Abstract
Purpose
Although first-trimester vaginal bleeding is an alarming symptom, few studies have investigated the prevalence and predictors of early bleeding. This study characterizes first trimester bleeding, setting aside bleeding that occurs at time of miscarriage.
Methods
Participants (n=4539) were women ages 18–45 enrolled in Right From the Start, a community-based pregnancy study (2000–2008). Bleeding information included timing, heaviness, duration, color, and associated pain, as well as recurrence risk in subsequent pregnancies. Life table analyses were used to describe gestational timing of bleeding. Factors associated with bleeding were investigated using multiple logistic regression, with multiple imputation for missing data.
Results
Approximately one-fourth of participants (n=1207) reported bleeding (n=1656 episodes), but only 8% of women with bleeding reported heavy bleeding. Of the spotting and light bleeding episodes (n=1555), 28% were associated with pain. Among heavy episodes (n=100), 54% were associated with pain. Most episodes lasted less than 3 days, and most occurred between gestational weeks 5–8. Twelve percent of women with bleeding and 13% of those without experienced miscarriage. Maternal characteristics associated with bleeding included fibroids and prior miscarriage.
Conclusions
Consistent with the hypothesis that bleeding is a marker for placental dysfunction, bleeding is most likely to be seen around the time of the luteal-placental shift.
INTRODUCTION
Although first-trimester vaginal bleeding is an alarming symptom (1–4), few studies have rigorously investigated the prevalence, timing, and predictors of bleeding. Estimates of bleeding prevalence in early pregnancy are imprecise and range from 7 to 24% (5–9). This wide range in estimates is likely due to differences in study design.
Only three reports have attempted to systematically describe early pregnancy bleeding patterns (5, 8, 10). These studies are limited by small sample (10), observation through only the eighth week of gestation (10), and recruitment and data collection late during pregnancy (5, 8). The latter two studies do not provide detailed information on first-trimester bleeding and exclude miscarriages (5, 8). Only one of these investigated maternal predictors of vaginal bleeding (5). This study found that women who were older, with passive smoking exposure, prior preterm birth, multiple prior elective terminations or with prior miscarriages were more likely to experience “intense” vaginal bleeding, measured by heaviness, duration, and an index of total blood loss.
We used data from Right From the Start, a community-based early pregnancy cohort, to characterize the patterns and predictors of early pregnancy bleeding, setting aside bleeding episodes that occur at the time of miscarriage.
MATERIALS AND METHODS
Study Population
Right From the Start (RFTS) is an ongoing cohort that began enrollment of pregnant women in 2000. Over time, the study has included three phases (RFTS 1, 2, and 3) and has been active in Galveston, Texas, Memphis and Nashville, Tennessee, and the Triangle region (including Raleigh, Durham, and Chapel Hill), North Carolina. Participants were at least 18 years old, spoke English or Spanish, had not used assisted reproductive technologies to conceive, and intended to carry the pregnancy to term. Women who were not yet pregnant but attempting to conceive could pre-enroll prior to pregnancy and were considered enrolled once they reported a positive pregnancy test. Pre-enrolled women must have been attempting pregnancy for fewer than six months (RFTS 1 and 2) or fewer than three months (RFTS 3) to be eligible. Women entered the study prior to twelve completed weeks of gestation (RFTS 1), prior to nine completed weeks of gestation (RFTS 2), or only pre-enrolled (RFTS 3). Formal enrollment occurred, on average, at 53 days of gestation for women who enrolled while pregnant (n=3581), and at 38 days of gestation for women who pre-enrolled in the study (n=958). Informed consent was obtained from each study subject in compliance with Institutional Review Boards.
Participants had an early pregnancy ultrasound to assess fetal viability and gestational age of the fetus. Gestational age was calculated using self-reported last menstrual period (LMP). If this was unavailable, ultrasound-based gestational age was used (n=15). Seventy-five percent of ultrasounds were completed by the end of the ninth week of gestation.
Telephone interviews were conducted to collect detailed information about the first trimester, including information about bleeding symptoms. All women, regardless of pregnancy outcome, provided this detailed information. In the first phase of the study (RFTS 1), two interviews were conducted: one shortly after enrollment during the first trimester, followed by a second interview around 20 weeks of pregnancy. Data from both of these interviews were compiled to reflect all first trimester information. Later phases of RFTS (RFTS 2, RFTS 3) collected detailed first trimester data in an interview at the end of the first trimester, no later than the 16th week of pregnancy. Average time of completion of this interview was during the fourteenth week of pregnancy. When miscarriage occurred before a scheduled interview, the interview was conducted as soon as possible after pregnancy loss.
Outcomes were self-reported, and prenatal records were obtained to verify the outcome. Live birth verification was obtained through linkage to state vital records. Miscarriage was defined as loss of a recognized pregnancy prior to twenty completed weeks. The date of a miscarriage was self-reported as the date of dilatation and evacuation or as the date of most severe bleeding.
