Abstract
Context
While fatigue is a common and distressing symptom, a well-specified definition of fatigue is lacking. One of the least well-defined aspects of fatigue is its quality, which might reflect the underlying pathophysiology.
Objectives
To identify qualities of fatigue and assess whether they are associated with distinct chronic conditions.
Methods
We identified five fatigue qualities in the literature, two mental and three physical, and selected representative items from those available in our data from a prospective cohort of 495 community-dwelling primary care patients aged 65 years or older. We then examined the prevalence of each quality, the correlations among qualities, and the association of fatigue qualities with health and functional status, including chronic conditions.
Results
Fatigue was very common among older primary care patients, with 70% reporting any fatigue and 43% reporting feeling tired most of the time, and was associated with worse health and functional status. Physical fatigue qualities were more common than mental qualities. Correlations among fatigue qualities were 0.09–0.27 and did not support the mental versus physical classification. Different fatigue qualities were not well explained by older adults’ underlying chronic conditions. Rather the cumulative number of fatigue qualities was associated with worse health and function.
Conclusion
These first steps in exploring fatigue qualities suggest that different fatigue qualities could represent disparate manifestations of a common underlying etiology, while not ruling out distinct underlying pathophysiologies.
Keywords: Fatigue, older adults, chronic conditions
Introduction
Fatigue is common among older adults (1), is distressing, and is associated with functional decline and mortality (2–5). Despite this, the underlying causes and treatment of fatigue are not well understood. While clinicians and patients easily recognize fatigue when they see or experience it, the lack of a well-specified definition of fatigue is a major barrier to further research on the etiology, diagnosis, and treatment of this important symptom (6,7). A first step in developing a definition of fatigue is agreeing upon a set of domains required to describe the clinical phenomenon of fatigue. Although the numerous scales that have been developed to measure fatigue assess a wide variety of domains or aspects of fatigue, there is minimal consistency across measures in the specific domains addressed. Given that fatigue is first and foremost a symptom, the traditional dimensions of symptoms taught in medical history-taking may be a good starting point. These dimensions (location, chronology, precipitating and palliating factors, quality, severity, and associated symptoms (8)) have provided a framework for the assessment and management of pain (9,10), another subjective and difficult to study symptom. Diagnostic criteria for fatigue-related diseases, such as chronic fatigue syndrome (11), already take in to account many of these dimensions. One of the least explored and most difficult to define dimensions of fatigue is quality.
The quality of a symptom is its character or nature; for example, qualities of pain include aching, cramping, dull, sharp or burning. The quality of pain can be used to identify the underlying pathophysiology and to guide treatment. For example, burning or shooting pain is often neuropathic, and may be treated with anticonvulsants or tricyclic antidepressants, whereas aching pain is likely to be nociceptive and to respond to standard analgesics (10). The number or types of fatigue qualities have not been well defined. Some measurement scales for fatigue consider it a unidimensional construct, while others have specified different qualities of fatigue, such as physical (e.g., lack of energy, sleepiness) and mental (e.g., apathy and inability to concentrate) (12–18).
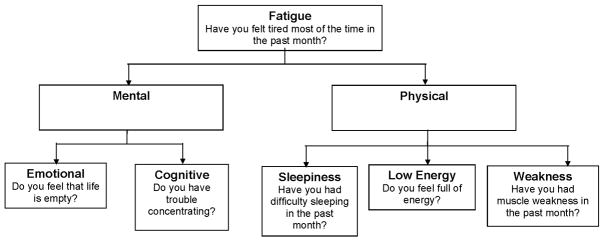
Defining qualities of fatigue is important because different qualities of fatigue may represent distinct underlying pathophysiologies. Alternatively, different qualities may represent diverse manifestations of a common underlying problem or set of problems. Our goal in this research was to identify qualities of fatigue and assess whether they were associated with distinct patterns of clinical characteristics. To identify fatigue qualities, we reviewed the literature and evaluated the existing fatigue scales (6, 12–20). Based on this evidence, we developed a model of fatigue qualities with two groups of qualities: mental, comprising cognitive and emotional qualities, and physical, comprising sleepiness, lack of energy, and weakness (Figure 1). To assess the potential relationship between fatigue qualities and underlying pathophysiology, we examined the correlations among the various qualities of fatigue and the association of clinical characteristics with specific qualities of fatigue. If fatigue qualities reflect distinct underlying pathophysiologies, we would expect low correlations among fatigue types and distinctive patterns of clinical characteristics associated with each quality.
