Abstract
Plasmacytoid dendritic cells (pDC) are the body’s main source of IFN-α, but, unlike classical myeloid DC (myDC), they lack phagocytic activity and are generally perceived as playing only a minor role in Ag processing and presentation. We show that murine pDC, as well as myDC, express Fcγ receptors (CD16/CD32) and can use these receptors to acquire Ag from immune complexes (IC), resulting in the induction of robust Ag-specific CD4+ and CD8+ T cell responses. IC-loaded pDC stimulate CD4 + T cells to proliferate and secrete a mixture of IL-4 and IFN-γ, and they induce CD8+ T cells to secrete IL-10 as well as IFN-γ. In contrast, IC-loaded myDC induce both CD4+ and CD8+ T cells to secrete mainly IFN-γ. These results indicate that pDC can shape an immune response by acquiring and processing opsonized Ag, leading to a predominantly Th2 response.
Plasmacytoid dendritic cells (pDC)4 are the body’s first line of defense against invading pathogens, and can efficiently fight viruses through their high and immediate production of IFN-α (1). In their resting state, pDC do not resemble classical myeloid dendritic cells (myDC), with long dendritic protrusions, but rather have a rounded morphology more like lymphocytes (2, 3). Unlike myDC, pDC do not phagocytose or take up exogenous protein Ags efficiently, mainly because they lack DEC-205 and mannose receptors (4). In addition, they express low levels of MHC Ags and accessory molecules (1–3). In turn, this leads to weak activation of naive Ag-specific T cells. However, endogenous Ags in the form of self-Ags or viral products may be efficiently presented by pDC, suggesting a role for these cells in both viral defense and maintenance of tolerance in the steady state (5, 6). Moreover, pDC can activate Ag-experienced and memory T cells (7), further indicating a role for these cells in shaping the adaptive immune response.
In addition to acquiring Ags via phagocytosis and pinocytosis, monocytes, macrophages, and myDC express and take up Ags in immune complexes (IC) via their FcγR (8), resulting in Ag processing and presentation to T cells. Such opsonized Ags were described more than a century ago (9), and opsonization is known to play a central role in the induction of innate immune responses to various pathogens. However, despite the well-established role of pDC in innate immunity, their ability to acquire and process opsonized Ags is unknown. This led us to ask whether pDC, which express FcγR (CD16/CD32, FcγRIII/FcγRII), can take up exogenous Ags via this receptor, and, if so, whether such Ags would be processed in a manner that promotes Ag-specific T cell activation.
Materials and Methods
Mice
Female or male C57BL/6, OT-I, and OT-II OVA-TCR transgenic (OVA-TCR Tg) mice were obtained from The Jackson Laboratory and used at 8–12 wk of age. OT-I and OT-II mice crossed onto RAG−/− background were purchased from Taconic Farms and bred in-house. All experiments were performed under institutional guidelines according to an approved protocol.
Reagents
All fluorochrome-labeled Abs were purchased from BD Pharmingen. IFN-α was detected using a kit from R&D Systems, according to the manufacturer’s instructions. IL-6 and TNF-α were detected using Abs purchased from eBioscience. Mouse anti-chicken OVA (IgG1) was obtained as ascites from Sigma-Aldrich. The Ab was dialyzed and filtered before the titer was confirmed in direct OVA ELISA. Sterile aliquots were stored at −20°C until used. DQ-OVA, a self-quenched dye emitting green fluorescence upon degradation, and CFSE were purchased from Invitrogen. LPS, PMA, ionomycin, and brefeldin A were all purchased from Sigma-Aldrich. OVA323–339 (ISQAVHAAHAEINEAGR) and OVA257–264 (SIINFEKL) were synthesized at the University of Pittsburgh and purified using HPLC. OVA/Anti-OVA-IC were prepared by incubating DQ-OVA (100 µg/ml) with mouse-anti-OVA (titer 1:20) for 1 h at 37°C. The complexes were used at a final concentration of 10 µg/ml DQ-OVA and 1/200 dilution of the Ab.
Uptake of IC in vivo
To determine uptake of IC by dendritic cells (DC) in vivo, mice were injected s.c. in the footpad with 50 µl of DQ-OVA-containing IC. Five to 24 h later, mice were sacrificed, and draining and nondraining lymph nodes were harvested. Single-cell suspensions were prepared in the presence of collagenase IV (Worthington Biochemical), washed, and stained with directly conjugated Abs. Cells were analyzed by four-color flow cytometry using anti-CD11c allophycocyanin, anti-CD19 PE, anti-CD3 PE, and IC measured in the FITC channel. B220 PE-Cy5 and CD11b PE-Cy5 were used to detect pDC and myDC, respectively.
Cell preparation and flow cytometry
Bone marrow-derived DC were prepared, as described by Gilliet et al. (10). Briefly, bone marrow cells were obtained by flushing the femur and tibia using a 23G needle. After red cell lysis, total leukocytes were cultured in HEPES-buffered RPMI 1640 supplemented with 5% FCS, antibiotics, 2 mM l-glutamine, 5 × 10−5 M 2-ME, nonessential amino acids, Na-pyruvate, and 100 ng/ml human rFlt3 ligand. Cultures were performed at 1 × 106 cells/ml in six-well plates (Costar). After 10 days, DC were harvested, and after washing, directly conjugated anti-B220 allophycocyanin and anti-CD11b allophycocyanin-Cy7 Abs were added and cells were further incubated on ice for 15 min. FcR-blocking Abs were deliberately excluded from the cell preparation protocol, because they would have interfered with our subsequent studies. After final washing, cells were sorted on a FACSVantage (BD Biosciences). Purity exceeded 96% for both pDC and mDC Postsort analyses showed all cells expressed the mouse DC marker CD11c. OVA-specific CD4+ T cells were isolated from the spleens of OT-II (C57BL/6) mice. Spleens were dispersed into single-cell suspensions and, after lysis of red cells, anti-CD4 Ab-conjugated magnetic beads (Miltenyi Biotec) were added and cells were further incubated for 20 min at 4°C Purity was >90%. T cells were labeled with CFSE (10 µM) for 10 min at 37°C, washed, and resuspended in HEPES-buffered RPMI 1640 supplemented with 5% FCS, antibiotics, and 2 mM l-glutamine.
