study is that the differences in expression found between normal and asthmatic cells were not statistically significant after correcting for multiple comparisons, and we elected to test the validity of the microarray findings by conducting additional independent experiments that were analyzed by qRT-PCR. Overall, the two techniques demonstrated very good correlation both in terms of direction and magnitude of changes. Moreover, gene expression changes in six additional PBE cell cultures tested in preliminary studies using HG Focus GeneChips were consistent with those discussed in this paper. In addition to mRNA expression, we confirmed that protein expression of three secreted cytokines (IL1B, IL6 and IL8) was induced in cell culture media after RV infection, consistent with microarray and qRT-PCR results. Due to the limited number of replicates, it should be acknowledged that the chances of a type II statistical error are high, and there certainly could be small asthma-related differences in the RV-induced gene expression patterns that were not detected in our study.
In addition to confirmation of our findings by statistical and quantitative means, we compared them with those of other published studies involving microarray analysis. Gene expression profiles in nasal epithelial scrapings after experimental RV16 infection of normal volunteers have demonstrated up-regulation of chemokines, signaling molecules, interferon-responsive genes and antivirals, and a number of these factors (CCL20, SOCS1, SOCS3 and OASL) were also identified in our study of isolated epithelial cells.36 Microarrays have recently been used to analyze inflammatory responses in asthma after allergen challenge, neuropeptide stimulation, and corticosteroid resistance.37–39 In spite of significant differences between cell types and/or stimulus we found overlap between RV-induced changes in gene expression, and those found in brushings of mild asthmatics after allergen challenge (GOS2; IL1RN, IL1B, IL8, SERPINB2, MMP10 and SPARC),37 after neuropeptide stimulation of epithelial cells (INHBA, MMP10, EGR1, SERPINB2, FOSL1, CXCL2, IL8 and PTGS2),38 and in bronchoalveolar lavage cells from subjects with corticosteroid-resistant asthma (IL6, TNF, IL1B, CCL20, IL8, CXCL1, CXCL2, CXCL3, EGR1 and TNFAIP3).39 Taken together, these similarities at transcriptional level could demonstrate the existence of some common mechanisms of asthma.
Overall, we demonstrated similar RV replication rates and transcriptional response to RV1A in normal and asthmatic PBE cells. These findings suggest that factors outside of the epithelial cell, such as airway inflammation and abnormal airway structure and physiology, are important contributors to more severe clinical outcomes of common cold infections in asthma. Even so, our studies identified a subset of epithelial cell genes that were differentially expressed in asthma, compared to normal subjects with functions related to inflammatory pathways and regulation of airway repair and extracellular matrix. Further characterization of these potential asthma-related differences in the epithelial cell response to viral infection should provide a better understanding of molecular mechanisms of virus-induced asthma exacerbations.
METHODS
Cell culture and viral infection
Human PBE cells were obtained from the bronchial brushings of normal and asthmatic individuals (Table 1). Subjects in the asthma group were required to have doctor-diagnosed asthma, and either metacholine PC20 ≤ 8 mg/ml, or at least 12% reversibility in FEV1 after administration of albuterol. Prick skin testing was performed using a panel of 15 common allergens, including grass and tree pollens, dust, dog and cat hair, and a positive response was defined as a wheal size greater than the histamine negative control. Cells were grown at 37°C (5% CO2)in bronchial epithelial growth medium (BEGM, Lonza, Walkersville, MD). Purified and concentrated RV1A was diluted in BEGM with a reduced concentration of hydrocortisone (10−8 M) just before infection. One six-well plate of PBE cells from each patient was either infected with RV1A (10 PFU/cell), or mock-infected with medium alone. At collection (16 h p.i.), cell monolayers were washed three times with phosphate buffered saline and lysed by adding TRIzol Reagent (Invitrogen, Carlsbad, CA). Supernatant and cell lysate samples were stored in microcentrifuge tubes at −80°C until RNA isolation. Detailed information about the cell culture and infection procedures is provided in the Supplementary Materials online. Preliminary experiments to determine the optimal virus dose (MOI of 2, 10, and 50 PFU per cell) and time p.i. (8, 16 and 24 h) were conducted with PBE cells obtained by enzymatic digestion of bronchi from two lung transplants40 and used at passages 2–3. Cells were grown in bronchial epithelial growth media and infected with RV1A as described above.
