Abstract
The two-component signaling systems (TCSs), which mediate the histidine-aspartate signaling, control diverse biological processes of many organisms, including cell division, cell growth and proliferation and responses to environmental stimuli and growth regulators. We have provided in planta evidence that the cytokinin (CK) responsive TCS mediates abscisic acid (ABA) and osmotic stress responses. By using loss-of-function approach we have demonstrated that the three cytokinin (CK) receptor histidine kinases AHK2, AHK3 and AHK4/CRE1 act as negative regulators in ABA, drought and high salinity stress signalings. Genome-wide expression profiling of the stress-tolerant <ahk2,3> double mutant suggested that CK receptor kinases mediate osmotic stress response in both an ABA-dependent and ABA-independent manner. Additionally, we showed evidence for the role of CK in mediating stress responses, judging from the fact that AHK4 requires the CK to function as a negative regulator in osmotic stress response. Our results suggested that cross-talk exists among CK, ABA and osmotic stress signaling pathways, and that CK signaling and CK metabolism may play crucial roles not only in plant growth and development but also in osmotic stress signaling.
Key words: two-component systems, osmotic stress, abscisic acid, cytokinin, microarray
Water deficit and high salinity stress limit crop productivity worldwide. In response to these stresses, plants activate a number of defense mechanisms that function to increase tolerance to the adverse conditions.1 Phosphorylation, which is mediated by two-component systems (TCSs) or histidine-to-aspartate (His-Asp) phosphorelays, is a key mechanism for stress signal transduction in a cell.2 A great number of TCSs have been identified and characterized not only in many prokaryotic organisms but also in key plant species including Arabidopsis thaliana, rice (Oryza sativa) and Lotus japonicus.3–7 Increasing evidence has indicated that the Arabidopsis TCS pathways are involved in response to environmental stimuli, ethylene signaling, light perception, circadian rhythm and cytokinin (CK)-dependent processes which include shoot and root development, vascular defferentiation and leaf senescence.4,8–10 Genome-wide analysis supports the existence of eight histidine kinases (HKs) in Arabidopsis. Among the HKs, ETR1 and ERS1 act as ethylene receptor HKs, while others including AHK1/ATHK1, AHK2, AHK3, AHK4/CRE1, CKI1 and AHK5/CKI2 are nonethylene receptors.10 AHK1 has been shown to function as positive regulator in abscisic acid (ABA) and osmotic stress signalings in both ABA-dependent and ABA-independent pathways.11,12 CKI1 is implicated in megagametophyte development, and together with AHK2 and AHK3 it is also required for vascular bundle formation in Arabidopsis.13,14 AHK5 has been shown to be involved in root elongation through an ETR1-dependent ABA and ethylene signaling pathway.15 AHK5 may also have function in mediating H2O2-dependent processes in stomatal guard cells.16
In Arabidopsis, CK signaling is mediated by a multi-step phosphorelay, which is comprised of sensor HKs (AHKs), phosphotransfers (AHPs) and response regulators (ARRs).10,17 Analysis of ahk2, ahk3 and ahk4 single, double and triple mutants suggest that the AHK2, AHK3 and AHK4 function as CK receptor HKs, and act as positive regulators in CK signaling and plant growth.18–20 Interestingly, AHK4 exhibits a dual function depending on the presence or absence of CK. In the presence of CK, AHK4 phosphorylates the AHP. Conversely, it removes phosphate from AHP in the absence of CK.21 The five authentic AHPs (AHP1-AHP5) and a pseudo AHP (AHP6) are involved in mediating the transfer of the phosphoryl group from the AHKs to the ARRs.17 Analysis of ahp multiple mutants indicate that most of the AHPs act as redundant, positive regulators of CK signaling and affect many aspects of plant development.22 In contrast, the pseudo AHP6 plays an inhibitory role in CK signaling.23 As for the ARRs, the typical ARRs are classified into either type-A (9 members) or type-B (11 members) or type-C (2 members).10,17 The 11 type-B ARRs, which are not induced by CK, are transcription factors that contain receiver domain and a large C-terminal region harboring a Myb-like DNA-binding domain and a glutamine-rich domain. Analysis of multiple type-B arr mutants demonstrates that type-B ARRs act as positive regulators of CK signaling.24 By contrast, most of type-A ARRs, which have short C-terminal domains and are rapidly transcriptionally upregulated by CK treatment, are partially redundant negative regulators of CK signaling.10,17
Since AHK1 was first discovered to function in osmotic stress response by its ability to complement the function of the yeast SLN1 histidine kinase in the <sln1 sho1> yeast double mutant, which is lethal under high-osmolarity conditions due to the disruption of both SLNI and SHO1,25 we were interested in testing whether the cytokinin receptor AHK2, AHK3 and AHK4 have catalytic activity similar to those of AHK1 and SLN1 under high-salinity conditions. Therefore, we introduced the AHK2, AHK3 and AHK4 cDNAs into the <sln1 sho1> mutant for testing their possible function in complementation of SLN1 in high-osmolarity conditions.25,26 Yeast transformants containing AHK2, AHK3 grew as well as those having AHK1 or SLN1 on minimal medium containing 0.3 M NaCl. When AHK4 was introduced into the yeast mutant, transformants could grow under high-salt concentration only in the presence of CK. Our results indicated that the CK receptor HKs were able to complement the function of SLN1 similar to AHK1, giving rise to high-osmolarity tolerance to the <sln1 sho1> mutant.