A total of 5 × 104 DC and 2 × 105 T cells was cultured in duplicate wells in 96-well round-bottom plates at a final volume of 200 µl. Cultures were supplemented with either OVA-IC or DQ-OVA. Control cultures containing OVA peptide were set up in parallel.
In vivo T cell activation
Sorted DC subsets were incubated together with OVA-IC overnight at 37°C. After washing in PBS, 1–2 × 106 DC pulsed with IC were injected s.c. in the footpad. OVA Tg, splenic T cells were isolated using either anti-CD4- or anti-CD8-conjugated magnetic beads (Miltenyi Biotec) and labeled with CFSE (1 µM). A total of 8–10 × 106 T cells was administered by i.v. injection in the tail. Three to 4 days later, mice were sacrificed, and draining popliteal and superficial inguinal nodes were harvested and CD4+ or CD8+ T cells were reisolated using MACS beads, stained with directly fluorochrome-labeled Abs, and analyzed by flow cytometry.
CTL assay
For determination of CTL activity, EG7-OVA were labeled with a high concentration of CFSE (1 µM/ml), and control EL-4 tumor cells were labeled with a lower CFSE concentration (0.1 µM/ml) (11), mixed in equal parts, and used as target cells. Graded numbers of effector OT-I T cells, either unstimulated or cultured for 6 days with pDC-IC or myDC-IC, were added to the labeled target cells and incubated overnight at 37°C. Percentage of specific lysis of target cells was determined using FACS according to the following formula: ratio = (percentage of CFSElow/CFSEhigh); percentage of specific lysis = (1 − (ratio unprimed/ratio primed)) × 100.
ELISA for OVA-IC
Mouse anti-chicken OVA (Sigma-Aldrich) was dialyzed against PBS, sterile filtered, and stored at −20°C. ELISA plates (Costar) were coated with DQ-OVA (10 µg/ml) in PBS at 100 µl/well and incubated overnight at 4°C. The plates were blocked for 1 h at 37°C using 3% BSA in PBS-Tween 20, and washed, and serial dilutions of mouse-anti-OVA mAb were added to duplicate wells. After incubation overnight at 4°C, the plates were washed and biotinylated F(ab′)2 goat anti-mouse IgG (Jackson Immuno-Research Laboratories) was added. After 1-h incubation at room temperature, the plate was washed and extravidin-peroxidase (Sigma-Aldrich) was added. After an additional 1-h incubation and washing, the plates were developed using tetramethylbenzidine substrate (Kirkegaard & Perry Laboratories). Ab titer was determined as highest OD450 (typically 1:10,000).
Statistical analysis
For comparisons between groups, Student’s t test was performed.
Results
pDC express FcγR and capture IC in vivo
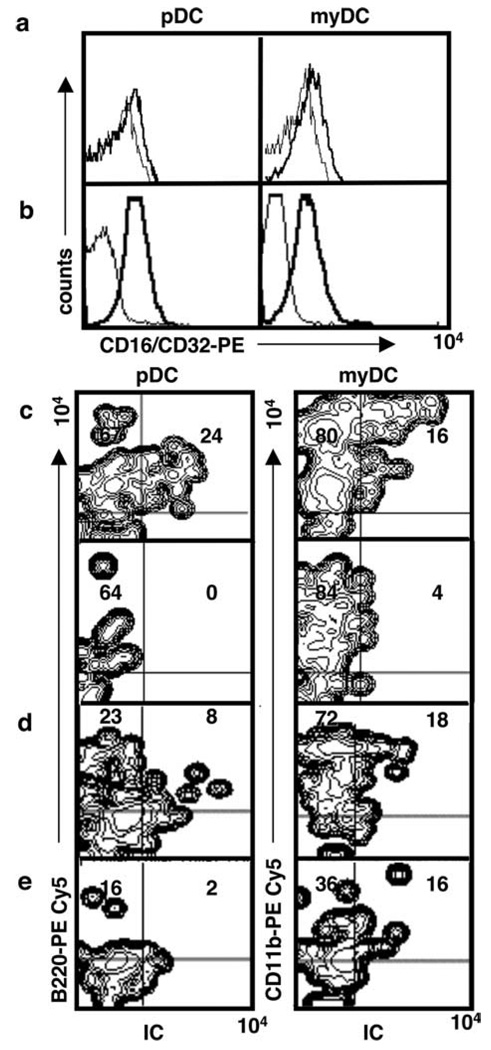
As shown in Fig. 1a, freshly isolated splenic pDC and myDC express FcγR (CD16/CD32) at low levels (Fig. 1a). DC from lymph nodes expressed somewhat higher levels of FcγR (Fig. 1b). To determine whether pDC can use their FcγR to take up opsonized Ag, we injected one footpad of mice with IC containing anti-OVA Ab and DQ-OVA, a self-quenched fluorescent OVA conjugate exhibiting green fluorescence after proteolytic degradation. Control mice were injected with DQ-OVA alone. Draining popliteal and superficial inguinal lymph nodes were harvested at different time points (5–24 h postinjection), and single-cell suspensions were stained and analyzed by four-color flow cytometry. As shown in Fig. 1c, both pDC and myDC acquired opsonized Ag. Uptake was detectable at 5 h, peaked at 8 h, and declined markedly at 12–24 h postinjection (Fig. 1d). No staining of pDC or myDC was seen in the contralateral lymph nodes of IC-injected mice (Fig. 1c), nor was any staining detected in pDC in mice injected with DQ-OVA alone (Fig. 1e).