Figure 1.
Model of fatigue qualities. Survey items used to assess each quality are presented under each quality.
Methods
Overview
Subjects for this cross-sectional study were recruited from two primary care clinics (a Medicare Health Maintenance Organization and a Veterans Affairs clinic) during April-October of 1996. The purpose of the original prospective cohort study was to determine the feasibility and effectiveness of physical performance measures as predictors of health and functional outcomes in the primary care setting. The present study used data from the baseline assessment. The study was approved by the Institutional Review Boards of the relevant institutions. Study methods, described in detail elsewhere (21, 22), are summarized below.
Subjects
Eligible community-dwelling adults aged 65 years or older from two primary care clinics serving a common geographic region in a major U.S. metropolitan area were recruited. Clinic patients were eligible if they were cognitively intact (Mini-Mental State Examination [MMSE] (23) score ≥ 24) or mildly impaired (MMSE 16–23) with a caregiver, were able to walk four meters, and had a gait speed between 0.2 and 1.3 meters per second (m/s). Participants who used assistive devices were included.
Quality of Fatigue
Using the model of fatigue qualities shown in Figure 1, we selected representative items from the data set (Table 1). Because “tiredness” was the most common synonym presented for fatigue in existing scales (2,12,13, 24–26), we also present data on participant responses to the question “Do you feel tired most of the time?” as a general measure of fatigue. When multiple items were available to represent a fatigue quality, we selected the most representative item, defined as the item most highly correlated with the sum of the candidate items (27). As shown in Table 1, we identified three candidate items for the emotional, cognitive, and sleepiness qualities, two items for lack of energy, and one item for weakness. Feeling tired much of the time was the item most highly correlated with the sum of the other items and was also the most prevalent item. The items selected as most representative are highlighted in bold in Table 1 and presented in Figure 1. All subsequent analyses are based on these five items and the general fatigue item.
Table 1.
Candidate Questions for Each Fatigue Quality
| Fatigue Quality | Candidate Questionsa | Answer Indicating Fatigue | Prevalence | Correlation with Quality Total | Correlation with Overall Total |
|---|---|---|---|---|---|
| General | Do you feel tired much of the time? | Yes | 212 (43) | n/a | 0.49 |
| Emotional | Do you feel that life is empty? | Yes | 38 (8) | 0.36 | 0.35 |
| Do you often get bored? | Yes | 114 (23) | 0.33 | 0.34 | |
| Do you find life very exciting? | No | 143 (29) | 0.33 | 0.35 | |
| Cognitive | Do you have confused thinking? | Yes | 74 (15) | 0.38 | 0.37 |
| Do you have memory loss? | Yes | 176 (36) | 0.38 | 0.30 | |
| Do you have trouble concentrating? | Yes | 65 (13) | 0.42 | 0.44 | |
| Sleepiness | Do you have a sleep problem? | Yes | 30 (6) | 0.13 | 0.12 |
| Do you have difficulty sleeping? | Yes | 200 (40) | 0.20 | 0.28 | |
| Do you enjoy getting up in the morning? | No | 78 (16) | 0.15 | 0.36 | |
| Weakness | Do you have muscle weakness? | Yes | 142 (29) | n/a | 0.32 |
| Lack of Energy | Do you feel full of energy? | No | 206 (42) | 0.33 | 0.48 |
| Is it hard for you to get started on new projects? | Yes | 153 (31) | 0.33 | 0.39 | |
The item selected as most representative of the quality is bolded.
Measures
Participant assessments included demographic characteristics, cognition (23), and self-reported physician-diagnosed chronic conditions (28). Depressive symptoms were assessed with the Geriatric Depression Scale (29). Self-rated health was assessed with the 5-level ordinal global health item from the Medical Outcomes Study (SF-36) (25). Functional status was assessed with the 100-point physical function index (PFI) of the SF-36 (25) and a 16-item basic and instrumental activities of daily living (ADL) scale from the National Health Interview Survey (NHIS), which counts the number of tasks performed without difficulty (30). Physical performance was measured with the 12-point Short Physical Performance Battery (SPPB) (31, 32), and usual gait speed was assessed over a four-meter course. Inter-rater and test-retest reliability for the measures used in this study were found to be excellent, with intra-class correlations (ICC) generally over 0.9 (22).
