Abstract
The sugar alcohol mannitol and it’s catabolic enzyme mannitol dehydrogenase (MTD), in addition to welldocumented roles in metabolism and osmoprotection, may play roles in hostpathogen interactions. Research suggests that in response to the mannitol that pathogenic fungi secrete to suppress reactive oxygen-mediated host defenses, plants make MTD to catabolize fungal mannitol. Yet previous work suggested that pathogen-secreted mannitol is extracellular, while in healthy plants MTD is cytoplasmic. We have presented results showing that the normally cytoplasmic MTD is exported into the cell wall or extracellular space in response to the endogenous inducer of plant defense responses salicylic acid (SA). This SA-induced secretion is insensitive to brefeldin A, an inhibitor of Golgimediated protein transport. Together with the absence of MTD in Golgi stacks and the lack of a documented extracellular targeting sequence in the MTD protein, this suggests MTD is secreted by a non-Golgi, pathogen-activated secretion mechanism in plants. Here we discuss the potential significance of non-Golgi secretion in response to stress.
Key words: protein secretion, mannitol metabolism, plant-pathogen interaction, extracellular space, apoplast
Introduction
Because the cell wall is a major interface between plant cells and their environment, the rapid, regulated secretion of specific proteins into this extracellular space (the apoplast) is an important defense response.1 Secretion of defense proteins in both plants and animals was originally thought to be solely via an endoplasmic reticulum (ER)/Golgi-mediated pathway, with an N-terminal signal peptide directing the protein to the ER for routing, modification and subsequent secretion via the Golgi. However, the existence of alternate secretion mechanisms was suggested when Auron et al.2 reported that interleukin 1 (IL1β), a cytokine with no signal peptide, was secreted from human monocytes in response to Staphylococcus. Since then, numerous Golgi-independent or “leaderless” eukaryotic secretion mechanisms have been reported, and the importance of these pathways, particularly in response to stress, is well established. 3 Although non-Golgi secretion has been described in many other eukaryotes, our report that the normally cytoplasmic enzyme, mannitol dehydrogenase (MTD) is secreted by tobacco in response to salicylic acid (SA) is one of the few reports suggesting that non-classical secretion also occurs in plants.4
The regulated conversion of mannitol to mannose by MTD in the cytosol of plants such as celery (Apium graveolens) allows mannitol to be used as both a metabolite and an osmoprotectant.5 There is now compelling evidence that mannitol and MTD also play roles in plant-pathogen interactions. Mannitol’s antioxidant activity6,7 together with its secretion by fungal pathogens8–10 allows these pathogens to use mannitol to quench reactive oxygen species (ROS) that signal plant defenses. In response, pathogen-induced plant MTD was hypothesized to catabolize this mannitol, thus protecting the host’s ROS-mediated defenses.5,11,12 However, while fungal mannitol secreted during infection is found in the extracellular space,8,11 MTD is cytoplasmic in uninfected celery.13 If MTD catabolizes fungal mannitol, then mannitol and MTD must somehow become co-localized. In Cheng et al.4 we demonstrated that MTD is exported to the apoplast in response to SA, an endogenous inducer of many plant defense responses. This is of special significance because MTD lacks a recognized ER/Golgi signal sequence, and its SA-induced secretion is not inhibited by an inhibitor of Golgi-mediated protein transport, brefeldin A. Thus, contrary to the model that PR-protein secretion occurs only via the ER/Golgi pathway, secretion of MTD appears to be by a non- Golgi mechanism.