FIGURE 1.
pDC and myDC express FcγR (CD16/CD32) and take up IC in vivo. a and b, Expression of FcγR (CD16/CD32) on DC subsets isolated from spleen (a) or lymph nodes (b). c, IC containing fluorescent DQ-OVA was injected into the left footpads of mice and, after 8 h, draining popliteal and superficial inguinal lymph nodes were isolated. Contralateral (nondraining) popliteal lymph nodes served as control. d, Uptake of IC OVA-IC at 5 h postinjection. e, Uptake of DQ-OVA 8 h postinjection. IC or DQ-OVA uptake was analyzed by four-color flow cytometry. Gates were set on CD11c+ DC, excluding cells in the PE channel, dead cells, and debris. IC uptake was measured in the FITC channel, and pDC or myDC were detected using PE-Cy5-conjugated mAb to B220 and CD11b, respectively. Data are representative of three independent experiments.
pDC exposed to IC in vitro take up Ag and trigger Ag-specific CD4+ T cell activation
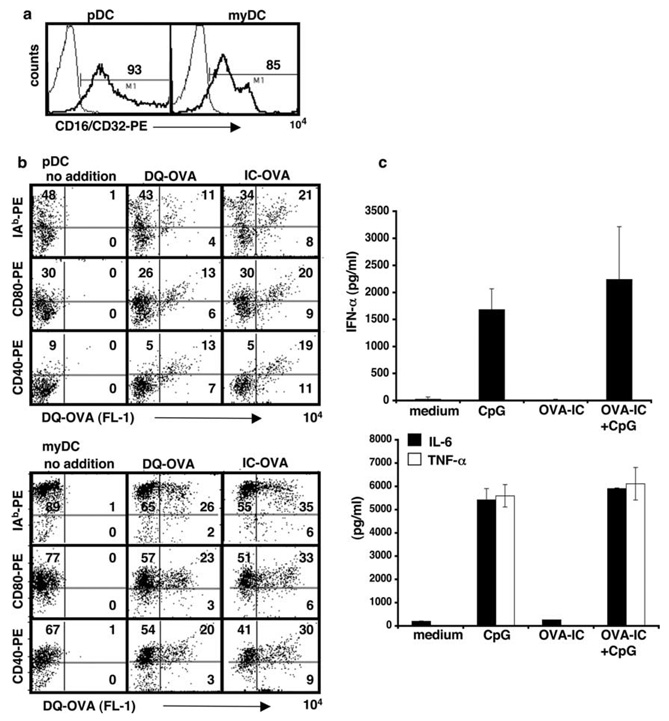
To evaluate the effects of IC on pDC, we purified pDC and myDC from bone marrow cells cultured for 10 days in medium containing Flt3 ligand. FcγR expression was higher on these DC than on DC analyzed immediately after their isolation from spleen or lymph nodes (Fig. 2a). We incubated each population with either DQ-OVA or OVA-IC for 24 h, after which the cells were examined by flow cytometry for evidence of Ag uptake and for changes in selected surface markers. Ag uptake was clearly demonstrated in pDC as well as myDC, although uptake was greater in myDC (Fig. 2b). For both DC subsets, Ag uptake was accompanied by cellular activation, based on up-regulation of MHC class II (I-Ab), CD80, and CD40 (Fig. 2b). Some Ag uptake and cellular activation of pDC occurred when OVA was provided as free protein; however, more Ag acquisition and cellular stimulation were seen when the pDC were exposed to OVA-IC. We also examined IFN-α secretion, the hallmark of pDC activation. pDC were cultured with OVA-IC alone or with both OVA-IC and a TLR agonist, CpG 2336. In addition, we examined IL-6 and TNF-α, two proinflammatory cytokines produced by pDC. As shown in Fig. 2c, in the absence of TLR agonists, OVA-IC did not induce IFN-α production, not did they produce IL-6 or TNF-α.
FIGURE 2.
Exposure of pDC to IC leads to Ag uptake in vitro. a, Expression of FcγR (CD16/CD32) on bone marrow-derived, FACS-sorted DC subsets. b, FACS-sorted pDC and myDC were incubated overnight together with OVA-IC consisting of DQ-OVA and mouse IgG1 anti-OVA Ab, DQ-OVA, or medium alone. Cells were washed and stained for I-Ab, CD80, and CD40. Data are representative of at least five independent experiments. c, IC do not induce IFN-α, IL-6, or TNF-α production in pDC in the absence of a TLR agonist (CpG 2336). A total of 1 × 105 pDC was cultured either in medium alone, or together with IC-OVA, CpG, or IC-OVA plus CpG for 48 h. Culture supernatants were harvested and analyzed by ELISA.
