Abstract
The activation of the phenylpropanoid pathway in plants by environmental stimuli is one of the most universal biochemical stress responses known. In tomato plant, rubbing applied to a young internode inhibit elongation of the rubbed internode and his neighboring one. These morphological changes were correlated with an increase in lignification enzyme activities, phenylalanine ammonia-lyase (PAL), cinnamyl alcohol dehydrogenase (CAD) and peroxidases (POD), 24 hours after rubbing of the forth internode. Furthermore, a decrease in indole-3-acetic acid (IAA) content was detected in the rubbed internode and the upper one. Taken together, our results suggest that decrease in rubbed internode length is a consequence of IAA oxidation, increases in enzyme activities (PAL, CAD and POD), and cell wall rigidification associated with induction of lignification process.
Key words: Mechanical stimulation, PAL, CAD, POD, IAA
In their environment, plants are constantly submitted to several stimuli such as wind, rain and wounding. The growth response of plants to such stimuli was termed thigmomorphogenesis and was observed in a wide range of plants.1–3 The most common thigmomorphogenetic response is a retardation of tissue elongation accompanied by an increase in thickness.4 The plant response to mechanical perturbation is mainly restricted to the young developing internode, since no influence can be detected when the internode has reached its final length.5,6 These plant growth modifications, which characterize thigmomorphogenesis, are related to biochemical events associated with lignification process7 and ethylene production.8,9
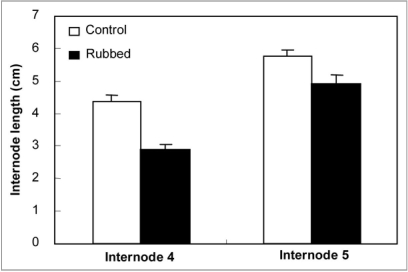
In tomato plant the length of internodes 4 (N4) and 5 (N5) was measured 14 days after rubbing of the fourth internode. Results reported in Figure 1 show that rubbing led to a significant reduction of elongation of the stressed internode (N4) (decrease of N4 length from 4.3 cm in the control plant to 2.9 in the rubbed one). This effect was not limited to the rubbed area but affected also the elongation of the neighboring internodes (N5) that were shorter in rubbed plants than in control ones.
Figure 1.
Internode lengths of control and rubbed plants measured 14 day after mechanical stress applied to the fourth internode. Standard errors are indicated by vertical bars.
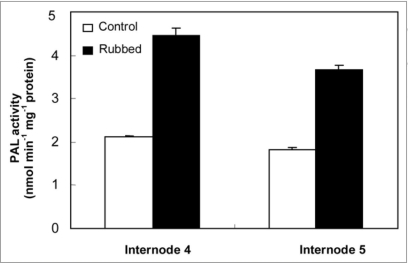
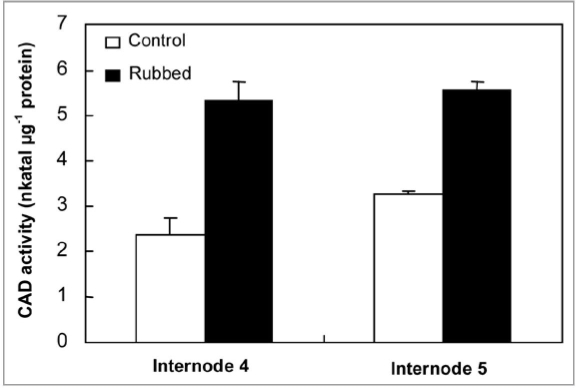
Results reported in Figure 2 show an increase in PAL activity in both internodes N4 and N5, 24 hours after mechanical stress application as compared with corresponding controls. CAD activity was also investigated in N4 and N5, 24 h after rubbing of the fourth internode. Results presented in Figure 3 show that mechanical stress application induces a strong increase of CAD activity in the rubbed internode N4 (5.3 nkatal μg-1 protein) with an approximately two-fold increase when compared to control tomato internodes (2.3 nkatal μg-1 protein). Further, CAD activity in N5 was also increased in the rubbed internode (5.538 nkatal μg-1 protein) as compared with the control one (3.256 nkatal μg-1 protein).
Figure 2.
PAL activity of internode 4, and 5 in control and rubbed plants 24 h after rubbing of the fourth internode. Standard errors are indicated by vertical bars.
Figure 3.
CAD activity of internode 4, and 5 in control and rubbed plants 24 h after rubbing of the fourth internode. Standard errors are indicated by vertical bars.
