Abstract
Intraventricular hemorrhage is a common complication of preterm infants. Mutations in the type IV procollagen gene, COL4A1, are associated with cerebral small vessel disease with hemorrhage in adults and fetuses. We report a rare variant in COL4A1 associated with intraventricular hemorrhage in dizygotic preterm twins. These results expand the spectrum of diseases attributable to mutations in type IV procollagens.
Intraventricular hemorrhage (IVH) is a major cause of adverse outcome for very low birth weight (VLBW) preterm neonates.1 Ten to 20% of these infants have IVH, and nearly 75% of children who survive Grade 3 or 4 lesions, involving either acute distension of the cerebral ventricular system with blood (Grade 3) or parenchymal infarction (Grade 4), develop mental retardation and/or cerebral palsy.2 A familial susceptibility for IVH in VLBW preterm twins has been suggested, although no causative genes have been identified.3
IVH has been attributed to alterations in cerebral blood flow to the immature germinal matrix microvasculature. COL4A1 is a gene that encodes type IV collagen α-chain 1. This is 1 of 6 α-chains that contribute to type IV collagen, the principal component of basement membranes. Along with COL4A2, COL4A1 forms a heterotrimer, [(α1(IV)]2[(α2(IV)], that is ubiquitously expressed during early mammalian development.4,5 Truncating mutations in mouse Col4a1 result in cerebral hemorrhage in both neonatal and adult mice. Missense mutations have been reported in human infants with congenital porencephaly and adults with cerebral small vessel disease,6–8 and recently a heterozygous missense mutation was found in 2 siblings with fetal hemorrhagic stroke.9
We searched for COL4A1 variants in 41 preterm infants presenting with IVH and identified a rare heterozygous duplication (a genetic mutation resulting in the insertion of a string of nucleic acids on 1 of 2 alleles) within a highly conserved residue in COL4A1 in dizygotic twins affected with IVH.
Case Reports
Patients IVH-018 and IVH-019 were dizygotic twins, born at 24 weeks’ gestation. Delivery was complicated by chorioamnionitis and preterm labor to a 29-year-old G2 P0 AB1 female. The fetuses received antenatal steroids, and delivery was by spontaneous vaginal delivery. The parents had no related significant medical history.
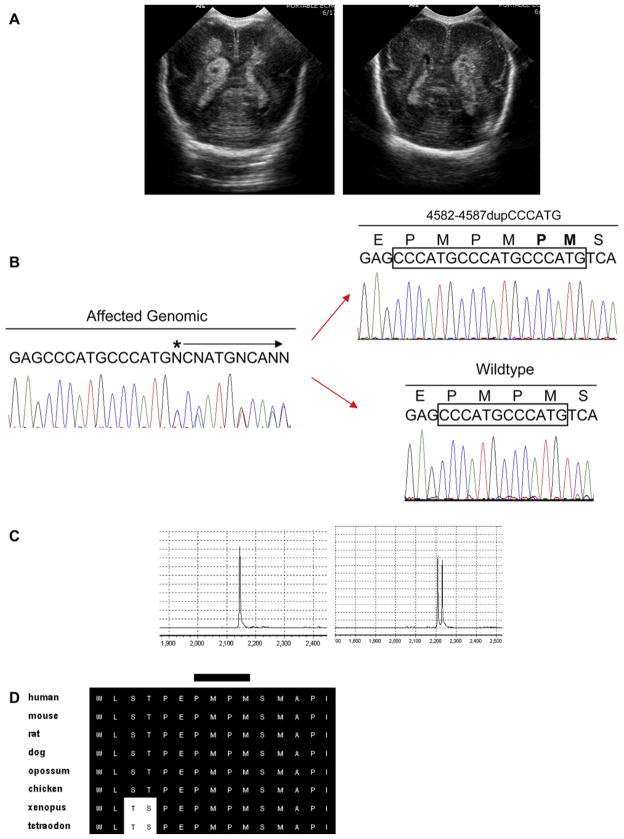
Twin A, a girl, had a birth weight of 720 g; Apgar scores were 1 at 1 minute and 1 at 5 minutes. She was intubated in the delivery room and received surfactant and low-dose indomethacin. She was found to have a Grade 3 IVH on the second postnatal day (Figure, A). Her course was complicated by respiratory distress syndrome, hyperbilirubinemia, bronchopulmonary dysplasia, and retinopathy of prematurity.
Figure.
COL4A1 mutation. A, Representative transcranial ultrasound images through the lateral ventricles of patient IVH-018 (left) and IVH-019 (right) are shown. Hyperechogeneity within the lateral ventricles and germinal matrix (arrows) can be seen representing grade III and IV hemorrhage, respectively. B, Direct sequencing of genomic DNA (left panel) reveals a suspected insertion mutation. Subsequent T/A cloning of the PCR amplicon demonstrates the c.4582–4587dupCCCATG mutation. C, Examples of TGCE chromatograms representing a double-peak (right), suggesting a heteroduplex, in both patients, as compared with the normal, homoduplex found in a representative control (left). D, Sequence alignment of the NC1 domain of COL4A1 shows high conservation across species. The site of the duplication is marked with a bar.