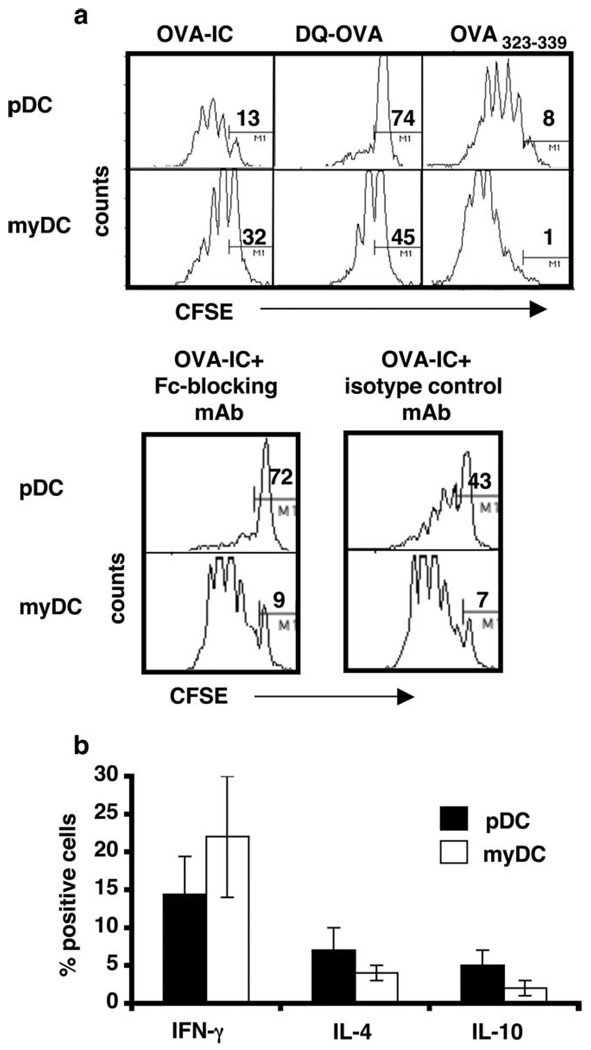
To determine whether Ag uptake by pDC leads to processing and presentation of antigenic peptides, we cultured sorted pDC or myDC together with CFSE-labeled CD4+ OT-II T cells in the presence of OVA-IC, DQ-OVA, or an immunodominant I-Ab/d OVA-peptide (OVA323–339). After 4 days, the T cells were stained for CD25 and analyzed by flow cytometry for evidence of proliferation. Fig. 3a shows that CD4+ T cells proliferated in response to both pDC and myDC pulsed with IC (pDC-IC and myDC-IC). pDC incubated with OVA alone induced poor T cell stimulation, whereas OVA-pulsed myDC induced T cell activation similar to that generated by myDC-IC. Addition of an FcγR-blocking Ab at the time pDC were pulsed with IC resulted in inhibition of pDC-IC-, but not myDC-IC-induced T cell proliferation. As expected, pDC or myDC pulsed with OVA323–339 strongly promoted T cell proliferation. Fig. 3b shows that a proportion of the CD4+ T cells cultured with pDC-IC or myDC-IC produced IFN-γ. Fewer T cells produced IL-4 and IL-10, but such cells were more frequent in pDC-IC/T cell cultures than myDC-IC/T cell cultures. Both pDC-IC and myDC-IC promoted T cell proliferation in these cultures, with the number of cell divisions indicated by progressive reduction in intracellular CFSE content.
FIGURE 3.
a, Exposure of pDC to IC leads to T cell activation in vitro. A total of 5 × 104 FACS-sorted pDC or myDC was cultured for 4 days with OVA-IC, DQ-OVA, or OVA323–339 peptide, together with 1 × 105 CFSE-labeled CD4+ OVA-Tg T cells (OT-II). Cells were stained for CD25 and analyzed using a FACSCalibur. Numbers indicate percentage of cells not dividing. Lower panel, OVA-IC and FcγR-blocking Ab (2.4G2, 25 µg/ml) or isotype control. b, Four-day cultures of CD4+ T cells and DC-IC were examined for IFN-γ, IL-4, and IL-10 production by intracellular staining with cytokine-specific Abs. Cellular divisions were determined using CFSE. Data shown are representative of three independent experiments.
pDC-IC promote Ag-specific CD4+ T cell proliferation and cytokine production in vivo
We extended our investigations to examine whether opsonized Ag processed by pDC could also induce T cell activation in vivo. Sorted pDC and myDC from C57BL/6 mice were incubated with OVA-IC overnight at 37°C. After washing, pDC-IC or myDC-IC (1–2 × 106) were injected s.c. in the footpads of syngeneic animals. Immediately thereafter, CFSE-labeled, MACS-isolated splenic CD4+ T cells (10 × 106) from OT-II (OVA-TCR Tg) mice were injected i.v. in the tail. Three to 4 days after T cell administration, draining popliteal and superficial inguinal lymph nodes were harvested, stained, and analyzed using multicolor flow cytometry. As shown in Fig. 4, both pDC-IC and myDC-IC induced T cell activation in vivo, as indicated by up-regulation of CCR7, as well as T cell proliferation. T cells from control animals given PBS did not proliferate. To examine cytokine production by T cells activated in vivo with DC-IC, we injected mice with pDC-IC or myDC-IC, as above. After 4 days, draining lymph nodes were harvested and CD4+ T cells were isolated and stimulated with PMA and ionomycin before undergoing analysis for intracellular cytokines. The results show that T cells exposed to pDC-IC in vivo displayed a predominantly Th2 cytokine profile, with more cells producing IL-4 than IFN-γ, whereas T cells from animals given myDC-IC displayed a predominantly Th1 response (Fig. 4b).
FIGURE 4.
FcγR-mediated Ag uptake by pDC leads to CD4+ T cell activation and cytokine production in vivo. A total of 1 × 106 FACS-sorted DC subsets incubated with OVA-IC overnight was injected into the footpad, and CFSE-labeled, CD4+ OVA-Tg T cells (10 × 106) were injected i.v. in the base of the tail. a, After 3–4 days, draining lymph nodes were harvested and stained for CCR7. Fine line indicates T cell control. b, Lymph node T cells were analyzed for their production of cytokines after stimulation with PMA and ionomycin in vitro. OVA-Tg CD4+ T cells were gated based on forward and side scatter and TCR Vα2 expression, and IFN-γ- and IL-4-secreting cells were identified by intracellular staining. *, Indicates p < 0.05. Results are representative of three independent experiments.