Types of Non-Golgi Secretion
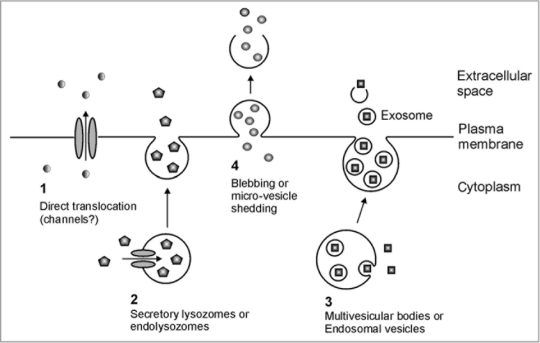
The existence of leaderless secretion in animals is well accepted, and four general mechanisms have been proposed (Fig. 1): (1) Translocation directly across the plasma membrane: proteins such as fibroblast growth factor 1 (FGF-1)14 and IL1α15 are translocated directly across the plasma membrane via the chaperonin-like action of hydrophobic “release complexes” or through transmembrane channels. (2) Endolysosomal pathways: normally cytoplasmic proteins such as IL-1β16 and HMBG1,17 are transported into intracellular vesicles called endolysosomes via protein-conducting channels such as ATP-binding cassette (ABC) transporters. These endolysosomes then fuse with the cell membrane to release the proteins into the apoplast. (3) Exosome-mediated secretion: first intracellular vesicles called endosomes are generated by inward budding from the plasma membrane. Cytosolic proteins, such as HSP90 are then packaged into the endosomes by further inward budding to form multivesicular bodies. These next fuse with the plasma membrane to release the included, formerly cytosolic components.18 (4) Membrane blebbing or microvesicle shedding: cytosolic secretory transglutaminase and galectins19 are proposed to be packaged directly into membrane-derived blebs, or protrusions. The blebs detach and the formerly cytosolic components are released when the blebs rupture.
Figure 1.
Proposed mechanisms for non-Golgi secretion of normally cytoplasmic proteins in animals. (1) Translocation directly across the plasma membrane. (2) Endolysozomal or secretory lysosomal pathway. (3) Intra-endosomal vesicle or multi-vesicular body pathway. (4) Membrane blebbing or micro-vesicle shedding. Adapted from Nickel and Rabouille.3
Non-classically secreted proteins appear to be first modified in response a stress mediated signal, enabling them to interact with the relevant secretion machinery.20 These proteins are then apparently transported across the membranes in a folded or native configuration like FGF2.21 As a result, the “targeting signal” is an integrated feature of the protein’s three-dimensional structure rather than a simple linear amino acid sequence.
Why Non-Golgi Secretion?
Proteins might be secreted by nonclassical mechanisms for a number of reasons. For instance, non-Golgi secretion would be necessary if the presence of a protein in the ER/Golgi would disrupt ER functioning. For example, in animal cells the non-Golgi secretion of galectin 1 keeps this β-galactose-binding lectin from binding glycolipids and glycoproteins in the lumen of the Golgi/ER.22 Non-Golgi secretion could also be desirable if a protein has multiple functions, with each occurring in a different cellular compartment. HMGB1 (High mobility group box 1), for example, is normally found in the nucleus, where it mediates DNAbinding complex assembly. Upon bacterial induction of monocytes, HMGB1 is secreted into the extracellular space where it acts as an endotoxin mediator or a cell differentiation signal.23 As previously documented,5 MTD has normal roles in central metabolism and osmoregulation in the cytoplasm. When mobilized during pathogen attack, however, MTD has a very different role in the extracellular space. As MTD’s normal role in central metabolism requires cytoplasmic localization, a nonclassical mechanism is needed to mediate its subsequent secretion in response to pathogen attack.
There are a growing number of indications that non-Golgi mechanisms are involved in the secretion of proteins other than MTD in plants. For instance, although extracellular SODs (ecSOD’s) containing a classical signal peptide are well known in animals, plant SODs lack such a signal sequence, and were long thought to be absent from the apoplast. This raised questions about how superoxide was converted to H2O2 in the apoplast during the early stages of defense responses. In fact, many researchers are now reporting that SOD activity is present in the apoplast of stress or pathogen-induced plants.24–28
Subsequent mass spectrophotometric analyses of SA-induced protein secretion have not only confirmed the presence of SOD in the secretome,29–31 but suggest that a large number of proteins whose regulated secretion occurs soon after SA treatment lack a classical signal peptide.31 Finally, this is consistent with the report that pathogen-induced apoplastic H2O2 production in Arabidopsis is insensitive to BFA.32 Taken together, the preponderance of data suggests the existence of a novel, pathogen-activated, protein secretion mechanism that mediates pathogeninduced secretion of normally cytoplasmic enzymes in plants.