Women who had their last menstrual period before July 14, 2008 were included. Exclusions from the sample include: women who did not complete the first trimester interview (n=170), women missing both LMP and ultrasound-based gestational age (n=2), women missing enrollment dates (n=1), women whose miscarriage occurred on same day as the enrollment day (n=4), and women with ectopic pregnancies (n=6). Women could enroll during more than one pregnancy, but only the first enrollment was included (n=238 subsequent pregnancies excluded). A total of 4539 pregnancies contributed to the analysis.
Variable definitions
Bleeding information
Participants reported the total number of bleeding episodes experienced during the first trimester and detailed information about the timing, heaviness, color, duration, and pain associated with the first three episodes (see Appendix). If bleeding stopped for at least two days and then started again, this was considered two separate episodes of bleeding. Duration was reported in days. Heaviness was defined according to the heaviest bleeding in an episode, and was compared to heaviness of usual menses. A ‘spotting’ episode was only noticed when wiping, a ‘light bleeding’ episode was defined as having the heaviest day(s) of flow being lighter than the heavy flow of a usual menstrual period, and a heavy bleeding episode was defined as having the heaviest day(s) of flow as heavy or heavier than the heavy flow of a usual menstrual period. Participants could describe the color of each episode as ‘pink,’ ‘red,’ or ‘brown.’ If participants reported bleeding-associated pain, they were asked to characterize the pain as mild, moderate, or severe.
This analysis focused on bleeding episodes that occur during the first trimester, regardless of whether a miscarriage occurs. To exclude bleeding that occurred at the time of miscarriage, we did not include any bleeding episodes that ended within 4 days of a miscarriage. This cutpoint was varied in later sensitivity analyses, and the choice of cutpoint was not found to affect results.
Other characteristics
Data collected at the first trimester interview included demographic factors (age, race/ethnicity, education, marital status, percent of poverty level [according to the 2008 poverty guidelines, accounting for number of persons in household]), pre-pregnancy body mass index, usual menstrual cycle length in days, maternal morbidities (reproductive tract infections during pregnancy and diabetes), maternal behaviors (active and passive smoking, prenatal vitamin use, alcohol intake, caffeine intake), and prior obstetric history (parity, gravidity, history of miscarriage, induced abortion, or preterm birth). The early pregnancy ultrasound included systematic screening for uterine fibroids (≥ 0.5 cm diameter) (11).
Women were classified according to whether or not they were treated for one or more of the following infections at any time from LMP to the end of the first trimester: yeast infection, urinary tract infection, bacterial vaginosis, pelvic inflammatory disease, chlamydia, trichomonas, gonorrhea, syphilis, genital warts, or outbreaks of genital herpes. Women with pre-existing diabetes or who had gestational diabetes in a previous pregnancy were classified as having diabetes. Women who smoked cigarettes during pregnancy were identified as smokers. Passive smoking was defined according to whether an individual in the participant’s household was a regular smoker. Women who reported drinking any alcoholic beverages during the pregnancy were classified as being exposed to alcohol.
Data Analysis
All analyses were conducted in Stata, version 9.2 (College Station, Texas).
Descriptive analyses
Episodes were categorized into completed weeks of pregnancy based on the day in which an episode began. We used life table analyses to calculate the proportion of women with bleeding for each week of gestation during the first trimester. The same was repeated for women with heavy bleeding only. Participants were censored at time of miscarriage (n=464), induced abortion (n=14), or interview when it occurred before the end of the first trimester (n=114). Miscarriage and induced abortion dates were based on participant self-report. The distribution of episodes by heaviness, duration, color, and associated pain were examined descriptively for all bleeding episodes.
Exploratory modeling
Maternal characteristics predicting the presence/absence of bleeding were evaluated using exploratory logistic models. All covariates were included in initial models and the least important (highest P-value) was sequentially removed if the P-value from the likelihood ratio test was greater than 0.15. The final reduced model included those variables with an associated P-value less than 0.15. The purpose of these analyses was to identify potential associations between maternal characteristics and bleeding, not to determine risk factors based on statistical significance alone; thus, a relaxed P-value cutoff was used in order to identify all maternal characteristics potentially associated with bleeding.
Predictors of the heaviness of bleeding (based on each woman’s heaviest episode) were evaluated using a generalized multinomial logistic model, with a reference group of “no bleeding” and index categories of “spotting,” “light bleeding,” and “heavy bleeding.” We followed the same strategy previously described to identify variables predicting the heaviness of bleeding, while requiring the previously identified predictors of any bleeding to remain in the model. For the multinomial logistic model, likelihood ratio tests were conducted to determine whether categories of heaviness could be combined. If heaviness levels were not different from each other at an a priori P-value of 0.15, the categories were combined.
Multiple imputation procedures (Stata, Imputation with Chained Equations (12)) were used so that participants with missing covariate data could be included. This method imputes missing values of a variable from a posterior distribution based on a regression of the non-missing values of the variable on all other predictors in the model. Five imputation cycles were used. Most variables were missing for less than 3% of the sample, but poverty level, fibroid status, and cycle length were missing for 4.0%, 6.9% and 16.9% of the sample, respectively.