pDC can cross-present OVA to CD8+ T cells
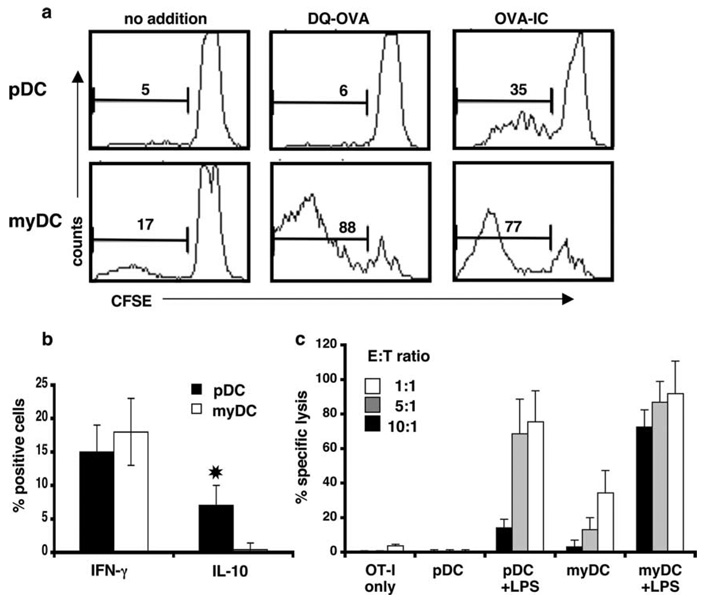
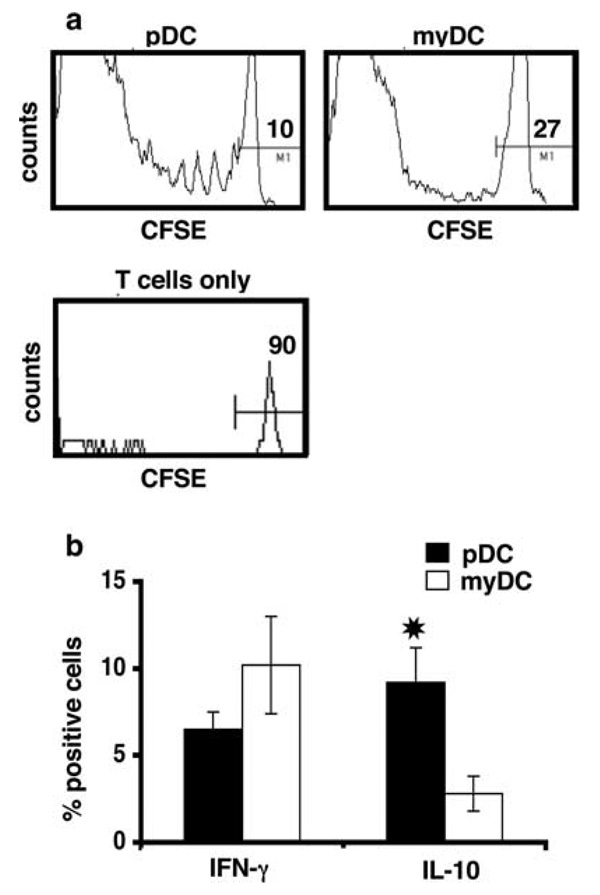
Cross-presentation of Ag to CD8+ T cells is an important property of myDC, particularly CD8αα+ myDC (12, 13). To examine whether pDC can cross-present opsonized Ag, we incubated pDC-IC with CFSE-labeled, MACS-enriched, CD8+ OT-I T cells for 4–5 days, as above. As shown in Fig. 5a, OT-I T cell proliferation was induced by pDC-IC, but not by pDC that had been cultured with OVA alone. In contrast, myDC cultured with either OVA-IC or OVA alone stimulated equivalent OT-I activation. In both cases, the responding T cells had a CD44+ memory pheno-type. Although T cell proliferation was greater with myDC-IC than pDC-IC on a per cell basis, a substantial proportion of the CD8 + T cells underwent division when cultured with pDC-IC, as compared with CD8+ T cells cultured with pDC and OVA alone. pDC-IC also induced cytokine production by cocultured CD8+ T cells. Both IFN-γ- and IL-10-producing cells were generated, with approximately twice as many cells producing IFN-γ than IL-10 (Fig. 5b). Although a larger proportion of CD8+ T cells cultured with myDC-IC produced IFN-γ, there were few, if any, IL-10-producing cells in the myDC-IC cultures. To determine whether OT-I T cells activated by pDC-IC could kill OVA-transfected tumor cells, we cultured OT-I T cells together with pDC-IC or myDC-IC for 6 days. After washing, the T cells were incubated overnight with CFSE-labeled EL-4 cells or EL-4 cells expressing OVA (EG7-OVA) to analyze their cytotoxic activity. As shown in Fig. 5c, myDC-IC induced CTL capable of lysing EG7-OVA cells, whereas pDC-IC did not. In the presence of LPS, pDC-IC, as well as myDC-IC, induced CTL in a dose-dependent manner, and, at ratios of 5:1 or greater, CTL induction by pDC-IC was only slightly less efficient than that induced by myDC-IC.
FIGURE 5.
pDC can cross-present IC-derived Ag to CD8+ T cells in vitro. a, FACS-sorted DC subsets (5 × 104) were cultured in the presence of OVA-IC (consisting of DQ-OVA plus anti-OVA), DQ-OVA, or medium alone together with CFSE-labeled CD8+ OVA-Tg (OT-I) T cells (1 × 105) for 4 days. CD8+ cells were analyzed using a FACSCalibur. b, OT-I T cells cultured with DC-IC were examined for IFN-γ and IL-10 production by intracellular staining. *, Indicates p < 0.05. Data shown are representative of three independent experiments. c, OT-I T cells alone, or stimulated for 6 days with either pDC-IC or myDC-IC, were cultured at increasing numbers together with a fixed number of target EG7-OVA and EL4 tumor cells labeled with high and low concentrations of CFSE, respectively, in the absence or presence of LPS. After overnight incubation, the percentage of lysis was determined by FACS, as described in Materials and Methods. Data shown are representative of three independent experiments.