Acknowledgements
This work was supported by grants from the US Department of Agriculture and the North Carolina Agricultural Service to J.D.W. and D.M.P.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/10304
References
- 1.Grant M, Lamb C. Systemic immunity. Curr Opin Plant Biol. 2006;9:414–420. doi: 10.1016/j.pbi.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 2.Auron PE, Warner SJ, Webb AC, Cannon JG, Bernheim HA, McAdam KJ, et al. Studies on the molecular nature of human interleukin 1. J Immunol. 1987;138:1447–1456. [PubMed] [Google Scholar]
- 3.Nickel W, Rabouille C. Mechanisms of regulated unconventional protein secretion. Nature Rev Mol Cell Biol. 2009;10:148–155. doi: 10.1038/nrm2617. [DOI] [PubMed] [Google Scholar]
- 4.Cheng F-y, Zamski E, Guo W-w, Pharr DM, Williamson JD. Salicylic acid stimulates secretion of the normally symplastic enzyme mannitol dehydrogenase (MTD): a possible defense against mannitol secreting fungal pathogens. Planta. 2009;230:1093–1103. doi: 10.1007/s00425-009-1006-3. [DOI] [PubMed] [Google Scholar]
- 5.Stoop JMH, Williamson JD, Pharr DM. Mannitol metabolism in plants: a method for coping with stress. Trends Plant Sci. 1996;1:139–144. [Google Scholar]
- 6.Shen B, Jensen RG, Bohnert HJ. Increased resistance to oxidative stress in transgenic plants by targeting mannitol biosynthesis to the chloroplast. Plant Physiol. 1997;113:1177–1183. doi: 10.1104/pp.113.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Voegele RT, Hahn M, Lohaus G, Link T, Heiser I, Mendgen K. Possible roles for mannitol and mannitol dehydrogenase in the biotrophic plant pathogen Uromyces fabae. Plant Physiol. 2005;137:190–198. doi: 10.1104/pp.104.051839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jennings DB, Ehrenshaft M, Pharr DM, Williamson JD. Roles for mannitol and mannitol dehydrogenase in active oxygen mediated plant defense. Proc Natl Acad Sci USA. 1998;95:15129–15133. doi: 10.1073/pnas.95.25.15129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joosten MHAJ, Hendrickx LJM, de Wit PJGM. Carbohydrate composition of apoplastic fluids isolated from tomato leaves inoculated with virulent or avirulent races of Cladosporium fulvum. Eur J Plant Pathol. 1990;96:103–112. [Google Scholar]
- 10.Vélë H, Glassbrook NJ, Daub ME. Mannitol biosynthesis is required for plant pathogenicity by Alternaria alternata. FEMS Microbiol Lett. 2008;285:122–129. doi: 10.1111/j.1574-6968.2008.01224.x. [DOI] [PubMed] [Google Scholar]
- 11.Williamson JD, Stoop JMH, Massel MO, Conkling MA, Pharr DM. Sequence analysis of a mannitol dehydrogenase cDNA from plants suggests a function for the PR protein ELI3. Proc Natl Acad Sci USA. 1995;92:7148–7152. doi: 10.1073/pnas.92.16.7148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jennings DB, Daub ME, Pharr DM, Williamson JD. Constitutive expression of a celery mannitol dehydrogenase in tobacco enhances resistance to the mannitol-secreting fungal pathogen Alternaria alternata. Plant J. 2002;32:41–49. doi: 10.1046/j.1365-313x.2001.01399.x. [DOI] [PubMed] [Google Scholar]
- 13.Zamski E, Yamamoto YT, Williamson JD, Conkling MA, Pharr DM. Immunolocalization of mannitol dehydrogenase in celery plants and cells. Plant Physiol. 1996;112:931–938. doi: 10.1104/pp.112.3.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jackson A, Friedman S, Zhan X, Engleka KA, Forough R, Maciag T. Heat shock induces the release of fibroblast growth factor 1 from NIH 3T3 cells. Proc Natl Acad Sci USA. 1992;89:10691–10695. doi: 10.1073/pnas.89.22.10691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watanabe N, Kobayashi Y. Selective release of a processed form of interleukin 1alpha. Cytokine. 1994;6:597–601. doi: 10.1016/1043-4666(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 16.Andrei C, Dazzi C, Lotti L, Torrisi MR, Chimini G, Rubartelli A. The secretory route of the leaderless protein interleukin 1beta involves exocytosis of endolysosome-related vesicles. Mol Biol Cell. 1999;10:1463–1475. doi: 10.1091/mbc.10.5.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gardella S, Andrei C, Ferrera D, Lotti LV, Torrisi MR, Bianchi ME, et al. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesiclemediated secretory pathway. EMBO Rep. 2002;3:995–1001. doi: 10.1093/embo-reports/kvf198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu X, Harris SL, Levine AJ. The regulation of exosome secretion: a novel function of the p53 protein. Cancer Res. 2006;66:4795–4801. doi: 10.1158/0008-5472.CAN-05-4579. [DOI] [PubMed] [Google Scholar]