Next, to examine the potential functions of the CK receptor HKs in abiotic stress signaling, we initially analyzed the expression patterns of genes encoding CK receptor HKs under various stresses and hormone treatments. The transcripts of all three AHK2, AHK3 and AHK4 were rapidly induced by dehydration. Expression of AHK2 also appeared to be influenced by NaCl and ABA treatments. Furthermore, induction of the AHK3 mRNA was observed during high salinity and perhaps cold stress. These results suggested that these CK receptor HKs play an important role not only in CK response but also in stress response.
To further confirm the stress responsive role of CK receptor HKs in osmotic stress responses, we first compared the level of drought and salt stress tolerance of the ahk2, ahk3 and ahk4 single mutants, as well as the <ahk2,3> double mutant, to WT plants. The results showed a strong drought and salinity tolerance for both ahk2 and ahk3 single mutants. The <ahk2,3> mutant was even more tolerant to drought and salt stresses than the respective single ones, suggesting a combinatory function of AHK2 and AHK3 in osmotic stress signaling. Both the ahk4 mutant and WT responded similarly to drought and salt stresses without CK. However, in the presence of CK, the ahk4 mutant displayed a strong salt stresstolerant phenotype. In the absence of CK, AHK4 is locked in its phosphatase form, exhibiting phosphatase activity instead of phosphorylation activity.21 In the presence of CK, AHK4 changes to its HK form in a CK-dependent manner and can function as a negative regulator of stress signaling. To our knowledge, this is the first direct evidence to demonstrate the involvement of CK in stress signaling as a potential mediator.
These results collectively suggested that all of the AHK2, AHK3 and AHK4 HKs function in stress responses and that they act as negative regulators. Comparative genome-wide expression analysis of <ahk2,3> double mutant and WT plants identified many stress- and/or ABA-responsive genes which are upregulated in <ahk2,3> mutant. These results together with ABA-sensitive phenotype of the ahk2, ahk3 and ahk4 single mutants suggested that AHK2, AHK3 and AHK4 act as negative regulators in both ABA-dependent and ABA-independent pathways.
The fact that the CK receptor AHK2, AHK3 and AHK4 are involved in ABA and osmotic stress signalings as negative regulators strongly indicates that there are cross-talks among CK, ABA and stress signaling pathways. Moreover, our results indicate that CK mediates stress response, demonstrating that CK signaling and CK metabolism may play crucial roles not only in plant growth and development but also in abiotic stress signaling. Recent reports suggested that CKs may be an important signal traveling from roots to shoots, and ABA:CK ratios in xylem sap are important for stress signaling.27,28 How does the CK affect plant stress response and how do stresses affect CK metabolism, i.e., how do the CK contents are changed under different stress conditions, analysis of the relationship between CK metabolism and stress responses may provide answers to these questions. Functional analysis of genes involved in CK metabolisms, such as genes encoding adenosine phosphate-isopentenyltransferases, which are involved in CK biosynthesis,29 and genes encoding CK oxidases, which are involved in CK degradation,30 in stress response will unravel the regulatory role of CK in stress signaling.
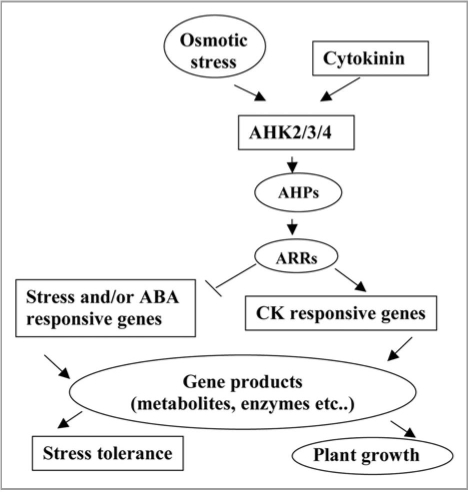
By analogy to the CK responsive signaling, we suggest that the stress signaling is mediated by the multistep His-Asp phosphorelay (Fig. 1). However, the functions of the AHPs and ARRs in stress response as well as their downstream genes of the phosphorelay remain still unknown. Investigation of various combinations of ahp and arr mutants may aid in identifying those AHP and ARR genes involved in stress signaling. Discovery of mechanisms of activation and the targets of the downstream components of the CK responsive phosphorelay in stress signaling is important and challenging goal for study of regulatory network of plant stress response and plant growth.
