Abstract
Co-localization of mitochondria with chloroplasts in plant cells has long been noticed as beneficial interactions of the organelles to active photosynthesis. Recently, we have found that mitochondria in mesophyll cells of Arabidopsis thaliana expressing mitochondrion-targeted green fluorescent protein (GFP) change their distribution in a light-dependent manner. Mitochondria occupy the periclinal and anticlinal regions of palisade cells under weak and strong blue light, respectively. Redistributed mitochondria seem to be rendered static through co-localization with chloroplasts. Here we further demonstrated that distribution patterns of mitochondria, together with chloroplasts, returned back to those of dark-adapted state during dark incubation after blue-light illumination. Reversible association of the two organelles may underlie flexible adaptation of plants to environmental fluctuations.
Key words: Arabidopsis thaliana, blue light, chloroplast, green fluorescent protein, mesophyll cell, mitochondrion, organelle positioning
Highly dynamic cell organelles, mitochondria, are responsible not only for energy production, but also for cellular metabolism, cell growth and survival as well as gene regulations.1,2 Appropriate intracellular positioning and distribution of mitochondria contribute to proper organelle functions and are essential for cell signaling.3,4 In plant cells operating photosynthesis, the co-localization of mitochondria with chloroplasts has been a well known phenomenon for a long period of time.5,6,7 Physical contact of mitochondria with chloroplasts may provide a means to transfer genetic information from the organelle genome,8 as well as to exchange metabolite components; a process required for the maintenance of efficient photosynthesis.9,10,11
Using Arabidopsis thaliana stably expressing mitochondrion-targeted GFP,12 we have recently examined a different aspect of mitochondria positioning. Although mitochondria in leaf mesophyll cells are highly motile under dark condition, mitochondria change their intracellular positions in response to light illumination.13 The pattern of light-dependent positioning of mitochondria seems to be essentially identical to that of chloroplasts.14 Mitochondria occupy the periclinal regions under weak blue light (wBL; 470 nm, 4 µmol m−2s−1) and the anticlinal regions under strong blue light (sBL; 100 µmol m−2s−1), respectively. A gradual increase in the number of static mitochondria located in the vicinity of chloroplasts in the periclinal regions with time period of wBL illumination clearly demonstrates that the co-localization of these two organelles is a light-induced phenomenon.13
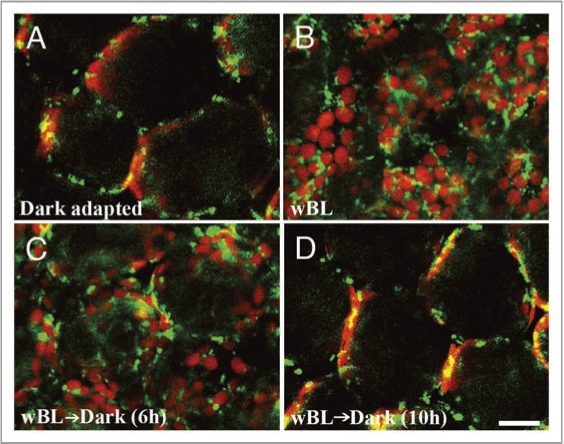
In the present study, to ask whether the light-dependent positioning of mitochondria is reversible or not, a time course of mitochondria redistribution was examined transferring the sample leaves from light to dark conditions. The representative results (Fig. 1) clearly show that mitochondria re-changed their positions within several hours of dark treatment. Immediately after dark adaptation, mitochondria in the palisade mesophyll cells were distributed randomly throughout the cytoplasm (Fig. 1A and ref. 13). Chloroplasts were distributed along the inner periclinal walls and the lower half of the anticlinal walls. On the contrary, mitochondria accumulated along the outer (Fig. 1B) and inner periclinal walls when illuminated with wBL. Chloroplast position was also along the outer and inner periclinal walls. Many of the mitochondria located near the chloroplasts lost their motility. When wBL-illuminated leaves were transferred back to dark condition, the numbers of mitochondria and chloroplasts present on the periclinal regions began to decrease within several hours (Fig. 1C). After 10 h dark treatment, distribution patterns of mitochondria as well as chloroplasts almost recovered to those of dark-adapted cells (Fig. 1D).
Figure 1.
Distribution of mitochondria and chloroplasts on the outer periclinal regions of palisade mesophyll cells of A. thaliana under different light conditions. Mitochondria (green; GFP) and chloroplasts (red; chlorophyll autofluorescence) were visualized with confocal microscopy after dark adaptation (A), immediately after wBL (470 nm, 4 µmol m−2s−1) illumination for 4 h (B), after dark treatment for 6 h (C) and 10 h (D) following the 4-h wBL illumination, respectively. Bar = 50 µm.