RESULTS
Most of the 4,539 women in this study were 25–34 years and self-identified as white, black, or Hispanic (Table 1). Participants were generally of high educational attainment. About half were nulliparous. Pregnancy ended in miscarriage for 12% of women and about two-thirds them reported some bleeding during pregnancy. After excluding bleeding episodes that ended within 4 days of miscarriage, 25% of women with miscarriage reported at least one episode of bleeding during the first trimester, similar to the proportion of women without miscarriage who reported bleeding during pregnancy (27%). However, heavy bleeding was more common in pregnancies that miscarried (13). Of those reporting bleeding, 71% reported only one episode (n=856); 20% reported two episodes (n=241); and 9%, three or more (n=110).
Table 1.
Participants of Right From the Start, 2000–2008 (n=4539).
| n | % | |
|---|---|---|
| Age | ||
| 18–27 years | 1792 | 39.5 |
| 28–34 years | 2083 | 45.9 |
| 35–45 years | 664 | 14.6 |
| Missing | 0 | |
| Race/ethnicity | ||
| White, non-Hispanic | 3020 | 66.6 |
| Black, non-Hispanic | 967 | 21.3 |
| Hispanic ethnicity | 341 | 7.5 |
| Other | 204 | 4.5 |
| Missing | 7 | |
| Education | ||
| High school or less | 899 | 19.8 |
| Some college | 822 | 18.1 |
| College or more | 2817 | 62.1 |
| Missing | 1 | |
| Smoking | ||
| No | 3948 | 87.3 |
| Yes | 576 | 12.7 |
| Missing | 15 | |
| Parity | ||
| Nulliparous | 2113 | 47.5 |
| Primiparous | 1549 | 34.8 |
| Multiparous | 789 | 17.7 |
| Missing | 88 | |
| Bleeding | ||
| None | 3311 | 73.3 |
| Any bleeding | 1207 | 26.7 |
| Missing | 21 | |
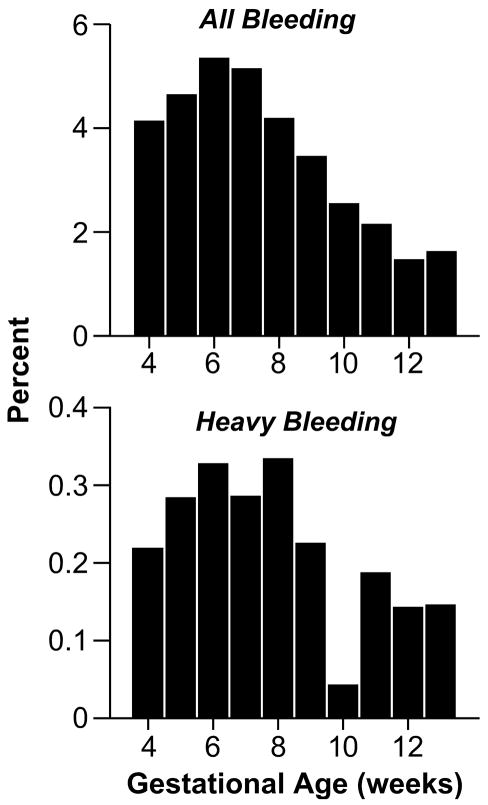
Our bleeding data began at gestational week 4, and bleeding episodes occurred during all subsequent weeks of the first trimester, peaking around the sixth and seventh week of pregnancy (Figure 1). Heavy episodes showed a similar pattern, and the peak extended throughout a longer period of the first trimester.
Figure 1. Percent with Bleeding Among All Pregnancies that Reach Each Completed Gestational Week Through the First Trimester, Right From the Start, 2000–2008 (n=4539).
All bleeding: 1656 Episodes of Spotting, Light, or Heavy Bleeding Reported by 1207 Women.
Heavy bleeding: 100 Heavy Bleeding Episodes Reported by 97 Women.
Most episodes were characterized as ‘spotting only’ (75.6%), and the majority were painless (70.7%) (Table 2). Heavy episodes comprised fewer than 10% of reported episodes. Half of all episodes persisted for only one day, 30% were of two or three days duration, and approximately 20% of episodes continued for more than 3 days. Heavy episodes were more likely to be painful, of longer duration, and red in color (Table 2).
Table 2.
Descriptive Characterization of All Bleeding Episodes (n=1656 Episodes from 1207 Women) and Episodes of Heavy Bleeding (n=100 Episodes from 97 Women) of Participants in Right From the Start, 2000–2008.