Statistical Analysis
We present the candidate items for each fatigue quality, their prevalences, and their correlation with the sum of the other candidate items with each quality and overall. We present the prevalence of each quality of fatigue and evaluate the correlation between qualities using Spearman coefficients. To assess the interrelationships among the fatigue qualities and the general item, we performed maximum likelihood factor analyses of the five items with and without tiredness. To determine how many factors to retain, we used an eigenvalue > 1 and examination of scree plots of the eigenvalues. Because out items had binary responses, we used a tetrachoric correlation matrix in the factor analyses (33). The characteristics of participants reporting each individual fatigue quality and of participants reporting 1, 2, or ≥3 fatigue qualities were compared using Chi-square tests for dichotomous variables and the Kruskal-Wallace test for continuous variables. Because of the exploratory nature of these analyses, we did not correct the P-values for multiple comparisons. We determined the independent association between individual fatigue qualities and each chronic condition category by using multiple logistic regression to calculate odds ratios and 95% confidence intervals, which are presented graphically. To determine if the strength of the associations between the fatigue qualities and each condition differed among qualities, we used the TEST statement in PROC LOGISTIC. If an overall difference was noted, we used post hoc tests to identify the specific differences among the qualities. All tests were two-tailed with alpha=0.05, and SAS® version 9.2 (SAS Institute, Cary, North Carolina) was used for all analyses.
Results
The study population, which included the 495 participants who had baseline data on all fatigue qualities, had an average age of 74 years and was 44% female and 80% white (Table 2, column 1). The relatively low proportion of women was a result of the predominantly male population recruited from one of our two sites (VA primary care). Seventy percent of the participants (348/495) reported at least one fatigue quality. The most prevalent fatigue quality was low energy, reported by 42% of participants, while the least common was emotional fatigue, reported by 8% (Table 3, column 1). The physical fatigue qualities (sleepiness, low energy, and weakness) were all more common than the mental fatigue qualities (emotional and cognitive). Correlations between the physical fatigue qualities ranged from 0.13 to 0.20, while the correlation between the mental qualities was 0.27. Correlations between the mental and physical qualities were similar in magnitude, ranging from 0.09 to 0.27. Correlations of each subtype with tiredness tended to be greater, ranging from 0.12 for emotional fatigue to 0.45 for low energy.
Table 2.
Participant Characteristics by Fatigue Presencea
| Fatigue |
||||
|---|---|---|---|---|
| Characteristic | Total n=495 | No n=147 | Any n=348 | P-value |
| Age, years | 74.0±5.7 | 73.9±6.1 | 74.1±5.6 | 0.63 |
| Female | 217 (44) | 51 (34) | 166 (48) | 0.008 |
| White | 395 (80) | 110 (75) | 285 (82) | 0.07 |
| Number of chronic condition typesb | 2.2±1.3 | 1.7±1.2 | 2.4±1.2 | <0.001 |
| Cardiovascular | 112 (23) | 25 (17) | 87 (25) | 0.05 |
| Neurological | 53 (11) | 9 (6) | 44 (13) | 0.03 |
| Musculoskeletal | 351 (71) | 91 (62) | 260 (75) | 0.004 |
| Pulmonary | 118 (24) | 20 (14) | 98 (28) | <0.001 |
| Diabetes | 87 (18) | 14 (10) | 73 (21) | 0.002 |
| Cancer | 114 (23) | 32 (22) | 82 (24) | 0.66 |
| Visual | 263 (53) | 65 (44) | 198 (57) | 0.01 |
| General | 103 (21) | 19 (13) | 84 (24) | 0.005 |
| Cognitive function (MMSEc) | 27.5±2.3 | 27.3±2.5 | 27.5±2.3 | 0.32 |
| Depressive symptoms (GDSd) | 2.3±2.8 | 0.5±0.8 | 3.1±2.9 | <0.001 |
| Fair or poor self-rated health | 110 (22) | 10 (7) | 100 (29) | <0.001 |
| SF-36 PFIe | 63±30 | 81±22 | 57±29 | <0.001 |
| NHIS ADLf | 14.2±2.2 | 15.4±1.0 | 13.7±2.3 | <0.001 |
| SPPBa\g | 8.4±2.6 | 9.2±2.1 | 8.0±2.8 | <0.001 |
| Gait speed, meters/second | 0.88±0.24 | 0.95±0.22 | 0.84±0.24 | <0.001 |
Values represent n (%) for dichotomous variables and mean ± standard deviation for continuous variables.