Optimization of rhinovirus infection procedure for microarray analysis
The minor group RV1A was chosen for this study because minor group viruses infect a much larger percentage of cultured epithelial cells compared to major group viruses,40 and RV1A and RV16 strains have been shown to induce similar expression changes in host cells in vitro.5 We carried out preliminary experiments to establish the optimal infectious dose of the virus and time p.i. that is the most informative for microarray analysis. The major criterion was to have a productive infection with clear CPE in host cells in parallel with sufficient total RNA yield and quality for use as starting material in GeneChip analysis. Previous studies from our laboratory using HG Focus array (Affymetrix, Santa Clara, CA) and cells from three normal subjects demonstrated that the maximal gene expression changes were observed 16 h p.i., and the vast majority of mRNAs up-regulated earlier (4 and 8 h p.i.) remained induced at later time points (unpublished data). We then used this time point to compare different virus doses of infection in PBE cells from two normal lung donors. Infection at MOI of 10 PFU per cell caused distinctive CPE with more than 50% of cells rounded and detached while producing sufficient amount of total RNA (≥10 μg) from adherent cells suitable for GeneChip hybridization (Supplementary Figure 2 online).
RNA extraction and microarray hybridization
Total RNA was isolated from the frozen TRIzol lysates according to manufacturer’s protocol, and then purified by the RNeasy Mini Kit (Qiagen, Hilden, Germany). A total of 10 μg of purified total RNA samples were submitted to University of Wisconsin-Madison Gene Expression Center (Madison, WI) for labeling and hybridization. Following all appropriate protocols and procedures for eukaryotic total RNA quality control, labeling and fragmentation, the biotin labeled cRNA samples were hybridized to either the Human Genome Focus GeneChip Array (samples 1–6) or Human Genome U133 Plus 2.0 GeneChip arrays (samples 7–18) (Affymetrix) according to the manufacturer’s protocols.
Microarray data analysis
The CEL files extracted and processed with Affymetrix GeneChip Operating software (GCOS) were analyzed using Bioconductor41 package “affy” based on R 2.4.1 statistical software (www.r-project.org). Log2-transformed expression values across all the chips were extracted using the Robust Multichip Average (RMA) method.42 Processing with RMA involved background correction, probe level quantile normalization across all the chips and expression summarization. Statistical analysis for detecting differentially expressed genes in two sample comparisons involved either a paired t-test or a two independent sample t-test. We used the Benjamini-Hocberg false discovery rate controlling procedure to account for multiple testing.43 Details are provided in the online supplement. Hierarchical clustering using centroid linkage method was performed based on the selected gene sets using Cluster 3.044 (Human Genome Center, Institute of Medical Science, University of Tokyo, Japan) and visualized using JavaTreeView 1.1.145 (http://jtreeview.sourceforge.net). The microarray data have been submitted to Gene Expression Omnibus (GEO, http://www.ncbi.nlm.nih.gov/projects/geo/) and assigned the accession number GSE13396.
Functional analysis of differentially expressed genes
Annotation and functional clustering of selected probe sets was performed using the DAVID (National Cancer Institute at Frederick, Frederick, MD) web-accessible program.46 Genes with multiple corresponding probe sets were analyzed only when all probe sets demonstrated consistent changes in the same direction (up- or downregulation). The data were analyzed using the “Gene Functional Classification” tool using the “High” classification stringency setting.
Additionally, pathway analysis was performed using Gene Set Enrichment Analysis (GSEA) software47 that determines whether an a priori defined set of genes shows statistically significant differences between two biological states (e.g. mock and virus infection). We have performed GSEA on our pre-ranked list of genes for each comparison of interest. The genes were ranked based on their t-test statistics and GSEA was run in the weighted mode. The main feature of this type of analysis is that it can detect subtle changes present in the data set.
Quantitative RT-PCR (qRT-PCR) validation of microarray results
First-strand cDNAsynthesis was performed using the RT2 First Strand Kit (SuperArray, Frederick, MD). Human RT2 RNA QC PCR Array (SuperArray) was used to assess the quality and integrity of purified RNAs. A total of 11 selected genes differentially expressed in asthma were targeted using custom-designed RT2 Profiler™ PCR Array (SuperArray). The list of target genes, amplicon size and reference positions of SuperArray primers are shown in Supplementary Table S5 online. Expression of 11 additional genes was tested using primers shown in Supplementary Table S6 online. RT2 Real-Time SYBR Green/ROX PCR master mix (SuperArray) was used to perform the reactions. Fold differences were determined by the 2−ΔΔCt method. RV RNA was quantified in supernatants and adherent cells after infection using the two primers and probe described previously.48 Additional details on qRT-PCR are provided in the online supplement.