We also examined the ability of pDC-IC and myDC-IC to cross-present Ag in vivo by injecting CFSE-labeled OT-I T cells into mice that had received one or the other DC-IC subset. Two days after T cell administration, total CD8+ T cells were isolated from draining lymph nodes and analyzed for evidence of proliferation by CFSE staining and for intracellular IFN-γ. As shown in Fig. 6a, both pDC-IC and myDC-IC induced CD8+ T cell activation. When stimulated in vitro with PMA and ionomycin, the CD8+ T cells from mice that had received pDC-IC produced mainly IL-10, whereas the CD8+ T cells from mice that had received myDC-IC produced mainly IFN-γ (Fig. 6b). In combination with our results (Fig. 4b) showing that pDC-IC also stimulated production of Th2-type cytokines from CD4+ T cells, these data suggest that under noninflammatory conditions, pDC-IC mainly promote a Th2/tolerogenic environment in vivo.
FIGURE 6.
pDC can cross-present IC-derived Ag to CD8+ T cells in vivo. FACS-sorted DC subsets (1–2 × 106) incubated with OVA-IC overnight were injected in the footpad, and CFSE-labeled, CD8+ OVA-Tg T cells (8–10 × 106) were injected i.v. in the base of the tail. a, After 2 days, draining lymph nodes were harvested and CD8+ (OT-I) T cell activation was determined. b, Lymph node T cells were analyzed for their production of cytokines after restimulation with PMA and ionomycin. OVA-Tg CD8+ T cells were gated based on forward and side scatter and TCR Vα2 expression, and IFN-γ- and IL-10-producing cells were determined. *, Indicates p < 0.05. Data shown are representative of five independent experiments.
Discussion
We initiated this study with the objective of evaluating the Ag-presenting capacity of pDC. The extraordinary ability of myDC to present and cross-present Ags is well established (14, 15). However, evidence for exogenous Ag presentation by pDC has been limited.
Our data show that pDC, like myDC (16), are capable of acquiring opsonized Ags through their IgG1-binding FcR, leading to Ag presentation and cross-presentation in vivo. Recent reports describe a new type of lectin-binding receptor, Siglec-H, which is specifically expressed on pDC and can function in Ag uptake, leading to Ag presentation and cross-presentation (17, 18). It seems likely that Siglec-H and FcγR mediate the uptake of different types of Ags, thus providing pDC with multiple mechanisms for acquiring Ag and modulating the immune response.
Expression of FcγR was higher on pDC derived from cultured bone marrow than on freshly prepared spleen or lymph node cells, and this may partly explain why FcγR on murine pDC has not been described previously. In addition, we deliberately avoided the use of FcR-blocking Ab (clone 2.4G2) in our FACS-staining protocols, which would have prevented detection of FcR. Moreover, we focused our study on stimulating FcγRIII/FcγRII by using an Ab, 2.4G2, that recognizes an epitope shared by CD16 and CD32. This receptor mainly binds Abs of the IgG1, IgG2a, and IgG2b subclasses contained in IC (19). We used an IgG1 anti-OVA Ab to prepare OVA plus anti-OVA-IC, in part, because the IgG1 Abs consistently bind and stimulate other cells in vivo (20). Although both pDC and mDC express CD64 (FcγRI) at low levels, this receptor does not bind IgG1 Abs well in the mouse, in contrast to human CD64, which binds IgG1 with high affinity (19). Exposure of pDC to IC not only resulted in Ag uptake, but also cellular activation, as indicated by up-regulation of MHC class II and accessory molecules. Such activated pDC-IC, in turn, induced the growth and activation of Ag-specific CD4+ and CD8+ T cells. myDC, like pDC, acquired opsonized Ag, became activated, and stimulated Ag-specific T cells, confirming a previous report (16). MyDC also acquired and processed free protein relatively efficiently. By contrast, pDC that had been pulsed with free protein failed to stimulate T cell responses. This is probably explained both by the reduced ability of pDC to take up free Ag as opposed to opsonized Ag, and by their reduced cellular activation relative to myDC-IC. The mechanism responsible for IC-induced activation of pDC remains to be explored.
Surprisingly, the cytokine profile of pDC-IC-activated CD4+ T cells varied, depending on whether the cells were activated in vitro or in vivo. In vitro, pDC-IC induced mainly IFN-γ-producing Th1 cells, whereas in vivo pDC-IC induced mainly IL-4-producing Th2 cells. By contrast, myDC-IC promoted mainly Th1 cells in vitro and in vivo. The reason for this discrepancy is not clear from our data, but it is apparent that cells and soluble factors in addition to those present in our in vitro culture system can modulate the effects of pDC-IC in vivo. The absence of Th1-biasing factors could also favor the generation of a Th2 response by pDC-IC. In this regard, we were not able to detect IFN-α in supernatants from pDC cultured in the presence of IC (Fig. 2c), and pDC are more likely to polarize T cells toward a Th2 profile in the absence of this cytokine (21). Interactions between OX40L on pDC and OX40 on T cells may also contribute to a Th2 bias, as suggested by in vitro studies of human pDC (22).
pDC-IC activated Ag-specific CD8+ T cells as well as Ag-specific CD4+ T cells. Although a relatively high proportion of CD8+ T cells activated in vitro by pDC-IC produced IFN-γ, they did not differentiate into CTL effectors unless LPS, a TLR4 agonist, was added to the cultures. Of note, mouse pDC express almost all TLRs (23), whereas human pDC have a more restricted TLR repertoire, expressing mainly TLR 1, 2, 7, and 9 (1). One possible explanation for the failure of CTL development in our pDC cultures is that in the absence of TLR stimulation, IL-10-secreting CD8+ T cells, which were also induced in abundance by pDC-IC, blocked the development of CTL. IL-10 is known to inhibit CD8+ T cell activation and CTL formation (24, 25). Moreover, human pDC cross-linked with CD40L have been shown to induce IL-10-secreting CD8+ T cells in vitro, which inhibit the activation of naive CD8+ T cells (26, 27). Peptide-specific IL-10-secreting CD8+ regulatory T cells have been described in humans following immunization with peptide-pulsed immature myDC (28), and in patients with advanced HIV disease (29). It remains to be determined whether the IL-10-secreting CD8+ T cells generated in our pDC-IC cultures were responsible for preventing development of CTL and, if so, whether these cells have features in common with previously described CD8+ regulatory/suppressor cells.