Chronic condition categories include: cardiovascular (angina, heart failure, or heart attack), neurological (stroke or Parkinson’s disease), pulmonary (lung disease, emphysema, asthma, or bronchitis), musculoskeletal (arthritis, osteoporosis, broken bone, amputation, or joint replacement), diabetes, cancer, visual (cataracts or glaucoma), and general (depression, anxiety, emotional problem, sleep problem, or chronic pain).
Mini-Mental State Exam, range 0–30 with higher scores representing better cognition.
Geriatric Depression Score, range 0–15 with higher scores representing more depressive symptoms.
Medical Outcomes Survey Physical Function Index, range 0–100 with higher scores representing better function.
National Health Interview Survey Activities of Daily Living, range 0–16 with higher scores representing better function.
Short Physical Performance Battery, range 0–12 with higher scores representing better physical performance.
Table 3.
Spearman Correlations Between Different Qualities of Fatigue (n=495) a
| Fatigue Subtypes |
|||||||
|---|---|---|---|---|---|---|---|
| n (%) | Tiredness | Emotional Fatigue | Cognitive Fatigue | Sleepiness | Low Energy | Weakness | |
| Tiredness | 212 (43) | 1.0 | 0.12 | 0.22 | 0.24 | 0.45 | 0.37 |
| Emotional fatigue | 38 (8) | 1.0 | 0.27 | 0.09 | 0.20 | 0.14 | |
| Cognitive fatigue | 65 (13) | 1.0 | 0.09 | 0.27 | 0.19 | ||
| Sleepiness | 200 (40) | 1.0 | 0.13 | 0.20 | |||
| Low energy | 206 (42) | 1.0 | 0.16 | ||||
| Weakness | 142 (29) | 1.0 | |||||
All P<0.01 except correlations between emotional fatigue and sleepiness (P=0.05) and between cognitive fatigue and sleepiness (P=0.04).
In factor analysis of the five qualities both with and without tiredness, only a single factor was retained. For the five-item analysis, factor loadings ranged from 0.31 for sleepiness to 0.77 for trouble concentrating. When tiredness was included in the analysis, the factor loadings ranged from 0.39 for sleepiness to 0.81 for tiredness.
Participants who reported any quality of fatigue were more likely to be female and to report fair or poor self-rated health (Table 2). Presence of any fatigue was also associated with more chronic conditions, greater depressive symptoms, and worse functional status and physical performance; it was not associated with age.
The characteristics associated with reporting fatigue were similar across qualities, with variation in the strength but not in the direction of the associations (Table 4). Most qualities were associated with fair or poor self-rated health, more chronic conditions, greater depressive symptoms, and worse functional status and physical performance. The only gender differences in fatigue quality were a greater prevalence of sleepiness and tiredness in women compared to men. Whites were more likely to report low energy and tiredness than non-whites. The only fatigue quality associated with cognitive status was cognitive fatigue.
Table 4.
Association of Participant Characteristics with Individual Fatigue Qualitiesa
| Characteristic | Emotional | Cognitive | Sleepiness | Low Energy | Weakness | Tiredness |
|---|---|---|---|---|---|---|
| Age, years | ||||||
| Female | ↑↑ | ↑ | ||||
| White | ↑↑↑ | ↑ | ||||
| Number of chronic conditionsb | ↑↑↑ | ↑↑↑ | ↑↑↑ | ↑↑↑ | ↑↑↑ | |
| Cognitive function (MMSEc) | ↓↓↓ | |||||
| Depressive symptoms (GDSd) | ↑↑↑ | ↑↑↑ | ↑↑↑ | ↑↑↑ | ↑↑↑ | ↑↑↑ |
| Fair or poor self-rated health | ↑↑↑ | ↑↑↑ | ↑↑↑ | ↑↑↑ | ↑↑↑ | |
| SF-36 PFIe | ↓↓ | ↓↓↓ | ↓↓↓ | ↓↓↓ | ↓↓↓ | ↓↓↓ |
| NHIS ADLf | ↓↓↓ | ↓↓↓ | ↓↓↓ | ↓↓↓ | ↓↓↓ | ↓↓↓ |
| SPPBg | ↓ | ↓↓ | ↓ | ↓↓↓ | ↓↓↓ | ↓↓↓ |
| Gait speed, meters/second | ↓↓ | ↓↓↓ | ↓↓↓ | ↓↓↓ | ↓↓↓ | |
Arrows represent the direction and significance of the association: up arrows positive association; down arrows negative association; one arrow P<0.05; two arrows P<0.01; three arrows P<0.001.