| Bleeding characteristic | All episodes (n=1656) | Heavy bleeding episodes (n=100) | ||
|---|---|---|---|---|
| Heaviness | n | % | n | % |
| Spotting | 1,251 | 75.6 | -- | -- |
| Light bleeding | 304 | 18.4 | -- | -- |
| Heavy bleeding | 100 | 6.1 | -- | -- |
| Missing | 1 | |||
| Color | ||||
| Pink | 511 | 31.0 | 4 | 4.0 |
| Red | 419 | 25.4 | 84 | 84.0 |
| Brown | 717 | 43.5 | 12 | 12.0 |
| Missing | 9 | |||
| Pain | ||||
| None | 1,168 | 70.7 | 46 | 46.0 |
| Mild | 350 | 21.1 | 19 | 19.0 |
| Moderate | 100 | 6.0 | 19 | 19.0 |
| Severe | 35 | 2.1 | 16 | 16.0 |
| Missing | 3 | |||
| Duration | ||||
| 1 day | 852 | 51.5 | 38 | 38.0 |
| 2 days | 285 | 17.2 | 16 | 16.0 |
| 3 days | 195 | 11.8 | 7 | 7.0 |
| 4–6 days | 161 | 9.7 | 14 | 14.0 |
| 7+ days | 160 | 9.7 | 25 | 25.0 |
| Missing | 3 | |||
Of the women who reported bleeding, about 15% (n=151) reported an episode that occurred around the time of their expected menstrual period, but only 2% (n=3) of these were heavy episodes lasting more than 3 days. Women who reported more than one episode (n=351) had variable intervals between episodes. About half of all multiple episodes occurred less than fourteen days apart. Fewer than 3% of women (n=10) with multiple episodes reported episode intervals consistent with the timing and length of their usual menstrual cycles, and none of these had heaviness similar to usual menses.
Table 3 shows the factors predictive of bleeding. Maternal age (particularly between 28 and 34 years), more years of education, long (≥34 days) and short (<27 days) cycle length, fibroids, infection, pre-existing or prior gestational diabetes, nulliparity, history of prior miscarriage, and history of induced abortion were predictors of bleeding. The strongest predictor based on strength of association was history of miscarriage. Body mass index, race/ethnicity, marital status, percent poverty level, active or passive smoking, prenatal vitamin use, alcohol intake, caffeine intake, gravidity, and prior preterm birth, were not predictors.
Table 3.
Predictors of the Occurrence of Bleeding, Right From the Start, 2000–2008 (n=4539).
| n | % | Unadjusted OR | 95% CI | P | Adjusted OR* | 95% CI | P | |
|---|---|---|---|---|---|---|---|---|
| Age | <0.01 | <0.01 | ||||||
| 18–27 years | 1792 | 39.5 | 1.0 | 1.0 | ||||
| 28–34 years | 2083 | 45.9 | 1.4 | 1.2, 1.6 | 1.3 | 1.1, 1.6 | ||
| 35–45 years | 664 | 14.6 | 1.2 | 1.0, 1.5 | 1.1 | 0.9, 1.4 | ||
| Education | <0.01 | 0.11 | ||||||
| ≤ High school | 899 | 19.8 | 1.0 | 1.0 | ||||
| Some college | 822 | 18.1 | 1.3 | 1.0, 1.6 | 1.2 | 0.9, 1.5 | ||
| College or more | 2817 | 62.1 | 1.4 | 1.2, 1.6 | 1.2 | 1.0, 1.5 | ||
| Cycle length | <0.01 | <0.01 | ||||||
| <27 days | 667 | 17.7 | 1.3 | 1.1, 1.6 | 1.4 | 1.1, 1.6 | ||
| 27–33 days | 2793 | 74.1 | 1.0 | 1.0 | ||||
| ≥ 34 days | 312 | 8.3 | 1.3 | 1.0, 1.6 | 1.3 | 1.0, 1.6 | ||
| Infection | 0.11 | 0.06 | ||||||
| No | 3442 | 76.2 | 1.0 | 1.0 | ||||
| Yes | 1076 | 23.8 | 1.1 | 1.0, 1.3 | 1.2 | 1.0, 1.4 | ||
| Fibroids | <0.01 | 0.01 | ||||||
| No | 3753 | 88.8 | 1.0 | 1.0 | ||||
| Yes | 472 | 11.2 | 1.4 | 1.2, 1.8 | 1.3 | 1.0, 1.6 | ||
| Diabetes | 0.15 | 0.07 | ||||||
| None | 4381 | 97.0 | 1.0 | 1.0 | ||||
| Pre-existing/prior | 137 | 3.0 | 1.3 | 0.9, 1.9 | 1.4 | 1.0, 2.1 | ||
| Parity | <0.01 | <0.01 | ||||||
| ≥ 1 live birth | 2338 | 52.5 | 1.0 | 1.0 | ||||
| Nulliparous | 2113 | 47.5 | 1.2 | 1.1, 1.4 | 1.3 | 1.2, 1.5 | ||
| Miscarriage history | 0.03 | 0.01 | ||||||
| None | 3457 | 77.7 | 1.0 | 1.0 | ||||
| One | 795 | 17.9 | 1.2 | 1.0, 1.4 | 1.2 | 1.0, 1.4 | ||
| Multiple | 199 | 4.5 | 1.4 | 1.0, 1.9 | 1.5 | 1.1, 2.1 | ||
| Induced abortion history | <0.01 | <0.01 | ||||||
| None | 3729 | 83.7 | 1.0 | 1.0 | ||||
| One | 546 | 12.3 | 1.4 | 1.1, 1.6 | 1.3 | 1.1, 1.6 | ||
| Multiple | 179 | 4.0 | 1.4 | 1.0, 1.9 | 1.4 | 1.0, 1.9 |
CI Confidence interval; OR Odds ratio
Factors are adjusted for all other variables in the table.