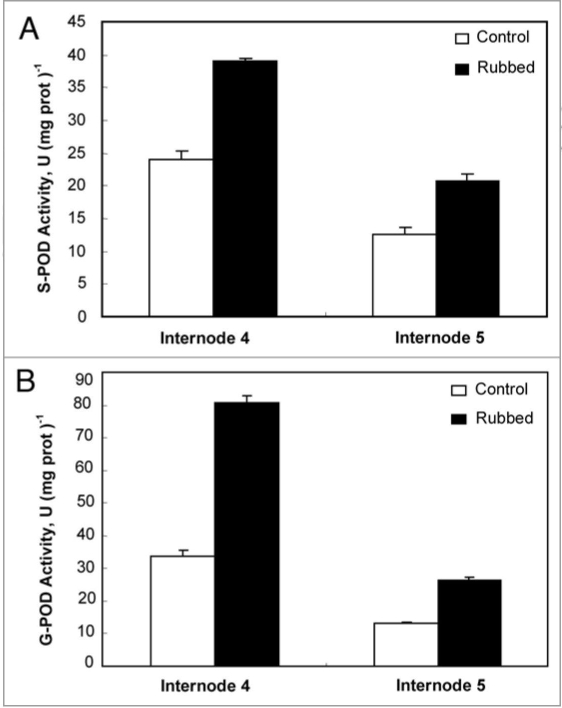
Syringaldazine (S-POD) and gaïacol (G-POD) peroxidase activities were measured in tomato N4 and N5. Results reported in Figure 4 show an increase in soluble peroxidase activity with both substrates in the rubbed internode N4 as compared with control plant. Enhancement in peroxidase activities in N4 was more pronounced with gaïacol (80.7 U) as an electron donor than syringaldazine (33.8 U). Similar results were observed in internode 5 as compared with control one (Fig. 4).
Figure 4.
(A) Syringaldazine-POD (Syr-POD) activity of internode 4 and 5 in control and rubbed plants 24 h after rubbing of the fourth internode. Standard errors are indicated by vertical bars. (B) Gaiacol-POD (G-POD) activity of internode 4 and 5 in control and rubbed plants 24 h after rubbing of the fourth internode. Standard errors are indicated by vertical bars.
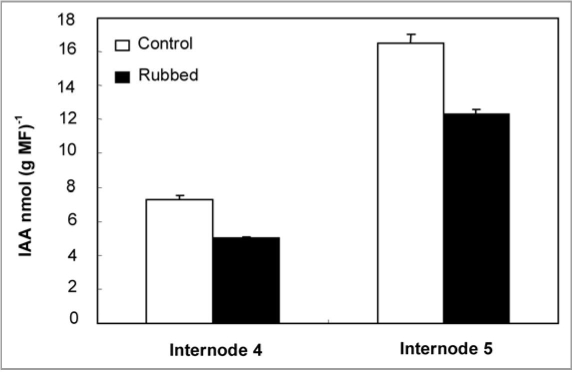
IAA was quantified in control and rubbed plant internodes 24 h after rubbing of the fourth internode. Results reported in figure 5 show that in control sample and as expected, the content of IAA was found to be higher in the younger internode (N5) as compared to the older one (N4). Rubbing led to a significant decrease in IAA levels in N4 (5.06 nmol g−1 MF−1) as compared with corresponding controls (7.27 nmol g−1 MF−1). Similar results were observed in internode 5, where IAA content was reduced from 16.52 nmol g−1 MF−1 in control internode to 12.35 nmol g−1 MF−1 in the rubbed internode (Fig. 5).
Figure 5.
IAA Level of internode 4 and 5 in control and rubbed plants 24 h after rubbing of the fourth internode. Standard errors are indicated by vertical bars.
The results reported here establish an evident correlation between growth limitation of the rubbed internode and their degree of lignification, the increase in lignification enzymes activities and auxin degradation after mechanical stress application.