Of eight categories: cardiovascular, neurological, pulmonary, musculoskeletal, diabetes, cancer, visual, and general.
Mini-Mental State Exam, range 0–30 with higher scores representing better cognition.
Geriatric Depression Score, range 0–15 with higher scores representing more depressive symptoms.
Medical Outcomes Survey Physical Function Index, range 0–100 with higher scores representing better function.
National Health Interview Survey Activities of Daily Living, range 0–16 with higher scores representing better function.
Short Physical Performance Battery, range 0–12 with higher scores representing better physical performance.
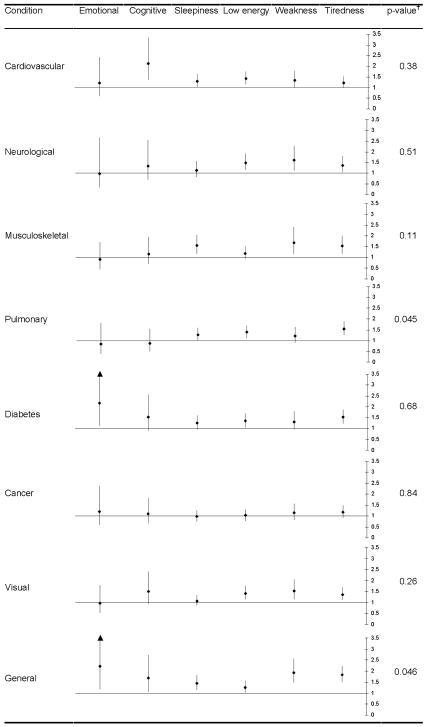
In order to examine the pattern of associations between fatigue qualities and chronic disease, we compared the relative odds of having each category of chronic disease across fatigue qualities (Figure 2). Most of the associations with specific diseases are similar across fatigue qualities. While cognitive fatigue had a stronger association with cardiovascular conditions and emotional fatigue had a stronger association with diabetes compared to the other qualities, these differences were not statistically significant. There was a significant difference overall among qualities in their association with pulmonary conditions; post hoc analyses of these associations with pulmonary disease indicated that there was no difference between emotional and cognitive fatigue (χ2=0.06, df=1, P=0.81), and no difference among the physical qualities (χ2=1.28, df=2, P=0.53), but that there was a significant difference between the mental and physical groups (χ2=7.43, df=1, P=0.006). There was also a significant difference overall among qualities in their association with general conditions, which included psychiatric problems, sleep disorders, and chronic pain. However, post hoc pairwise comparisons between qualities’ associations with general conditions revealed a significant difference only between low energy and weakness (χ2=5.28, df=1, P=0.022).
Figure 2.
Association of individual chronic disease categories with fatigue qualities. Odds ratios and 95% confidence intervals representing the independent association of each fatigue type with a chronic condition are presented. Chronic condition categories include: cardiovascular (angina, heart failure, or heart attack), neurological (stroke or Parkinson’s disease), pulmonary (lung disease, emphysema, asthma, or bronchitis), musculoskeletal (arthritis, osteoporosis, broken bone, amputation, or joint replacement), diabetes, cancer, visual (cataracts or glaucoma), and general (depression, anxiety, emotional problem, sleep problem, or chronic pain). P-values represent a Chi-square test of an overall difference among qualities in the strength of their associations with each condition.
Among the 348 participants who reported any fatigue, 161 (46%) reported one quality, 104 (30%) two qualities, and 83 (24%) three or more qualities. Comparing these three groups, participants reporting more fatigue qualities had more chronic conditions (means 2.0, 2.6, 2.8; P<0.001), worse cognition (mean MMSE 27.8, 27.7, 26.8; P=0.008), greater depressive symptoms (mean GDS 1.5, 3.6, 5.6; P<0.001), worse functional status (mean SF-36 PFI 67, 53, 41; P<0.001), and worse physical performance (mean SPPB 8.6, 7.7, 7.1; P<0.001). In general, the prevalence of chronic conditions increased as the number of fatigue qualities increased; this association was statistically significant for cardiovascular (18%, 27%, 36%; P=0.007), musculoskeletal (68%, 81%, 80%; P=0.04), and general conditions (14%, 31%, 35%; P<0.001).