What are the implications of these findings? Although pDC respond rapidly to invading pathogens by secreting IFN-α, and hence are a key component of the innate immune system, our data suggest that pDC can play an important role in adaptive immunity as well. The results show clearly that pDC have the capacity to function as true Ag-processing and -presenting cells, inducing effector as well as memory T cells. On the one hand, by promoting immune effector activity, pDC-IC may play an important role in the clearance of pathogens or tumors. In addition, through their induction of Th2 and regulatory T cells, pDC-IC can modulate the immune response, potentially averting excessive cellular immunity and related tissue damage. In contrast, inopportune inhibition of the immune system by pDC-IC-activated regulatory cells could prevent clearance of pathogens or tumors, whereas induction of immunity to allergens or autoantigens might contribute to allergic or autoimmune disorders. For example, patients with systemic lupus erythematosus have circulating IC containing small nuclear RNA and anti-small nuclear RNA autoantibody; recent studies have shown that pDC can acquire such IC via their FcγR, resulting in stimulation of TLR7 and 8 and production of IFN-α (30). Because IFN-α is thought to play a fundamental role in the pathogenesis of systemic lupus erythematosus, IC-mediated activation of pDC may be a key initiation factor in this disease. Thus, the consequences of IC uptake by pDC appear to depend on the nature of the Ag as well as the presence or absence of other factors (e.g., TLR ligands) in the environment that help determine whether these cells induce a proinflammatory or anti-inflammatory/tolerogenic response.
Acknowledgments
We thank Drs. R. G. Morris and R. de Waal Malefyt for critical reading of the manuscript.
Footnotes
This work was supported by Grants AR051748 (to E.G.E.) and CA80006 (to R.S.N.).
Abbreviations used in this paper: pDC, plasmacytoid dendritic cell; DC, dendritic cell; IC, immune complex; myDC, myeloid DC; Tg, transgenic.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Liu Y-J. IPC: professional type 1 interferon-producing cells and plasma-cytoid dendritic cell precursors. Annu. Rev. Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- 2.Asselin-Paturel C, Boonstra A, Dalod M, Durand I, Yessaad N, Dezutter-Dambuyant C, Vicari A, O’Garra A, Biron C, Briere F, Trinchieri G. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat. Immunol. 2001;2:1144–1150. doi: 10.1038/ni736. [DOI] [PubMed] [Google Scholar]
- 3.Björck P. Isolation and characterization of plasmacytoid dendritic cells from Flt3 ligand and granulocyte-macrophage colony-stimulating factor-treated mice. Blood. 2001;98:3520–3526. doi: 10.1182/blood.v98.13.3520. [DOI] [PubMed] [Google Scholar]
- 4.O’Keeffe M, Hochrein H, Vremec D, Caminschi I, Miller JL, Anders EM, Wu L, Lahoud MH, Henri S, Scott B, et al. Mouse plasmacytoid cells: long-lived cells, heterogeneous in surface phenotype and function, that differentiate into CD8+ dendritic cells only after microbial stimulus. J. Exp. Med. 2002;196:1307–1319. doi: 10.1084/jem.20021031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinman RM, Hawinger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu. Rev. Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 6.Salio M, Palmowski MJ, Atzberger A, Hermans IF, Cerundolo V. CpG-matured murine plasmacytoid dendritic cells are capable of in vivo priming of functional CD8 T cell responses to endogenous but not exogenous antigens. J. Exp. Med. 2004;199:567–579. doi: 10.1084/jem.20031059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krug A, Veeraswamy R, Pekosz A, Kanagawa O, Unanue ER, Colonna M, Cella M. Interferon-producing cells fail to induce proliferation of native T cells but can promote expansion and T helper 1 differentiation of antigen-experienced unpolarized T cells. J. Exp. Med. 2003;197:899–906. doi: 10.1084/jem.20021091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amigorena S, Bonnerot C. Fc receptors for IgG and antigen presentation on MHC class I and class II. Semin. Immunol. 1999;11:385–390. doi: 10.1006/smim.1999.0196. [DOI] [PubMed] [Google Scholar]
- 9.Wright AE, Douglas SR. An experimental investigation of the role of the body fluids in connection with phagocytosis. Proc. R. Soc. London. 1903;72:357–270. doi: 10.1093/clinids/11.5.827. [DOI] [PubMed] [Google Scholar]
- 10.Gilliet M, Boostra A, Paturel C, Antonenko S, Xu XL, Trinchieri G, O’Garra A, Liu Y-L. The development of murine plasmacytoid dendritic cell precursors is differentially regulated by Flt3-ligand and granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 2002;195:953–958. doi: 10.1084/jem.20020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curtsinger JM, Lins DC, Mescher MF. Signal 3 determines tolerance versus full activation of naive CD8 T-cells: dissociating proliferation and development of effector functions. J. Exp. Med. 2003;197:1141–1151. doi: 10.1084/jem.20021910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Den Haan JMM, Bevan MJ. Constitutive versus activation dependent cross-presentation of immune complexes by CD8+ and CD8− dendritic cells. J. Exp. Med. 2002;196:817–827. doi: 10.1084/jem.20020295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pooley JL, Heath WR, Shortman K. Cutting edge: intravenous soluble antigen is presented to CD4 T cells by CD8− dendritic cells, but cross-presented to CD8 T cells by CD8+ dendritic cells. J. Immunol. 2001;166:5327–5330. doi: 10.4049/jimmunol.166.9.5327. [DOI] [PubMed] [Google Scholar]