Protein analysis
Supernatants from rhinovirus- and mock-infected cell cultures were assayed for IL8, IL1B and IL6 proteins. IL8 chemokine concentrations were determined by sandwich ELISA using anti-human IL8 monoclonal antibody in combination with biotinylated polyclonal detection antibody and recombinant IL-8 protein as the standard (R&D systems, Minneapolis, MN). IL1B and IL6 cytokine levels were assessed using human IL1B and IL6 Beadmates™ assays (Millipore, Temecula, CA) according to the manufacturer’s instructions. Luminex 100 (Luminex Corporation, Austin, TX) instrument was used to run plates and generate quantitative data. Sensitivity of the IL1B and IL6 assays for the protocol used was 8.2 pgml−1.
Statistical analysis
Student’s t-test was used to determine the statistical significance of the data. Significance was defined at P<0.05. Statistic calculations were carried out by SigmaPlot 11.0 software (Systat Software, San Jose, CA).
Supplementary Material
Acknowledgments
We thank Dr. Wai-Ming Lee for providing us the purified and concentrated human rhinovirus strain 1A, and Ms. Kathryn Schmit for her technical assistance with qRT-PCR testing. This work was supported by grants NIAID/NIH U19 AI070503-01, NHLBI/NIH P01 HL70831-01 and NHLBI R01HL080412 from National Institutes of Health (Bethesda, MD).
Footnotes
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/mi
DISCLOSURE
The authors declared no conflict of interest.
References
- 1.Corne JM, et al. Frequency, severity, and duration of rhinovirus infections in asthmatic and non-asthmatic individuals: a longitudinal cohort study. Lancet. 2002;359:831–834. doi: 10.1016/S0140-6736(02)07953-9. [DOI] [PubMed] [Google Scholar]
- 2.van Elden LJ, et al. Enhanced severity of virus associated lower respiratory tract disease in asthma patients may not be associated with delayed viral clearance and increased viral load in the upper respiratory tract. J Clin Virol. 2008;41:116–121. doi: 10.1016/j.jcv.2007.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hansbro NG, Horvat JC, Wark PA, Hansbro PM. Understanding the mechanisms of viral induced asthma: new therapeutic directions. Pharmacol Ther. 2008;117:313–353. doi: 10.1016/j.pharmthera.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kallal LE, Lukacs NW. The role of chemokines in virus-associated asthma exacerbations. Curr Allergy Asthma Rep. 2008;8:443–450. doi: 10.1007/s11882-008-0084-9. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, et al. Rhinovirus induces airway epithelial gene expression through double-stranded RNA and IFN-dependent pathways. Am J Respir Cell Mol Biol. 2006;34:192–203. doi: 10.1165/rcmb.2004-0417OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wark PA, et al. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005;201:937–947. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Contoli M, et al. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med. 2006;12:1023–1026. doi: 10.1038/nm1462. [DOI] [PubMed] [Google Scholar]
- 8.Amineva SP, Aminev AG, Palmenberg AC, Gern JE. Rhinovirus 3C protease precursors 3CD and 3CD’ localize to the nuclei of infected cells. J Gen Virol. 2004;85:2969–2979. doi: 10.1099/vir.0.80164-0. [DOI] [PubMed] [Google Scholar]
- 9.Gustin KE, Sarnow P. Inhibition of nuclear import and alteration of nuclear pore complex composition by rhinovirus. J Virol. 2002;76:8787–8796. doi: 10.1128/JVI.76.17.8787-8796.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopez-Souza N, et al. Resistance of differentiated human airway epithelium to infection by rhinovirus. Am J Physiol Lung Cell Mol Physiol. 2004;286:L373–L381. doi: 10.1152/ajplung.00300.2003. [DOI] [PubMed] [Google Scholar]
- 11.Jakiela B, Brockman-Schneider R, Amineva S, Lee WM, Gern JE. Basal cells of differentiated bronchial epithelium are more susceptible to rhinovirus infection. Am J Respir Cell Mol Biol. 2008;38:517–523. doi: 10.1165/rcmb.2007-0050OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neznanov N, et al. Proteolytic cleavage of the p65-RelA subunit of NF-kappaB during poliovirus infection. J Biol Chem. 2005;280:24153–24158. doi: 10.1074/jbc.M502303200. [DOI] [PubMed] [Google Scholar]