Discussion
Reports of a variety of fatigue qualities were common among this cohort of older primary care patients. The correlations among the fatigue qualities were small to moderate (34), consistent with the hypothesis that they reflect distinct underlying pathophysiologies. However, the patterns of association with chronic disease were similar across fatigue qualities. The cumulative number rather than the type of fatigue qualities reported was correlated with degree of morbidity. Taken together, these first steps in exploring fatigue qualities suggest that different fatigue qualities could represent disparate manifestations of a common underlying etiology, while not ruling out distinct underlying pathophysiologies.
Fatigue was very common among older primary care patients, with 70% reporting any fatigue and 43% reporting feeling tired most of the time. Cross-sectionally, fatigue was associated with worse health and functional status. Physical fatigue qualities were more common than mental qualities. Correlations among the fatigue qualities did not support the mental versus physical classification. The majority of older adults reporting fatigue report multiple qualities, and measures of health and function worsened as the number of qualities reported increased.
If fatigue qualities reflected distinct underlying pathophysiologies, we might have hypothesized that musculoskeletal disorders might be most strongly associated with weakness or cardiopulmonary disease most strongly associated with low energy. While musculoskeletal disease was associated with a high relative risk of weakness, the increase in risk was similar for sleepiness and tiredness. Pulmonary conditions were associated with increased risk of low energy, although the risk increase was greater for tiredness. Cardiac disease was associated with the greatest increase in risk of cognitive fatigue. Our results instead suggest that the various qualities of fatigue may all represent disparate manifestations of a common underlying process, such as increased inflammation or disordered homeostasis. Another possibility is that a common underlying subjective sensation of fatigue is described in varying ways by different older adults, influenced not only by individual characteristics but also potentially by cultural differences. Our results do not rule out the possibility, however, that different qualities reflect distinct pathophysiologies that do not correlate with chronic conditions.
Our study population represents an older primary care population with a high burden of chronic disease. Compared with palliative care populations (35, 36), they have higher functional status and fewer depressive symptoms. Relationships among fatigue qualities may be different in palliative care. In addition, our participants were mostly white, which may limit the generalizability of these findings, although the percentage of whites in our study is similar to that among older adults in the United States (81%) (37). Although our data was collected in 1996, fatigue remains a common problem among older adults (4, 38, 39).
Our study has several additional limitations. First, our assessments of fatigue subtypes were drawn from preexisting health status measures, and may not optimally capture each subtype. Second, although these are the qualities of fatigue most often mentioned in the literature (6, 12–20), there may be others qualities that that we have not captured, such as lack of endurance. Third, our measures simply assessed general symptoms, rather than symptoms related to a specific level or type of activity. Fourth, our ability to assess the correlations among qualities was limited by the dichotomous nature of our fatigue measures. Finally, we did not have data available on subclinical disease (40), inflammatory markers (40–42), or other potential physiologic correlates of fatigue (43, 44), and so we cannot draw any conclusions about the underlying pathophysiology of distinct fatigue qualities.
In conclusion, older adults report a variety of symptoms that might represent distinct qualities of fatigue, but these qualities are not well explained by underlying chronic conditions. Rather the cumulative number of fatigue qualities is associated with worse health and function. These results suggest that fatigue qualities represent disparate manifestations of a common pathophysiology rather than multiple underlying pathophysiologies.