Figure 1.
Signal tranduction network in stress responses and plant growth.
Acknowledgements
Funding supports from Grants-in-Aid (Start-up) for Scientific Research, Ministry of Education, Culture, Sports, Science and Technology of Japan (No. 21870046) and from Yokohama Institute Director Discretionary Funds (2009) are gratefully appreciated.
Abbreviations
- ABA
abscisic acid
- CK
cytokinin
- HK
histidine kinase
- TCS
two-component system
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/10411
References
- 1.Yamaguchi-Shinozaki K, Shinozaki K. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu Rev Plant Biol. 2006;57:781–803. doi: 10.1146/annurev.arplant.57.032905.105444. [DOI] [PubMed] [Google Scholar]
- 2.Urao T, Yamaguchi-Shinozaki K, Shinozaki K. Plant histidine kinases: an emerging picture of two-component signal transduction in hormone and environmental responses. Sci STKE. 2001;109:18. doi: 10.1126/stke.2001.109.re18. [DOI] [PubMed] [Google Scholar]
- 3.Koretke KK, Lupas AN, Warren PV, Rosenberg M, Brown JR. Evolution of two-component signal transduction. Mol Biol Evol. 2000;17:1956–1970. doi: 10.1093/oxfordjournals.molbev.a026297. [DOI] [PubMed] [Google Scholar]
- 4.Hwang I, Chen HC, Sheen J. Two-component signal transduction pathways in Arabidopsis. Plant Physiol. 2002;129:500–515. doi: 10.1104/pp.005504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pareek A, Singh A, Kumar M, Kushwaha HR, Lynn AM, Singla-Pareek SL. Whole-genome analysis of Oryza sativa reveals similar architecture of two-component signaling machinery with Arabidopsis. Plant Physiol. 2006;142:380–397. doi: 10.1104/pp.106.086371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pils B, Heyl A. Unraveling the evolution of cytokinin signaling. Plant Physiol. 2009;151:782–791. doi: 10.1104/pp.109.139188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishida K, Niwa Y, Yamashino T, Mizuno T. A genome-wide compilation of the two-component systems in Lotus japonicus. DNA Res. 2009;16:237–247. doi: 10.1093/dnares/dsp012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kakimoto T. Perception and signal transduction of cytokinins. Annu Rev Plant Biol. 2003;54:605–627. doi: 10.1146/annurev.arplant.54.031902.134802. [DOI] [PubMed] [Google Scholar]
- 9.Mizuno T. Two-component phosphorelay signal transduction systems in plants: from hormone responses to circadian rhythms. Biosci Biotechnol Biochem. 2005;69:2263–2276. doi: 10.1271/bbb.69.2263. [DOI] [PubMed] [Google Scholar]
- 10.Schaller GE, Kieber JJ, Shiu SH. Two-component signaling elements and histidyl-aspartyl phosphorelays. The Arabidopsis Book. 2008. doi: 10.1199/tab.0112. [DOI] [PMC free article] [PubMed]
- 11.Tran L-SP, Urao T, Qin F, Maruyama K, Kakimoto T, Shinozaki K, et al. Functional analysis of AHK1/ATHK1 and cytokinin receptor histidine kinases in response to abscisic acid, drought and salt stress in Arabidopsis. Proc Natl Acad Sci USA. 2007;104:20623–20628. doi: 10.1073/pnas.0706547105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wohlbach DJ, Quirino BF, Sussman MR. Analysis of the Arabidopsis histidine kinase ATHK1 reveals a connection between vegetative osmotic stress sensing and seed maturation. Plant Cell. 2008;20:1101–1117. doi: 10.1105/tpc.107.055871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pischke MS, Jones LG, Otsuga D, Fernandez DE, Drews GN, Sussman MR. An Arabidopsis histidine kinase is essential for megagametogenesis. Proc Natl Acad Sci USA. 2002;99:15800–15805. doi: 10.1073/pnas.232580499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hejátko J, Ryu H, Kim GT, Dobesova R, Choi S, Choi SM, et al. The histidine kinases CYTOKININ-INDEPENDENT1 and ARABIDOPSIS HISTIDINE KINASE2 and 3 regulate vascular tissue development in Arabidopsis shoots. Plant Cell. 2009;21:2008–2021. doi: 10.1105/tpc.109.066696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwami A, Yamashino T, Tanaka Y, Sakakibara H, Kakimoto T, Sato S, et al. AHK5 histidine kinase regulates root elongation through an ETR1-dependent abscisic acid and ethylene signaling pathway in Arabidopsis thaliana. Plant Cell Physiol. 2007;48:375–380. doi: 10.1093/pcp/pcl065. [DOI] [PubMed] [Google Scholar]