- 19.Aumüller G, Wilhelm B, Seitz J. Apocrine secretion—fact or artifact? Ann Anat. 1999;181:437–446. doi: 10.1016/S0940-9602(99)80020-X. [DOI] [PubMed] [Google Scholar]
- 20.Denny PW, Gokool S, Russell DG, Field MC, Smith DF. Acylation-dependent protein export in Leishmania. J Biol Chem. 2000;275:11017–11025. doi: 10.1074/jbc.275.15.11017. [DOI] [PubMed] [Google Scholar]
- 21.Backhaus R, Zehe C, Wegehingel S, Kehlenbach A, Schwappach B, Nickel W. Unconventional protein secretion: membrane translocation of FGF-2 does not require protein unfolding. J Cell Sci. 2004;117:1727–1736. doi: 10.1242/jcs.01027. [DOI] [PubMed] [Google Scholar]
- 22.Hughes RC. Secretion of the galectin family of mammalian carbohydrate-binding proteins. Biochim Biophys Acta. 1999;1473:172–185. doi: 10.1016/s0304-4165(99)00177-4. [DOI] [PubMed] [Google Scholar]
- 23.Müller S, Scaffidi P, Degryse B, Bonaldi T, Ronfani L, Agresti A, et al. The double life of HMGB1 chromatin protein: architectural factor and extracellular signal. EMBO J. 2001;20:4337–4340. doi: 10.1093/emboj/20.16.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Streller S, Wingsle G. Pinus sylvestris (L.) needles contain extracellular Cu/Zn superoxide dismutase. Planta. 1994;192:195–201. [PubMed] [Google Scholar]
- 25.Vanacker H, Carver T, Foyer CH. Pathogen-induced changes in the antioxidant status of the apoplast in barley leaves. Plant Physiol. 1998;117:1103–1114. doi: 10.1104/pp.117.3.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hernández JA, Ferrer MA, Jiménez A, Barceló AR, Sevilla F. Antioxidant systems and O2/H2O2 production in the apoplast of pea leaves. Its relation with salt-induced necrotic lesions in minor veins. Plant Physiol. 2001;127:817–831. doi: 10.1104/pp.010188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karpinska B, Karlsson M, Schinkel H, Streller S, Süss KH, Melzer M, et al. A novel superoxide dismutase with a high isoelectric point in higher plants. Expression, regulation, and protein localization. Plant Physiol. 2001;126:1668–1677. doi: 10.1104/pp.126.4.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patykowski J, Urbanek H. Activity of enzymes related to H2O2 generation and metabolism in leaf apoplastic fraction of tomato leaves infected with Botrytis cinerea. J Phytopathol. 2003;151:153–161. [Google Scholar]
- 29.Slabas AR, Ndimba B, Simon WJ, Chivasa S. Proteomic analysis of the Arabidopsis cell wall reveals unexpected proteins with new cellular locations. Biochem Soc Trans. 2004;32:524–528. doi: 10.1042/BST0320524. [DOI] [PubMed] [Google Scholar]
- 30.Oh IS, Park AR, Bae MS, Kwon SJ, Kim YS, Lee JE, et al. Secretome analysis reveals an Arabidopsis lipase involved in defense against Alternaria brassicicola. Plant Cell. 2005;17:2832–2847. doi: 10.1105/tpc.105.034819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng FY, Blackburn K, Lin YM, Goshe MB, Williamson JD. Absolute protein quantification by LC/MSE for global analysis of salicylic acid-induced plant protein secretion responses. J Proteome Res. 2009;8:82–93. doi: 10.1021/pr800649s. [DOI] [PubMed] [Google Scholar]
- 32.Davies DR, Bindschedler LV, Strickland TS, Bolwell GP. Production of reactive oxygen species in Arabidopsis thaliana cell suspension cultures in response to an elicitor from Fusarium oxysporum: implications for basal resistance. J Exp Bot. 2006;57:1817–1827. doi: 10.1093/jxb/erj216. [DOI] [PubMed] [Google Scholar]