To our knowledge, this may be the first report that directly demonstrates that wBL regulates mitochondria and chloroplast positioning in a reversible manner, though the nuclei in A. thaliana leaf cells were also found to reverse their positions when transferred from sBL to dark conditions.15 Reversible regulation of organelle positioning in leaf cells should play critical roles in adaptation of plants to highly fluctuating light conditions in the nature. Since distribution patterns of mitochondria under wBL and sBL are identical to those of chloroplasts, we can assume that phototropins, the BL receptors for chloroplast photo-relocation movement,16 may have some role in the redistribution of mitochondria. On the other hand, we also found that red light exhibited a significant effect on mitochondria positioning (Islam et al. 2009), suggesting an involvement of photosynthesis. These possibilities are now under investigation.
Acknowledgements
M.S.I. was supported by MEXT, Japan.
Abbreviations
- GFP
green fluorescent protein
- sBL
strong blue light
- wBL
weak blue light
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/10410
References
- 1.Park MK, Ashby MC, Erdemli G, Petersen OH, Tepikin AV. Perinuclear, perigranular and sub-plasmalemmal mitochondria have distinct functions in the regulation of cellular calcium transport. EMBO J. 2001;20:1863–1874. doi: 10.1093/emboj/20.8.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Logan DC. Mitochondrial dynamics. New Phytologist. 2003;160:463–478. doi: 10.1046/j.1469-8137.2003.00918.x. [DOI] [PubMed] [Google Scholar]
- 3.Logan DC, Scott I, Tobin AK. The genetic control of plant mitochondrial morphology and dynamics. Plant J. 2003;36:500–509. doi: 10.1046/j.1365-313x.2003.01894.x. [DOI] [PubMed] [Google Scholar]
- 4.Hollenbeck PJ, Saxton WM. The axonal transport of mitochondria. J Cell Sci. 2005;118:5411–5419. doi: 10.1242/jcs.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frederick SE, Newcomb EH. Microbody-like organelles in leaf cells. Science. 1969;163:1353–1355. doi: 10.1126/science.163.3873.1353. [DOI] [PubMed] [Google Scholar]
- 6.Logan DC, Leaver CJ. Mitochondria-targeted GFP highlights the heterogeneity of mitochondrial shape, size and movement within living plant cells. J Exp Bot. 2000;51:865–871. [PubMed] [Google Scholar]
- 7.Takagi S. Actin-based photo-orientation movement of chloroplasts in plant cells. J Exp Biol. 2003;206:1963–1969. doi: 10.1242/jeb.00215. [DOI] [PubMed] [Google Scholar]
- 8.Unseld M, Marienfeld JR, Brandt P, Brennicke A. The mitochondrial genome of Arabidopsis thaliana contains 57 genes in 366,924 nucleotides. Nat Genet. 1997;15:57–61. doi: 10.1038/ng0197-57. [DOI] [PubMed] [Google Scholar]
- 9.Raghavendra AS, Padmasree K. Beneficial interactions of mitochondrial metabolism with photosynthetic carbon assimilation. Trends Plant Sci. 2003;8:546–553. doi: 10.1016/j.tplants.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 10.Noctor G, De Paepe R, Foyer CH. Mitochondrial redox biology and homeostasis in plants. Trends Plant Sci. 2007;12:125–134. doi: 10.1016/j.tplants.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Noguchi K, Yoshida K. Interaction between photosynthesis and respiration in illuminated leaves. Mitochondrion. 2008;8:87–99. doi: 10.1016/j.mito.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Niwa Y, Hirano T, Yoshimoto K, Shimizu M, Kobayashi H. Non-invasive quantitative detection and applications of non-toxic, S65T-type green fluorescent protein in living plants. Plant J. 1999;18:455–463. doi: 10.1046/j.1365-313x.1999.00464.x. [DOI] [PubMed] [Google Scholar]
- 13.Islam MS, Niwa Y, Takagi S. Light-dependent intracellular positioning of mitochondria in Arabidopsis thaliana mesophyll cells. Plant Cell Physiol. 2009;50:1032–1040. doi: 10.1093/pcp/pcp054. [DOI] [PubMed] [Google Scholar]
- 14.Trojan A, Gabrys H. Chloroplast distribution in Arabidopsis thaliana (L.) depends on light conditions during growth. Plant Physiol. 1996;111:419–425. doi: 10.1104/pp.111.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwabuchi K, Sakai T, Takagi S. Blue light-dependent nuclear positioning in Arabidopsis thaliana leaf cells. Plant Cell Physiol. 2007;48:1291–1298. doi: 10.1093/pcp/pcm095. [DOI] [PubMed] [Google Scholar]
- 16.Wada M, Kagawa T, Sato Y. Chloroplast movement. Annu Rev Plant Biol. 2003;54:455–468. doi: 10.1146/annurev.arplant.54.031902.135023. [DOI] [PubMed] [Google Scholar]