When assessing heaviness of bleeding, likelihood ratio tests indicated that the categories of spotting and light bleeding could be constrained to have the same coefficients (X2 =14.7, df=12, p=0.26), whereas the coefficients for the light and heavy bleeding categories were substantially different and could not be constrained to be the same (X2=25.5, df=12, p=0.01). Because of this, the spotting and light bleeding categories were combined for the multinomial analysis.
Table 4 shows the potentially important predictors of bleeding heaviness: maternal age, long/short menstrual cycle length, fibroids, infection, pre-existing or prior gestational diabetes, smoking, alcohol intake, nulliparity, and history of miscarriage and induced abortion. Estimates for some categories are highly imprecise due to the small number of observations within some strata of heavy bleeding. Most factors showed a stronger relationship with heavy bleeding compared with light bleeding, but education, parity, and induced abortion history appeared to be associated primarily with light bleeding. Of note, active smoking in pregnancy tended to be inversely associated with spotting/light bleeding (Odds ratio (OR) 0.84, 95% confidence interval (CI): 0.66, 1.05) but positively associated with heavy bleeding (OR 1.42, 95% CI: 0.81, 2.47). No dose-response was seen for number of cigarettes (data not shown). Alcohol exposure was predictive of heavy bleeding only (OR 1.60, 95% CI: 1.03, 2.48). Smoking and alcohol variables were not important predictors of ‘any’ bleeding.
Table 4.
Predictors of Bleeding Heaviness, Right from the Start, 2000–2008 (n=4539).
| Unadjusted model |
Adjusted model* |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No bleeding |
Spotting/Light Bleeding |
Heavy Bleeding |
Spotting/Light Bleeding |
Heavy Bleeding |
||||||||||
| n | OR | n | OR | 95% CI | n | OR | 95% CI | p | OR | 95% CI | OR | 95% CI | p | |
| Age | <0.01 | 0.01 | ||||||||||||
| 18–27 years | 1367 | 1.0 | 372 | 1.0 | 40 | 1.0 | 1.0 | 1.0 | ||||||
| 28–34 years | 1462 | 1.0 | 570 | 1.4 | 1.2, 1.7 | 45 | 1.1 | 0.7, 1.6 | 1.4 | 1.14, 1.61 | 1.1 | 0.7, 1.9 | ||
| 35–45 years | 482 | 1.0 | 167 | 1.3 | 1.0, 1.6 | 12 | 0.9 | 0.5, 1.7 | 1.2 | 0.91, 1.47 | 0.8 | 0.4, 1.6 | ||
| Education | <0.01 | 0.20 | ||||||||||||
| ≤High school | 693 | 1.0 | 173 | 1.0 | 25 | 1.0 | 1.0 | 1.0 | ||||||
| Some college | 601 | 1.0 | 197 | 1.3 | 1.1, 1.7 | 21 | 1.0 | 0.5, 1.8 | 1.2 | 0.95, 1.53 | 1.0 | 0.5, 1.8 | ||
| College or more | 2016 | 1.0 | 739 | 1.5 | 1.2, 1.8 | 51 | 0.7 | 0.4, 1.1 | 1.3 | 1.02, 1.57 | 0.8 | 0.4, 1.4 | ||
| Cycle length | <0.01 | <0.01 | ||||||||||||
| <27 days | 457 | 1.0 | 195 | 1.3 | 1.1, 1.6 | 15 | 1.3 | 0.8, 2.2 | 1.4 | 1.14, 1.67 | 1.3 | 0.8, 2.2 | ||
| 27–33 days | 2085 | 1.0 | 651 | 1.0 | 55 | 1.0 | 1.0 | 1.0 | ||||||
| ≥34 days | 217 | 1.0 | 89 | 1.3 | 1.0, 1.8 | 5 | 1.1 | 0.4, 2.7 | 1.3 | 1.01, 1.77 | 1.2 | 0.5, 3.0 | ||
| Infection | 0.24 | 0.18 | ||||||||||||
| No | 2542 | 1.0 | 831 | 1.0 | 69 | 1.0 | 1.0 | 1.0 | ||||||
| Yes | 769 | 1.0 | 278 | 1.1 | 0.9, 1.3 | 28 | 1.3 | 0.9, 2.1 | 1.2 | 0.99, 1.36 | 1.2 | 0.8, 1.9 | ||
| Fibroids | <0.01 | 0.07 | ||||||||||||
| No | 2765 | 1.0 | 895 | 1.0 | 73 | 1.0 | 1.0 | 1.0 | ||||||
| Yes | 316 | 1.0 | 143 | 1.4 | 1.1, 1.7 | 13 | 1.5 | 0.8, 2.7 | 1.2 | 1.00, 1.54 | 1.5 | 0.8, 2.9 | ||
| Diabetes | 0.27 | 0.19 | ||||||||||||
| None | 3218 | 1.0 | 1070 | 1.0 | 92 | 1.0 | 1.0 | 1.0 | ||||||
| Prior diabetes | 93 | 1.0 | 39 | 1.3 | 0.9, 1.8 | 5 | 1.9 | 0.8, 4.7 | 1.4 | 0.93, 2.02 | 1.8 | 0.7, 4.6 | ||
| Parity | <0.01 | <0.01 | ||||||||||||
| ≥1 live birth | 1752 | 1.0 | 530 | 1.0 | 56 | 1.0 | 1.0 | 1.00 | ||||||
| Nulliparous | 1496 | 1.0 | 567 | 1.3 | 1.1, 1.5 | 40 | 0.9 | 0.6, 1.3 | 1.4 | 1.18, 1.59 | 0.9 | 0.6, 1.4 | ||
| Miscarriage history | 0.02 | 0.01 | ||||||||||||
| None | 2552 | 1.0 | 831 | 1.0 | 64 | 1.0 | 1.0 | 1.0 | ||||||