- 16.Desikan R, Horák J, Chaban C, Mira-Rodado V, Witthöft J, Elgass K, et al. The histidine kinase AHK5 integrates endogenous and environmental signals in Arabidopsis guard cells. PLoS One. 2008;3:2491. doi: 10.1371/journal.pone.0002491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.To JPC, Kieber JJ. Cytokinin signaling: two-components and more. Trends Plant Sci. 2008;13:85–92. doi: 10.1016/j.tplants.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Higuchi M, Pischke MS, Mahonen AP, Miyawaki K, Hashimoto Y, Seki M, et al. In planta functions of the Arabidopsis cytokinin receptor family. Proc Natl Acad Sci USA. 2004;101:8821–8826. doi: 10.1073/pnas.0402887101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishimura C, Ohashi Y, Sato S, Kato T, Tabata S, Ueguchi C. Histidine kinase homologs that act as cytokinin receptors possess overlapping functions in the regulation of shoot and root growth in Arabidopsis. Plant Cell. 2004;16:1365–1377. doi: 10.1105/tpc.021477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riefler M, Novak O, Strnad M, Schmülling T. Arabidopsis cytokinin receptor mutants reveal functions in shoot growth, leaf senescence, seed size, germination, root development and cytokinin metabolism. Plant Cell. 2006;18:40–54. doi: 10.1105/tpc.105.037796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mähönen AP, Higuchi M, Törmäkangas K, Miyawaki K, Pischke MS, Sussman MR, et al. Cytokinins regulate a bidirectional phosphorelay network in Arabidopsis. Curr Biol. 2006;16:1116–1122. doi: 10.1016/j.cub.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 22.Hutchison CE, Li J, Argueso C, Gonzalez M, Lee E, Lewis MW, et al. The Arabidopsis histidine phosphotransfer proteins are redundant positive regulators of cytokinin signaling. Plant Cell. 2006;18:3073–3087. doi: 10.1105/tpc.106.045674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mähönen AP, Bishopp A, Higuchi M, Nieminen KM, Kinoshita K, Tormakangas K, et al. Cytokinin signaling and its inhibitor AHP6 regulate cell fate during vascular development. Science. 2006;311:94–98. doi: 10.1126/science.1118875. [DOI] [PubMed] [Google Scholar]
- 24.Argyros RD, Mathews DE, Chiang YH, Palmer CM, Thibault DM, Etheridge N, et al. Type B response regulators of Arabidopsis play key roles in cytokinin signaling and plant development. Plant Cell. 2008;20:2102–2116. doi: 10.1105/tpc.108.059584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Urao T, Yakubov B, Satoh R, Yamaguchi-Shinozaki K, Seki M, Hirayama T, et al. A transmembrane hybrid-type histidine kinase in Arabidopsis functions as an osmosensor. Plant Cell. 1999;11:1743–1754. doi: 10.1105/tpc.11.9.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reiser V, Raitt DC, Saito H. Yeast osmosensor SLN1 and plant cytokinin receptor Cre1 respond to changes in turgor pressure. J Cell Biol. 2003;161:1035–1040. doi: 10.1083/jcb.200301099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alvarez S, Marsh EL, Schroeder SG, Schachtman DP. Metabolomic and proteomic changes in the xylem sap of maize under drought. Plant Cell Environ. 2008;31:325–340. doi: 10.1111/j.1365-3040.2007.01770.x. [DOI] [PubMed] [Google Scholar]
- 28.Schachtman DP, Goodger JQ. Chemical root to shoot signaling under drought. Trends Plant Sci. 2008;13:281–287. doi: 10.1016/j.tplants.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 29.Hirose N, Takei K, Kuroha T, Kamada-Nobusada T, Hayashi H, Sakakibara H. Regulation of cytokinin biosynthesis, compartmentalization and translocation. J Exp Bot. 2008;59:75–83. doi: 10.1093/jxb/erm157. [DOI] [PubMed] [Google Scholar]
- 30.Werner T, Köllmer I, Bartrina I, Holst K, Schmülling T. New insights into the biology of cytokinin degradation. Plant Biol. 2006;8:371–381. doi: 10.1055/s-2006-923928. [DOI] [PubMed] [Google Scholar]