- 13.Kotla S, Peng T, Bumgarner RE, Gustin KE. Attenuation of the type I interferon response in cells infected with human rhinovirus. Virology. 2008;374:399–410. doi: 10.1016/j.virol.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 14.Lopez-Souza N, et al. In vitro susceptibility to rhinovirus infection is greater for bronchial than for nasal airway epithelial cells in human subjects. J Allergy Clin Immunol. 2009;123:1384–1390. doi: 10.1016/j.jaci.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyles DS. Cytopathogenesis and inhibition of host gene expression by RNA viruses. Microbiol Mol Biol Rev. 2000;64:709–724. doi: 10.1128/mmbr.64.4.709-724.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newcomb DC, et al. Cooperative effects of rhinovirus and TNF-{alpha} on airway epithelial cell chemokine expression. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1021–L1028. doi: 10.1152/ajplung.00060.2007. [DOI] [PubMed] [Google Scholar]
- 17.Gern JE, Vrtis R, Grindle KA, Swenson C, Busse WW. Relationship of upper and lower airway cytokines to outcome of experimental rhinovirus infection. Am J Respir Crit Care Med. 2000;162:2226–2231. doi: 10.1164/ajrccm.162.6.2003019. [DOI] [PubMed] [Google Scholar]
- 18.Dodge IL, Carr MW, Cernadas M, Brenner MB. IL-6 production by pulmonary dendritic cells impedes Th1 immune responses. J Immunol. 2003;170:4457–4464. doi: 10.4049/jimmunol.170.9.4457. [DOI] [PubMed] [Google Scholar]
- 19.Doganci A, et al. The IL-6R alpha chain controls lung CD4+CD25+ Treg development and function during allergic airway inflammation in vivo. J Clin Invest. 2005;115:313–325. doi: 10.1172/JCI22433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cates EC, et al. Intranasal exposure of mice to house dust mite elicits allergic airway inflammation via a GM-CSF-mediated mechanism. J Immunol. 2004;173:6384–6392. doi: 10.4049/jimmunol.173.10.6384. [DOI] [PubMed] [Google Scholar]
- 21.Yamashita N, et al. Attenuation of airway hyperresponsiveness in a murine asthma model by neutralization of granulocyte-macrophage colony-stimulating factor (GM-CSF) Cell Immunol. 2002;219:92–97. doi: 10.1016/s0008-8749(02)00565-8. [DOI] [PubMed] [Google Scholar]
- 22.Vos JB, et al. Transcriptional response of bronchial epithelial cells to Pseudomonas aeruginosa: identification of early mediators of host defense. Physiol Genomics. 2005;21:324–336. doi: 10.1152/physiolgenomics.00289.2004. [DOI] [PubMed] [Google Scholar]
- 23.Silverman ES, et al. The transcription factor early growth-response factor 1 modulates tumor necrosis factor-alpha, immunoglobulin E, and airway responsiveness in mice. Am J Respir Crit Care Med. 2001;163:778–785. doi: 10.1164/ajrccm.163.3.2003123. [DOI] [PubMed] [Google Scholar]
- 24.Ingram JL, et al. Opposing actions of Stat1 and Stat6 on IL-13-induced up-regulation of early growth response-1 and platelet-derived growth factor ligands in pulmonary fibroblasts. J Immunol. 2006;177:4141–4148. doi: 10.4049/jimmunol.177.6.4141. [DOI] [PubMed] [Google Scholar]
- 25.Adiseshaiah P, Vaz M, Machireddy N, Kalvakolanu DV, Reddy SP. A Fra-1-dependent, matrix metalloproteinase driven EGFR activation promotes human lung epithelial cell motility and invasion. J Cell Physiol. 2008;216:405–412. doi: 10.1002/jcp.21410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szczeklik W, Sanak M, Szczeklik A. Functional effects and gender association of COX-2 gene polymorphism G-765C in bronchial asthma. J Allergy Clin Immunol. 2004;114:248–253. doi: 10.1016/j.jaci.2004.05.030. [DOI] [PubMed] [Google Scholar]
- 27.Buckova D, Izakovicova HL, Vacha J. Polymorphism 4G/5G in the plasminogen activator inhibitor-1 (PAI-1) gene is associated with IgE-mediated allergic diseases and asthma in the Czech population. Allergy. 2002;57:446–448. doi: 10.1034/j.1398-9995.2002.03582.x. [DOI] [PubMed] [Google Scholar]