Acknowledgments
Dr. Hardy was funded by the Beeson Career Development Award (K23 AG030977); the Pittsburgh Claude D. Pepper Older Americans Independence Center (P30 AG-024827); and the Hartford Foundation’s Pittsburgh Center of Excellence in Geriatric Medicine. Dr. Studenski is funded by the National Institute on Aging (K07 AG023641). The original study for which these data were collected was funded by Merck Research Laboratories.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Liao S, Ferrell BA. Fatigue in an older population. J Am Geriatr Soc. 2000;48:426–430. doi: 10.1111/j.1532-5415.2000.tb04702.x. [DOI] [PubMed] [Google Scholar]
- 2.Avlund K, Damsgaard MT, Sakari-Rantala R, Laukkanen P, Schroll M. Tiredness in daily activities among nondisabled old people as determinant of onset of disability. J Clin Epidemiol. 2002;55:965–973. doi: 10.1016/s0895-4356(02)00463-8. [DOI] [PubMed] [Google Scholar]
- 3.Hardy SE, Studenski SA. Fatigue and function over three years among older adults. J Gerontol A Biol Sci Med Sci. 2008;63:1389–1392. doi: 10.1093/gerona/63.12.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vestergaard S, Nayfield S, Patel K, et al. Fatigue in a representative population of older persons and its association with functional impairment, functional limitation and disability. J Gerontol A Biol Sci Med Sci. 2009;64:76–82. doi: 10.1093/gerona/gln017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hardy SE, Studenski SA. Fatigue predicts mortality among older adults. J Am Geriatr Soc. 2008;56:1910–1914. doi: 10.1111/j.1532-5415.2008.01957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poluri A, Mores J, Cook DB, Findley TW, Cristian A. Fatigue in the elderly population. Phys Med Rehabil Clin N Am. 2005;16:91–108. doi: 10.1016/j.pmr.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Working Group on Functional Outcome Measures for Clinical Trials. Indications, labeling, and outcomes assessment for drugs aimed at improving functional status in older persons: a conversation between aging researchers and FDA regulators. J Gerontol A Biol Sci Med Sci. 2009;64A:487–491. doi: 10.1093/gerona/gln042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swartz MH. Textbook of physical diagnosis: History and examination. 4. Philadelphia: WB Saunders; 2002. [Google Scholar]
- 9.Institute for Clinical Systems Improvement (ICSI) Assessment and management of chronic pain. Bloomington, MN: ICSI; 2008. [Google Scholar]
- 10.Institute for Clinical Systems Improvement (ICSI) Assessment and management of acute pain. Bloomington, MN: ICSI; 2008. [Google Scholar]
- 11.Fukuda K, Straus SE, Hickie I, et al. The chronic fatigue syndrome: a comprehensive approach to its definition and study. Ann Intern Med. 1994;121:953–959. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- 12.Okuyama T, Akechi T, Kugaya A, et al. Development and validation of the cancer fatigue scale: a brief, three-dimensional, self-rating scale for assessment of fatigue in cancer patients. J Pain Symptom Manage. 2000;19:5–15. doi: 10.1016/s0885-3924(99)00138-4. [DOI] [PubMed] [Google Scholar]
- 13.Piper BF, Dibble SL, Dodd MJ, et al. The revised Piper Fatigue Scale: psychometric evaluation in women with breast cancer. Oncol Nurs Forum. 1998;25:677–684. [PubMed] [Google Scholar]
- 14.Smets EMA, Garssen B, Bonke B, De Haes JCJM. The multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39:315. doi: 10.1016/0022-3999(94)00125-o. [DOI] [PubMed] [Google Scholar]
- 15.Stein KD, Martin SC, Hann DM, Jacobsen PB. A multidimensional measure of fatigue for use with cancer patients. Cancer Pract. 1998;6:143–152. doi: 10.1046/j.1523-5394.1998.006003143.x. [DOI] [PubMed] [Google Scholar]
- 16.Holley SK. Evaluating patient distress from cancer-related fatigue: an instrument development study. Oncol Nurs Forum. 2000;27:1425–1431. [PubMed] [Google Scholar]
- 17.Fisk JD, Ritvo PG, Ross L, et al. Measuring the functional impact of fatigue: initial validation of the fatigue impact scale. Clin Infect Dis. 1994;18 (Suppl 1):S79–83. doi: 10.1093/clinids/18.supplement_1.s79. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz AL. The Schwartz Cancer Fatigue Scale: testing reliability and validity. Oncol Nurs Forum. 1998;25:711–717. [PubMed] [Google Scholar]
- 19.Whitehead L. The measurement of fatigue in chronic illness: a systematic review of unidimensional and multidimensional fatigue measures. J Pain Symptom Manage. 2009;37:107–128. doi: 10.1016/j.jpainsymman.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 20.Chalder T, Berelowitz G, Pawlikowska T, et al. Development of a fatigue scale. J Psychosom Res. 1993;37:147–153. doi: 10.1016/0022-3999(93)90081-p. [DOI] [PubMed] [Google Scholar]