| One | 564 | 1.0 | 207 | 1.1 | 0.9, 1.3 | 24 | 1.7 | 1.0, 2.7 | 1.2 | 0.98, 1.40 | 1.6 | 1.0, 2.7 | ||
| Two or more | 132 | 1.0 | 59 | 1.4 | 1.0, 1.9 | 8 | 2.5 | 1.2, 5.2 | 1.5 | 1.02, 2.04 | 2.5 | 1.1, 5.4 | ||
| Induced abortion history | <0.01 | <0.01 | ||||||||||||
| None | 2757 | 1.0 | 880 | 1.0 | 79 | 1.0 | 1.0 | 1.0 | ||||||
| One or more | 491 | 1.0 | 217 | 1.4 | 1.2, 1.7 | 17 | 1.2 | 0.7, 2.1 | 1.4 | 1.18, 1.71 | 1.0 | 0.6, 1.8 | ||
| Smoking | 0.01 | 0.12 | ||||||||||||
| No | 2876 | 1.0 | 989 | 1.0 | 77 | 1.0 | 1.0 | 1.0 | ||||||
| Yes | 435 | 1.0 | 120 | 0.8 | 0.6, 1.0 | 20 | 1.7 | 1.0, 2.8 | 0.8 | 0.66, 1.05 | 1.4 | 0.8, 2.5 | ||
| Alcohol exposure | 0.14 | 0.09 | ||||||||||||
| No | 1483 | 1.0 | 483 | 1.0 | 34 | 1.0 | 1.0 | 1.0 | ||||||
| Yes | 1826 | 1.0 | 625 | 1.1 | 0.9, 1.2 | 63 | 1.5 | 1.0, 2.3 | 1.0 | 0.85, 1.13 | 1.6 | 1.0, 2.5 | ||
CI Confidence interval; OR Odds ratio
Factors are adjusted for all other variables in the table.
We re-analyzed subsets of the data to evaluate potential biases. Our descriptive characterization of the timing of bleeding in the first trimester was robust to restrictions in the study population based on when women enrolled. When considering predictors of bleeding, we compared the results of the model with multiple imputation to the model with no missing data for any covariates, the results did not change substantially, though confidence intervals around estimates were wider for the model without missing data, as expected given the reduced sample size. We restricted to participants in their first pregnancy (n=1,527) and also to women whose pregnancies progressed beyond the first trimester (n=3,960) and found no substantial changes in model estimates.
DISCUSSION
We provide new evidence that first trimester bleeding episodes tend to peak during the sixth and seventh weeks. Different characteristics of bleeding tend to cluster together. Heavy bleeding episodes (similar or heavier than those of a woman’s normal menses) are more likely to be associated with pain, longer duration, bright red color, and presence of multiple episodes, while spotting episodes are more likely to occur in isolation and be of shorter duration and without pain. This suggests that heavy bleeding may arise from different underlying biologic events than spotting.
Though the causes of bleeding in later pregnancy have been investigated (due to placenta previa, abruption, or infection), there has been little investigation of first trimester bleeding (9, 14, 15). It is interesting that the peak in bleeding episodes coincides with the development of a hormonally functional placenta. In very early pregnancy, the corpus luteum produces progesterone. The shift from luteal production to placental production of progesterone occurs by the seventh week of pregnancy and can result in a temporary reduction in progesterone levels if the placenta is not producing sufficiently (16). Decreasing levels of progesterone are associated with the onset of menses outside of pregnancy; similarly, during pregnancy, decreasing levels may trigger an episode of vaginal bleeding and limit successful maintenance of the pregnancy. Thus, bleeding at this time in pregnancy may signal that the early placenta has not developed optimally.
A first-trimester bleeding episode may also occur due to premature onset of maternal-fetal circulation or abnormal formation of placental membranes (17). In early pregnancy, the maternal spiral arteries are blocked by a trophoblastic shell, maintaining a low oxygen environment for fetal development until the ninth or tenth week of gestation when maternal-fetal circulation begins (17). Premature onset of maternal-fetal circulation may be associated with first trimester bleeding episodes (17, 18). Such bleeding episodes may serve as a marker of an improperly developing placenta. Placental dysfunction has been suggested to play a causal role in adverse pregnancy outcomes, including miscarriage and pre-eclampsia (18).