- 14.Guermonprez P, Valladeau J, Zitvogel L, Thery C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu. Rev. Immunol. 2002;20:621–667. doi: 10.1146/annurev.immunol.20.100301.064828. [DOI] [PubMed] [Google Scholar]
- 15.Schnorrer P, Behrens GM, Wilson NS, Pooley JL, Smith CM, El-Sukkari D, Davey G, Kupresanin F, Li M, Maraskovsky E, et al. The dominant role of CD8+ dendritic cells in cross-presentation is not dictated by antigen capture. Proc. Natl. Acad. Sci. USA. 2006;103:10729–10734. doi: 10.1073/pnas.0601956103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Regnault A, Lankar D, Lacabanne V, Rodriguez A, Thery C, Rescigno M, Saito T, Verbeek S, Bonnerot C, Ricciardi-Castagnoli P, Amigorena S. Fc-γ receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J. Exp. Med. 1999;189:371–380. doi: 10.1084/jem.189.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blasius AL, Cella M, Maldonado J, Takai T, Colonna M. Siglec-H is an IPC-specific receptor that modulates type I IFN secretion through DAP12. Blood. 2006;107:2474–2476. doi: 10.1182/blood-2005-09-3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J, Raper A, Sugita N, Hingorani R, Salio M, Palmowski MJ, Cerundolo V, Crocker PR. Characterization of Siglec-H as a novel endocytic receptor expressed on murine plasmacytoid dendritic cell precursors. Blood. 2006;107:3600–3608. doi: 10.1182/blood-2005-09-3842. [DOI] [PubMed] [Google Scholar]
- 19.Gessner JE, Heiken H, Tamm A, Schmidt RE. The IgG Fc receptor family. Ann. Hematol. 1998;76:231–248. doi: 10.1007/s002770050396. [DOI] [PubMed] [Google Scholar]
- 20.Nimmerjahn F, Ravetch JV. Fcγ receptors: old friends and new family members. Immunity. 2006;24:19–28. doi: 10.1016/j.immuni.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 21.Björck P. Dendritic cells exposed to herpes simplex virus in vivo do not produce IFN-α after rechallenge with virus in vitro and exhibit decreased T cell alloreactivity. J. Immunol. 2004;172:5396–5404. doi: 10.4049/jimmunol.172.9.5396. [DOI] [PubMed] [Google Scholar]
- 22.Ito T, Amakawa T, Inaba M, Hori T, Ota M, Nakamura K, Takebayashi M, Miyaji M, Yoshimura T, Inaba K, Fukuhara S. Plasmacytoid dendritic cells regulate Th1 cell responses through OX40 ligand and type I IFNs. J. Immunol. 2004;172:4253–4259. doi: 10.4049/jimmunol.172.7.4253. [DOI] [PubMed] [Google Scholar]
- 23.Edwards AD, Diebold SS, Slack EM, Tomizawa H, Hemmi H, Kaisho T, Akira S, Reis e Sousa C. Toll-like receptor expression in murine DC subsets: lack of TLR7 expression by CD8α DC correlates with unresponsiveness to imidazoquinolines. Eur. J. Immunol. 2003;33:827–833. doi: 10.1002/eji.200323797. [DOI] [PubMed] [Google Scholar]
- 24.Steinbrink K, Jonuleit H, Muller G, Schuler G, Knop J, Enk AH. Interleukin-10 treated human dendritic cells induce a melanoma-antigen-specific anergy in CD8+ T cells resulting in a failure to lyse tumor cells. Blood. 1999;93:1634–1642. [PubMed] [Google Scholar]
- 25.Steinbrink K, Graulich E, Kunsch S, Knop J, Enk AH. CD4+ and CD8+ anergic T cells induced by interleukin-10-treated human dendritic cells display antigen-specific suppressor activity. Blood. 2002;99:2468–2476. doi: 10.1182/blood.v99.7.2468. [DOI] [PubMed] [Google Scholar]
- 26.Gilliet M, Liu Y-J. Generation of human CD8 T regulatory cells by CD40 ligand-activated plasmacytoid dendritic cells. J. Exp. Med. 2002;195:695–704. doi: 10.1084/jem.20011603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilliet M, Liu Y-J. Human plasmacytoid dendritic cells and the induction of T-regulatory cells. Hum. Immunol. 2002;63:1149–1155. doi: 10.1016/s0198-8859(02)00753-x. [DOI] [PubMed] [Google Scholar]
- 28.Dhodakpar MD, Steinman RM. Antigen-bearing immature dendritic cells induce peptide-specific CD8+ regulatory T cells in vivo in humans. Blood. 2002;100:174–177. doi: 10.1182/blood.v100.1.174. [DOI] [PubMed] [Google Scholar]
- 29.Elrefaei M, Ventura FL, Baker CA, Clark CR, Bangsberg DR, Cao H. HIV-specific IL-10-positive CD8+ T cells suppress cytolysis and IL-2 production by CD8+ T cells. J. Immunol. 2007;178:3265–3271. doi: 10.4049/jimmunol.178.5.3265. [DOI] [PubMed] [Google Scholar]
- 30.Vollmer J, Tluk S, Schmitz S, Hamm S, Jurk M, Forsbach A, Akira S, Kelly KM, Reeves WH, Bauer S, Krieg AM. Immune stimulation mediated by autoantigen binding sites within small nuclear RNAs involves Toll-like receptors 7 and 8. J. Exp. Med. 2005;202:1575–1585. doi: 10.1084/jem.20051696. [DOI] [PMC free article] [PubMed] [Google Scholar]