Auxin seems to be involved in thigmomorphogenesis.10 It was proposed that MIS (Mechanically-induced stress) has opposite effects on auxin levels in the two species studied to date, Phaseolus vulgaris10 and Bryonia dioica.11,12 Auxin level as measured by bioassay, increased in Phaseolus vulgaris following rubbing of the stem.10 It was proposed that a build up of auxin may result from the reduced polar transport of IAA at the rubbed internode, causing a build up of IAA in the stem tissue. Exogenous IAA did not reverse the MIS inhibition of growth in Phaseolus vulgaris and high levels of IAA retarded growth in non-stressed plants.10 Thus, retardation of extension growth in Phaseolus vulgaris may have been caused by high levels of endogenous auxin and the increase in stem diameter by increased ethylene production.4 However, ethylene increases radial growth only if auxin is present.13
Boyer11 reported a decrease in auxinlike activity in Bryonia dioica following MIS and this was confirmed in the same species by Hofinger et al.12 who reported a decrease in IAA using gas chromatography-mass spectrometry. Auxin catabolism was accompanied with changes in both soluble and ionically bound cell wall basic peroxidases14 and the appearance of an additional peroxidase. This can suggest that in Bryonia, auxin catabolism is hastened by mechanical stimulated peroxidase. In addition, Boyer et al.15 reported that lithium pre-treatment prevents both thigmomorphogenesis and appearance of specific cathodic isoperoxidase in Bryonia plants subjected to MIS. This is give further credence to the possibility that the peroxidase-auxin system is involved in Bryonia thigmomorphogenesis. In addition, ethylene increases peroxidase activity which reduces the auxin content in the tissue to a level low enough not to support normal growth. We have evidence that decrease of auxin level contribute to mechanism leading to tomato internode inhibition subjected to mechanical stress.
Growth inhibition has been suggested to be the result of tissues lignification.6 As the initial enzyme in the monolignol biosynthesis pathway, PAL has a direct influence on lignin accumulation.16 The characteristics of lignin differ among cell wall tissues and plant organs.17 It comprises polyphenolic polymers derived from the oxidative polymerization of different monolignols, including p-coumaryl, coniferyl and sinapyl alcohols via a side pathway of phenylalanine metabolism leading to lignin synthesis.18 The increase in lignin content in the rubbed tomato internode could be a response mechanism to mechanical damage caused by rubbing.3 It is known that plants create a natural barrier that includes lignin and suberin synthesis, components directly linked to support systems.19,20
The increase in lignin content of rubbed tomato internode3 is paralleled by a rise in CAD activity and whilst such direct proportionality between CAD activity and lignin accumulation does not always agree with the results in the literature, it clearly is responding in ways similar to those of the other enzymes in the pathway.21
Mechanical stress-induced membrane depolarization would generate different species of free radicals and peroxides, which in turn initiate lipid peroxidation.22 The degradation of cell membranes is suggested to bring about rapid changes in ionic flux, especially release of K+ which would result in an enhanced endogenous Ca/K ratio and in leakage of solutes, among them electron donors such as ascorbic acid and phenolic substances. The increased intracellular relative calcium level activated secretion of basic peroxidases23 into the free space where, in association with the electron donors and may be with the circulating IAA, they eliminate the peroxides, and facilitated binding of basic peroxidases to membrane structures allowing a role as 1-aminocyclopropane-1-carboxylic acid (ACC)-oxidases. The resulting IAA and ACC oxidase-mediated changes in ethylene production24 would further induce (this time through the protein synthesis machinery) an increase in activity of phenylalanine ammonia-lyase and peroxidases. The resulting lignification and cell wall rigidification determines the growth response of tomato internode to the mechanical stress.
Abbreviations
- PAL
phenylalanine ammonia-lyase
- CAD
cinnamyl alcohol dehydrogenase
- POD
peroxidases
- IAA
auxine
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/10302
References
- 1.Jaffe MJ. Thigmomorphogenesis: the response of plant growth and development to mechanical stimulation with special reference to Bryonia dioica. Planta. 1973;114:143–157. doi: 10.1007/BF00387472. [DOI] [PubMed] [Google Scholar]
- 2.Braam J, Davis RW. Rain, wind and touch-induced expression of calmodulin and calmodulin-related genes in Arabidopsis. Cell. 1990;60:357–364. doi: 10.1016/0092-8674(90)90587-5. [DOI] [PubMed] [Google Scholar]