Our results show that early pregnancy bleeding rarely mimics the bleeding of menses. Gestational age misdating has been previously attributed to early pregnancy bleeding;(19) however, the majority of women in our study reported bleeding that was light, of short duration, and rarely resembled the heaviness of a usual menstrual cycle. Gestational age dating was verified by ultrasound, and a validation substudy of ongoing pregnancies in RFTS 1 found that the average difference between gestational age based on self-reported LMP and ultrasound was less than one day (20). Based on these results, it is unlikely that many women misdate their pregnancy by mistaking early pregnancy bleeding for their LMP, consistent with the results of a previous study (10). We found little evidence that cyclic, menstrual-like bleeding occurs during the first trimester. Few women reported intervals between episodes consistent with menstrual cycling, and none of these reported heaviness similar to their menses.
In our exploratory analyses, several maternal characteristics emerged as important predictors of the presence of bleeding, outlined in Tables 3 and 4. Of note, education, nulliparity, and previous induced abortion may be important as measures of reporting sensitivity because they were associated with increased reporting of spotting or light bleeding but not heavy bleeding. Maternal behaviors, such as active smoking and alcohol intake during pregnancy, were associated with heavy, but not light, bleeding. Smoking was also inversely associated with light bleeding, likely related to decreased reporting of spotting and light bleeding episodes among smokers. Overall, our results were consistent with previous reports and also provide new information about maternal characteristics that may be associated with bleeding.
We acknowledge important limitations. Our results are based on women’s recall of their bleeding during the early months of pregnancy, although a separate analysis found that a subgroup who volunteered to prospectively record bleeding information throughout early pregnancy showed high reporting accuracy at interview (21). However, even in this group expected to represent a best-case scenario, recall accuracy was not perfect. Our study is also limited by small sample size for low-prevalence predictors, such as diabetes. The decreased precision of these estimates was particularly evident in the analysis of predictors of bleeding heaviness.
Despite the limitations, our study provides important new information about bleeding in early pregnancy, including data on the timing, heaviness, duration, color, and overall number of episodes. The generalizability of our findings is strengthened by the community recruitment that did not exclude participants whose pregnancies terminated prior to twenty weeks of gestation. In addition, timing of gestational age was verified with ultrasound, which increases the confidence in our findings regarding the gestational timing of bleeding. The ultrasound also provided unique information about uterine fibroids not available to other perinatal researchers. Further research is needed to systematically study bleeding in mid to late pregnancy with the same detail.
In conclusion, spotting or light bleeding episodes are common symptoms in early pregnancy. Heavy bleeding is much less common. Whether both light and heavy bleeding arise from the same mechanisms or have different etiologies is an important question for future research. Future steps include investigating the relationship between bleeding episodes, early pregnancy biology, placental pathophysiology, and pregnancy outcomes.
Acknowledgments
The field research was supported in part by grants from the National Institute of Child and Human Development (5R01HD043883 and 5R01HD049675) and the American Water Works Association Research Foundation (2579). Additional funds were provided by the National Institute of Environmental Health Sciences (Intramural Research Program and P30ES10126).
We thank Dr. Olga Basso and Dr. Shannon Laughlin for helpful comments and Dr. Sue Edelstein for assistance with graphics.
Abbreviations
- CI
Confidence interval
- LMP
Last menstrual period
- NC
North Carolina
- OR
Odds ratio
- RFTS
Right From the Start
- TN
Tennessee
- TX
Texas
Appendix
Questions about bleeding in first trimester interview
H8a. Since you got pregnant, have you had any bleeding or spotting with blood?
H8b. Did the bleeding or spotting start at the time you expected your menstrual period?
H9a. As best as you can remember when did you start to bleed or spot for the first/2nd/3rd time? M/d/y
H9b. (if H9a ‘don’t know’) Do you remember what week that was (1st, 2nd, 3rd, 4th, etc)
H10a. Compare this spotting/bleeding to amount of bleeding you usually have: on the day of your heaviest spotting or bleeding in the 1st/2nd/3rd episode, would you describe the bleeding as light, lighter than heavy flow, like heavy flow, more than heavy flow
H10b. What color was the blood, was it generally red, pink, or brown?
H10c. How many days did it last? If it stopped for at least 2 days and started again, consider this a separate episode.
H11a. Did you have any pain during the time you had spotting or bleeding?
H11b. Overall, would you describe the pain as mild, moderate, or severe?