Twin B, a boy, also weighed 720 g; his Apgar scores were 2 at 1 minute and 5 at 5 minutes. He was also intubated in the delivery room and was treated with surfactant and early low-dose indomethacin. Twin B was diagnosed with Grade 4 IVH on the second postnatal day (Figure, A), and his course was complicated by respiratory distress syndrome, late-onset coagulase-negative staphylococcal sepsis, patent ductus arteriosus, hyperbilirubinemia, and retinopathy of prematurity.
Genetic Analysis
This study was performed at Yale University School of Medicine and was approved by the institutional review board and Human Investigations Committee. Written parental permission was obtained for the study protocol.
Mutation screening of the COL4A1 gene in the twins was part of a project to resequence this gene in 41 pre-term infants presenting with IVH. Primers for polymerase chain reaction (PCR) flanking each of the exons for COL4A1 (OMIM 120130; NM_001845) were designed using the program PRIMER3 (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi). PCR amplicons were generated from patient-derived genomic DNA using standard procedures. Sequence data were analyzed using Sequencer software (Genecodes, Ann Arbor, Michigan).
A c.4582–4586dupCCCATG insertion in exon 4 was identified in both twins (Figure, B). This mutation results in a third proline-methionine repeat inserted into the highly conserved noncollagenous (NC1) domain. None of the other 39 infants with IVH had this mutation. The zygosity of twins IVH-018 and IVH-019 was confirmed using a Gen-eChip mapping 10 K array (Affymetrix Inc, Santa Clara, California) containing 11,555 single nucleotide polymorphism (SNP) markers for genotype analysis, according to the company’s protocols. Affymetrix Micro-Array Suite 5.0 software was used to obtain raw microarray feature intensities, the results of which were processed to derive SNP genotypes using the Affymetrix Genotyping Tools software package (GTYPE; Affymetrix, Santa Clara, California).
The mother and maternal grandmother of the twins were identified as heterozygous carriers of this mutation through resequencing of their COL4A1 loci. Neither the mother nor the maternal grandmother had a history of neonatal IVH, prematurity, intracranial hemorrhage, or stroke. Brain MRI imaging on both was normal (data not shown).
A control group of 450 unaffected individuals (900 chromosomes) was evaluated for the c.4582–4586dupCCCATG mutation using temperature gradient capillary electrophoresis (TGCE) as previously described10 followed by sequencing of putative heterozygotes. As shown by a representative control TGCE result (Figure, C), none of the control groups’ 900 chromosomes carried this variant, demonstrating that it is rare in the general population.
Discussion
We report a putative functional mutation in preterm infants with neonatal IVH. Previous findings demonstrated heterozygous mutations in COL4A1 associated with fetal hemorrhagic stroke. A murine model recapitulated perinatal IVH, and there was an absence of the identified mutation in 900 control chromosomes. These findings and the highly conserved nature of the amino acids at the point of substitution all strongly point to a role for rare COL4A1 mutations in IVH. The finding of this variant in 1 set of twins of 41 cases of IVH suggest that the condition is likely to be genetically heterogeneous.
The COL4A1 mutation that we report results in the insertion of 2 amino acids into the highly conserved NC1 domain (Figure, D). Mutations in the NC1 domain in type IV and other collagens were shown to be detrimental to trimer and hexamer formation and consequently to basement membrane stability.11,12 Furthermore, there are no known insertions, deletions, or copy number variations (CNVs) within the NC1 domain of type IV collagen.
Mice and adult humans with mutations in COL4A1 have cerebral hemorrhages within a wide spectrum of phenotypic severity and variability.6,7,13 The Col4a1 mutations leading to hemorrhage in newborn mice were thought to be in the context of trauma (birth), although adult mice also had cerebral hemorrhages.6 Adult humans with COL4A1 mutations have hemorrhagic strokes and white matter abnormalities7 as well as intracranial aneurysms.8 The context and severity of the phenotype probably depends on other genetic interactions, environmental factors, and constitutional stressors like prematurity. Of note, the presence of basal ganglia hemorrhages in mice and human subjects suggests that small perforator branches of the cerebral vessels are probably weakened by the mutated collagen.7 This vascular dynamic is similar to the germinal matrix of the premature infant.
Although the functional consequences of the c.4582–4586dupCCCATG mutation remain to be elucidated, these findings, along with the previous demonstration of heterozygous mutations leading to a range of vascular phenotypes, suggest that it is not likely to be an incidental finding. Both functional studies and resequencing of larger groups of patients are necessary to confirm these results. Nonetheless, as survival among vulnerable VLBW infants continues to improve, identifying contributory genetic factors and their connected pathophysiologic mechanisms that increase risk for IVH will lead researchers to better strategies for prevention and treatment of an important morbidity.