- 3.Saidi I, Ammar S, Demont-Caulet N, Thévenin J, Lapierre C, Bouzid S, et al. Thigmomorphogenesis in Solanum lycopersicum: Morphological and biochemical responses in stem after mechanical stimulation. Plant Sci. 2009;177:1–6. doi: 10.4161/psb.5.2.10302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biro RL, Hunt ER, Erner T, Jaff MJ. Thigmomorphogenesis: changes in cell division and elongation in the internodes of mechanically perturbed or ethrel-treated bean plants. Ann Bot. 1980;45:655–664. [Google Scholar]
- 5.Erner Y, Biro R, Jaffe MJ. Thigmomorphogenesis: evidence for a translocable thigmomorphogenetic factor induced by mechanical perturbation in beans (Phaseolus vulgaris. L) Physiol Plant. 1980;50:21–25. [Google Scholar]
- 6.Dépège N, Thonat C, Coutand C, Julien JL, Boyer N. Morphological responses and molecular modification in tomato plants after mechanical stimulation. Plant Cell Physiol. 1997;38:1127–1134. doi: 10.1093/oxfordjournals.pcp.a029097. [DOI] [PubMed] [Google Scholar]
- 7.De Jaegher G, Boyer N, Gaspar T. Thigmomorphogenesis in Bryonia dioica: change in soluble and wall peroxidase, phenylalanine ammonialyase activity, cellulose, lignin content and monomeric constituents. Plant Growth Regul. 1985;3:133–148. [Google Scholar]
- 8.Boyer N. Corrélation de croissance dans les plantes à vrilles. Ann Sci Nat Bot. 1973;14:303–306. (Fre). [Google Scholar]
- 9.Boyer N, Desbiez MO, Hofinger M, Gaspar T. Effect of lithium on thigmomorphogenesis in Bryonia dioica. Ethylene production and sensitivity. Plant Physiol. 1983;72:522–525. doi: 10.1104/pp.72.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erner Y, Jaffe MJ. Thigmomorphogenesis: The involvement of auxin and abscisic acid in growth retardation due to mechanical perturbation. Plant Cell Physiol. 1982;23:935–941. [Google Scholar]
- 11.Boyer N. Modifications de la croissance de la tige de Bryone (Bryonia dioica) à la suite d’irritations tactiles. C R Acad Sc Paris. 1967;264:2114–2117. (Fre). [Google Scholar]
- 12.Hofinger M, Chappelle B, Boyer N, Gaspar T. GC-MS identification and titration of IAA in mechanically perturbed Bryonia dioica. Plant Physiol. 1979:63. [Google Scholar]
- 13.Osborne DJ. The ethylene, regulation of cell growth in specific target tissue of plants. In: Wareing PF, editor. Plant Growth Substances. London.: Academic Press; 1982. pp. 279–290. [Google Scholar]
- 14.Grambow HJ, Langenbeck-Schwich B. The relationship between oxidase activity, peroxidase activity hydrogen peroxide, and phenolic compounds in the degradation of indole-3-acetic acid in vitro. Planta. 1983;157:131–137. doi: 10.1007/BF00393646. [DOI] [PubMed] [Google Scholar]
- 15.Boyer N, Gaspar T, Lamond M. Modification des isoperoxydases et de l’allongement des entre-noeuds de Bryone à la suite des irritations mécaniques. Z Pflanzenphysiol. 1979;93:459–470. (Fre). [Google Scholar]
- 16.Campos R, Nonogaki H, Suslow T, Saltveit ME. Isolation and characterization of a wound inducible phenylalanine ammonia-lyase gene (LsPAL1) from Romaine lettuce leaves. Physiol Plant. 2004;121:429–438. [Google Scholar]
- 17.Grabber JH, Ralph J, Lapierre C, Barriere Y. Genetic and molecular basis of grass cell-wall degradability I. Lignin-cell wall matrix interactions. C R Biol. 2004;327:455–465. doi: 10.1016/j.crvi.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 18.Whetten R, Sederoff R. Lignin biosynthesis. Plant Cell. 1995;7:1001–1013. doi: 10.1105/tpc.7.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vance CP, Kirk TK, Sherwood RT. Lignification as a mechanism of disease resistance. Ann Rev Phytopathol. 1980;18:259–288. [Google Scholar]
- 20.Aquino-Bolañosa EN, Mercado-Silva E. Effects of polyphenol oxidase and peroxidase activity, phenolics and lignin content on the browning of cut jicama. Postharv Biol Technol. 2004;33:275–283. [Google Scholar]
- 21.Mitchell H, Hall L, Barber MS. Elicitor-induced cinnamyl alcohol dehydrogenase activity in lignifying wheat (Triticum aestivum L.) leaves. Plant Physiol. 1994;104:551–556. doi: 10.1104/pp.104.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dépège N, Varenne M, Boyer N. Induction of oxidative stress and GPX-like protein activation in tomato plants after mechanical stimulation. Physiol Plant. 2000;10:209–214. [Google Scholar]
- 23.Van huystee RB, Esnault R. Reflections on peanut peroxidase regulation of growth. Plant Perox Newslett. 1995;6:8–10. [Google Scholar]
- 24.Biro RL, Jaffe MJ. Thigmomorphogenesis: Ethylene evolution and its role in the changes observed in mechanically perturbed bean plants. Physiol Plant. 1984;62:289–296. doi: 10.1111/j.1399-3054.1984.tb05925.x. [DOI] [PubMed] [Google Scholar]