- 28.Zhu G, et al. Polymorphisms in the endothelin-1 (EDN1) are associated with asthma in two populations. Genes Immun. 2008;9:23–29. doi: 10.1038/sj.gene.6364441. [DOI] [PubMed] [Google Scholar]
- 29.Woodruff PG, et al. Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proc Natl Acad Sci U S A. 2007;104:15858–15863. doi: 10.1073/pnas.0707413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramadas RA, et al. IL-1 Receptor antagonist as a positional candidate gene in a murine model of allergic asthma. Immunogenetics. 2006;58:851–855. doi: 10.1007/s00251-006-0146-x. [DOI] [PubMed] [Google Scholar]
- 31.Kumar A, Gupta V, Changotra H, Sarin BC, Sehajpal PK. Tumor necrosis factor - alpha and transforming growth factor - beta1 polymorphisms in bronchial asthma. Indian J Med Sci. 2008;62:323–330. [PubMed] [Google Scholar]
- 32.Kamali-Sarvestani E, Ghayomi MA, Nekoee A. Association of TNF-alpha -308 G/A and IL-4 -589 C/T gene promoter polymorphisms with asthma susceptibility in the south of Iran. J Investig Allergol Clin Immunol. 2007;17:361–366. [PubMed] [Google Scholar]
- 33.Lachheb J, Chelbi H, Hamzaoui K, Hamzaoui A. Association between RANTES polymorphisms and asthma severity among Tunisian children. Hum Immunol. 2007;68:675–680. doi: 10.1016/j.humimm.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 34.Harada M, et al. Functional polymorphism in the suppressor of cytokine signaling 1 gene associated with adult asthma. Am J Respir Cell Mol Biol. 2007;36:491–496. doi: 10.1165/rcmb.2006-0090OC. [DOI] [PubMed] [Google Scholar]
- 35.Ekmekcioglu S, Mumm JB, Udtha M, Chada S, Grimm EA. Killing of human melanoma cells induced by activation of class I interferon-regulated signaling pathways via MDA-7/IL-24. Cytokine. 2008;43:34–44. doi: 10.1016/j.cyto.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Proud D, et al. Gene expression profiles during in vivo human rhinovirus infection: insights into the host response. Am J Respir Crit Care Med. 2008;178:962–968. doi: 10.1164/rccm.200805-670OC. [DOI] [PubMed] [Google Scholar]
- 37.Lilly CM, Tateno H, Oguma T, Israel E, Sonna LA. Effects of allergen challenge on airway epithelial cell gene expression. Am J Respir Crit Care Med. 2005;171:579–586. doi: 10.1164/rccm.200404-532OC. [DOI] [PubMed] [Google Scholar]
- 38.Vendelin J, et al. Downstream target genes of the neuropeptide S-NPSR1 pathway. Hum Mol Genet. 2006;15:2923–2935. doi: 10.1093/hmg/ddl234. [DOI] [PubMed] [Google Scholar]
- 39.Goleva E, et al. Corticosteroid-resistant asthma is associated with classical antimicrobial activation of airway macrophages. J Allergy Clin Immunol. 2008;122:550–559. doi: 10.1016/j.jaci.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schroth MK, et al. Rhinovirus replication causes RANTES production in primary bronchial epithelial cells. Am J Respir Cell Mol Biol. 1999;20:1220–1228. doi: 10.1165/ajrcmb.20.6.3261. [DOI] [PubMed] [Google Scholar]
- 41.Gentleman RC, et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Irizarry RA, et al. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003;31:e15. doi: 10.1093/nar/gng015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Statist Soc Ser B. 1995;57:289–300. [Google Scholar]
- 44.de Hoon MJ, Imoto S, Nolan J, Miyano S. Open source clustering software. Bioinformatics. 2004;20:1453–1454. doi: 10.1093/bioinformatics/bth078. [DOI] [PubMed] [Google Scholar]
- 45.Saldanha AJ. Java Treeview--extensible visualization of microarray data. Bioinformatics. 2004;20:3246–3248. doi: 10.1093/bioinformatics/bth349. [DOI] [PubMed] [Google Scholar]
- 46.Dennis G, Jr, et al. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003;4:3. [PubMed] [Google Scholar]
- 47.Subramanian A, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mosser AG, et al. Quantitative and qualitative analysis of rhinovirus infection in bronchial tissues. Am J Respir Crit Care Med. 2005;171:645–651. doi: 10.1164/rccm.200407-970OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.