- 21.Perera S, Studenski S, Chandler JM, Guralnik JM. Magnitude and patterns of decline in health and function in 1 year affect subsequent 5-year survival. J Gerontol A Biol Sci Med Sci. 2005;60:894–900. doi: 10.1093/gerona/60.7.894. [DOI] [PubMed] [Google Scholar]
- 22.Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51:314–322. doi: 10.1046/j.1532-5415.2003.51104.x. [DOI] [PubMed] [Google Scholar]
- 23.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state:” a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 24.Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13:63–74. doi: 10.1016/s0885-3924(96)00274-6. [DOI] [PubMed] [Google Scholar]
- 25.Ware JJ, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 26.Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85:1186–1196. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 27.Yu CH. An introduction to computing and interpreting Cronbach coefficient alpha in SAS. Proceedings of the Twenty-Sixth Annual SAS Users Group International Conference; Cary, NC: SAS Institute Inc; 2001. pp. 246–26. Paper. [Google Scholar]
- 28.Rigler SK, Studenski S, Wallace D, Reker DM, Duncan PW. Co-morbidity adjustment for functional outcomes in community-dwelling older adults. Clin Rehabil. 2002;16:420–428. doi: 10.1191/0269215502cr515oa. [DOI] [PubMed] [Google Scholar]
- 29.Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS). Recent evidence and development of a shorter version. In: Brink TL, editor. Clinical gerontology: A guide to assessment and intervention. New York: The Haworth Press, Inc; 1986. pp. 165–173. [Google Scholar]
- 30.Fitti JE, Kovar MG. Vital & health statistics, series 2, no. 2. DHSS Pub. NO. PHS 87–1323. Hyattsville, MD: DHHS; 1987. The supplement on aging to the 1984 National Health Interview Survey; pp. 1–115. [PubMed] [Google Scholar]
- 31.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–231. doi: 10.1093/gerona/55.4.m221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 33.Woods CM. Factor analysis of scales composed of binary items: illustration with the Maudsley Obsessional Compulsive Inventory. J Psychopathol Behav Assess. 2002;24:215–223. [Google Scholar]
- 34.Cohen J. Statistical power analysis for the behavioral sciences. 2. Mahwah, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- 35.Reeve JL, Lloyd-Williams M, Dowrick C. Revisiting depression in palliative care settings: the need to focus on clinical utility over validity. Palliat Med. 2008;22:383–391. doi: 10.1177/0269216307087953. [DOI] [PubMed] [Google Scholar]
- 36.Chochinov HM, Hack T, Hassard T, et al. Dignity in the terminally ill: a cross-sectional, cohort study. Lancet. 2002;360:2026–2030. doi: 10.1016/S0140-6736(02)12022-8. [DOI] [PubMed] [Google Scholar]
- 37.Administration on Aging. Minority aging. Vol. 2009. Washington, DC: Department of Health and Human Services; 2007. [Google Scholar]
- 38.Goldman SE, Ancoli-Israel S, Boudreau R, et al. Sleep problems and associated daytime fatigue in community dwelling older individuals. J Gerontol A Biol Sci Med Sci. 2008;63A:1069–1075. doi: 10.1093/gerona/63.10.1069. [DOI] [PubMed] [Google Scholar]
- 39.Santos-Eggimann B, Cuenoud P, Spagnoli J, Junod J. Prevalence of frailty in middle-aged and older community-dwelling Europeans living in 10 countries. J Gerontol A Biol Sci Med Sci. 2009;64A:675–681. doi: 10.1093/gerona/glp012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walston J, Hadley EC, Ferrucci L, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54:991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 41.Bautmans I, Njemini R, Lambert M, Demanet C, Mets T. Circulating acute phase mediators and skeletal muscle performance in hospitalized geriatric patients. J Gerontol A Biol Sci Med Sci. 2005;60:361–367. doi: 10.1093/gerona/60.3.361. [DOI] [PubMed] [Google Scholar]
- 42.Varadhan R, Walston J, Cappola AR, et al. Higher levels and blunted diurnal variation of cortisol in frail older women. J Gerontol A Biol Sci Med Sci. 2008;63:190–195. doi: 10.1093/gerona/63.2.190. [DOI] [PubMed] [Google Scholar]
- 43.Chaves PHM, Semba RD, Leng SX, et al. Impact of anemia and cardiovascular disease on frailty status of community-dwelling older women: the Women’s Health and Aging Studies I and II. J Gerontol A Biol Sci Med Sci. 2005;60:729–735. doi: 10.1093/gerona/60.6.729. [DOI] [PubMed] [Google Scholar]
- 44.Ruggiero C, Ferrucci L. The endeavor of high maintenance homeostasis: resting metabolic rate and the legacy of longevity. J Gerontol A Biol Sci Med Sci. 2006;61:466–471. doi: 10.1093/gerona/61.5.466. [DOI] [PMC free article] [PubMed] [Google Scholar]