H12. Did you have a 2nd/3rd time when you had spotting or bleeding? (start back at H9a)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ananth CV, Savitz DA. Vaginal bleeding and adverse reproductive outcomes: a meta-analysis. Paediatr Perinat Epidemiol. 1994;8:62–78. doi: 10.1111/j.1365-3016.1994.tb00436.x. [DOI] [PubMed] [Google Scholar]
- 2.Williams MA, Mittendorf R, Lieberman E, Monson RR. Adverse infant outcomes associated with first-trimester vaginal bleeding. Obstet Gynecol. 1991;78:14–8. [PubMed] [Google Scholar]
- 3.Berkowitz GS, Harlap S, Beck GJ, Freeman DH, Baras M. Early gestational bleeding and pregnancy outcome: a multivariable analysis. Int J Epidemiol. 1983;12:165–73. doi: 10.1093/ije/12.2.165. [DOI] [PubMed] [Google Scholar]
- 4.Weiss JL, Malone FD, Vidaver J, et al. Threatened abortion: A risk factor for poor pregnancy outcome, a population-based screening study. Am J Obstet Gynecol. 2004;190:745–50. doi: 10.1016/j.ajog.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 5.Yang J, Savitz DA, Dole N, et al. Predictors of vaginal bleeding during the first two trimesters of pregnancy. Paediatr Perinat Epidemiol. 2005;19:276–83. doi: 10.1111/j.1365-3016.2005.00655.x. [DOI] [PubMed] [Google Scholar]
- 6.Batzofin JH, Fielding WL, Friedman EA. Effect of vaginal bleeding in early pregnancy on outcome. Obstet Gynecol. 1984;63:515–8. [PubMed] [Google Scholar]
- 7.Sipila P, Hartikainen-Sorri AL, Oja H, Von Wendt L. Perinatal outcome of pregnancies complicated by vaginal bleeding. Br J Obstet Gynaecol. 1992;99:959–63. doi: 10.1111/j.1471-0528.1992.tb13697.x. [DOI] [PubMed] [Google Scholar]
- 8.Axelsen SM, Henriksen TB, Hedegaard M, Secher NJ. Characteristics of vaginal bleeding during pregnancy. Eur J Obstet Gynecol Reprod Biol. 1995;63:131–4. doi: 10.1016/0301-2115(95)02236-8. [DOI] [PubMed] [Google Scholar]
- 9.French JI, McGregor JA, Draper D, Parker R, McFee J. Gestational bleeding, bacterial vaginosis, and common reproductive tract infections: risk for preterm birth and benefit of treatment. Obstet Gynecol. 1999;93:715–24. doi: 10.1016/s0029-7844(98)00557-2. [DOI] [PubMed] [Google Scholar]
- 10.Harville EW, Wilcox AJ, Baird DD, Weinberg CR. Vaginal bleeding in very early pregnancy. Hum Reprod. 2003;18:1944–7. doi: 10.1093/humrep/deg379. [DOI] [PubMed] [Google Scholar]
- 11.Laughlin SK, Baird DD, Savitz DA, Herring AH, Hartmann KE. Prevalence of Uterine Leiomyomas in the First Trimester of Pregnancy: An ultrasound screening study. Obstetrics and Gynecology. 2009 doi: 10.1097/AOG.0b013e318197bbaf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Royston P. Multiple imputation of missing values: update. The Stata Journal. 2005;5:188–201. [Google Scholar]
- 13.Hasan R, Baird DD, Herring AH, Olshan AF, Jonsson Funk ML, Hartmann KE. Association between first-trimester vaginal bleeding and miscarriage. Obstet Gynecol. 2009;114:860–7. doi: 10.1097/AOG.0b013e3181b79796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomez R, Romero R, Nien JK, et al. Idiopathic vaginal bleeding during pregnancy as the only clinical manifestation of intrauterine infection. J Matern Fetal Neonatal Med. 2005;18:31–7. doi: 10.1080/14767050500217863. [DOI] [PubMed] [Google Scholar]
- 15.Williams MA, Hickok DE, Zingheim RW, Mittendorf R, Kimelman J, Mahony BS. Low birth weight and preterm delivery in relation to early-gestation vaginal bleeding and elevated maternal serum alpha-fetoprotein. Obstet Gynecol. 1992;80:745–9. [PubMed] [Google Scholar]
- 16.Csapo AI, Pulkkinen M. Indispensability of the human corpus luteum in the maintenance of early pregnancy. Luteectomy evidence Obstet Gynecol Surv. 1978;33:69–81. doi: 10.1097/00006254-197802000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Jauniaux E, Johns J, Burton GJ. The role of ultrasound imaging in diagnosing and investigating early pregnancy failure. Ultrasound Obstet Gynecol. 2005;25:613–24. doi: 10.1002/uog.1892. [DOI] [PubMed] [Google Scholar]
- 18.Jauniaux E, Poston L, Burton GJ. Placental-related diseases of pregnancy: Involvement of oxidative stress and implications in human evolution. Hum Reprod Update. 2006;12:747–55. doi: 10.1093/humupd/dml016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gjessing HK, Skjaerven R, Wilcox AJ. Errors in gestational age: evidence of bleeding early in pregnancy. Am J Public Health. 1999;89:213–8. doi: 10.2105/ajph.89.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffman CS, Messer LC, Mendola P, Savitz DA, Herring AH, Hartmann KE. Comparison of gestational age at birth based on last menstrual period and ultrasound during the first trimester. Paediatric and Perinatal Epidemiology. 2008;22(6):587–96. doi: 10.1111/j.1365-3016.2008.00965.x. [DOI] [PubMed] [Google Scholar]
- 21.Hasan R, Jonsson Funk ML, Herring AH, Olshan AF, Hartmann KE, Baird DD. Accuracy of reporting bleeding during pregnancy. Paediatr Perinat Epidemiol. 2010;24:31–34. doi: 10.1111/j.1365-3016.2009.01089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]