Acknowledgments
The authors thank Drs Deborah Hirtz, Walter Allan, and Betty Vohr for scientific advice and Drs Fatih Bayrakli, Mohamad Bydon, Charles C. Duncan, and Matthew W. State for assistance in preparing the manuscript.
Supported by grants NS053l865, NS 27116, and U24 NS051869 from the National Institutes of Health and the Yale Program on Neurogenetics. R.P.L. is an investigator of the Howard Hughes Medical Institute. The authors declare no conflicts of interest.
- IVH
Intraventricular hemorrhage
- VLBW
Very low birth weight
References
- 1.Neubauer AP, Voss W, Kattner E. Outcome of extremely low birth weight survivors at school age: the influence of perinatal parameters on neurodevelopment. Eur J Pediatr. 2008;167:87–95. doi: 10.1007/s00431-007-0435-x. [DOI] [PubMed] [Google Scholar]
- 2.Sherlock RL, Synnes AR, Grunau RE, Holsti L, Hubber-Richard P, Johannesen D, et al. Long term outcome after neonatal intraparenchymal echodensities with porencephaly. Arch Dis Child Fetal Neonatal Ed. 2008;93:F127–31. doi: 10.1136/adc.2006.110726. [DOI] [PubMed] [Google Scholar]
- 3.Bhandari V, Bizzarro MJ, Shetta A, Zhong X, Page GP, Zhang H, et al. Familial and genetic susceptibility to major neonatal morbidities in pre-term twins. Pediatrics. 2006;117:1901–6. doi: 10.1542/peds.2005-1414. [DOI] [PubMed] [Google Scholar]
- 4.Favor J, Gloeckner CJ, Janik D, Klempt M, Neuhauser-Klaus A, Pretsch W, et al. Type IV procollagen missense mutations associated with defects of the eye, vascular stability, brain, kidney function and embryonic or postnatal viability in the mouse, Mus musculus: an extension of the Col4a1 allelic series and the identification of the first 2 Col4a2 mutant alleles. Genetics. 2007;175:725–36. doi: 10.1534/genetics.106.064733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sado Y, Kagawa M, Naito I, Ueki Y, Seki T, Momota R, et al. Organization and expression of basement membrane collagen IV genes and their roles in human disorders. J Biochem (Tokyo) 1998;123:767–76. doi: 10.1093/oxfordjournals.jbchem.a022003. [DOI] [PubMed] [Google Scholar]
- 6.Gould DB, Phalan C, Breedveld GJ, van Mil SE, Smith RS, Schimenti JC, et al. Mutations in Col4a1 cause perinatal cerebral hemorrhage and porencephaly. Science. 2005;308:1167–71. doi: 10.1126/science.1109418. [DOI] [PubMed] [Google Scholar]
- 7.Breedveld G, de Coo IF, Lequin MH, Arts WF, Heutink P, Gould DB, et al. Novel mutations in three families confirm a major role of COL4A1 in hereditary porencephaly. J Med Genet. 2006;43:490–5. doi: 10.1136/jmg.2005.035584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plaisier E, Gribouval O, Alamowitch S, Mougenot B, Prost C, Verpont MC, et al. COL4A1 mutations and hereditary angiopathy, nephropathy, aneurysms, and muscle cramps. N Engl J Med. 2007;357:2687–95. doi: 10.1056/NEJMoa071906. [DOI] [PubMed] [Google Scholar]
- 9.De Vries LS, Koopman C, Groenendaal F, Schooneveld MV, Verheijen FW, Verbeek E, et al. COL4A1 mutation in two preterm siblings with antenatal onset of parenchymal hemorrhage. Ann Neurol. 2008 doi: 10.1002/ana.21525. In press. [DOI] [PubMed] [Google Scholar]
- 10.Abelson JF, Kwan KY, O’Roak BJ, Baek DY, Stillman AA, Morgan TM, et al. Sequence variants in SLITRK1 are associated with Tourette’s syndvrome. Science. 2005;310:317–20. doi: 10.1126/science.1116502. [DOI] [PubMed] [Google Scholar]
- 11.Bateman JF, Wilson R, Freddi S, Lamande SR, Savarirayan R. Mutations of COL10A1 in Schmid metaphyseal chondrodysplasia. Hum Mutat. 2005;25:525–34. doi: 10.1002/humu.20183. [DOI] [PubMed] [Google Scholar]
- 12.Borza DB, Bondar O, Ninomiya Y, Sado Y, Naito I, Todd P, et al. The NC1 domain of collagen IV encodes a novel network composed of the alpha 1, alpha 2, alpha 5, and alpha 6 chains in smooth muscle basement membranes. J Biol Chem. 2001;276:28532–40. doi: 10.1074/jbc.M103690200. [DOI] [PubMed] [Google Scholar]
- 13.Poschl E, Schlotzer-Schrehardt U, Brachvogel B, Saito K, Ninomiya Y, Mayer U. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development. 2004;131:1619–28. doi: 10.1242/dev.01037. [DOI] [PubMed] [Google Scholar]